Abstract
Background
Temporal trends of the impact of social determinants on cardiovascular outcomes of cancer patients has not been previously studied.
Objectives
This study examined social disparities in cardiovascular mortality of people with and without cancer in the US population between 1999 and 2019.
Methods
Primary cardiovascular deaths were identified from the Multiple Cause of Death database and grouped by cancer status. The cancer cohort was subcategorized into breast, lung, prostate, colorectal, and haematological. The number of cardiovascular deaths, crude cardiovascular mortality rate, cardiovascular age-adjusted mortality rate (AAMR), and percentage change in cardiovascular AAMR were calculated by cancer status and cancer type, and stratified by sex, race, ethnicity, and urban-rural setting.
Results
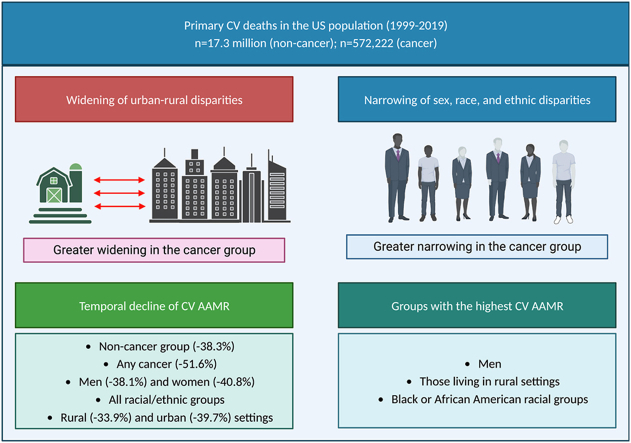
17.9 million cardiovascular deaths were analysed. Of these, 572,222 occurred in patients with a record of cancer. The cancer cohort were older and included more men and White racial groups. Regardless of cancer status, cardiovascular AAMR was higher in men, rural settings, and Black or African American races. Cardiovascular AAMR declined over time, with greater reduction in those with cancer (−51.6% vs −38.3%); the greatest reductions were in colorectal (−68.4%), prostate (−60.0%), and breast (−58.8%) cancers. Sex, race, and ethnic disparities reduced over time, with greater narrowing in the cancer cohort. There was increase in urban-rural disparities, which appeared greater in those with cancer.
Conclusions
While most social disparities narrowed over time, urban-rural disparities widened, with greater increase in those with cancer. Healthcare plans should incorporate strategies for reduction of health inequality equitable access to cardio-oncology services.
Keywords: Cardio-oncology, Social determinants of health, Race, Urbanisation, Women's health
Graphical abstract
Illustrative overview of study findings, Central illustration footnote. AAMR: age adjusted mortality rate; CV: cardiovascular.
1. Introduction
A large body of evidence highlights the role of social determinants in driving health inequalities [1]. The structure of societies leads to striking disparities in circumstances of daily life and inequalities in power, money, and resources within and across populations [2,3]. The social gradient in health is a major problem across all societies [1].
Cardiovascular disease (CVD) and cancer are the two most important non-communicable causes of disability and ill health worldwide [4]. Social gradients determine prevention, diagnosis, and treatment of both conditions. The role of social determinants in driving poorer cardiovascular health is widely recognised in both historic [5] and contemporary literature [6,7]. In the context of cancer, social disadvantage and racial disparities have been previously recognised and related to differential access to screening programmes, timely diagnosis, treatments, and specialist care [8]. In the United States of America (USA), Black racial groups have the highest death rate and shortest life expectancy of any racial group for most cancers [9]. Indeed, structural racism remains a fundamental cause of health disparities in the USA [10]. Furthermore, social gradients shape the distribution of risk factors such as obesity, diabetes, and hypertension [11], which have been shown to drive both CVD and cancer [12].
Recent reports highlight health disparities in the intersection of cancer and CVD. Existing work indicates greater baseline cardiovascular risk in patients with cancer from disadvantaged backgrounds [13], which contributes importantly to disparities in occurrence of cancer treatment-related cardiotoxicities [14,15]. Factors such as differential access to preventive therapies, cardiovascular screening, and cardio-oncology services are further drivers of these health inequalities. Whilst several reports have called for action on tackling inequalities in cardio-oncology [13,16] [[13], [16], [17], [18]] [[13], [16], [17], [18]], such disparities have not been characterised at a population level. There are little data on the temporal trends of the social determinants of health on cardiovascular outcomes of patients with cancer, and it is not known whether such disparities are magnified in this cohort compared to patients without cancer. This information is essential to guide future healthcare provisions towards reducing health inequalities.
We compared the distribution and burden of cardiovascular mortality in patients with and without cancer in the US population between 1999 and 2019, considering temporal trends and differential patterns by sex, race, ethnicity, and place of residence (urban vs rural).
2. Methods
The data underlying this article are available through the Centres for Disease Control and Prevention Wide-Ranging, Online Data for Epidemiologic Research (CDC WONDER) resource [19], which may be accessed at: https://wonder.cdc.gov/mcd-icd10.html. The analysis uses anonymized data. Ethical approval was not required.
2.1. Setting and study population
The Multiple Cause of Death database comprises mortality causes and population counts for all US counties from 1999 to 2019. The data are based on death certificates for US residents. The cause of death information is extracted from the death certificate. Each death certificate contains a single underlying cause of death and up to 20 contributing causes, completed by physicians. The causes of death are classified in accordance with the International Classification of Disease, Tenth Revision (ICD-10) for deaths occurring in 1999 and beyond. This information is linked to basic demographic data. The number of deaths, crude mortality rates, age-adjusted mortality rates (AAMR), and 95% confidence intervals for mortality rates can be obtained by cause of death, place of residence, age, race, Hispanic ethnic origin, sex, and year. Age-adjusted mortality rates are weighted averages of the age-specific mortality rates, where the weights represent a fixed population by age. They are used to compare relative mortality risk within groups and over time. An AAMR represents the mortality rate that would have existed had the age-specific rates of the particular year prevailed in a population whose age distribution was the same as that of the fixed population. The year "2000 U S. standard" is the default population selection for the calculation of AAMRs in CDC WONDER.
2.2. Analysis sample
All primary cardiovascular deaths, defined as decedents with any cardiovascular disease entered as the underlying cause of death (ICD-10 codes: I00–I99) were selected and grouped into those with and without cancer, based on record of any cancer (ICD-10 codes: C00–C97) as a contributory cause of death. Those with cancer were further subcategorized into the following groups: breast (ICD-10 codes: C50), lung (ICD-10 codes: C34), prostate (ICD-10 codes: C61), colorectal (ICD-10 codes: C18–C20), and haematological (ICD-10 codes: C81–C96). These cancer categories were selected to capture the most prevalent cancers in the US population, as per latest national statistics [20].
2.3. Social determinants of health
The following social determinants of health were considered: sex, race, ethnicity, and place of residence (a proxy for socio-economic status). Sex was defined based on the label attached to the death certificate (two available categories: men, women). The CDC WONDER resource provides bridged-race population data within four racial categories: Asian or Pacific Islander, Black or African American, American Indian or Alaska Native, White. The estimates result from bridging the 31 race categories used in Census 2000, as indicated in the Office of Management and Budget standards for the collection of data on race and ethnicity, to the four specific racial categories specified under the 1997 Office of Management and Budget standards for the collection of data on race and ethnicity. This information is updated annually from the National Center for Health Statistics bridged-race population estimates [21]. Data is also available on Hispanic ethnic origin (Hispanic or Latino, not Hispanic or Latino). In the CDC wonder database each death is associated with an urbanisation category based on the county of the person's legal residence. Each county is classified as one of six categories (based on the 2013 National Center for Health Statistics Urban-Rural Scheme for Counties): Large Central Metro, Large Fringe Metro, Medium Metro, Small Metro (Urban counties), Micropolitan, or Non-Core (Rural counties).
2.4. Statistical analysis
The total number of primary cardiovascular deaths, crude cardiovascular mortality rate, and cardiovascular AAMR was calculated for people without cancer, those with any cancer, and by cancer type. Cardiovascular mortality rates were stratified by cancer type and then by each of the social determinants of health: sex (men, women), race (Asian or Pacific Islander, Black or African American, American Indian or Alaska Native, White), Hispanic ethnic origin (Hispanic or Latino, not Hispanic or Latino), and place of residence (urban, rural). To examine temporal trends, percentage change in cardiovascular AAMR between 1999 and 2019 was calculated. Percentage change is calculated as the difference between cardiovascular AAMR in 1999 and 2019 expressed as a percentage. Data was analysed using Microsoft Excel.
3. Results
3.1. Overall population characteristics
The analysis sample included 17.9 million primary cardiovascular deaths, of these 3.2% (n = 572,222) occurred in patients with cancer recorded as a contributing cause of death (Table 1). The age distribution was broadly similar in those with and without a record of cancer, with most deaths occurring in the oldest age group; however, there was a greater skew towards older ages in those with record of cancer. The male to female split was near equal amongst those without cancer, with slight preponderance of women (51.5%). The reverse was observed in the cancer group with men comprising 58.0% of deaths. There was a slightly greater proportion of White racial groups and individuals who were “not Hispanic or Latino” ethnicity in those with (vs without) cancer. Across both the cancer and non-cancer groups, a greater proportion of deaths was recorded in urban compared to rural settings.
Table 1.
Baseline characteristics and crude cardiovascular mortality in patients with and without record of cancer between 1999 and 2019.
| Number of deaths |
Crude mortality ratea |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Whole sample | No cancer | Any cancer | Whole sample | No cancer | Any cancer | ||||
| Overall | 17,855,050 | % | 17,282,828 | % | 572,222 | % | 278.4 | 269.5 | 8.9 |
| Age | |||||||||
| Under 55 | 1,388,193 | 7.8 | 1,371,569 | 7.9 | 16,624 | 2.9 | 28.8 | 28.5 | 0.3 |
| 55–74 | 4,655,596 | 26.1 | 4,501,100 | 26.0 | 154,496 | 27.0 | 387.3 | 374.4 | 12.9 |
| 75+ | 11,810,351 | 66.1 | 11,409,258 | 66.0 | 401,093 | 70.1 | 2990.7 | 2846.0 | 144.7 |
| Sex | |||||||||
| Men | 8,716,264 | 48.8 | 8,384,266 | 48.5 | 331,998 | 58.0 | 276.3 | 265.8 | 10.5 |
| Women | 9,138,786 | 51.2 | 8,898,562 | 51.5 | 240,224 | 42.0 | 280.2 | 272.8 | 7.4 |
| Place of residence | |||||||||
| Urban | 14,355,191 | 80.4 | 13,893,650 | 80.4 | 461,541 | 80.7 | 263.1 | 254.6 | 8.5 |
| Rural | 3,499,859 | 19.6 | 3,389,178 | 19.6 | 110,681 | 19.3 | 364.2 | 352.7 | 11.5 |
| Race | |||||||||
| White | 15,271,788 | 85.5 | 14,772,766 | 85.5 | 499,022 | 87.2 | 298.7 | 288.9 | 9.8 |
| Black | 2,133,918 | 12.0 | 2,073,293 | 12.0 | 60,625 | 10.6 | 244.8 | 237.8 | 7.0 |
| Native | 80,632 | 0.5 | 78,565 | 0.5 | 2067 | 0.4 | 96.9 | 94.4 | 2.5 |
| Asian | 368,712 | 2.1 | 358,204 | 2.1 | 10,508 | 1.8 | 105.5 | 102.5 | 3.0 |
| Hispanic origin | 0.0 | 0.0 | |||||||
| Hispanic or Latino | 898,419 | 5.0 | 876,290 | 5.1 | 22,129 | 3.9 | 88.4 | 86.2 | 2.2 |
| Not Hispanic or Latino | 16,906,440 | 94.7 | 16,357,790 | 94.6 | 548,650 | 95.9 | 313.0 | 302.8 | 10.2 |
Per 100,000 population.
The crude cardiovascular mortality rate attributed to patients with cancer was 8.9 per 100,000 population and 269.5 per 100,000 in those without cancer (Table 1). Among the cancer categories, the highest crude cardiovascular mortality rate was related to those with prostate (1.6 per 100,000 population) and haematological (1.3 per 100,000 population) cancers (Supplementary Table 1).
3.2. Cardiovascular AAMR by cancer status
Between 1999 and 2019, there was a declining trend of cardiovascular AAMR across the whole sample, with a notably greater decline in those with a record of any cancer (−51.6%) compared to those without cancer (−38.8%). Across cancer types, the greatest percentage declines in cardiovascular AAMR were attributed to colorectal (−68.4%), prostate (−60.0%), and breast (−58.8%) cancer (Table 2). The greater temporal reduction in cardiovascular AAMR related to cancer was consistent across all social determinants of health considered. That is, across all strata (sex, race, ethnicity, place of residence) those with cancer had greater percentage decline in cardiovascular AAMR than those without cancer.
Table 2.
Age-adjusted cardiovascular disease mortality stratified by cancer site and race.
| Whole sample |
White |
Black |
Native |
Asian |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1999 | 2019 | % change | 1999 | 2019 | % change | 1999 | 2019 | % change | 1999 | 2019 | % change | 1999 | 2019 | % change | |
| Overall | 350.8 | 214.6 | −38.8% | 343.3 | 211.7 | −38.3% | 450.0 | 277.3 | −38.4% | 263.7 | 142.0 | −46.2% | 225.0 | 123.7 | −45.0% |
| No Cancer | 338.0 | 208.4 | −38.3% | 330.6 | 205.4 | −37.9% | 434.3 | 270.1 | −37.8% | 256.2 | 138.1 | −46.1% | 217.0 | 120.6 | −44.4% |
| Any cancer | 12.8 | 6.2 | −51.6% | 12.7 | 6.3 | −50.4% | 15.7 | 7.2 | −54.1% | 7.5 | 3.9 | −48.0% | 8.0 | 3.1 | −61.3% |
| Breast | 1.7 | 0.7 | −58.8% | 1.7 | 0.7 | −58.8% | 1.9 | 1.0 | −47.4% | N/A | N/A | N/A | 0.8 | 0.3 | −62.5% |
| Lung | 1.7 | 0.9 | −47.1% | 1.8 | 0.9 | −50% | 1.9 | 1.0 | −47.4% | N/A | 0.6 | N/A | 1.0 | 0.5 | −50.0% |
| Colorectal | 1.9 | 0.6 | −68.4% | 1.9 | 0.6 | −68.4% | 2.2 | 0.7 | −68.2% | N/A | 0.7 | N/A | 1.0 | 0.3 | −70.0% |
| Haematological | 1.7 | 1.0 | −41.2% | 1.7 | 1.0 | −41.2% | 1.6 | 1.0 | −37.5% | N/A | 0.6 | N/A | 0.9 | 0.6 | −33.3% |
| Prostate | 2.5 | 1.0 | −60.0% | 2.4 | 1.0 | −58.3% | 4.1 | 1.7 | −58.5% | N/A | N/A | N/A | 1.5 | 0.4 | −73.3% |
Table 2 footnote*per 100,000 population.
3.3. Cardiovascular AAMR by cancer status and race
Black or African American racial groups had the highest cardiovascular AAMR regardless of cancer status throughout the entire study period (Table 2). The only exception was in cases with haematological cancer, where cardiovascular AAMR was similar between White and Black or African American races. Asian and Pacific Islander race had the lowest cardiovascular AAMR of all racial groups regardless of cancer status or type.
There was a declining trend of cardiovascular AAMR consistent across those with and without cancer, all cancer types considered, and among all racial groups (Table 2, Fig. 1, Fig. 2).
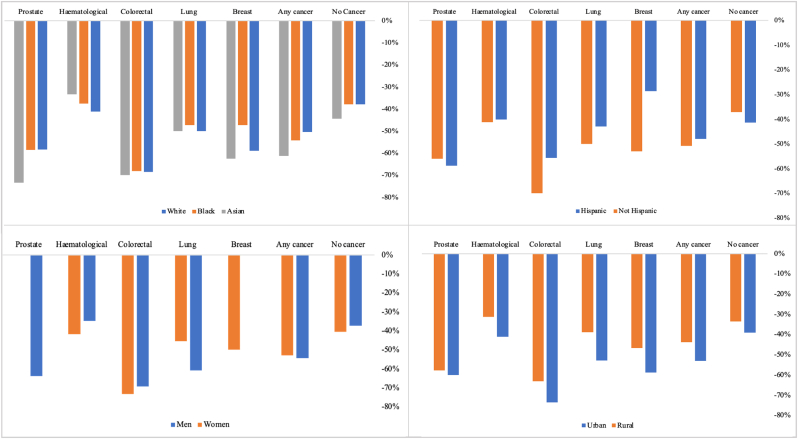
Fig. 1.
Percentage change in cardiovascular AAMR by cancer status and social determinants of health, between 1999 and 2019
Fig. 1footnote. AAMR: age adjusted mortality rate. *Inadequate data for American Indian or Alaska Native racial groups.
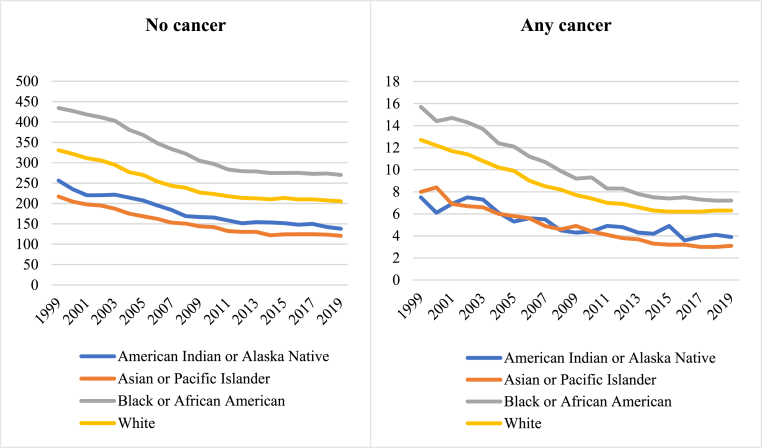
Fig. 2.
Temporal trends of cardiovascular AAMR in the cancer and non-cancer groups stratified by racial group between 1999 and 2019
Fig. 2footnote. AAMR: age adjusted mortality rate. *Inadequate data for American Indian or Alaska Native racial groups.
Over the study period, racial disparities in cardiovascular mortality narrowed in both cancer and non-cancer groups. Racial disparities reduced to a greater extent in those with cancer, largely driven by greater reduction in cardiovascular AAMR of Black or African American races with cancer (−54.1%) vs those without cancer (−37.8%). The narrowing of racial disparities was most marked in colorectal cancer (Supplementary Figure 1); within this subset, cardiovascular AAMR of Black or African American races reduced from 2.2 to 0.7 per 100,000 population (Table 2). Similarly, there was notable narrowing of racial disparities in those with prostate cancer with marked reduction of cardiovascular AAMR in Black or African American races in this group (4.1–1.7 per 100,000 population).
In terms of percentage change in cardiovascular AAMR, Black or African American races without cancer had the lowest percentage reduction (−37.8%) of all the racial groups considered (Table 2). Among the cancer group, Native American and Alaska Natives had the lowest percentage reduction in cardiovascular AAMR (−48.0%) while Asian and Pacific Islander races had the greatest percentage decline (−61.3%).
3.4. Cardiovascular AAMR by cancer status and Hispanic ethnicity
Throughout the study period, the “not Hispanic or Latino” ethnic group had higher cardiovascular AAMR than the “Hispanic or Latino” ethnic group, across both cancer and non-cancer groups (Table 3). There was temporal decline in cardiovascular AAMR in both ethnic groups regardless of cancer status (Table 3, Figs. 1, Figure 3). There was narrowing of ethnic disparities in cardiovascular AAMR in the cancer and non-cancer groups, with greater narrowing in those with cancer.
Table 3.
Age-adjusted cardiovascular disease mortality stratified by cancer site and Hispanic ethnic origin.
| Whole sample |
Hispanic or Latino |
Not Hispanic or Latino |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 1999 | 2019 | % change | 1999 | 2019 | % change | 1999 | 2019 | % change | |
| Overall | 350.8 | 214.6 | −38.8% | 269.0 | 157.2 | −41.6% | 353.7 | 220.5 | −37.7% |
| No cancer | 338.0 | 208.4 | −38.3% | 261.5 | 153.3 | −41.4% | 340.7 | 214.1 | −37.2% |
| Any cancer | 12.8 | 6.2 | −51.6% | 7.5 | 3.9 | −48.0% | 13.0 | 6.4 | −50.0% |
| Breast | 1.7 | 0.7 | −58.8% | 0.7 | 0.5 | −28.6% | 1.7 | 0.8 | −52.9% |
| Lung | 1.7 | 0.9 | −47.1% | 0.7 | 0.4 | −42.9% | 1.8 | 0.9 | −50.0% |
| Colorectal | 1.9 | 0.6 | −68.4% | 0.9 | 0.4 | −55.6% | 2.0 | 0.6 | −70.0% |
| Haematological | 1.7 | 1.0 | −41.2% | 1.0 | 0.6 | −40.0% | 1.7 | 1.0 | −41.2% |
| Prostate | 2.5 | 1.0 | −60.0% | 1.7 | 0.7 | −58.8% | 2.5 | 1.1 | −56.0% |
Table 3 footnote*per 100,000 population.
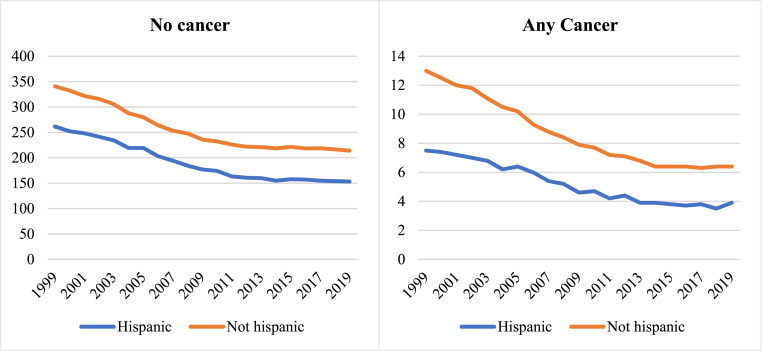
Fig. 3.
Temporal trends of cardiovascular AAMR in the cancer and non-cancer groups stratified by Hispanic or Latino ethnic group between 1999 to 2019
Fig. 3footnote. AAMR: age adjusted mortality rate.
The narrowing of ethnic disparities in the cancer groups was mostly due to decline in cardiovascular AAMR in the “not Hispanic or Latino” group. Across all cancer types, there was greater rate of temporal decline in cardiovascular AAMR in the “not Hispanic or Latino” group compared to “Hispanic or Latino” ethnicities (−50.0% vs −48.0%). The greatest disparity in degree of cardiovascular AAMR decline was in those with breast cancer, with 52.9% reduction in the “not Hispanic or Latino” group compared to 28.6% in those of “Hispanic or Latino” ethnicities (Table 3, Supplementary Figure 2). There were similarly greater reductions in cardiovascular AAMR in the “not Hispanic or Latino” ethnicities in those with a record of colorectal (−70.0% vs −55.6%) and lung (−50.0% vs −42.9%) cancer.
3.5. Cardiovascular AAMR by cancer status and sex
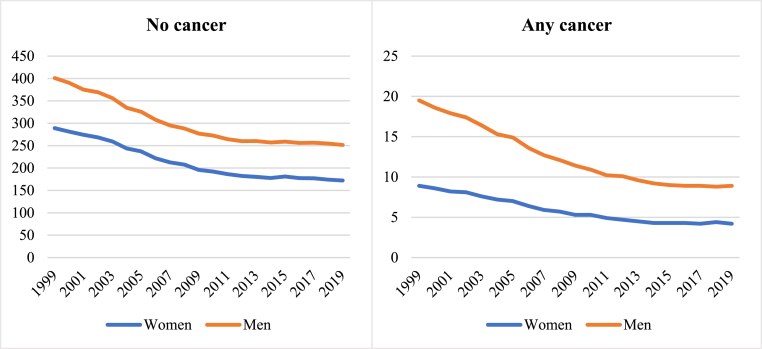
Throughout the study period, regardless of cancer status or type, men had higher cardiovascular AAMR than women (Table 4). There was a declining temporal trend of cardiovascular AAMR for both men and women, consistent in those with and without cancer and across all cancer types considered (Table 4, Figs. 1, Figure 4, Supplementary Figure 3).
Table 4.
Age-adjusted cardiovascular disease mortality stratified by cancer site and sex.
| Whole sample |
Men |
Women |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 1999 | 2019 | % change | 1999 | 2019 | % change | 1999 | 2019 | % change | |
| Overall | 350.8 | 214.6 | −38.8% | 420.5 | 260.4 | −38.1% | 297.9 | 176.4 | −40.8% |
| No cancer | 338.0 | 208.4 | −38.3% | 401.0 | 251.5 | −37.3% | 289.0 | 172.2 | −40.4% |
| Any cancer | 12.8 | 6.2 | −51.6% | 19.5 | 8.9 | −54.4% | 8.9 | 4.2 | −52.8% |
| Breast | 1.7 | 0.7 | −58.8% | <0.1 | <0.1 | N/A | 2.6 | 1.3 | −50.0% |
| Lung | 1.7 | 0.9 | −47.1% | 2.8 | 1.1 | −60.7% | 1.1 | 0.6 | −45.5% |
| Colorectal | 1.9 | 0.6 | −68.4% | 2.6 | 0.8 | −69.2% | 1.5 | 0.4 | −73.3% |
| Haematological | 1.7 | 1.0 | −41.2% | 2.3 | 1.5 | −34.8% | 1.2 | 0.7 | −41.7% |
| Prostate | 2.5 | 1.0 | −60.0% | 7.2 | 2.6 | −63.9% | N/A | N/A | N/A |
Table 4 footnote*per 100,000 population.
Fig. 4.
Temporal trends of cardiovascular AAMR in the cancer and non-cancer groups stratified by sex between 1999 and 2019
Fig. 4footnote. AAMR: age adjusted mortality rate.
Over the study period, sex disparities in cardiovascular AAMR reduced in both the cancer and non-cancer groups (Table 4). The narrowing of sex disparities in the cancer group was almost entirely due to greater rate of cardiovascular AAMR reduction in men compared to women, which was most marked in the case of lung (−60.7% vs −45.5%) cancer.
3.6. Cardiovascular AAMR by cancer status and place of residence
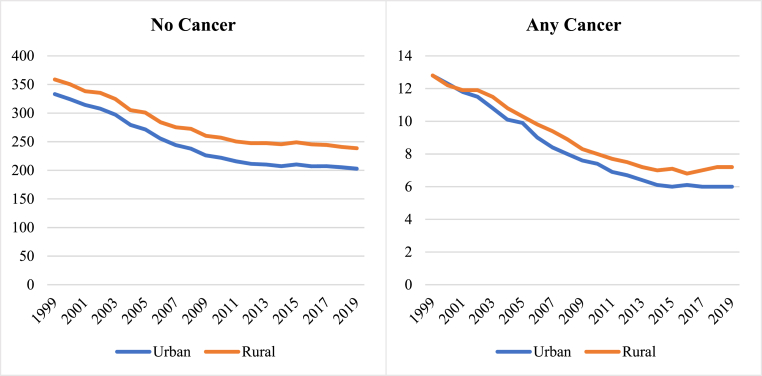
In 1999, cardiovascular AAMR was higher in rural compared to urban (358.8 vs 333.2 per 100,000 population) settings in those without record of cancer as a contributing cause of death. In those with a record of cancer, there was no urban-rural difference in cardiovascular AAMR (12.8 per 100,000 population in both settings) at that time. Between 1999 and 2019, cardiovascular AAMR declined in both urban and rural settings, consistent amongst those with and without cancer (Table 5, Figs. 1 and 5). Over the study period, urban-rural disparities widened across both cancer and non-cancer groups, with greater increase in those with cancer (Table 5). Urban-rural disparities were most marked in those with lung and colorectal cancer (Table 5, Supplementary Figure 4).
Table 5.
Age-adjusted cardiovascular disease mortality stratified by cancer site and urban/rural place of residence.
| Whole sample | Urban | Rural | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1999 | 2019 | % change | 1999 | 2019 | % change | 1999 | 2019 | % change | |
| Overall | 350.8 | 214.6 | −38.8% | 346.0 | 208.8 | −39.7% | 371.6 | 245.8 | −33.9% |
| No cancer | 338.0 | 208.4 | −38.3% | 333.2 | 202.8 | −39.1% | 358.8 | 238.6 | −33.5% |
| Any cancer | 12.8 | 6.2 | −51.6% | 12.8 | 6.0 | −53.1% | 12.8 | 7.2 | −43.8% |
| Breast | 1.7 | 0.7 | −58.8% | 1.7 | 0.7 | −58.8% | 1.5 | 0.8 | −46.7% |
| Lung | 1.7 | 0.9 | −47.1% | 1.7 | 0.8 | −52.9% | 1.8 | 1.1 | −38.9% |
| Colorectal | 1.9 | 0.6 | −68.4% | 1.9 | 0.5 | −73.7% | 1.9 | 0.7 | −63.2% |
| Haematological | 1.7 | 1.0 | −41.2% | 1.7 | 1.0 | −41.2% | 1.6 | 1.1 | −31.3% |
| Prostate | 2.5 | 1.0 | −60.0% | 2.5 | 1.0 | −60.0% | 2.6 | 1.1 | −57.7% |
Table 5 footnote*per 100,000 population.
Fig. 5.
Temporal trends of cardiovascular AAMR in the cancer and non-cancer groups stratified by urban-rural setting between 1999 and 2019
Fig. 5footnote. AAMR: age adjusted mortality rate.
4. Discussion
In this large nationally representative sample of 17.9 million primary cardiovascular deaths recorded between 1999 and 2019, differential mortality distributions by cancer status and social determinants of health were identified. Compared to those without a record of cancer, the cancer group were older, included more men, White racial groups, and individuals who were not of Hispanic or Latino ethnic origin. Regardless of cancer status, higher cardiovascular AAMR was observed in Black or African American racial groups (vs all other racial groups), men (vs women), and in rural (vs urban) settings.
We observed a declining temporal trend of cardiovascular mortality over the period of study across those with and without cancer, all cancer types, and all social determinant stratifications considered. Those with record of cancer had a greater narrowing in the cardiovascular mortality gap amongst the different social determinants of health than those without cancer. Among the cancer subcategories, the greatest cardiovascular mortality declines were in those with colorectal, prostate, and breast cancer; individuals with haematological cancer had the lowest decline.
Disparities by sex, race, and ethnicity narrowed over the study period in both the non-cancer and cancer groups, but with greater narrowing in the cancer group. On the other hand, there was increase in urban-rural disparities, which appeared greater in those with cancer. These findings corroborate those of a recent analysis of the Surveillance, Epidemiology, and End Results (SEERS) database [22], and provides additional insight by including a wider range of potential social determinants of health.
We observed older ages among cardiovascular deaths in individuals with record of cancer compared to those without cancer. This is likely foremost due to lower occurrence of cancer in younger people and second, because non-cardiac causes of death are more common in younger cancer patients. Living with cancer to older ages where cardiovascular death may occur is, in some ways, a positive prognostic indicator with regards cancer survival. That is, it is during cancer survivorship where CVD may overtake cancer as the cause of death in patients with past cancer [23]. We found a slightly disproportionately higher number of White racial and ethnic individuals in the cancer group. This may reflect poorer cancer outcomes in other racial and ethnic groups [8], who do not survive long enough to experience cardiovascular disease as a primary cause of death. This may also be augmented by delayed or unrecognised cancer diagnosis in other racial and ethnic groups due to poorer access to the health system. In our analysis, Black and African American individuals had the highest cardiovascular AAMR of all racial group considered across both cancer and non-cancer groups; this observation is consistent with existing reports of racial disparities in overall cardiovascular mortality in the US population [24]. Men had greater cardiovascular AAMR than women, regardless of cancer status and consistent across all cancer categories (except breast cancer). Adding to the well-established reports on higher cardiovascular mortality rate in men within the general population [25], our results indicate that these sex-differential pattens extend to individuals with cancer.
We observed declining trend of cardiovascular mortality across cancer and non-cancer groups. Similar trends have been reported in recent analyses of overall cardiovascular mortality in US [24] and European [25] populations. Our findings add to existing work by demonstrating declining cardiovascular mortality in those with cancer as a contributory cause, which was substantially greater than the decline in the non-cancer group. The greater reduction in cardiovascular mortality in those with cancer may reflect greater awareness of the cardiovascular healthcare needs of this group and the emergence of dedicated services to monitor and treat these patients. Furthermore, patients with cancer are more likely to have regular contact with healthcare professionals with greater opportunity for optimising preventive strategies and early disease detection. Whilst there have been advances in general cardiovascular care, the introduction of cardio-oncology services represents a step change in the quality of care and attention dedicated to patients with cancer [26].
Over the study period, sex, race, and ethnic disparities in cardiovascular mortality narrowed in both those with and without cancer, with greater reduction of disparities in the cancer group. This may reflect success of cardiovascular care pathways in those who have regular medical contact as part of a cancer diagnosis. Greater attention to risk factor control, early diagnosis, and treatment of cardiovascular disease as part of such programmes is likely to importantly alter cardiovascular mortality risk of patients with cancer, which, at least to some extent, counteracts healthcare disparities that would occur otherwise.
An important observation from our study was urban-rural disparities in cardiovascular mortality, which were observed across cancer and non-cancer groups. Such disparities have been previously described in the US population with regards cardiovascular mortality [27] and disease-specific cardiovascular outcomes [28]. Our analysis confirms these previous observations in an up-to-date analysis. Importantly, we present new information in demonstrating that urban-rural disparities in cardiovascular mortality are increased in people with cancer. Furthermore, we observed widening of urban-rural disparities, which was also more marked in the cancer group. These disparities likely reflect differential healthcare access in urban and rural settings. The augmented urban-rural disparities in the cancer group highlights the importance of access to specialist services for these patients. Currently cardio-oncology services are limited to large quaternary centres, to which most patients in a rural setting would have limited access. Our results support efforts towards more equitable access to cardio-oncology.
The greatest temporal cardiovascular mortality decline was observed among colorectal cancer patients. This may indicate both greater improvement in survival from colorectal cancer and better recognition and treatment of cardiotoxicity of related therapies (e.g., 5-flurouracil). Breast cancer deaths also declined substantially (third greatest decline), which may reflect greater awareness of anthracycline induced cardiomyopathy and the introduction of screening programmes for detection of trastuzumab cardiotoxicity [26]. Notably, among those with breast cancer, Black and African American individuals had the smallest cardiovascular AAMR decline of all racial groups considered. This is in keeping with poorer cardiovascular outcomes highlighted in Black populations [24], which are augmented in Black women [29], and have also been reported in small clinical groups in the context of breast cancer [15]. Our analysis adds to these observations by demonstrating similar trends in a large national sample. Among the racial groups considered within the cancer group, the smallest temporal decline in cardiovascular AAMR was in American Indian and Native Alaska racial groups; these individuals are historically underserved and adverse cardiovascular outcomes in this group have been highlighted in recent reports [30]. Our results suggest that these disparities extend into cardio-oncology outcomes, supporting dedicated strategies to reach these communities in this context.
5. Clinical and public health implications
This analysis demonstrates health disparities at the intersection of cancer and cardiology care. Our results support existing international calls for addressing health inequalities at a population level [31,32]. Whilst the presented analysis focuses on cardiovascular mortality of cancer patients, it is clear that our results represent a fraction of the picture which has led to this endpoint. In order to make meaningful change in the state of health of populations, it is important to appreciate the lifecourse impact of social determinants of health which culminate in adverse health outcomes. As healthcare providers we should actively advocate public health policies which address the root causes, “the causes of the causes”, of ill health. In this way, we may alleviate the population burden of both cancer and cardiovascular disease. At an individual patient level, we should be mindful of the disparities highlighted in our study at all stages of cardio-oncology care and adopt strategies to communicate, engage, and treat diverse communities.
A key finding from our analysis is augmented and widening urban-rural disparities in cardiovascular mortality among individuals with cancer compared to those without cancer. This likely reflects differential access to healthcare services, in particular those with cardio-oncology expertise. Other disparities observed in our study may also be explained, at least in part, by inequal access to appropriate specialist healthcare. These observations may be viewed as a testament to the clinical value of cardio-oncology care. Going forward, promoting equitable access to cardio-oncology services should be a priority for the cardiology community. This will include development of practical and cost-effective models to establish and maintain high quality cardio-oncology programmes in different settings and with different levels of resources. The publication of cardio-oncology clinical practice guidelines goes some way in addressing standardisation of care in this setting [33]. Other factors such as introduction and/or expansion of cardio-oncology fellowship programmes to provide appropriate training and innovative approaches to care delivery (e.g., virtual clinics) should be explored as strategies to improve access to high quality care [34].
6. Conclusions
In this large nationally representative dataset, we examined cardiovascular mortality in people with and without record of cancer and described differential patterns by key social determinants of health. Integrated whole system approaches should be taken to tackle clinical and social inequalities driving differential cardiovascular outcomes of cancer patients. There is need for concerted multidisciplinary efforts to reach socially disadvantaged groups and to establish high quality cardio-oncology care across different settings.
Author Contribution Statement
ZRE, OK, and MAM conceptualised the work. ZRE and MAM designed the statistical analysis plan. OK performed the data analysis. ZRE wrote the manuscript. MAM and OK provided revisions to the manuscript. MAM provided overall supervision and is the guarantor of the work. All co-authors provided critical review of the manuscript and approved the final version.
Strengths and limitations
As we used death certification data, there was near complete capture of data from US residents over the study period. We cannot comment on non-residents (e.g. non-resident aliens and nationals living abroad); these individuals may be particularly disadvantaged in terms of life circumstances and access to healthcare who were not captured in our dataset. The standardised recording of causes of death according to ICD-10 codes permitted comparisons across groups and time. Whilst the capture of cardiovascular disease as the underlying cause of death is likely to be reliable, cancer as a contributory cause may be omitted, particularly in those with historic cancers, leading to underestimation of cardiovascular deaths related to cancer. We selected cardiovascular mortality as representing a hard cardiovascular outcome. A wider look at cardiovascular disease burden, access to cardiovascular care, and service utilisation may present a more complete picture of health inequalities and actionable areas. Furthermore, while we select key determinants of health (sex, race, ethnicity, place of residence), these are not exhaustive and many environmental, behavioural, and socio-political factors are not included in our analysis. The four racial groups in our analysis are created by bridging 31 racial groups extracted from census records. Our analysis cannot capture variations within these broad racial categories. The study does not assess several important social determinants such as income, education, occupation. Finally, we present a descriptive epidemiologic study, which serves to identify patterns, but cannot definitively explain observed phenomena.
Ethics statement
This study was conducted using anonymized routine health data. Ethical approval was not required.
Data availability
The data underlying this article are available through the CDC WONDER resource: https://wonder.cdc.gov/mcd.html.
Funding
ZR-E recognizes the National Institute for Health Research (NIHR) Integrated Academic Training programme which supports her Academic Clinical Lectureship post.
Conflict of interest
None declared
Handling editor: D Levy
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcrp.2023.200218.
Contributor Information
Teresa López-Fernández, Email: tlfernandez8@gmail.com.
Mamas A. Mamas, Email: mamasmamas1@yahoo.co.uk.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Marmot M. Social justice, epidemiology and health inequalities. Eur. J. Epidemiol. 2017;32:537–546. doi: 10.1007/S10654-017-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marmot M., Bell R. Social inequalities in health: a proper concern of epidemiology. Ann. Epidemiol. 2016;26:238–240. doi: 10.1016/j.annepidem.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organisation. Social determinants of health. https://www.who.int/health-topics/social-determinants-of-health#tab=tab_1 (accessed August 27, 2023).
- 4.Vos T., Lim S.S., Abbafati C., Abbas K.M., Abbasi M., Abbasifard M., et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rose G., Marmot M.G. Social class and coronary heart disease. Heart. 1981;45:13–19. doi: 10.1136/HRT.45.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Churchwell K., Elkind M.S.V., Benjamin R.M., Carson A.P., Chang E.K., Lawrence W., et al. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American heart association. Circulation. 2020;142:E454–E468. doi: 10.1161/CIR.0000000000000936. [DOI] [PubMed] [Google Scholar]
- 7.Powell-Wiley T.M., Baumer Y., Baah F.O., Baez A.S., Farmer N., Mahlobo C.T., et al. Social determinants of cardiovascular disease. Circ. Res. 2022;130:782–799. doi: 10.1161/CIRCRESAHA.121.319811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tucker-Seeley R.D. Social determinants of health and disparities in cancer care for Black people in the United States. JCO Oncol Pract. 2021;17:261–263. doi: 10.1200/OP.21.00229. [DOI] [PubMed] [Google Scholar]
- 9.DeSantis C.E., Miller K.D., Goding Sauer A., Jemal A., Siegel R.L. Cancer statistics for african Americans. CA Cancer J Clin 2019. 2019;69:211–233. doi: 10.3322/CAAC.21555. [DOI] [PubMed] [Google Scholar]
- 10.Churchwell K., Elkind M.S.V., Benjamin R.M., Carson A.P., Chang E.K., Lawrence W., et al. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American heart association. Circulation. 2020;142:E454–E468. doi: 10.1161/CIR.0000000000000936. [DOI] [PubMed] [Google Scholar]
- 11.Marmot M., Bell R. Social determinants and non-communicable diseases: time for integrated action. BMJ. 2019;364:l251. doi: 10.1136/bmj.l251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koene R.J., Prizment A.E., Blaes A., Konety S.H. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohman R.E., Yang E.H., Abel M.L. Inequity in cardio‐oncology: identifying disparities in cardiotoxicity and links to cardiac and cancer outcomes. J. Am. Heart Assoc. 2021;10 doi: 10.1161/JAHA.121.023852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasan S.P., Dinh K., Lombardo F., Kark J. Doxorubicin cardiotoxicity in african Americans. J. Natl. Med. Assoc. 2004;96:196–199. [PMC free article] [PubMed] [Google Scholar]
- 15.Litvak A., Batukbhai B., Russell S.D., Tsai H.L., Rosner G.L., Jeter S.C., et al. Racial disparities in the rate of cardiotoxicity of HER2-targeted therapies among women with early breast cancer. Cancer. 2018;124:1904. doi: 10.1002/CNCR.31260. –11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fazal M., Malisa J., Rhee J.W., Witteles R.M., Rodriguez F. Racial and ethnic disparities in cardio-oncology: a call to action. JACC CardioOncol. 2021;3:201–204. doi: 10.1016/J.JACCAO.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thotamgari S.R., Sheth A.R., Grewal U.S. Racial disparities in cardiovascular disease among patients with cancer in the United States: the elephant in the room. EClinicalMedicine. 2022;44 doi: 10.1016/j.eclinm.2022.101297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prasad P., Branch M., Asemota D., Elsayed R., Addison D., Brown S.-A. Cardio-oncology preventive care: racial and ethnic disparities. Curr Cardiovasc Risk Rep. 2020;14:18. doi: 10.1007/s12170-020-00650-8. [DOI] [Google Scholar]
- 19.Control C for D. Multiple Cause of Death 1999-2019. CDC WONDER Online Database 2020. https://wonder.cdc.gov/mcd-icd10.html (accessed November 10, 2020) .
- 20.National Cancer Institute. Undersranding Cancer: Cancer Statistics. https://www.cancer.gov/about-cancer/understanding/statistics (accessed August 27, 2023).
- 21.National Center for Health Statistics. National Vital Statistics System: U.S. Census Populations With Bridged Race Categories. https://www.cdc.gov/nchs/nvss/bridged_race.htm (accessed August 27, 2023).
- 22.Zhu C., Shi T., Jiang C., Liu B., Baldassarre L.A., Zarich S. Racial and ethnic disparities in all-cause and cardiovascular mortality among cancer patients in the U.S. JACC CardioOncol. 2023;5:55–66. doi: 10.1016/j.jaccao.2022.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strongman H., Gadd S., Matthews A.A., Mansfield K.E., Stanway S., Lyon A.R., et al. Does cardiovascular mortality overtake cancer mortality during cancer survivorship?: an English retrospective cohort study. JACC CardioOncol. 2022;4:113–123. doi: 10.1016/j.jaccao.2022.01.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyalwazi A.N., Loccoh E.C., Brewer L.C., Ofili E.O., Xu J., Song Y., et al. Disparities in cardiovascular mortality between Black and white adults in the United States, 1999 to 2019. Circulation. 2022;146:211–228. doi: 10.1161/CIRCULATIONAHA.122.060199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vardas P., Townsend N., Torbica A., Katus H., De Smedt D., et al. European Society of Cardiology: cardiovascular disease statistics 2021. Eur. Heart J. 2022;43:716–799. doi: 10.1093/eurheartj/ehab892. Timmis (Chair Writing Group) A. [DOI] [PubMed] [Google Scholar]
- 26.Tan L.L., Lyon A.R. Cardio-oncology for the general cardiologist. Heart. 2021;107:1254–1266. doi: 10.1136/heartjnl-2020-317871. [DOI] [PubMed] [Google Scholar]
- 27.Cross S.H., Mehra M.R., Bhatt D.L., Nasir K., O'Donnell C.J., Califf R.M., et al. Rural-urban differences in cardiovascular mortality in the US, 1999-2017. JAMA. 2020;323:1852–1854. doi: 10.1001/JAMA.2020.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loccoh E.C., Joynt Maddox K.E., Wang Y., Kazi D.S., Yeh R.W., Wadhera R.K. Rural-urban disparities in outcomes of myocardial infarction, heart failure, and stroke in the United States. J. Am. Coll. Cardiol. 2022;79:267–279. doi: 10.1016/J.JACC.2021.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chinn J.J., Martin I.K., Redmond N. Health equity among Black women in the United States. J Womens Health. 2021;30:212. doi: 10.1089/JWH.2020.8868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breathett K., Sims M., Gross M., Jackson E.A., Jones E.J., Navas-Acien A., et al. Cardiovascular health in American Indians and Alaska Natives: a scientific statement from the American heart association. Circulation. 2020;141:E948–E959. doi: 10.1161/CIR.0000000000000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organisation . Final Report of the Commission on Social Determinants of Health. 2008. Closing the gap in a generation: health equity through action on the social determinants of health.https://www.who.int/publications/i/item/WHO-IER-CSDH-08.1 [DOI] [PubMed] [Google Scholar]
- 32.Office of Disease Prevention and Health Promition. Healthy People 2030. https://health.gov/healthypeople (accessed August 27, 2023).
- 33.Lyon A.R., López-Fernández T., Couch L.S., Asteggiano R., Aznar M.C., Bergler-Klein J., et al. ESC guidelines on cardio-oncology developed in collaboration with the European hematology association (EHA), the European society for therapeutic radiology and oncology (ESTRO) and the international cardio-oncology society (IC-OS)developed by the task force on cardio-oncology of the European society of cardiology (ESC) Eur. Heart J. 2022;43:4229–4361. doi: 10.1093/EURHEARTJ/EHAC244. 2022. [DOI] [PubMed] [Google Scholar]
- 34.Sadler D., Chaulagain C., Alvarado B., Cubeddu R., Stone E., Samuel T., et al. Practical and cost-effective model to build and sustain a cardio-oncology program. Cardio-Oncology. 2020;6:9. doi: 10.1186/s40959-020-00063-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available through the CDC WONDER resource: https://wonder.cdc.gov/mcd.html.