This randomized clinical trial compares fitness improvements and control of cardiovascular risk factors among cancer survivors with cardiovascular disease and/or exposure to cardiotoxic cancer treatment who underwent center-based cardiac rehabilitation or usual care with community-based exercise training.
Key Points
Question
Does a structured and supervised cardio-oncology rehabilitation program improve cardiorespiratory fitness compared with a community-based exercise intervention?
Findings
In this randomized clinical trial including 75 cancer survivors with high cardiovascular risk, an 8-week cardio-oncology rehabilitation framework promoted superior, clinically meaningful improvements in peak oxygen consumption.
Meaning
The findings suggest that a cardio-oncology program is effective and has the potential within the established infrastructure of cardiac rehabilitation to be incorporated in the standard care of this population with complex and challenging needs.
Abstract
Importance
Cardiovascular disease is a leading cause of morbidity in cancer survivors, which makes strategies aimed at mitigating cardiovascular risk a subject of major contemporary importance.
Objective
To assess whether a center-based cardiac rehabilitation (CBCR) framework compared with usual care encompassing community-based exercise training (CBET) is superior for cardiorespiratory fitness improvement and cardiovascular risk factor control among cancer survivors with high cardiovascular risk.
Design, Setting, and Participants
This prospective, single-center, randomized clinical trial (CORE trial) included adult cancer survivors who had exposure to cardiotoxic cancer treatment and/or previous cardiovascular disease. Enrollment took place from March 1, 2021, to March 31, 2022. End points were assessed at baseline and after the 8-week intervention.
Interventions
Participants were randomly assigned in a 1:1 ratio to 8 weeks of CBCR or CBET. The combined aerobic and resistance exercise sessions were performed twice a week.
Main Outcomes and Measures
The powered primary efficacy measure was change in peak oxygen consumption (V̇o2) at 2 months. Secondary outcomes included handgrip maximal strength, functional performance, blood pressure (BP), body composition, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), lipid profile, plasma biomarker levels, physical activity (PA) levels, psychological distress, quality of life (QOL), and health literacy.
Results
A total of 75 participants completed the study (mean [SD] age, 53.6 [12.3] years; 58 [77.3%] female), with 38 in the CBCR group and 37 in the CBET group. Participants in CBCR achieved a greater mean (SD) increase in peak V̇o2 than those in CBET (2.1 [2.8] mL/kg/min vs 0.8 [2.5] mL/kg/min), with a between-group mean difference of 1.3 mL/kg/min (95% CI, 0.1-2.6 mL/kg/min; P = .03). Compared with the CBET group, the CBCR group also attained a greater mean (SD) reduction in systolic BP (−12.3 [11.8] mm Hg vs −1.9 [12.9] mm Hg; P < .001), diastolic BP (−5.0 [5.7] mm Hg vs −0.5 [7.0] mm Hg; P = .003), and BMI (−1.2 [0.9] vs 0.2 [0.7]; P < .001) and greater mean (SD) improvements in PA levels (1035.2 [735.7] metabolic equivalents [METs]/min/wk vs 34.1 [424.4] METs/min/wk; P < .001), QOL (14.0 [10.0] points vs 0.4 [12.9] points; P < .001), and health literacy scores (2.7 [1.6] points vs 0.1 [1.4] points; P < .001). Exercise adherence was significantly higher in the CBCR group than in the CBET group (mean [SD] sessions completed, 90.3% [11.8%] vs 68.4% [22.1%]; P < .001).
Conclusion and Relevance
The CORE trial showed that a cardio-oncology rehabilitation model among cancer survivors with high cardiovascular risk was associated with greater improvements in peak V̇o2 compared with usual care encompassing an exercise intervention in a community setting. The CBCR also showed superior results in exercise adherence, cardiovascular risk factor control, QOL, and health literacy.
Trial Registration
ClinicalTrials.gov Identifier: NCT05132998
Introduction
There is growing awareness about the increased risk of morbidity and mortality from noncancer causes among cancer survivors.1,2 The increased burden of cardiovascular risk factors (CVRFs) in association with aging, systemic cancer-related therapies, and organ-specific cardiovascular toxic effects makes cardiovascular disease (CVD) a leading cause of death and disability in this population.3,4,5,6,7 In addition, behavioral factors (such as sedentarism) associated with potential toxic effects of treatment can reduce functional capacity and cardiorespiratory fitness, which in turn, have been associated with increased cardiovascular morbidity.8,9 Therefore, identifying effective approaches for improving cardiorespiratory fitness and CVRF control in cancer survivors with increased cardiovascular risk has major contemporary relevance.
Although exercise training is recommended as an important strategy in the management of exercise intolerance and overall CVRF control among patients with several CVDs, only recently was a structured multidimensional model based on a cardiac rehabilitation (CR) framework proposed for patients with cancer.10,11,12,13,14 Recent data from a large noncontrolled study showed that exercise-based CR was associated with improve cardiorespiratory fitness and survival in patients with CVD and cancer.9 Also, 2 recent trials in populations with high cardiovascular risk showed that both a comprehensive lifestyle modification program (that included supervised exercise delivered in a CR setting) and an exercise training program alone improved cardiorespiratory fitness and arterial blood pressure control,15,16 albeit only the comprehensive lifestyle modification program promoted lifestyle changes leading to significant improvements in body composition and daily physical activity.15,16 These results are encouraging, as the existing infrastructure of CR may provide an opportunity to implement cardio-oncology rehabilitation programs targeting multiple health-promoting behaviors.
Notably, lifestyle changes encouraged by cardio-oncology rehabilitation have not been rigorously evaluated.9,17,18,19 A recent systematic review and meta-analysis of randomized and nonrandomized studies evaluating CR-based interventions in adult cancer survivors highlighted the small number of randomized trials, low-to-moderate reporting quality, and limited interpretation, reproducibility, and translation of current evidence into clinical practice.17 While physical exercise is a recognized evidence-based supportive care strategy with positive outcomes for cardiorespiratory fitness in cancer survivors, the overall effects of a multimodal rehabilitation approach in cancer survivors with previous CVD and/or those exposed to potentially cardiotoxic cancer treatment has not been clearly established.20,21,22,23 With this background, the Impact of a Comprehensive Cardiac Rehabilitation Framework Among High Cardiovascular Risk Cancer Survivors randomized clinical trial (RCT; CORE trial) was designed to address this issue by testing a center-based CR program (CBCR) compared with usual care encompassing community-based exercise.
Methods
Trial Overview
The CORE trial (NCT05132998) was a prospective, single-blinded RCT with a parallel 2-arm group designed to evaluate whether a 2-month medically supervised cardio-oncology rehabilitation intervention compared with usual care encompassing a community-based exercise training program (CBET) could achieve superior improvements in cardiorespiratory fitness, CVRF control, quality of life (QOL), and health literacy in cancer survivors with high cardiovascular risk.11 Detailed methods and the trial protocol have been published previously,24 and the statistical analysis plan and trial protocol are given in Supplement 1. Enrollment began March 1, 2021, and ended March 31, 2022. Potentially eligible participants were recruited by assistant physicians from a single hospital’s oncology and hematology departments and then referred to cardiopulmonary exercise stress testing prior to a final decision about eligibility. The study was approved by the Centro Hospitalar Vila Nova de Gaia/Espinho ethics committee; written informed consent was obtained from all participants. The study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Participants
Cancer survivors older than 18 years in follow-up after primary treatment with curative intent (completed at least 2 months prior to inclusion) were eligible. Additional inclusion criteria were the following factors associated with high cardiovascular risk11: (1) prior exposure to high-dose anthracycline (eg, doxorubicin ≥250 mg/m2) or high-dose radiotherapy (thoracic wall, ≥30 Gy), low-dose anthracycline, or anti-ERBB2 drugs alone plus 2 or more CVRFs25,26 and/or age 60 years or older at cancer treatment or receipt of low-dose anthracycline followed by anti-ERBB2 and/or (2) history of coronary artery disease, moderate valvular disease, or left ventricular ejection fraction less than 50%.24 Exclusion criteria included previous participation in a CR program, contraindications to exercise training, active cancer, or being considered unsuitable according to the judgment of the principal investigator (S.G.V.), namely due to expected inability to fulfill the proposed trial schedule.
Randomization and Allocation
Computer-based randomization (1:1) was generated using a permutated block design, with strata defined by age (<65 or ≥65 years) and sex. Allocation was concealed until the beginning of the intervention and communicated individually by telephone.24 Staff assessing outcome measures were blinded to participants’ group assignments.
Study Treatment
The detailed intervention protocol has been described in a previous report.24 Briefly, cancer survivors in the CBCR group received the core components of an outpatient CR program delivered by a multidisciplinary rehabilitation team (S.G.V., E.V.) in addition to standard care.25,26,27 This group underwent 2 center-based exercise training sessions per week at the existing hospital’s CR unit that were led by a physiotherapist and supervised by a physiatrist (S.G.V.). Each combined exercise session included a 10-minute warm-up, 30 to 40 minutes of aerobic exercise consisting of cycling and/or walking at 50% to 80% of the initial heart rate reserve (rating of 12 to 16 on the Borg scale), 10 to 15 minutes of dynamic resistance exercise, and 5 to 10 minutes of cooldown. The resistance training exercises were performed with free weights at 40% to 60% of the 1-repetition maximum, with 1 set of 10 to 15 repetitions increasing to 2 sets of 3 to 5 resistance exercises of the major muscle groups. Participants also received (1) an individualized plan delivered by a nutritionist addressing dietary goals to improve control of modifiable CVRFs, (2) a weekly group session (scheduled on the same days of the exercise sessions) on psychological management and lifestyle behavior change (addressing motivation for healthy lifestyle habits including regular physical activity), and (3) a monthly health educational group session raising awareness of the importance of control of CVRFs.
In the CBET group, nutritional and psychosocial management were provided as needed in the hospital setting as determined by the attending physician’s assessment and in accordance with standard care. Participants also received 2 combined exercise sessions per week conducted by an exercise physiologist (B.F.D.) certified in exercise for patients with cancer28 at a community-based facility with the same training intensity prescription as the CBCR group.21,22
Study Outcome Measures
The primary end point was cardiorespiratory fitness assessed by change in peak oxygen consumption (V̇o2) from baseline to 8 weeks, derived from a symptom-limited cardiopulmonary exercise stress test. Participants underwent a maximal graded treadmill test (electrocardiographic monitoring, Mortara X-Scribe; treadmill, Clinical 870 A [Medisoft]) using a modified version of the Bruce protocol.29 Ventilation, V̇o2, and carbon dioxide output were measured breath by breath using a metabolic analyzer (Blue Cherry Ergostik gas analyzer [Geratherm Respiratory GmbH]).
Secondary end points included muscle strength, CVRF control, inflammatory biomarkers, QOL, and health literacy. All end points were assessed at baseline and after the 8-week intervention at hospital facilities over 2 nonconsecutive days. Physical function was assessed by handgrip isometric maximal strength (digital hand dynamometer, Saehan model SH1001, DHD-1 [Saehan Corp])30 and the 1-minute sit-to-stand (STS) test.31 Regarding CVRF parameters, height, weight, waist and hip circumference, and body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) were measured, as was the percentage of fat mass and fat-free body mass with a calibrated digital body composition analyzer (InBody 270). Total cholesterol, low-density and high-density lipoprotein cholesterol, triglyceride, high-sensitivity C-reactive protein, and interleukin 6 levels were measured after a 12-hour fast, and resting arterial blood pressure (office values, in the sitting position) was assessed following current recommendations.32 Daily physical activity (International Physical Activity Questionnaire–Short Form),33 QOL (European Quality of Life 5 Dimensions [EQ-5D-5L] questionnaire),34 psychosocial distress (Hospital Anxiety and Depression Score [HADS]),35 and health literacy (Newest Vital Sign)36 were also assessed. Intervention adherence was registered as the total percentage of exercise sessions attended by participants, and adverse events were registered and classified according to Common Terminology Criteria for Adverse Events, version 5.0.37 A detailed description of the assessment procedures and outcome measures has been previously reported.24
Statistical Analysis
Sample size was based on previous results showing a moderate effect size (Cohen d = 0.6) for peak V̇o2 associated with CR interventions in patients with cardiovascular disease38,39 compared with exercise-based interventions delivered to patients with cancer.40 A 2-sided level of significance of α = 0.05 and a sample size of 72 participants (36 per group) provided 80% statistical power to demonstrate this effect in peak V̇o2. To accommodate for a 10% attrition rate, a total of 80 participants (40 in each group) was recruited (G*power, version 3.1 [University Düsseldorf]; independent t test; allocation ratio = 1). Exploratory and Shapiro-Wilk tests were performed to assess data distribution. Continuous variables are expressed as means with SDs or medians with IQRs for variables with skewed distributions; mean differences are expressed with their 2-sided 95% CIs. Categorical variables are presented as frequencies and percentages. Between-group differences at baseline and in the change from baseline to the end of the 8-week intervention were tested with unpaired t tests or Mann-Whitney U tests. Analysis of covariance was also used to adjust for exercise session adherence. Paired t tests were used for within-group comparisons from baseline to the end of the intervention. Between-group comparisons in categorical variables were compared with the Fisher exact test or the χ2 test, as appropriate. The level of significance was set as 2-sided P ≤ .05. All analyses were performed with SPSS, version 28.0 (IBM Corp).
Results
Participants
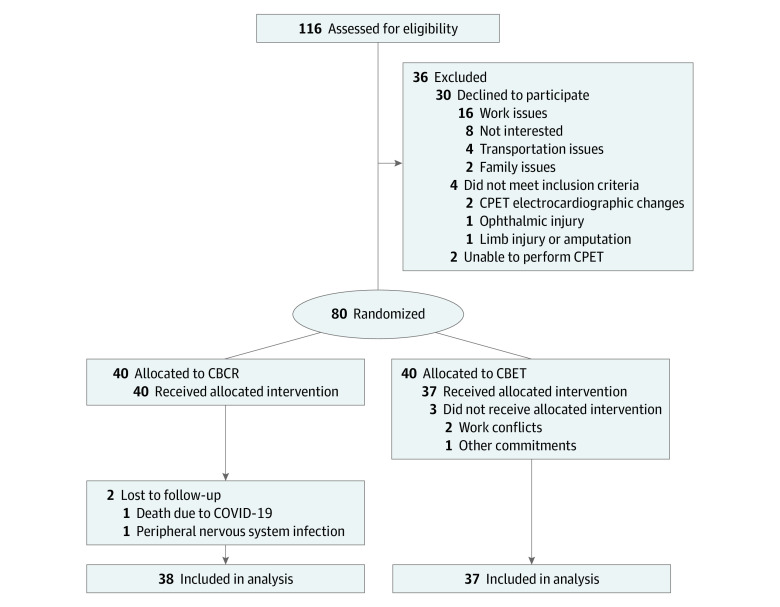
From a total of 116 participants who met eligibility criteria, 84 agreed to participate and completed screening assessments. Of these, 4 participants were excluded for not meeting inclusion criteria, and 80 individuals were randomized to CBCR (n = 40) or CBET (n = 40). The CONSORT diagram for study participants (Figure 1) describes the main reasons for not participating in the study. Of the 75 participants who completed follow-up assessments (38 in the CBCR group and 37 in the CBET group), 58 (77.3%) were female and 17 (22.7%) were male; the mean (SD) age was 53.6 (12.3) years. Participant characteristics are shown in Table 1.
Figure 1. CONSORT Diagram.
CBCR indicates center-based cardiac rehabilitation; CBET, community-based exercise training; CPET, cardiopulmonary exercise test.
Table 1. Baseline Characteristics of Participants.
| Characteristic | Patientsa | |
|---|---|---|
| CBCR (n = 38) | CBET (n = 37) | |
| Age, mean (SD), y | 54.5 (13.5) | 53.8 (10.7) |
| Sex | ||
| Female | 29 (76.3) | 29 (78.4) |
| Male | 9 (23.7) | 8 (21.6) |
| Marital status | ||
| Single | 3 (7.9) | 4 (10.8) |
| Married | 29 (76.3) | 27 (73.0) |
| Divorced | 4 (10.5) | 6 (16.2) |
| Widowed | 2 (5.3) | 0 |
| Highest educational level | ||
| Elementary school | 23 (60.5) | 15 (40.5) |
| High school | 12 (31.6) | 16 (43.2) |
| University graduate | 3 (7.9) | 5 (13.5) |
| Postgraduate degree | 0 | 1 (2.7) |
| Work situation | ||
| Employed | 23 (60.5) | 16 (43.2) |
| Unemployed | 7 (18.4) | 12 (32.4) |
| Retired | 8 (21.1) | 9 (24.3) |
| Type of cancer | ||
| Breast | 24 (63.1) | 26 (70.3) |
| Colorectal | 1 (2.6) | 1 (2.7) |
| Gastric | 1 (2.6) | 0 |
| Prostate | 1 (2.6) | 1 (2.7) |
| Lymphoma | 11 (28.9) | 9 (24.3) |
| Solid tumor | ||
| Any | 28 (73.7) | 27 (73.0) |
| Stage I | 12 (31.6) | 16 (43.2) |
| Stage II | 11 (28.9) | 5 (13.5) |
| Stage III | 3 (7.9) | 6 (16.2) |
| Stage IV | 2 (5.3) | 0 |
| Time elapsed between cancer diagnosis and study enrollment, median (IQR), mo | 27.0 (14.0-45.5) | 25.0 (17.5-38.5) |
| Cancer treatment | ||
| Chemotherapy | 36 (94.7) | 34 (91.9) |
| Anthracyclinesb | 33 (86.8) | 31 (83.8) |
| Cumulative dose of anthracyclines, mean (SD), mg/m2 | 265.9 (76.4) | 251.7 (44.9) |
| Thoracic radiotherapy | 24 (63.2) | 24 (64.9) |
| Radiotherapy dose, mean (SD), Gy | 46.7 (6.3) | 44.3 (7.4) |
| Surgery | 26 (68.4) | 26 (70.3) |
| Adjuvant hormonal therapy | ||
| Any | 22 (57.9) | 21 (56.8) |
| Tamoxifen | 6 (15.8) | 12 (32.4) |
| Tamoxifen plus goserelin | 3 (7.9) | 3 (8.1) |
| Exemestane plus goserelin | 1 (2.6) | 0 |
| Letrozol | 12 (31.6) | 6 (16.2) |
| Trastuzumab | 5 (13.2) | 2 (5.4) |
| Pertuzumab | 3 (7.9) | 1 (2.7) |
| Cardiovascular risk factors | ||
| Diabetes | 1 (2.6) | 2 (5.4) |
| Hypertension | 15 (39.5) | 13 (35.1) |
| Dyslipidemia | 19 (50.0) | 20 (54.0) |
| Smoking habits | 4 (10.5) | 5 (13.5) |
| Depression | 12 (31.6) | 9 (24.3) |
| Overweight | 18 (47.3) | 14 (37.8) |
| Obesity | 12 (31.6) | 10 (27.0) |
| Other comorbidities | ||
| Ischemic heart disease | 3 (7.9) | 4 (10.8) |
| Atrial fibrillation | 0 | 1 (2.7) |
| Heart failure | 4 (10.5) | 4 (10.8) |
| Valvular disease | 1 (2.6) | 0 |
| Implantable cardioverter-defibrillator | 1 (2.6) | 1 (2.7) |
| Respiratory diseasesc | 6 (15.8) | 5 (13.5) |
| Musculoskeletal diseased | 7 (18.4) | 7 (18.9) |
| Othere | 5 (13.2) | 4 (10.8) |
| Left ventricular ejection fraction, mean (SD) | 55.6 (12.4) | 55.6 (8.6) |
| Medication | ||
| Anticoagulants | 0 | 1 (2.7) |
| Antiplatelet therapy | 2 (5.3) | 3 (8.1) |
| β-Blockers | 5 (13.2) | 5 (13.5) |
| Anxiolytics | 11 (28.9) | 10 (27.0) |
| Antidepressants | 12 (31.6) | 9 (24.3) |
| Diuretics | 7 (18.4) | 7 (18.9) |
| Statins | 16 (42.1) | 14 (37.8) |
| Nitrates | 0 | 1 (2.7) |
| Fibrates | 2 (5.3) | 2 (5.4) |
| ACEI | 5 (13.2) | 6 (16.2) |
| ARA II | 4 (10.5) | 6 (16.2) |
| Sacubitril or valsartan | 2 (5.3) | 0 |
| Calcium channel blockers | 4 (10.5) | 1 (2.7) |
| Insulin | 0 | 1 (2.7) |
| Antidiabetic agentsf | 1 (2.6) | 2 (5.4) |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARA II, angiotensin II receptor antagonists; CBCR, center-based cardiac rehabilitation; CBET, community-based exercise treatment.
Data are presented as the number (percentage) of patients unless otherwise indicated.
Anthracyclines and radiotherapy were given sequentially.
Asthma and chronic obstructive pulmonary disease.
Degenerative joint disease.
Thyroid diseases, hepatitis, HIV infection, obstructive sleep apnea, chronic kidney disease, peripheral arterial disease, vertiginous syndrome.
Excluding insulin.
Cardiorespiratory Fitness and Physical Function
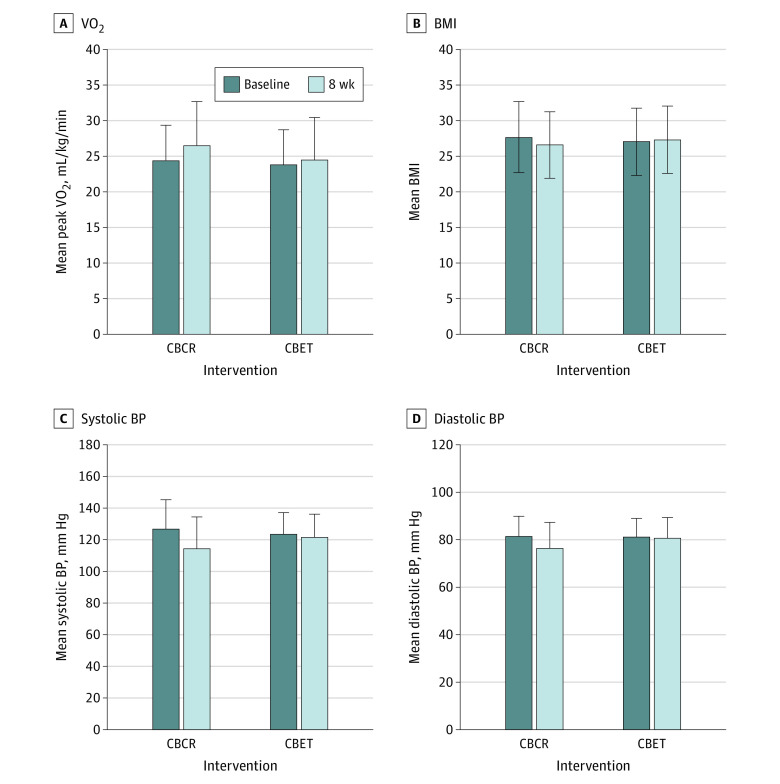
The peak V̇o2 improved in the CBCR group by a mean (SD) of 2.1 (2.8) mL/kg/min (P < .01) compared with 0.8 (2.5) mL/kg/min (P = .07) in the CBET group, with a significant between-group difference of 1.3 mL/kg/min (95% CI, 0.1-2.6 mL/kg/min; P = .03) (Figure 2). Individual peak V̇o2 changes from baseline to after intervention for the CBCR and CBET groups are provided in the eFigure in Supplement 2. The change in exercise test duration was also significantly different between groups by 58.6 seconds (95% CI, 28.9-88.3 seconds; P < .001), with a mean (SD) change of 105.8 (64.0) seconds in the CBCR group and 47.3 (64.2) seconds in the CBET group (Table 2).
Figure 2. Changes From Baseline to the End of Intervention.
Significant changes from baseline to the end of the intervention in peak oxygen consumption (V̇o2), body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), and blood pressure (BP) were observed in the center-based cardiac rehabilitation (CBCR) group, while these remained unchanged in the community-based exercise training (CBET) group, with significant between-group difference. Error bars represent SDs.
Table 2. Body Composition, Handgrip Strength, and Physical Function Changes After the 8-Week Intervention.
| Metric | CBCR (n = 38) | CBET (n = 37) | Between-group difference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | P value | Mean (SD) | P value | |||||||
| Baseline | Postintervention | Change from baseline | Baseline | Postintervention | Change from baseline | Mean (95% CI) | P value | |||
| Peak V̇o2, mL/kg/min | 24.4 (7.0) | 26.5 (7.5) | 2.1 (2.8) | <.001 | 23.8 (6.2) | 24.5 (6.0) | 0.8 (2.5) | .07 | 1.3 (0.1 to 2.6) | .03 |
| Exercise test duration, s | 583.4 (141.6) | 689.3 (142.9) | 105.8 (64.0) | <.001 | 574.0 (175.5) | 621.2 (156.6) | 47.3 (64.2) | <.001 | 58.6 (28.9 to 88.3) | <.001 |
| Weight, kg | 75.0 (14.0) | 72.1 (13.1) | −2.9 (2.6) | <.001 | 74.1 (14.8) | 74.4 (14.6) | 0.3 (1.6) | .22 | −3.2 (−4.2 to −2.2) | <.001 |
| BMI | 27.7 (5.0) | 26.6 (4.7) | −1.2 (0.9) | <.001 | 27.1 (4.7) | 27.3 (4.7) | 0.2 (0.7) | .10 | −1.4 (−1.7 to −1.0) | <.001 |
| Lean mass, kg | 25.6 (4.0) | 28.4 (5.5) | 2.8 (2.5) | <.001 | 25.6 (5.4) | 25.6 (5.5) | −0.1 (1.2) | .74 | 2.9 (2.0 to 3.8) | <.001 |
| Fat mass, kg | 27.8 (11.5) | 25.0 (10.8) | −2.9 (2.3) | <.001 | 26.9 (10.5) | 27.3 (10.7) | 0.4 (2.3) | .24 | −3.3 (−4.4 to −2.3) | <.001 |
| Waist circumference, cm | 99.2 (10.2) | 94.4 (9.8) | −4.8 (3.3) | <.001 | 97.4 (11.2) | 96.7 (11.4) | −0.7 (2.9) | .18 | −4.1 (−5.6 to −2.7) | <.001 |
| Hip circumference, cm | 106.8 (12.1) | 102.5 (10.8) | −4.3 (3.3) | <.001 | 103.8 (10.0) | 103.9 (9.6) | 0.1 (2.9) | .87 | −4.4 (−5.8 to −3.0) | <.001 |
| IHG, kgf | ||||||||||
| Dominant hand | 34.5 (7.2) | 37.2 (7.2) | 2.7 (2.4) | <.001 | 33.7 (9.7) | 35.3 (8.5) | 1.6 (3.4) | .01 | 1.0 (−0.3 to 2.4) | .13 |
| Nondominant hand | 31.6 (6.7) | 33.7 (7.0) | 2.1 (2.4) | <.001 | 31.6 (9.3) | 33.3 (8.4) | 1.6 (3.6) | .01 | 0.5 (−1.0 to 1.9) | .52 |
| Sit-to-stand test, repetitions in 60 s, No. | 32.0 (9.9) | 42.7 (12.5) | 10.7 (8.6) | <.001 | 30.9 (9.7) | 32.9 (10.6) | 2.0 (5.1) | .02 | 8.7 (5.5 to 12.0) | <.001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CBCR, center-based cardiac rehabilitation; CBET, community-based exercise treatment; IHG, isometric handgrip strength; kgf, kilogram-force; V̇o2, oxygen consumption.
Isometric handgrip strength improved in both groups, with no between-group differences in either the dominant (mean difference, 1.0 kg-force [kgf]; 95% CI, −0.3 to 2.4 kgf; P = .13) or nondominant (mean difference, 0.5 kgf; 95% CI, −1.0 to 1.9 kgf; P = .52) hand (Table 2). There was a significant between-group difference in the mean change in the number of repetitions per 60 seconds achieved in the STS test in favor of CBCR (8.7 repetitions; 95% CI, 5.5-12.0 repetitions; P < .01) (Table 2).
Cardiovascular Risk Factors
Compared with the CBET group, participants in the CBCR group had greater improvements in systolic (mean [SD] change, −12.3 [11.8] mm Hg vs −1.9 [12.9] mm Hg; P < .001) and diastolic (mean [SD] change, −5.0 [5.7] mm Hg vs −0.5 [7.0] mm Hg; P = .003) arterial blood pressure, heart rate, body composition (weight; BMI [mean (SD) change, −1.2 (0.9) vs 0.2 (0.7); P < .001]; lean and fat body mass; and waist and hip circumference), daily physical activity (mean [SD] increase, 1035.2 [735.7] metabolic equivalents [METs]/min/wk vs 34.1 [424.4] METs/min/wk; P < .001), and levels of total cholesterol, triglycerides, and low-density lipoprotein cholesterol, resulting in significant between-group differences in favor of the CBCR intervention (Table 2 and Table 3). The change in the HADS Anxiety and Depression Scale score was significantly different between groups by −1.8 points (95% CI, −2.9 to −0.6 points; P < .01) and −2.2 points (95% CI, −3.7 to −0.6 points; P < .01), respectively. There were no between-group differences in interleukin 6 or high-sensitivity C-reactive protein levels (Table 3); neither participants allocated to CBCR or CBET showed changes in circulating levels of these biomarkers.
Table 3. Blood Pressure, Heart Rate, Physical Activity, Lipid Profile, Inflammatory Markers, Psychological Symptoms, Health Literacy, and Health-Related Quality-of-Life Changes After the 8-Week Intervention.
| Metric | CBCR (n = 38) | CBET (n = 37) | Between-group difference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | P value | Mean (SD) | P value | |||||||
| Baseline | Postintervention | Change from baseline | Baseline | Postintervention | Change from baseline | Mean (95% CI) | P value | |||
| Resting SBP, mm Hg | 127.1 (18.9) | 114.8 (13.8) | −12.3 (11.8) | <.001 | 123.9 (20.2) | 122.0 (14.7) | −1.9 (12.9) | .39 | −10.4 (−16.1 to −4.7) | <.001 |
| Resting DBP, mm Hg | 81.5 (8.4) | 76.5 (7.8) | −5.0 (5.7) | <.001 | 81.4 (11.0) | 80.9 (8.7) | −0.5 (7.0) | .70 | −4.5 (−7.5 to −1.6) | .003 |
| Resting heart rate, bpm | 81.2 (13.6) | 75.3 (11.3) | −5.9 (6.5) | <.001 | 82.0 (13.3) | 83.7 (12.3) | 1.7 (8.2) | .22 | −7.6 (−11.0 to −4.2) | <.001 |
| Total cholesterol level, mg/dL | 182.1 (36.9) | 167.1 (26.3) | −15.0 (27.8) | .002 | 178.1 (33.1) | 186.7 (33.4) | 8.6 (32.6) | .12 | −23.6 (−37.6 to −9.7) | .001 |
| Triglyceride levels, mg/dL | 125.2 (60.0) | 93.0 (32.6) | −32.3 (40.4) | <.001 | 152.0 (105.6) | 154.9 (113.9) | 2.9 (68.0) | .80 | −35.2 (−60.8 to −9.5) | .008 |
| HDL cholesterol level, mg/dL | 53.3 (12.9) | 54.3 (10.5) | 1.0 (7.4) | .41 | 53.2 (13.3) | 52.6 (14.2) | −0.6 (7.5) | .60 | 1.6 (−1.8 to 5.1) | .34 |
| LDL cholesterol level, mg/dL | 107.0 (32.3) | 92.8 (24.5) | −14.2 (29.4) | .005 | 101.0 (32.3) | 105.1 (28.6) | 4.2 (21.6) | .25 | −18.3 (−30.2 to −6.5) | .003 |
| IL-6 level, pg/mL | 5.2 (6.2) | 4.8 (5.7) | −0.5 (2.0) | .19 | 3.6 (5.1) | 5.1 (5.5) | 1.5 (5.5) | .15 | −2.0 (−4.0 to 0.1) | .06 |
| HSCRP level, mg/dL | 0.35 (0.39) | 0.26 (0.29) | −0.08 (0.39) | .18 | 0.29 (0.46) | 0.41 (0.72) | 0.12 (0.66) | .35 | −0.2 (−0.5 to 0.1) | .12 |
| Physical activity, METs/min/wk | 349.6 (530.6) | 1384.7 (893.9) | 1035.2 (735.7) | <.001 | 519.7 (467.9) | 553.8 (608.1) | 34.1 (424.4) | .63 | 1001.1 (719.8 to 1282.8) | <.001 |
| HADS score | ||||||||||
| Anxiety | 7.9 (4.6) | 5.3 (3.9) | −2.6 (2.7) | <.001 | 8.2 (4.3) | 7.4 (4.2) | −0.8 (2.3) | .03 | −1.8 (−2.9 to −0.6) | .003 |
| Depression | 6.0 (4.4) | 3.3 (3.2) | −2.7 (3.0) | <.001 | 6.3 (4.0) | 5.8 (3.7) | −0.5 (2.2) | .21 | −2.2 (−3.7 to −0.6) | .009 |
| EQ-5D-5L health state score | 68.3 (15.2) | 82.3 (9.5) | 14.0 (10.0) | <.001 | 70.3 (15.7) | 70.7 (16.1) | 0.4 (12.9) | .85 | 13.6 (8.3 to 18.9) | <.001 |
| Health literacy scorea | 1.6 (1.8) | 4.3 (1.2) | 2.7 (1.6) | <.001 | 1.7 (2.0) | 1.8 (2.1) | 0.1 (1.4) | .81 | 2.6 (1.9 to 3.3) | <.001 |
Abbreviations: CBCR, center-based cardiac rehabilitation; CBET, community-based exercise treatment; DBP, diastolic blood pressure; EQ-5D-5L, European Quality of Life 5 Dimensions questionnaire; HADS, Hospital Anxiety and Depression Score; HDL, high-density lipoprotein; HSCRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; LDL, low-density lipoprotein; MET, metabolic equivalents; SBP, systolic blood pressure.
SI conversion factors: To convert HDL, LDL, and total cholesterol to mmol/L, multiply by 0.0259 and triglycerides to mmol/L, by 0.0113.
Assessed by the Newest Vital Sign instrument.
QOL and Health Literacy
The change in QOL was significantly different between groups, with a mean (SD) change in EQ-5D-5L health state score of 14.0 (10.0) points in the CBCR group and 0.4 (12.9) points in the CBET group (mean between-group difference, 13.6 points; 95% CI, 8.3-18.9 points; P < .001) (Table 3). The health literacy score significantly improved in the CBCR group (mean [SD] increase, 2.7 [1.6] points) but remained unchanged in the CBET group (mean [SD] increase, 0.1 [1.4] points) (P < .001), with a mean between-group difference of 2.6 points (95% CI, 1.9-3.3 points; P < .001) (Table 3).
Adherence and Safety
Participants in CBCR attended a mean (SD) of 90.3% (11.8%) of the exercise sessions compared with 68.4% (22.1%) in the CBET group (P < .01). The most frequent reasons for missing exercise sessions were related to family obligations (CBCR: 3.5% of total prescribed sessions vs CBET: 6.6%), scheduled examinations or medical appointments (CBCR: 3.5% vs CBET: 5.4%), medical conditions (including COVID-19 illness or isolation rules or reaction to the vaccine) (CBCR: 1.6% vs CBET: 4.9%), transportation difficulties (CBCR: 0.3% vs CBET: 0.5%), and no provided reason or other reason (CBCR: 0.8% vs CBET: 14.2%). Participants in the CBCR group attended all the educational and psychological management sessions. In the CBET group, 11 participants (29.7%) received nutritional follow-up and 24 (64.9%), psychological support. No serious adverse events related to exercise were reported. A detailed description regarding the compliance and safety of the CORE trial has been previously provided.41
Comparison of Changes Adjusted for Exercise Adherence
When adjusted for exercise adherence, the between-group differences in peak V̇o2 were not significant (1.48 mL/kg/min; 95% CI, −0.01 to 2.96 mL/kg/min; P = .05), while exercise test duration and physical function measures remained significant (eTable 1 in Supplement 2). Adjusted mean changes in CVRFs, QOL, HADS Anxiety and Depression Scale scores, and health literacy are provided in eTables 2 and 3 in Supplement 2; except for lipid profile, the results did not change after adjusting for exercise adherence.
Discussion
The CORE trial showed that a center-based comprehensive intervention following a CR model provided significant improvements in peak V̇o2 in cancer survivors at high cardiovascular risk compared with usual care encompassing community-based exercise. The CBCR was also associated with greater improvements in CVRF control. Although participants who received standard supportive care, which included exercise training as recommended for cancer survivors, improved physical function (namely in terms of exercise test duration, handgrip strength, and STS test), no significant changes were observed in body composition, lipid profile, arterial blood pressure, daily physical activity, overall QOL, or health literacy. It should be noted that differences in peak V̇o2 and lipid profile seemed to be influenced by exercise adherence; it is possible that the hospital setting and close monitoring of the CBCR program may have contributed to greater adherence.
From a clinical perspective, these data may be of paramount contemporary relevance, as they can expand the basis for discussing whether cancer survivors at high cardiovascular risk, especially those susceptible to late effects of cardiotoxic medical treatment and chest radiation, should be consistently referred to cardio-oncology rehabilitation programs.12,17,19 The CBCR intervention tested in the CORE trial could be streamlined in a larger scale (making use of the facilities, equipment, and health care professionals’ expertise already available), which may simplify the translation of the current evidence into clinical practice.
Although exercise is well established as a supportive care strategy for improving cardiorespiratory fitness during and after cancer treatment, evidence of the benefits of multimodal lifestyle modifications among this population is still scarce.20,42,43,44 In this regard, a recent review evaluated changes in cardiorespiratory fitness and CVRFs associated with CR-based interventions among cancer survivors.17 This analysis encompassed a total of 10 studies involving 685 participants, but it included only 1 RCT, and just 2 studies addressed CVRFs. The authors concluded that a multimodal CR program was associated with higher levels of cardiorespiratory fitness, however, emphasizing that more data were necessary to confirm the efficacy outcomes derived from the CR model approach.17 The improvements in peak V̇o2 (the primary end point in this trial) and muscle strength are clinically relevant, as these variables have been associated with a better prognosis.9,45 Indeed, physical fitness is recognized as a pivotal parameter in overall health assessment across different clinical settings, being associated with the incidence of treatment-related toxic effects and CVD burden as well as cancer-specific and cardiovascular mortality.9,45,46,47 Interestingly, an exploratory analysis suggested that patients with cancer and CVD achieving higher cardiorespiratory fitness improvements after CR completion may have a lower risk of death (adjusted HR, 0.41; 95% CI, 0.17-0.99) compared with patients with smaller changes.9
The clinical environment, lifestyle counseling, and multidisciplinary nature of CR may explain the positive changes in CVRF control and QOL in the CBCR group compared with the CBET group. Indeed, behavioral intervention and support may provide patients with the knowledge and tools to cope with their condition after curative cancer treatment. The significant health literacy improvements in the CBCR group should also be highlighted, as these could reflect a higher empowerment to understand and apply health information, possibly influencing lifestyle decisions.36 This impression is supported by the increased levels of daily physical activity, better lipid profile, less psychological distress, and improved QOL observed in the CBCR group. The increased levels of physical activity should be further underscored, as previous data derived from women with breast cancer have shown that those who achieved higher levels of physical activity had a reduced incidence of cardiovascular events.48
The results of the CORE trial support the concept that behavioral changes promoted by a multidisciplinary specialized team trained to deal with multiple comorbidities along with close medical management, as already recommended along the cardiovascular continuum, should be extended to cancer survivors at high cardiovascular risk.11,12,25,49 In a background in which the myriad complex links between CVD and cancer have been increasingly recognized, the results of the CORE trial reinforce the need for comprehensive tailored interventions to be included in supportive care across the span of cancer survivorship.11,12,13,50 These multidimensional interventions (eg, exercise, health education, dietary modification, and psychological counseling) could be provided on a background of existing resources in CR units, addressing cancer survivors’ specific needs, possibly followed by referrals to community programs aimed at maintaining supportive care.51
Limitations
Some limitations should be acknowledged when analyzing the present data. First, because CORE was conducted at a single site, there may be concerns about the generalizability of our findings. However, the participants were derived from a central hospital with broad representation of individuals with varied educational and cultural backgrounds. Second, although different cancers were represented, most patients had a history of breast cancer or lymphoma. Third, the small sample size limits the strength of the conclusions. Finally, the CORE trial was powered to detect potential effects in the primary end point of cardiorespiratory fitness as assessed by peak V̇o2 (considered the gold standard in this context), but it was not powered to detect potential effects in subgroups of interest (eg, men compared with women).
Conclusions
The CORE trial showed that a cardio-oncology rehabilitation model among cancer survivors at high cardiovascular risk promoted greater improvements in peak V̇o2 compared with usual care encompassing exercise training in a community setting. The CBCR group also had greater improvements in CVRF control, exercise adherence, QOL, and health literacy. These findings suggest that exercise training benefits and successful lifestyle changes can be best optimized by a comprehensive, multidisciplinary team within the established infrastructure of CR. Policy makers and health professionals should consider incorporating (with appropriate coverage) CR into the standard care of this population with complex and challenging health care needs.
Trial Protocol
eFigure. Individual Peak Oxygen Consumption Changes From Baseline to the End of the Intervention for Center-Based Cardiac Rehabilitation and Community-Based Exercise Training Groups
eTable 1. Changes in Cardiorespiratory Fitness, Body Composition, Handgrip Strength, and Physical Function
eTable 2. Changes in Blood Pressure, Heart Rate, Physical Activity, Lipid Profile, and Inflammatory Markers
eTable 3. Changes in Psychological Symptoms, Health Literacy, and Health-Related Quality Of Life
Data Sharing Statement
References
- 1.Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363-385. doi: 10.3322/caac.21565 [DOI] [PubMed] [Google Scholar]
- 2.Zaorsky NG, Churilla TM, Egleston BL, et al. Causes of death among cancer patients. Ann Oncol. 2017;28(2):400-407. doi: 10.1093/annonc/mdw604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyon AR, López-Fernández T, Couch LS, et al. ; ESC Scientific Document Group . 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43(41):4229-4361. doi: 10.1093/eurheartj/ehac244 [DOI] [PubMed] [Google Scholar]
- 4.Strongman H, Gadd S, Matthews A, et al. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet. 2019;394(10203):1041-1054. doi: 10.1016/S0140-6736(19)31674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyon AR, Dent S, Stanway S, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur J Heart Fail. 2020;22(11):1945-1960. doi: 10.1002/ejhf.1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paterson DI, Wiebe N, Cheung WY, et al. Incident cardiovascular disease among adults with cancer: a population-based cohort study. JACC CardioOncol. 2022;4(1):85-94. doi: 10.1016/j.jaccao.2022.01.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lau ES, Paniagua SM, Liu E, et al. Cardiovascular risk factors are associated with future cancer. JACC CardioOncol. 2021;3(1):48-58. doi: 10.1016/j.jaccao.2020.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Schoot GGF, Ormel HL, Westerink NL, et al. Optimal timing of a physical exercise intervention to improve cardiorespiratory fitness: during or after chemotherapy. JACC CardioOncol. 2022;4(4):491-503. doi: 10.1016/j.jaccao.2022.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williamson T, Moran C, Chirico D, et al. Cancer and cardiovascular disease: the impact of cardiac rehabilitation and cardiorespiratory fitness on survival. Int J Cardiol. 2021;343:139-145. doi: 10.1016/j.ijcard.2021.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott JM, Nilsen TS, Gupta D, Jones LW. Exercise therapy and cardiovascular toxicity in cancer. Circulation. 2018;137(11):1176-1191. doi: 10.1161/CIRCULATIONAHA.117.024671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilchrist SC, Barac A, Ades PA, et al. ; American Heart Association Exercise, Cardiac Rehabilitation, and Secondary Prevention Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and Council on Peripheral Vascular Disease . Cardio-oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from the American Heart Association. Circulation. 2019;139(21):e997-e1012. doi: 10.1161/CIR.0000000000000679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Ascenzi F, Anselmi F, Fiorentini C, Mannucci R, Bonifazi M, Mondillo S. The benefits of exercise in cancer patients and the criteria for exercise prescription in cardio-oncology. Eur J Prev Cardiol. 2021;28(7):725-735. doi: 10.1177/2047487319874900 [DOI] [PubMed] [Google Scholar]
- 13.Dittus KL, Lakoski SG, Savage PD, et al. Exercise-based oncology rehabilitation: leveraging the cardiac rehabilitation model. J Cardiopulm Rehabil Prev. 2015;35(2):130-139. doi: 10.1097/HCR.0000000000000091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Visseren FLJ, Mach F, Smulders YM, et al. ; ESC National Cardiac Societies; ESC Scientific Document Group . 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227-3337. doi: 10.1093/eurheartj/ehab484 [DOI] [PubMed] [Google Scholar]
- 15.Blumenthal JA, Sherwood A, Smith PJ, et al. Lifestyle modification for resistant hypertension: the TRIUMPH randomized clinical trial. Am Heart J. 2015;170(5):986-994.e5. doi: 10.1016/j.ahj.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopes S, Mesquita-Bastos J, Garcia C, et al. Effect of exercise training on ambulatory blood pressure among patients with resistant hypertension: a randomized clinical trial. JAMA Cardiol. 2021;6(11):1317-1323. doi: 10.1001/jamacardio.2021.2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fakhraei R, Peck BKin SS, Abdel-Qadir H, et al. Research quality and impact of cardiac rehabilitation in cancer survivors: a systematic review and meta-analysis. JACC CardioOncol. 2022;4(2):195-206. doi: 10.1016/j.jaccao.2022.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sase K, Kida K, Furukawa Y. Cardio-oncology rehabilitation—challenges and opportunities to improve cardiovascular outcomes in cancer patients and survivors. J Cardiol. 2020;76(6):559-567. doi: 10.1016/j.jjcc.2020.07.014 [DOI] [PubMed] [Google Scholar]
- 19.Cuthbertson CC, Pearce EE, Valle CG, Evenson KR. Cardiac rehabilitation programs for cancer survivors: a scoping review. Curr Epidemiol Rep. 2020;7(2):89-103. doi: 10.1007/s40471-020-00235-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott JM, Zabor EC, Schwitzer E, et al. Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J Clin Oncol. 2018;36(22):2297-2305. doi: 10.1200/JCO.2017.77.5809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ligibel JA, Bohlke K, May AM, et al. Exercise, diet, and weight management during cancer treatment: ASCO guideline. J Clin Oncol. 2022;40(22):2491-2507. doi: 10.1200/JCO.22.00687 [DOI] [PubMed] [Google Scholar]
- 22.Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375-2390. doi: 10.1249/MSS.0000000000002116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tevaarwerk A, Denlinger CS, Sanft T, et al. Survivorship, Version 1.2021. J Natl Compr Canc Netw. 2021;19(6):676-685. doi: 10.6004/jnccn.2021.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viamonte SG, Joaquim A, Alves A, et al. Impact of a comprehensive cardiac rehabilitation framework among high cardiovascular risk cancer survivors: protocol for the CORE trial. Int J Cardiol. 2023;371:384-390. doi: 10.1016/j.ijcard.2022.09.075 [DOI] [PubMed] [Google Scholar]
- 25.Abreu A, Frederix I, Dendale P, et al. Standardization and quality improvement of secondary prevention through cardiovascular rehabilitation programmes in Europe: the avenue towards EAPC accreditation programme: a position statement of the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology (EAPC). Eur J Prev Cardiol. 2021;28(5):496-509. doi: 10.1177/2047487320924912 [DOI] [PubMed] [Google Scholar]
- 26.Piepoli MF, Hoes AW, Agewall S, et al. ; ESC Scientific Document Group . 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315-2381. doi: 10.1093/eurheartj/ehw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Association of Cardiovascular and Pulmonary Rehabilitation . Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. 6th ed. Human Kinetics; 2021. [Google Scholar]
- 28.Home page. CanRehab. Accessed August 24, 2023. https://www.canrehab.com/
- 29.Mezzani A. Cardiopulmonary exercise testing: basics of methodology and measurements. Ann Am Thorac Soc. 2017;14(suppl 1):S3-S11. doi: 10.1513/AnnalsATS.201612-997FR [DOI] [PubMed] [Google Scholar]
- 30.Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423-429. doi: 10.1093/ageing/afr051 [DOI] [PubMed] [Google Scholar]
- 31.Strassmann A, Steurer-Stey C, Lana KD, et al. Population-based reference values for the 1-min sit-to-stand test. Int J Public Health. 2013;58(6):949-953. doi: 10.1007/s00038-013-0504-z [DOI] [PubMed] [Google Scholar]
- 32.Williams B, Mancia G, Spiering W, et al. ; ESC Scientific Document Group . 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021-3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 33.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381-1395. doi: 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 34.Ferreira PL, Antunes P, Ferreira LN, Pereira LN, Ramos-Goñi JM. A hybrid modelling approach for eliciting health state preferences: the Portuguese EQ-5D-5L value set. Qual Life Res. 2019;28(12):3163-3175. doi: 10.1007/s11136-019-02226-5 [DOI] [PubMed] [Google Scholar]
- 35.Pais-Ribeiro J, Silva I, Ferreira T, Martins A, Meneses R, Baltar M. Validation study of a Portuguese version of the Hospital Anxiety and Depression Scale. Psychol Health Med. 2007;12(2):225-235. doi: 10.1080/13548500500524088 [DOI] [PubMed] [Google Scholar]
- 36.Alves E, Costa AR, Moura-Ferreira P, Azevedo A, Lunet N. Health-related knowledge on hypertension among the Portuguese population: results from a population-based survey. Blood Press. 2018;27(4):194-199. doi: 10.1080/08037051.2018.1430503 [DOI] [PubMed] [Google Scholar]
- 37.Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0. US Department of Health and Human Services. November 27, 2017. Accessed August 25, 2023. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf
- 38.Gomes-Neto M, Durães AR, Reis HFCD, Neves VR, Martinez BP, Carvalho VO. High-intensity interval training versus moderate-intensity continuous training on exercise capacity and quality of life in patients with coronary artery disease: a systematic review and meta-analysis. Eur J Prev Cardiol. 2017;24(16):1696-1707. doi: 10.1177/2047487317728370 [DOI] [PubMed] [Google Scholar]
- 39.Valkeinen H, Aaltonen S, Kujala UM. Effects of exercise training on oxygen uptake in coronary heart disease: a systematic review and meta-analysis. Scand J Med Sci Sports. 2010;20(4):545-555. doi: 10.1111/j.1600-0838.2010.01133.x [DOI] [PubMed] [Google Scholar]
- 40.Sweegers MG, Altenburg TM, Brug J, et al. Effects and moderators of exercise on muscle strength, muscle function and aerobic fitness in patients with cancer: a meta-analysis of individual patient data. Br J Sports Med. 2019;53(13):812. doi: 10.1136/bjsports-2018-099191 [DOI] [PubMed] [Google Scholar]
- 41.Viamonte SG, Joaquim A, Alves A, et al. Adherence, safety, and satisfaction of a cardio-oncology rehabilitation program framework versus community exercise training for cancer survivors: findings from the CORE trial. Support Care Cancer. 2023;31(3):173. doi: 10.1007/s00520-023-07638-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones LW, Liang Y, Pituskin EN, et al. Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. Oncologist. 2011;16(1):112-120. doi: 10.1634/theoncologist.2010-0197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown JC, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferrer RA, Johnson BT. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20(1):123-133. doi: 10.1158/1055-9965.EPI-10-0988 [DOI] [PubMed] [Google Scholar]
- 44.Lin X, Zhang X, Guo J, et al. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2015;4(7):1-28. doi: 10.1161/JAHA.115.002014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Groarke JD, Payne DL, Claggett B, et al. Association of post-diagnosis cardiorespiratory fitness with cause-specific mortality in cancer. Eur Heart J Qual Care Clin Outcomes. 2020;6(4):315-322. doi: 10.1093/ehjqcco/qcaa015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vainshelboim B, Chan K, Chen Z, Myers J. Cardiorespiratory fitness and cancer in men with cardiovascular disease: analysis from the Veterans Exercise Testing Study. Eur J Prev Cardiol. 2021;28(7):715-721. doi: 10.1177/2047487320916595 [DOI] [PubMed] [Google Scholar]
- 47.Ross R, Blair SN, Arena R, et al. ; American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Cardiovascular and Stroke Nursing; Council on Functional Genomics and Translational Biology; Stroke Council . Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134(24):e653-e699. doi: 10.1161/CIR.0000000000000461 [DOI] [PubMed] [Google Scholar]
- 48.Jones LW, Habel LA, Weltzien E, et al. Exercise and risk of cardiovascular events in women with nonmetastatic breast cancer. J Clin Oncol. 2016;34(23):2743-2749. doi: 10.1200/JCO.2015.65.6603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vilela EM, Ladeiras-Lopes R, Joao A, et al. Current role and future perspectives of cardiac rehabilitation in coronary heart disease. World J Cardiol. 2021;13(12):695-709. doi: 10.4330/wjc.v13.i12.695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boudoulas KD, Triposkiadis F, Gumina R, Addison D, Iliescu C, Boudoulas H. Cardiovascular disease, cancer, and multimorbidity interactions: clinical implications. Cardiology. 2022;147(2):196-206. doi: 10.1159/000521680 [DOI] [PubMed] [Google Scholar]
- 51.Elad B, Habib M, Caspi O. Cardio-oncology rehabilitation—present and future perspectives. Life (Basel). 2022;12(7):1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure. Individual Peak Oxygen Consumption Changes From Baseline to the End of the Intervention for Center-Based Cardiac Rehabilitation and Community-Based Exercise Training Groups
eTable 1. Changes in Cardiorespiratory Fitness, Body Composition, Handgrip Strength, and Physical Function
eTable 2. Changes in Blood Pressure, Heart Rate, Physical Activity, Lipid Profile, and Inflammatory Markers
eTable 3. Changes in Psychological Symptoms, Health Literacy, and Health-Related Quality Of Life
Data Sharing Statement