Abstract
We isolated and sequenced a plasmid, named pIP1714 (4,978 bp), which specifies resistance to streptogramins A and B and the mixture of these compounds. pIP1714 was isolated from a Staphylococcus cohnii subsp. cohnii strain found in the environment of a hospital where pristinamycin was extensively used. Resistance to both compounds and related antibiotics is encoded by two novel, probably cotranscribed genes, (i) vatC, encoding a 212-amino-acid (aa) acetyltransferase that inactivates streptogramin A and that exhibits 58.2 to 69.8% aa identity with the Vat, VatB, and SatA proteins, and (ii) vgbB, encoding a 295-aa lactonase that inactivates streptogramin B and that shows 67% aa identity with the Vgb lactonase. pIP1714 includes a 2,985-bp fragment also found in two rolling-circle replication and mobilizable plasmids, pUB110 and pBC16, from gram-positive bacteria. In all three plasmids, the common fragment was delimited by two direct repeats of four nucleotides (GGGC) and included (i) putative genes closely related to repB, which encodes a replication protein, and to pre(mob), which encodes a protein required for conjugative mobilization and site-specific recombination, and (ii) sequences very similar to the double- and single-strand origins (dso, ssoU) and the recombination site, RSA. The antibiotic resistance genes repB and pre(mob) carried by each of these plasmids were found in the same transcriptional orientation.
Streptogramins and related antibiotics are produced by streptomycetes and are classified as A and B compounds according to their basic primary structure (9). Compounds of the A group, including streptogramin A (SgA), pristinamycin IIA (PIIA), virginiamycin M, mikamycin A, and synergistin A, are polyunsaturated cyclic macrolactones. Compounds of the B group, including streptogramin B (SgB), pristinamycin IB (PIB), virginiamycin S, mikamycin B, and synergistin B, are cyclic peptide macrolactones. A and B compounds are bacteriostatic when used separately, but they can act in synergy to become bactericidal, mainly against gram-positive bacteria. Natural mixtures such as pristinamycin (Pt), synergistin, virginiamycin, and mikamycin are used orally and topically. A semisynthetic injectable streptogramin (Synercid), consisting of a mixture of derivatives of A and B compounds (dalfopristin and quinupristin, respectively), is currently undergoing in vivo clinical trials and evaluation by the U.S. Food and Drug Administration (see the entire volumes of the Journal of Antimicrobial Chemotherapy [volume 30, Suppl. A, 1992, and volume 39 Suppl. A, 1997). In this study, pristinamycins (PIIA, PIB, and Pt) were used to evaluate the levels of resistance to A and B compounds and to the synergistic mixtures of these compounds. The MICs of dalfopristin and quinupristin, which are the derivatives of Pts, are similar to those of PIIA and PIB, respectively.
Staphylococcal resistance to synergistic mixtures of A and B compounds (Pt MICs, ≥2 μg/ml) is always associated with resistance to A compounds (PIIA MICs, ≥8 μg/ml) but not necessarily with resistance to B compounds (1). To date, five genes encoding resistance to A compounds have been isolated from staphylococcal and enterococcal plasmids. The genes vat (6), vatB (2), and satA (30) encode related acetyltransferases (47.7 to 60.8% amino acid [aa] identity) which inactivate A compounds. The staphylococcal genes vga (4) and vgaB (3) encode related ATP-binding proteins (48.3% aa identity) probably involved in the active efflux of A compounds. The distribution of these genes and the gene vgb (5), encoding a lactonase which inactivates B compounds, has been investigated with a collection of 53 staphylococcal isolates resistant to A compounds (PIIA MICs, ≥8 μg/ml) (1, 3, 23). Forty-eight staphylococcal isolates carried vga or a combination of two or three genes: vgaB-vatB, vga-vat, or vga-vat-vgb. None of these genes was detected in four Staphylococcus aureus isolates or in the single Staphylococcus cohnii subsp. cohnii strain tested (strain BM10711) (23).
We have reported on PCR experiments with degenerate primers corresponding to the conserved motifs III and IV in the acetyltransferases encoded by vat, vatB, and satA: a 147-bp DNA fragment was amplified from S. cohnii subsp. cohnii BM10711 (23). This fragment was used as a probe and hybridized with a plasmid of 5 kb, pIP1714, harbored by BM10711, suggesting that the plasmid carries a vat-related gene which we named vatC. We report herein the isolation and sequence of pIP1714. It carries a vgb-related gene, vgbB, in addition to vatC and a 2,985-bp fragment homologous to part of the rolling-circle and mobilizable staphylococcal plasmids pUB110 (24) and pBC16 (29), which has also been called pNS1981 (33).
MATERIALS AND METHODS
Bacterial strains and plasmids.
S. cohnii subsp. cohnii BM10711 was isolated from a cupboard in Douera Hospital in Algiers, Algeria (23). It is resistant to macrolides, lincosamides, streptogramins A and B and their mixture, β-lactams due to penicillinase production and the presence of the mecA gene, tetracycline, trimethoprim, sulfonamide, cadmium salts, sodium arsenate, ammonium bromide, and ethidium bromide. The 53 staphylococcal isolates resistant to A compounds screened for the presence of vatC and vgbB genes include BM10711 (23) and the 52 isolates described previously (1) (S. aureus, 32 isolates; Staphylococcus epidermidis, 14 isolates; Staphylococcus haemolyticus, 4 isolates; Staphylococcus simulans, 1 isolate; S. cohnii subsp. urealyticum, 1 isolate). S. aureus RN4220 (22) and Escherichia coli TG1 (16) were used as recipients.
Plasmids pIP1714 and pIP1742 (this study) were isolated from BM10711. The shuttle vector pOX7, also named pOX300 (14), consisting of pUC18 and pE194ts, was used as a vector in cloning experiments. pIP1740 and pIP1741 (this study) each contain an insert amplified from within the vatC and vgbB genes, respectively, and were used as intragenic probes.
Media.
Brain heart infusion broth and agar (Difco Laboratories, Detroit, Mich.) were used for staphylococcal growth. Susceptibility to antimicrobial agents was tested on Mueller-Hinton agar (Sanofi Diagnostics Pasteur, Marnes-la-Coquette, France). Trypticase soy broth or Trypticase soy agar (Difco Laboratories) was used for the detection of PIIA and PIB inactivation. E. coli was grown on Luria-Bertani broth as described previously (2).
Susceptibility to antimicrobial agents.
Susceptibility to antimicrobial agents was determined by a disk diffusion assay with commercially available antibiotic disks (Diagnostics Pasteur, Marne-la-Coquette, France) and disks containing 20 μg of PIIA, 40 μg of PIB, 0.2 μmol of cadmium acetate, 0.2 μmol of sodium arsenate, 0.2 μmol of mercuric nitrate, 200 μg of ethidium bromide, 200 μg of acriflavin, 200 μg of propamidine isethionate, or 10 μg of cetyltrimethylammonium bromide.
The MICs of PIIA, PIB, and Pt were determined with serial twofold dilutions of the antibiotics in Mueller-Hinton agar (15).
Detection of PIIA or PIB inactivation.
Inactivation of PIIA or PIB was investigated by the test described by Gots (17), with Micrococcus luteus ATCC 9341 grown on Trypticase soy agar supplemented with 0.2 μg of PIIA per ml or 2 μg of PIB per ml used as the indicator organism.
DNA isolation and analysis.
Total cellular DNA and plasmid DNA were isolated from staphylococcal strains and purified as described previously (12, 32). Plasmid DNA was extracted and purified from E. coli with the QIA-prep Spin plasmid kit from Qiagen (Hilden, Germany).
Restriction endonucleases were obtained from Amersham International (Little Chalfont, United Kingdom) or from Pharmacia (Uppsala, Sweden) and were used according to the manufacturers’ instructions. Native or digested DNA was analyzed by 0.7% (wt/vol) agarose gel electrophoresis, and the DNA fragments amplified by PCR were separated by electrophoresis in 4% (wt/vol) Nusieve agarose gels (FMC Products, Rockland, Maine) as described previously (2).
PCR.
DNA was amplified by PCR as described previously (2). The samples were subjected to a precycle of 3 min at 95°C and 2 min at 60°C and then 30 cycles of 20 s at 72°C, 20 s at 95°C, and 20 s at 60°C, followed by a final cycle of 1 min at 72°C.
The oligonucleotides used as primers were as follows: oligonucleotide O, 5′-ATGAATTCGCAAAATCAGCAAGG-3′ (the underlined sequence is the EcoRI site); oligonucleotide P, 5′-TCGTCTCGAGCTCTAGGTCC-3′ (the underlined sequence is the SacI site); oligonucleotide Q, 5′-CAGCAGTCTAGATCAGAGTGG-3′ (the underlined sequence is the XbaI site); and oligonucleotide R, 5′-CATACGGATCCATCTTTTCC-3′ (the underlined sequence is the BamHI site). The EcoRI and SacI sites in oligonucleotides O and P, respectively, were introduced to facilitate manipulation of the DNA fragment amplified with primer pair O-P. The XbaI and BamHI sites in oligonucleotides Q and R are present in the vgbB sequence. Primer pair O-P was designed to amplify a 581-bp DNA fragment from the vatC gene (this study), and primer pair Q-R was designed to amplify a 729-bp DNA fragment from the vgbB gene (this study).
Blotting and hybridization.
Hybridization of the DNA transferred onto Hybond-N+ membranes (Amersham International) was performed under stringent conditions as described previously (8).
DNA cloning and transformation.
Standard methods were used for DNA cloning (32). E. coli was transformed by the method of Hanahan (19) with selection on Luria-Bertani agar containing 100 μg of ampicillin per ml. S. aureus RN4220 was transformed by electroporation as described previously (2).
DNA sequencing.
An automated 373A DNA sequencer (Applied Biosystems, Inc.) and the protocol described by the manufacturer were used for sequencing. The sequencing reaction was performed by PCR amplification in a final volume of 20 μl with 500 ng of plasmid DNA, 10 pmol of primer, and 9.5 μl of a dye terminator premix. After heating at 94°C for 2 min, the reaction was carried out as follows: 25 cycles of 30 s at 94°C and 30 s at 55°C and then 4 min at 60°C (9600 thermal cycler; Perkin-Elmer). Excess dye terminators were removed with Quick Spin columns (Boehringer Mannheim). The samples were dried in a vacuum centrifuge and dissolved in 4 μl of a deionized mixture (5/1; vol/vol) of formamide and 50 mM EDTA (pH 8). The samples were loaded onto the sequencer and run for 12 h in a 4.5% denaturing acrylamide gel.
For the sequencing of vatC, the following primers were used: 5′-GAAATGGTTGGGAGAAGCATACC-3′, 5′-AATCGGCAGAATTACAAACG-3′, 5′-CAGCAATCGCGCCCGTTTG-3′, and 5′-CGTTCCCAATTTCCGTGTTACC-3′. For the sequencing of vgbB, the following primers were used: 5′-GTTTCTATGCTGATCTGAATC-3′, 5′-GGTCTAAATGGCGATATATGG-3′, 5′-GTCGTTTGTAATTCTGCCGATT-3′, and 5′-TTCGAATTCTTTTATCCTACC-3′.
Sequence analysis.
The aa sequence was analyzed with the Genetics Computer Group package. The aa sequences of vatC and vgbB were compared by using the program TFastA with those deduced from nucleotide sequences in the GenBank-EMBL Data Library. The aa sequences were aligned according to the algorithm in the Clustal V package.
Nucleotide sequence accession number.
The sequence of plasmid pIP1714 described in this paper has been deposited in the GenBank-EMBL Data Library under accession no. AFO15628.
RESULTS AND DISCUSSION
Isolation of plasmids pIP1714 and pIP1742 harbored by S. cohnii subsp. cohnii BM10711.
Two plasmids harbored by BM10711 were introduced separately by electroporation into recipient strain S. aureus RN4220. pIP1714 was found in a transformant selected on brain heart infusion agar supplemented with 10 μg of PIIA per ml, and pIP1742 was found in a transformant selected on brain heart infusion agar supplemented with 20 μg of PIB per ml. Plasmid pIP1714 conferred resistance to PIIA (MIC, 32 μg/ml) and PIB (MIC, 16 μg/ml), whereas pIP1742 conferred constitutive resistance to macrolides, lincosamides, and PIB (MIC, 64 μg/ml). The ability of the transformants carrying each of these two plasmids to inactivate PIIA and PIB was tested by the microbiological test described by Gots (17). The transformant harboring pIP1714 inactivated PIIA and PIB, whereas neither of these two antibiotics was inactivated by the transformant containing pIP1742.
Sequence of pIP1714.
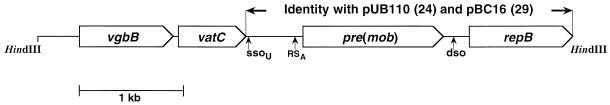
Cleavage of pIP1714 with HindIII generated a single fragment of 5 kb which was ligated into the HindIII site of pOX7 (14), giving pIP1715. The sequence of the HindIII insert (4,978 nucleotides [nt]) in pIP1715 was determined (Fig. 1) and is registered in the GenBank-EMBL Data Library under accession no. AFO15628. Four putative genes were detected, and all were in the same transcriptional orientation. Two of these genes are the same as the repB and pre(mob) genes in pUB110 (24) and pBC16 (29). One other gene is similar to the gene vgb (69.5% nt sequence identity) and was named vgbB, and the fourth gene is similar to the genes vat, vatB, and satA (71.7, 62.2, and 64.1% nt sequence identities, respectively) and was named vatC.
FIG. 1.
Physical map of the S. cohnii subsp. cohnii plasmid pIP1714 (4,978 bp).
The vgbB gene (885 nt) is delimited by a potential ATG start codon at nt 399 to 401 and a stop codon at nt 1284 to 1286. The potential start codon is preceded 7 nt upstream by a 5-nt putative ribosome-binding site (RBS; GGAGG). The free energy of association of the most stable structure between the putative RBS and the 3′ terminus of the 16S rRNA calculated as described by Tinoco et al. (34) is −60.2 kJ/mol. The G+C content of the gene is 38.9%. It encodes a 295-aa protein with a calculated molecular mass of 32.7 kDa. VgbB is very similar to the staphylococcal Vgb lactonase which inactivates B compounds (5) (67.0% identical aa and 81.9% similar aa). The common aa are distributed throughout the peptide chain (data not shown). No significant similarities were detected between the two SgB lactonases, Vgb and VgbB, and other peptide sequences in data banks. The molecular masses of Vgb and VgbB are similar to that of a lactonase in Actinoplanes missouriensis which inactivates virginiamycin B components (35 kDa) (20).
The putative gene vatC (636 nt) extends from an ATG start codon at nt 1307 to 1309 to the stop codon at nt 1943 to 1945. The start codon is preceded 10 nt upstream by an 8-nt putative RBS (TGGGAGTG). The free energy of association of the most stable structure between the putative RBS and the 3′ terminus of the 16S rRNA calculated as described by Tinoco et al. (34) is −44.3 kJ/mol. The G+C content of the gene is 36.3%. It encodes a 212-aa protein with a calculated molecular mass of 23.6 kDa; the sequence of this protein is similar to those of the three known SgA acetyltransferases with similar molecular masses: Vat (24.3 kDa; 69.8% identical aa and 83.5% similar aa), VatB (23.3 kDa; 58.2% identical aa and 77.4% similar aa), and SatA (23.3 kDa; 66.0% identical aa and 80.1% similar aa). These four SgA acetyltransferases have in common 48 identical aa and a repeated sequence of an isoleucine patch (13) also found in the peptide sequences of several homotrimer acetyltransferases which modify various substrates (25).
Putative −35 and −10 promoter sequences are present upstream from the vgbB gene but not upstream from the contiguous vatC gene, suggesting that the two genes may be coordinately transcribed. The reason why the staphylococcal vat-related genes (vat, vatB, and vatC) are regularly downstream from another gene encoding resistance to streptogramins (vgb, vgaB, and vgbB, respectively) and with which they appear to be cotranscribed remains unclear.
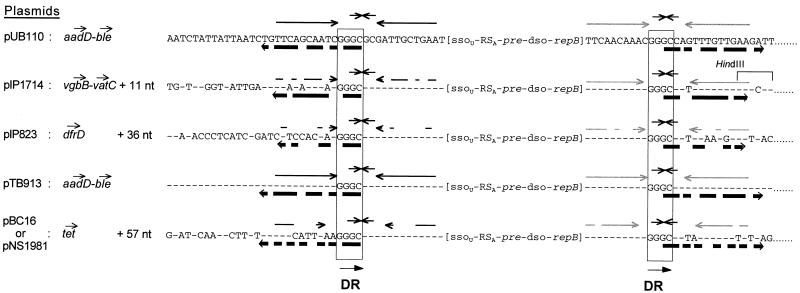
The region of pIP1714 located between nt 1984 and 4968 is the same as or very similar to regions in five rolling-circle replication and mobilizable plasmids (3.7 to 4.6 kb) previously isolated from strains belonging to gram-positive species: pUB110 from S. aureus (24), pBC16 from Bacillus cereus (29), pNS1981 from Bacillus subtilis (33), pTB913 from thermophilic Bacillus (27), and pIP823 from Listeria monocytogenes (7). These plasmids carry various antibiotic resistance genes. The similar regions include the replication pUB110-repB gene (24), recombination and mobilization pre(mob) genes (35), dso and ssoU sequences consisting of double-strand and single-strand replication origins, respectively (10, 11, 18, 35), and RSA sites corresponding to the origin transfer by mobilization (35). The similar regions of the six plasmids are delimited by two direct repeats (GGGC; see Fig. 2). In pUB110 and pNS1981, several inverted repeats overlapping part of the GGGC repeats were observed (31). Similar inverted repeats were also found in pIP1714, pBC16, pTB913, and pIP823, and those in pUB110 and pTB913 carrying the same antibiotic resistance genes (aadD and ble) exhibit the best matches (Fig. 2). These direct GGGC repeats and the multiple inverted, more or less perfect repeats including part of the GGGC sequences may be subject to site-specific breakage and joining, making this fragment available as a cassette to pick up antibiotic resistance genes and thus to trigger their transfer between gram-positive species via conjugative mobilization and/or interplasmid recombination (10, 18, 26–28).
FIG. 2.
Alignment of the nucleotide sequences flanking the ssoU sequences (previously named palU) and the repB gene of five plasmids: pUB110 (24), pIP1714 (this study), pIP823 (7), pTB913 (27), and pBC16 (29), also named pNS1981 (31). Dashes represent the nt which are identical to those of pUB110 (24). The direct GGGC repeats (DR) delimiting the conserved part of the plasmids are boxed, and the inverted repeats overlapping the direct repeats are indicated by arrows.
The RepB and pIP1714 Pre(Mob) proteins are similar to several other plasmid-encoded proteins present in the data bank, but the corresponding genes are not necessarily linked on the same plasmid. The plasmids encoding these proteins are between 1.8 and 8.7 kb and were found in strains belonging to various gram-positive genera (Bacillus, Lactococcus, Streptococcus).
The presence of highly related plasmids in staphylococci, bacilli, and L. monocytogenes is indicative of recent horizontal spread, probably facilitated by the high frequency of homologous and illegitimate recombination (18). Various pieces of evidence led Novick (26) to suggest that pUB110 is native to bacilli: its higher degree of stability and higher copy number in B. subtilis and the fact that it is the only staphylococcal rolling-circle replication plasmid for which there is functional lagging strand conversion. Moreover, Novick (26) and Polak and Novick (29) have reported that pBC16-like plasmids are widely distributed among aerobic spore-forming bacilli. Thus, it is likely that pIP1714 is also native to bacilli but that the genes encoding resistance to streptogramins may have been acquired from another source.
Distribution of the vgbB and vatC genes among 53 independent wild-type isolates resistant to the synergistic mixtures of A and B compounds.
Plasmids pIP1740 and pIP1741 were used as probes in hybridization experiments under stringent conditions. pIP1740 is pUC18 carrying, between the EcoRI and SacI sites, a vatC intragenic fragment of 581 bp amplified with the primer pair O-P and cleaved with the same enzymes. pIP1741 consists of the vgbB intragenic fragment of 729 bp amplified with the primer pair Q-R and cleaved with XbaI and BamHI inserted between the XbaI and BamHI sites of pUC18. The 53 staphylococcal isolates analyzed included S. cohnii subsp. cohnii BM10711 harboring pIP1714, which was used as a positive control. Both probes gave a strong positive signal with BM10711, whereas none of the 52 other isolates contained hybridizing nucleotide sequences.
The habitat of S. cohnii subsp. cohnii is human skin, where it produces small and transient populations (21). The presence of BM10711 on the top of a cupboard in a trauma ward of the Douera Hospital in Algiers therefore probably resulted from human contamination. Ptr S. cohnii subsp. urealyticum strains, usually found on humans and other primates, were isolated from the same environment (sheet, tray, needle) (23). The detection of Ptr S. cohnii strains in all the environmental samples of the hospital ward may result from their ability to persist because of an intrinsic resistance to environmental stresses and/or because of the substantial prevalence in this species of plasmids carrying streptogramin resistance genes. None of the Ptr staphylococci isolated from the pus of seven patients suffering from osteomyelitis and hospitalized in the same ward of Douera Hospital were S. cohnii species. The strains from these patients were S. aureus (six patients) and S. epidermidis (one patient), and the single Ptr staphylococcal strain isolated from a nurse’s hands was S. haemolyticus (23). The presence of Ptr S. cohnii strains on the skin flora of the patients treated with Pt cannot be ruled out, but because this species is rarely responsible for infectious diseases, the probability of finding such strains in pus samples is low. Note that in this ward, Pt was used extensively both by oral administration for the long-term treatment of chronic osteomyelitis and by inappropriate direct application of the powder to wounds. In Douera Hospital, the prevalence of Ptr staphylococci was higher (20%) than that elsewhere in Algeria (4.5%) and that in most French hospitals (≤5%). The relatively high prevalence of these strains was mostly a result of the independent selection of staphylococci of various taxa or types harboring diverse genes and plasmids conferring resistance to the mixtures of A and B compounds.
Conclusions.
The multiplicity of plasmids and genes conferring resistance to A and B compounds and to their mixtures, including quinuspristin-dalfopristin, must be kept in mind when administering these antibiotics for therapy or using them as additives in animal feed.
ACKNOWLEDGMENTS
We are grateful to Olivier Chesneau for valuable discussions and Catherine Tran for secretarial assistance.
REFERENCES
- 1.Allignet J, Aubert S, Morvan A, El Solh N. Distribution of the genes encoding resistance to streptogramin A and related compounds among the staphylococci resistant to these antibiotics. Antimicrob Agents Chemother. 1996;40:2523–2528. doi: 10.1128/aac.40.11.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allignet J, El Solh N. Diversity among the gram-positive acetyltransferases inactivating streptogramin A and structurally related compounds and characterization of a new staphylococcal determinant, vatB. Antimicrob Agents Chemother. 1995;39:2027–2036. doi: 10.1128/aac.39.9.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allignet J, El Solh N. Characterization of a new staphylococcal gene, vgaB, encoding a putative ABC transporter conferring resistance to streptogramin A and related compounds. Gene. 1997;202:133–138. doi: 10.1016/s0378-1119(97)00464-2. [DOI] [PubMed] [Google Scholar]
- 4.Allignet J, Loncle V, El Solh N. Sequence of a staphylococcal plasmid gene, vga, encoding a putative ATP-binding protein involved in resistance to virginiamycin A-like antibiotics. Gene. 1992;117:45–51. doi: 10.1016/0378-1119(92)90488-b. [DOI] [PubMed] [Google Scholar]
- 5.Allignet J, Loncle V, Mazodier P, El Solh N. Nucleotide sequence of a staphylococcal plasmid gene, vgb, encoding a hydrolase inactivating the B components of virginiamycin-like antibiotics. Plasmid. 1988;20:271–275. doi: 10.1016/0147-619x(88)90034-0. [DOI] [PubMed] [Google Scholar]
- 6.Allignet J, Loncle V, Simenel C, Delepierre M, El Solh N. Sequence of a staphylococcal gene, vat, encoding an acetyltransferase inactivating the A-type compounds of virginiamycin-like antibiotics. Gene. 1993;130:91–98. doi: 10.1016/0378-1119(93)90350-c. [DOI] [PubMed] [Google Scholar]
- 7.Charpentier E, Courvalin P. Emergence of the trimethoprim resistance gene dfrD in Listeria monocytogenes BM4293. Antimicrob Agents Chemother. 1997;41:1134–1136. doi: 10.1128/aac.41.5.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chesneau O, Allignet J, El Solh N. Thermonuclease gene as a target nucleotide sequence for specific recognition of Staphylococcus aureus. Mol Cell Probes. 1993;7:301–310. doi: 10.1006/mcpr.1993.1044. [DOI] [PubMed] [Google Scholar]
- 9.Cocito, C., M. Digiambattista, E. Nyssen, and P. Vannuffel. 1997. Inhibition of protein synthesis by streptogramins and related antibiotics. J. Antimicrob. Chemother. 39(Suppl. A):7–13. [DOI] [PubMed]
- 10.del Solar G, Moscoso M, Espinosa M. Rolling circle-replicating plasmids from gram-positive and gram-negative bacteria: a wall falls. Mol Microbiol. 1993;8:789–796. doi: 10.1111/j.1365-2958.1993.tb01625.x. [DOI] [PubMed] [Google Scholar]
- 11.Dempsey L A, Zhao A C, Khan S A. Localization of the start sites of lagging-strand replication of rolling-circle plasmids from gram-positive bacteria. Mol Microbiol. 1995;15:679–987. doi: 10.1111/j.1365-2958.1995.tb02377.x. [DOI] [PubMed] [Google Scholar]
- 12.Derbise A, Dyke K G H, El Solh N. Rearrangements in the staphylococcal β-lactamase-encoding plasmid, pIP1066, including a DNA inversion that generates two alternative transposons. Mol Microbiol. 1995;17:769–779. doi: 10.1111/j.1365-2958.1995.mmi_17040769.x. [DOI] [PubMed] [Google Scholar]
- 13.Dicker I B, Seetharam S. What is known about the structure and function of the Escherichia coli protein Fir A? Mol Microbiol. 1992;6:817–823. doi: 10.1111/j.1365-2958.1992.tb01532.x. [DOI] [PubMed] [Google Scholar]
- 14.Dyke K G H, Curnock S P. The nucleotide sequence of a small cryptic plasmid found in Staphylococcus aureus and its relationship to other plasmids. FEMS Microbiol Lett. 1989;43:209–215. doi: 10.1016/0378-1097(89)90040-2. [DOI] [PubMed] [Google Scholar]
- 15.Ericson H M, Sherris J C. Antibiotic susceptibility testing. Report of an international collaborative study. Acta Pathol Microbiol Scand Suppl. 1971;217:11–90. [PubMed] [Google Scholar]
- 16.Gibson T J. Ph.D. thesis. Cambridge, England: Cambridge University; 1984. [Google Scholar]
- 17.Gots J S. The detection of penicillinase-producing properties of microorganisms. Science. 1945;102:309. doi: 10.1126/science.102.2647.309. [DOI] [PubMed] [Google Scholar]
- 18.Gruss A, Ehrlich S D. The family of highly interrelated single-stranded deoxyribonucleic acid plasmids. Microbiol Rev. 1989;53:231–241. doi: 10.1128/mr.53.2.231-241.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 20.Hou C T, Perlman D, Schallock M R. Microbial transformation and properties of peptide antibiotics. VI. Purification and properties of a peptide lactonase hydrolyzing dihydrostaphylomycin S. J Antibiot. 1970;70:35–42. doi: 10.7164/antibiotics.23.35. [DOI] [PubMed] [Google Scholar]
- 21.Kloos W E, Wolfshohl J F. Staphylococcus cohnii subspecies: Staphylococcus cohnii subsp. cohnii subsp. nov. and Staphylococcus cohnii subsp. urealyticum subsp. nov. Int J Syst Bacteriol. 1991;41:284–289. doi: 10.1099/00207713-41-2-284. [DOI] [PubMed] [Google Scholar]
- 22.Kreiswirth B N, Lofdahl S, Bethey M J, O’Reilly M, Shlievert P M, Bergdoll M S, Novick R P. The toxic shock exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983;306:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 23.Liassine N, Allignet J, Morvan A, Aubert S, El Solh N. Analysis of pristinamycin-resistant staphylococci selected in an Algerian hospital by the extensive and inappropriate use of pristinamycin (Pt) Zentbl Bakteriol Parasitenkd Infektkrankh Hyg Abt I Orig. 1997;286:389–399. doi: 10.1016/s0934-8840(97)80097-7. [DOI] [PubMed] [Google Scholar]
- 24.McKenzie T, Hoshino T, Tanaka T, Sueoka N. The nucleotide sequence of pUB110: some salient features in relation to replication and its regulation. Plasmid. 1986;15:93–103. doi: 10.1016/0147-619x(86)90046-6. [DOI] [PubMed] [Google Scholar]
- 25.Murray I A, Shaw W V. O-Acetyltransferases for chloramphenicol and other natural products. Antimicrob Agents Chemother. 1997;41:1–6. doi: 10.1128/aac.41.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novick R P. Staphylococcal plasmids and their replication. Annu Rev Microbiol. 1989;43:537–565. doi: 10.1146/annurev.mi.43.100189.002541. [DOI] [PubMed] [Google Scholar]
- 27.Oskam L, Hillenga D J, Venema G, Bron S. The integrated state of the rolling-circle plasmid pTB913 in the composite Bacillus plasmid pTB19. Mol Gen Genet. 1992;233:462–468. doi: 10.1007/BF00265444. [DOI] [PubMed] [Google Scholar]
- 28.Perkins J B, Youngman P. Streptococcus plasmid pAMα1 is a composite of two separable replicons, one of which is closely related to Bacillus plasmid pBC16. J Bacteriol. 1983;155:607–615. doi: 10.1128/jb.155.2.607-615.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polak J, Novick R P. Closely related plasmids from Staphylococcus aureus and soil bacilli. Plasmid. 1982;7:152–162. doi: 10.1016/0147-619x(82)90074-9. [DOI] [PubMed] [Google Scholar]
- 30.Rende-Fournier R, Leclercq R, Galimand M, Duval J, Courvalin P. Identification of the satA gene encoding a streptogramin A acetyltransferase in Enterococcus faecium BM4145. Antimicrob Agents Chemother. 1993;37:2119–2125. doi: 10.1128/aac.37.10.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakaguchi R, Shishido K. A unique DNA structure of the junction of homologous and nonhomologous regions between tetracycline-resistance plasmid pNS1981 and kanamycin-resistance plasmid pUB110. Nucleic Acids Res. 1987;15:7202. doi: 10.1093/nar/15.17.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 33.Shishido K, Noguchi N, Kim C, Ando T. Isolation of a tetracycline-resistance plasmid excised from a chromosomal DNA sequence in Bacillus subtilis. Plasmid. 1983;10:224–234. doi: 10.1016/0147-619x(83)90036-7. [DOI] [PubMed] [Google Scholar]
- 34.Tinoco I, Jr, Borer P N, Dengler B, Levine M D, Uhlenbeck O C, Crothers D M, Gralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973;246:40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- 35.van der Lelie D, Bron S, Venema G, Oskam L. Similarity of minus origins of replication and flanking open reading frames of plasmids pUB110, pTB913 and pMV158. Nucleic Acids Res. 1989;17:7283–7294. doi: 10.1093/nar/17.18.7283. [DOI] [PMC free article] [PubMed] [Google Scholar]