Abstract
Data System. The UK Department of Health and Social Care funded the REal-time Assessment of Community Transmission-2 (REACT-2) study to estimate community prevalence of SARS-CoV-2 IgG (immunoglobulin G) antibodies in England.
Data Collection/Processing. We obtained random cross-sectional samples of adults from the National Health Service (NHS) patient list (near-universal coverage). We sent participants a lateral flow immunoassay (LFIA) self-test, and they reported the result online. Overall, 905 991 tests were performed (28.9% response) over 6 rounds of data collection (June 2020–May 2021).
Data Analysis/Dissemination. We produced weighted estimates of LFIA test positivity (validated against neutralizing antibodies), adjusted for test performance, at local, regional, and national levels and by age, sex, and ethnic group and area-level deprivation score. In each round, fieldwork occurred over 2 weeks, with results reported to policymakers the following week. We disseminated results as preprints and peer-reviewed journal publications.
Public Health Implications. REACT-2 estimated the scale and variation in antibody prevalence over time. Community self-testing and -reporting produced rapid insights into the changing course of the pandemic and the impact of vaccine rollout, with implications for future surveillance. (Am J Public Health. 2023;113(11):1201–1209. https://doi.org/10.2105/AJPH.2023.307381)
The REal-time Assessment of Community Transmission-2 (REACT-2) study sought to provide reliable and timely estimates of the prevalence of antibodies to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection from random samples of England’s adult population.
DATA SYSTEM
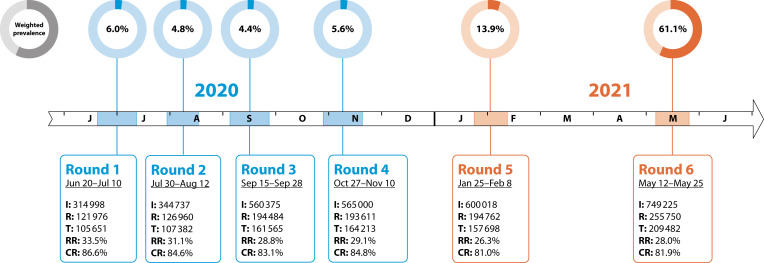
This study involved 6 rounds of data collection: from June 20, 2020, to May 25, 2021 (Figure 1).
FIGURE 1—
REACT-2 Study Timeline From June 20, 2020, to May 25, 2021, Over 6 Rounds of Data Collection: England
Note. CR = completion rate (tests/registrations); I = invitations sent; R = registrations; RR = response rate (tests/invitations); T = lateral flow immunoassay tests completed. CR is defined by the number of completed tests over the number of kits sent out and the prevalence of antibody positivity, adjusted for test characteristics and weighted to England’s adult population. Note the reported response rates are conservative because (1) not all invitations would have been received (or opened) by the potential participants, and (2) recruitment was stopped once the required sample size had been reached.
Name and Sponsor
The REACT-2 study was funded by the Department of Health and Social Care in England and sponsored by Imperial College London.
Purpose
We aimed to estimate the number and distribution of SARS-CoV-2 infections during the first and second waves of the COVID-19 pandemic in England by place and person, identify trends in antibody positivity, and subsequently measure the impact of vaccine rollout on population antibody prevalence.
Public Health Significance
REACT-2 was established following the first wave of the COVID-19 pandemic in England when little was known about the extent of SARS-CoV-2 transmission in the community because of limited access to diagnostic testing outside hospital settings. We provided estimates of cumulative community prevalence of SARS-CoV-2 IgG (immunoglobulin G) antibody test positivity with a rapid test and identified groups at highest risk of infection. In addition, we estimated the total number of individuals in England who had been infected and the infection fatality ratio overall and by age, sex, and ethnic group. REACT-2 was designed to provide repeated snapshots of the cumulative prevalence of test positivity for antibodies above the threshold of the rapid test initially from infection and later from vaccination. These data fed directly into the government through written and verbal reports to a weekly data debrief group of the UK Health Security Agency (previously Public Health England) to inform the public health response.
DATA COLLECTION/ PROCESSING
We invited random samples of adults in the community to use at-home testing with a finger prick lateral flow immunoassay (LFIA) device and to report the results along with demographic, behavioral, and clinical details in an online or telephone survey.
Data Sources and Collection Mode
Source population
We invited random cross-sectional samples of individuals aged 18 years and older in England to participate. Our sample frame was individuals on the National Health Service (NHS) patient list, which includes name, address, age, and sex of everyone registered with a general practitioner in England (almost the entire population).
Survey instruments
We collected data through a Web-based survey instrument designed and piloted with public input and hosted by our logistics partner, Ipsos (Paris, France). We mailed an invitation letter to named individuals, who were directed to an online or telephone registration site where they could consent to the study. The registration form confirmed date of birth and gathered additional information on household size and composition, occupation, education, and ethnic group (see the Appendix, available as a supplement to the online version of this article at http://www.ajph.org). We asked eligible people (which was everyone except those with possible bleeding risk from use of a lancet) for their e-mail address and mobile telephone number. Following registration, we sent participants a self-test LFIA kit, an instruction booklet linked to an online video, and a link to a Web site (or telephone option) to complete a further user survey once they had completed the test. The survey instruments are available on the study Web site (https://bit.ly/44eyByr).
Finger prick antibody test
We selected the LFIA (Fortress Diagnostics, Antrim, Northern Ireland) after we evaluated its performance characteristics (sensitivity and specificity) against predefined criteria for detection of SARS-CoV-2 IgG.1,2 The LFIA uses the structural spike (S) protein of the virus as the target antigen for antibody-based detection. We initially evaluated it for (1) sensitivity in an NHS health care worker cohort known to have been infected with SARS-CoV-2, as confirmed by RT-PCR (reverse transcription–polymerase chain reaction), at least 21 days earlier and who were not hospitalized; and (2) specificity using 500 prepandemic sera. Compared with results from at least 1 of 2 in-house ELISAs (enzyme-linked immunosorbent assay), sensitivity and specificity of finger prick blood self-test were 84.4% (95% confidence interval [CI] = 70.5%, 93.5%) and 98.6% (95% CI = 97.1%, 99.4%), respectively.1
The in-house ELISAs used were the spike protein ELISA (S-ELISA) and a hybrid spike protein receptor–binding domain double antigen–bridging assay.3 Further validation of the LFIA showed equivalent performance in an occupational cohort of people who were not health care workers4 and a cohort consisting of health care workers and renal transplant patients, all of whom self-tested after they were vaccinated.5 We also compared the self-test LFIA to a commercially available quantitative assay in 3758 participants, a majority of whom had been vaccinated or reported previous infection. The LFIA was less sensitive than the laboratory assay, being positive in 73.9% compared with 96.4% of participants; however, in a subset of 250 samples, the LFIA correlated better with live virus neutralization.6
Testing and reporting
Graphic designers specializing in health care designed the testing kit, instruction booklet, and video, with input from 300 public volunteers in a pilot study, which identified the need for improvements in elements of the kit, instructions, and interpretation of results. This was followed by a larger pilot study of more than 14 000 randomly selected members of the public, which showed high levels of acceptability and usability.7 Using the instructions provided, participants carried out the LFIA using a finger prick capillary blood sample, read the results, and reported them in the survey along with additional sociodemographic, behavioral, and clinical details (see the Appendix, available as a supplement to the online version of this article at http://www.ajph.org). We asked participants to upload a photograph of the completed test.
Ethical Procedures
Ethics
Participants gave individual consent to participate either online or by telephone. We obtained approval for use of the test kit from the Medicines and Healthcare Products Regulatory Agency (https://bit.ly/3qu6Lk9), with the caveat that the test was to be clearly labeled as for research purposes only and that participants were given advice not to change their behavior because of the result.
Public involvement
A public advisory panel provided input on the design, conduct, and dissemination of the study, and lay members sit on a data access committee governing further access to the data.
Population and Geographic Coverage
Population
The target population was England’s adult population aged 18 years and older. We aimed to provide data at the lower-tier local authority area (LTLA) level in England to aid local administrative and public health response to the pandemic. We included data for 316 of the 317 LTLAs in England (excluding Isles of Scilly), and by combining the 2 smallest with neighboring areas we report on 315 areas. We also provide national and regional estimates of antibody positivity and prevalence estimates for key demographic subgroups, including by age, ethnic group, socioeconomic status (as determined by an area-level deprivation score), and occupation. Estimates of weighted prevalence over the 6 rounds of the study are shown in Figure 1.
Sampling frame
The sampling frame was all adults 18 years and older who were registered with an NHS general practitioner in England. The NHS England holds this information, which provides near-complete coverage of the resident population.
Sampling strategy
We obtained random samples from the NHS patient list and mailed individual invitations. We stratified the sample by LTLA to achieve similar numbers of participants in each local area. For round 6 (May 2021), we adjusted the sampling to achieve a boost of 70 000 people in age groups 55 to 64 and 65 to 74 years to include additional numbers after their first and second vaccinations, because vaccines were rolled out in order of decreasing age starting in December 2020.8
Unit of Data Collection and Sample Size
Unit of data collection
We collected data at the individual level. The samples were nonoverlapping until the final boosted round, when some overlap with earlier rounds occurred, with 4950 people taking part twice over the 6 rounds.
Sample size and response rates
Over the 6 rounds of data collection from June 20, 2020, to May 25, 2021, 905 991 completed tests were included from 3 134 353 invitations, giving an overall response rate (number of completed tests/number of invitations sent out) of 28.9%. The response rate varied by round (range = 26.3%–33.5%), with completed tests ranging from 105 651 to 209 482 per round (Figure 1). The response rate also varied by sex, age, region, and deprivation score (Table A, available as a supplement to the online version of this article at http://www.ajph.org).
Sample size determination
In rounds 1 to 5, we aimed for 100 000 completed tests per round to provide meaningful information on England’s 315 LTLAs. The highest levels of uncertainty were in populations with low prevalence, where the point antibody positivity could be so low that there were no positive tests in that area. With a total of 100 000 completed tests, we were able to exclude (95% confidence) a prevalence of more than 1.7% in each LTLA recording zero positive tests. In round 6, we aimed for a total sample size of 240 000 test results, including, as noted, a boost of 70 000 people in age groups 55 to 64 and 65 to 74 years powered to detect a clinically important difference in outcome (relative risk = 0.5 for hospitalization) between individuals who tested positive and those who tested negative.
Completeness
By design, we aimed for approximately equal numbers of participants in England’s 315 LTLAs. The achieved samples at the LTLA level ranged from 200 to 598 in rounds 1 to 5 and 517 to 802 in round 6 with the boosted sample. We achieved sufficient data by round to estimate prevalence by age, region, and other key demographic groups, including ethnic group, deprivation index, and occupation.
Generalizability
Our study had a lower response among men, the youngest and oldest groups, people from minority ethnic groups, and those in more deprived areas (Table A). Unequal participation is observed in almost all population surveys. To account for the differential response, we weighted the data at each round to represent England as a whole, although this may not fully correct estimates.
Surveillance Design
This was a serial cross-sectional design, randomly selected, with largely nonoverlapping samples across 6 rounds of the study. The key was our use of at-home self-testing and results reporting from a point-of-care rapid test, which enabled us to obtain results at scale and disseminate them quickly. Most data collected were reported by participants, including history of COVID-19, comorbidities, and vaccination. However, where we had specific consent for data linkage, we were able to link to routine health data to confirm vaccination status and obtain outcome data (i.e., hospitalizations, deaths).
Frequency of Data Collection
The study was initially commissioned to estimate the total number of people who had been infected with SARS-CoV-2 in the first wave in England, which peaked in March 2020 and decreased rapidly after the introduction of a strict lockdown on March 23.9 The first round took place at the end of June 2020, followed by 3 more rounds2–4 at 6-week intervals in July and August as well as September and October 2020 (Figure 1). There was a 2-week reporting window for participants to upload their results, and the overwhelming majority performed the test and reported the results in the first few days of those periods. The final 2 rounds took place after a gap of 3 and 4 months (January and May 2021). We timed the rounds to capture the prevalence and trends in population antibody positivity: (1) after the first wave (rounds 1 and 2), (2) during the emergence of the second wave (rounds 3 and 4), and (3) to assess the impact of vaccination (rounds 5 and 6). We did not commission any further rounds.
Key Data Elements and Data Quality/Editing
Prevalence estimates
We calculated prevalence as the proportion of individuals with a positive IgG test result on the LFIA, adjusted for test performance using
| (1) |
where p is the adjusted proportion positive and q is the observed proportion positive.10
We weighted prevalence estimates (and 95% CIs) to account for the geographic sample design and for variation in response rates to be representative of the population (aged ≥ 18 years) of England (Table A). In our approach we used random iterative method weighting11 to adjust to population estimates for age, sex, index of multiple deprivation decile,12 LTLA, and ethnic group. We based the weighting approach on that described in Elliott et al.13 but for 7 rather than 9 age categories.
We used logistic regression to identify sociodemographic variation in antibody positivity by estimating the odds ratio (OR). An OR greater than 1 indicated that the group was more likely to have higher prevalence of antibody test positivity relative to the reference group per sociodemographic variable. We adjusted models for age, sex, and region as well as for ethnic group, deprivation score, household size, and occupation.
We estimated the infection fatality ratio from the total number of COVID-19 deaths among adults in England14 divided by our estimate of the total number of SARS-CoV-2 infections since the start of the pandemic until mid-July 2020. We estimated this by multiplying the weighted and adjusted antibody prevalence by the midyear population size at aged 18 years and older in England. We obtained an overall infection fatality ratio estimate of 0.90% (95% CI = 0.86, 0.94) as well as estimates stratified by age, sex, and ethnic group.15
LFIA self-testing procedure
The LFIA requires a blood sample from a finger prick and produces a test result after 10 to 15 minutes. The test kits sent to participants included 1 LFIA device, 1 bottle of buffer solution, 2 pressure-activated 23-gauge lancets, 1 alcohol wipe, and a 1-milliliter plastic pipette, alongside an instruction booklet with a link to an online video.
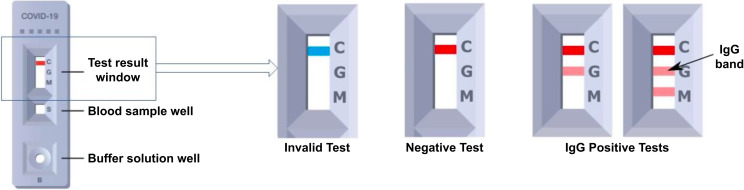
The key visual features of the Fortress SARS-CoV-2 LFIA device include the test result window and blood sample well (Figure 2). The result window has an initially blue control line, which will remain if the test is unsuccessful (i.e., invalid). In a successful test, the control line turns red, and if IgG antibodies are present in the blood sample above a threshold, a secondary line will appear below the control. There is also a line indicating IgM (immunoglobulin M), but this performed poorly in our initial laboratory evaluation and was not analyzed. We provided participants with detailed instructions on how to record the result in the questionnaire response as either negative, Ig M positive, Ig G positive, IgG and IgM positive, or invalid. We informed participants that results were not reliable at an individual level.
FIGURE 2—
Diagram of Lateral Flow Immunoassay (LFIA) Kit With Guide to Reading and Reporting the Result: England, June 20, 2020–May 25, 2021
Note. IgG = immunoglobin G. The detail of the test result window indicates what invalid, negative, and positive results look like.
Data security
We transferred data securely from Ipsos to Imperial College London and held them on secure servers in an ISO27001 environment managed by the School of Public Health. We assigned study participants a study ID and stripped data of identifying information for the statistical analyses; only a few named and designated individuals have access to identifying information, in line with a published privacy policy (see Privacy Notice Imperial College London: https://bit.ly/3YDT1Qp and Department of Health and Social Care: https://bit.ly/3skKHJf) and compliant with the UK Data Protection Act 2018, which is the UK implementation of the General Data Protection Regulation (https://www.gov.uk/data-protection).
Managing disclosure risks
To protect confidentiality, we do not release individual data, and we suppress tabular data if there are fewer than 5 entries in a cell where 1 or more person is positive for SARS-CoV-2 IgG on LFIA.
DATA ANALYSIS/DISSEMINATION
We fed the results of the REACT-2 study each round through written and verbal reports to a weekly data debrief group of the UK Health Security Agency (previously Public Health England) to provide situational awareness and inform public health policy. In addition, we placed REACT-2 data and results in the public domain in near real time (through preprints and media press releases), thus informing both the public and the international scientific community of emerging data on the prevalence of SARS-CoV-2 antibody test positivity.
Interpretation Issues
During the study period, we observed a gradual fall in response rates: from a high of 33.5% in round 1 (June 2020), which was carried out following the first wave in England, to 26.3% in round 5 (January 2021), which was conducted in the early stages of vaccine rollout. In round 6, the response rate rose to 28.0%, reflecting the boosted sample of individuals aged 55 to 74 years, who generally had high response rates to our surveys. Our surveys also had a lower response rate among people from minority ethnic groups and those in more deprived areas. We reweighted the sample in each round to account for differential variation in response to be representative of England’s population (≥ 18 years) as a whole, although this may not have overcome unknown participation biases.
We used a qualitative (yes/no) at-home self-administered LFIA on a finger prick capillary blood sample instead of more resource-intensive gold standard quantitative laboratory tests performed on venous blood samples. To demonstrate the validity of this approach, we conducted extensive evaluation of the selected LFIA, which showed it to have acceptable performance (sensitivity and specificity) compared with confirmatory laboratory tests.1 We took steps to measure and improve usability, including ability to perform and read an LFIA test at home.4,7 By adjusting our survey results for known LFIA performance, we demonstrated that, despite not meeting regulatory standards for clinical use in individuals, self-testing and -reporting using LFIAs provide a valid tool for obtaining reliable community-wide prevalence estimates in a cost-effective manner, rapidly, and at scale.
For those with a self-reported clinical history of confirmed or suspected COVID-19, there was a potential for reporting bias because respondents were not blinded to their test results; however, there was high concordance of self-test with clinician-read results. To support ongoing quality assurance for the self-tests, we designed an automated lateral flow analysis computerized pipeline using machine learning, computer vision techniques, and signal-processing algorithms to analyze the uploaded images of the test16; we found high concordance with reported self-test results.
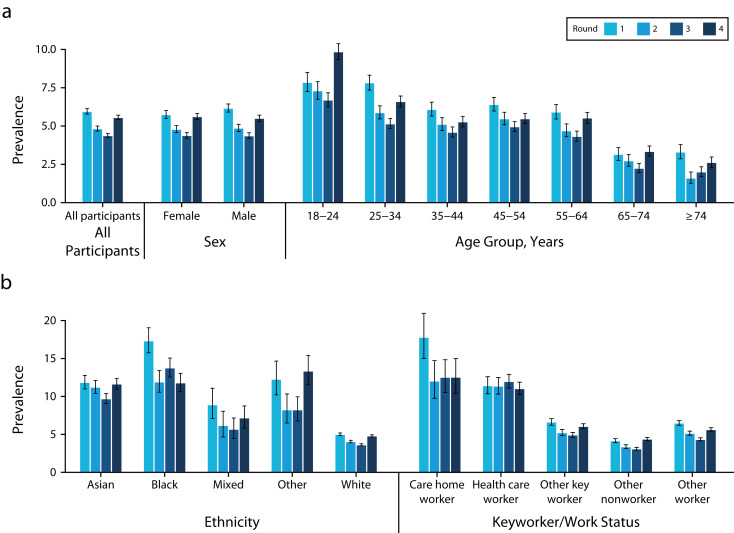
Our study demonstrated a substantial decrease (26.5%) in population antibody test positivity over 3 months between rounds 1 and 3 (June 20–September 28, 2020), indicating antibody waning 3 and 6 months after the first wave of infections (Figure 3).17 To exclude the possibility that that this could be attributable to differences in LFIA batch, we compared the laboratory performance of the LFIAs used in rounds 1 and 2 (where we had seen the strongest decrease in positive tests) and found no difference between the 2 rounds.
FIGURE 3—
Antibody Prevalence With Confidence Intervals by Round for Rounds 1–4 (before vaccination), in the Sample (a) Overall and Stratified by Sex and Age, and (b) Stratified by Ethnic Group and Employment: England, June 20, 2020–May 25, 2021
Note. Estimates were adjusted and weighted except for employment where data were not available for weighting.
Linkage Ability
Data linkage (based on unique NHS number) to vaccination status (i.e., vaccine type and date) and outcome data (i.e., hospitalizations, deaths) is available for participants who consented to linkage to their health records.
Data Release/Accessibility
Access to REACT-2 individual-level data is restricted to protect participants’ anonymity. Summary statistics, descriptive tables, and code from REACT-2 are available on Github (https://bit.ly/3EC15be), and study materials for each round are on the study Web site (https://bit.ly/3sgrybg).
Key References and Other Information
We published our initial protocol18 and our key findings during the 11 months of fieldwork,15,17,19–21 including clinical and laboratory evaluation of antibody tests and feasibility studies of at-home self-testing and -reporting using LFIAs2,5–7,16 in preprints and peer-reviewed journal publications. Links to all our publications are given on the study Web site (https://bit.ly/3KPg8l4) and included for reference in the appendix.
PUBLIC HEALTH IMPLICATIONS
REACT-2 provided reliable and robust estimates of population prevalence of SARS-CoV-2 IgG antibody test positivity during the first 2 waves of the COVID-19 pandemic and the initial stages of vaccine rollout in England. It demonstrated high feasibility and acceptability of using at-home self-administered LFIA tests (self-reported and uploaded photo for verification) as a means of providing reliable, cost-effective, community-wide prevalence estimates rapidly and at scale. This contrasts with the use of quantitative laboratory assays, which require blood to be collected, transported, and processed in a laboratory.
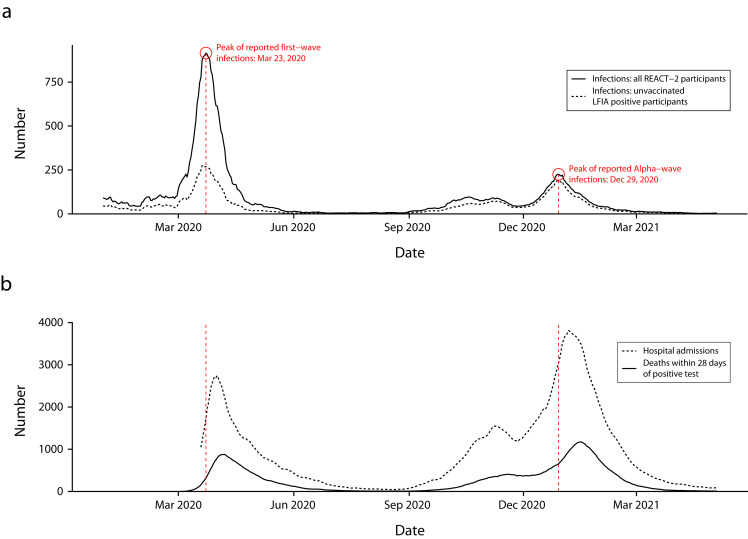
REACT-2 confirmed early reports that SARS-CoV-2 disproportionately affected people from disadvantaged and minority ethnic groups in England, as well as health and care workers (Figure 3), suggesting that the higher hospitalization and mortality from COVID-19 in these population groups reflected higher rates of infection. We found no difference in the estimated infection fatality ratio between people of broad ethnic categories (Black, Asian, White) when stratified by age and sex.15 Based on participant responses to questions about onset of previous COVID-19 symptoms, we were able to reconstruct a pandemic curve for infection in early 2020 that closely matched but slightly predated the curves of hospitalizations and deaths.15 This gives context validity and provides an indication of the size and shape of the first and second waves (Figure 4). The pandemic curve was replicated in each round, providing further validation of the approach.15,17,19,20
FIGURE 4—
Reconstruction of COVID-19 Pandemic Curve by (a) Week of Symptom Onset Reported by REACT-2 Participants, Alongside (b) National Data on Admissions and Deaths From COVID-19: England, June 20, 2020–May 25, 2021
Note. LFIA = lateral flow immunoassay; REACT-2 = REal-time Assessment of Community Transmission-2. In part a, the solid line includes date of onset for all cases of COVID-19 reported by participants, and the dashed line is limited to those who had a positive LFIA test result in the REACT-2 study.
We also provided timely information on changes in the prevalence of antibody positivity over time as a result of both natural infection and vaccination (Figure 1). The observed decrease in population antibody positivity following the first wave (Figure 3) supported emerging data on SARS-CoV-2 that indicated a decrease over time in antibody levels (i.e., waning) in a proportion of individuals followed in longitudinal studies.22 Before vaccination, we observed waning of 26.5% over 3 months, with the biggest decrease in older people.17 In the later rounds, by tracking antibody test positivity to COVID-19 following vaccination and showing differential waning, our study provided key data underpinning vaccination policy and contributed to recommendations regarding groups who might benefit from additional vaccine doses.20,21
Finally, the success of REACT-2 was strengthened by rapid public involvement at every stage. Public volunteers and a diverse advisory panel provided input into the design and conduct of the study. Their desire to support the national response shows that public involvement is both possible and necessary during periods of emergency response.
Antibody self-testing at home is feasible and acceptable and can provide essential data to policymakers within days. To roll this out quickly in future pandemics, it is important to invest in the necessary technologies and infrastructure,23 including test production, implementation logistics, and study design and data analysis.
ACKNOWLEDGMENTS
This study was funded by the Department of Health and Social Care in England. H. Ward acknowledges support from the National Institute for Health and Care Research (NIHR) Imperial Biomedical Research Centre, and the NIHR Applied Research Collaboration Northwest London. C. A. Donnelly acknowledges support from the Medical Research Council Centre for Global Infectious Disease Analysis, the NIHR Health Protection Research Unit in Emerging and Zoonotic Infections, and the NIHR-funded Vaccine Efficacy Evaluation for Priority Emerging Diseases (grant PR-OD-1017-20007). M. Chadeau-Hyam acknowledges support from Cancer Research UK (grant 22184), H2020-EXPANSE (Horizon 2020 grant 874627), and H2020-LongITools (Horizon 2020 grant 874739). G. S. Cooke is supported by an NIHR professorship. P. Elliott acknowledges support from Health Data Research UK, the NIHR Imperial Biomedical Research Centre, NIHR Health Protection Research Units in Chemical and Radiation Threats and Hazards (grant NIHR-200922), and Environmental Exposures and Health (grant NIHR-200880), the British Heart Foundation Centre for Research Excellence at Imperial College London (grant RE/18/4/34215), and the UK Dementia Research Institute at Imperial College London (grant MC_PC_17114).
We thank key collaborators on this work: K. Beaver, S. Clemens, G. Welch, N. Gilby, K. Ward, G. Pantelidou, and K. Pickering at Ipsos; G. Fontana and J. Alford at the Institute of Global Health Innovation at Imperial College London; E. Johnson, R. Elliott, G. Blakoe, M. Piggin, and H. Johnson at the School of Public Health, Imperial College London, UK. We also thank the REACT Public Advisory Panel and all participants in the study. We thank the National Health Service for access to the patient register, and the Department of Health and Social Care for logistical support.
Note. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of this article.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
HUMAN PARTICIPANT PROTECTION
The REACT-2 study received ethical approval from the South Central Berkshire B Research Ethics Committee, UK. Participants gave individual consent to participate either online or by telephone.
See also Tancredi and Chiolero, p. 1143.
REFERENCES
- 1.Flower B, Brown JC, Simmons B, et al. Clinical and laboratory evaluation of SARS-CoV-2 lateral flow assays for use in a national COVID-19 seroprevalence survey. Thorax. 2020;75(12):1082–1088. doi: 10.1136/thoraxjnl-2020-215732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moshe M, Daunt A, Flower B, et al. SARS-CoV-2 lateral flow assays for possible use in national COVID-19 seroprevalence surveys (REACT 2): diagnostic accuracy study. BMJ. 2021;372(423):n423. doi: 10.1136/bmj.n423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan M, Rosadas C, Katsanovskaja K, et al. Simple, sensitive, specific self-sampling assay secures SARS-CoV-2 antibody signals in sero-prevalence and post-vaccine studies. Sci Rep. 2022;12(1):1885. doi: 10.1038/s41598-022-05640-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies B, Araghi M, Moshe M, et al. Acceptability, usability, and performance of lateral flow immunoassay tests for severe acute respiratory syndrome coronavirus 2 antibodies: REACT-2 study of self-testing in nonhealthcare key workers. Open Forum Infect Dis. 2021;8(11):ofab496. doi: 10.1093/ofid/ofab496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cann A, Clarke C, Brown J, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody lateral flow assay for antibody prevalence studies following vaccination: a diagnostic accuracy study. Wellcome Open Res. 2021;6:358. doi: 10.12688/wellcomeopenres.17231.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atchison CJ, Moshe M, Brown JC, et al. Validity of self-testing at home with rapid severe acute respiratory syndrome coronavirus 2 antibody detection by lateral flow immunoassay. Clin Infect Dis. 2023;76(4):658–666. doi: 10.1093/cid/ciac629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atchison C, Pristerà P, Cooper E, et al. Usability and acceptability of home-based self-testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies for population surveillance. Clin Infect Dis. 2021;72(9):e384–e393. doi: 10.1093/cid/ciaa1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. UK Department of Health and Social Care. UK COVID-19 vaccines delivery plan. January. 2023;13 https://www.gov.uk/government/publications/uk-covid-19-vaccines-delivery-plan/uk-covid-19-vaccines-delivery-plan [Google Scholar]
- 9.UK Prime Minister’s Office. Prime minister’s statement on coronavirus (COVID-19) 2020. p. 23.https://www.gov.uk/government/speeches/pm-address-to-the-nation-on-coronavirus-23-march-2020
- 10.Diggle PJ. Estimating prevalence using an imperfect test. Epidemiol Res Int. 2011;608719 doi: 10.1155/2011/608719. [DOI] [Google Scholar]
- 11.Sharot T. Weighting survey results. J Mark Res Soc. 1986;28(3):269–284. [Google Scholar]
- 12.McLennan D, Noble S, Noble M, Plunkett E, Wright G, Gutacker N.The English Indices of Deprivation 2019. 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/833951/IoD2019_Technical_Report.pdf
- 13.Elliott P, Whitaker M, Tang D, et al. Design and implementation of a national SARS-CoV-2 monitoring program in England: REACT-1 study. Am J Public Health. 2023;113(5):545–554. doi: 10.2105/AJPH.2023.307230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Public Health England. Excess mortality in England. 2020. https://fingertips.phe.org.uk/static-reports/mortality-surveillance/excess-mortality-in-england-week-ending-17-Jul-2020.html
- 15.Ward H, Atchison C, Whitaker M, et al. SARS-CoV-2 antibody prevalence in England following the first peak of the pandemic. Nat Commun. 2021;12(1):905. doi: 10.1038/s41467-021-21237-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong NCK, Meshkinfamfard S, Turbé V, et al. Machine learning to support visual auditing of home-based lateral flow immunoassay self-test results for SARS-CoV-2 antibodies. Commun Med (Lond). 2022;2:78. doi: 10.1038/s43856-022-00146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward H, Cooke GS, Atchison C, et al. Prevalence of antibody positivity to SARS-CoV-2 following the first peak of infection in England: serial cross-sectional studies of 365,000 adults. Lancet Reg Health Eur. 2021;4:100098. doi: 10.1016/j.lanepe.2021.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riley S, Atchison C, Ashby D, et al. REal-time Assessment of Community Transmission (REACT) of SARS-CoV-2 virus: study protocol. Wellcome Open Res. 2021;5:200. doi: 10.12688/wellcomeopenres.16228.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward H, Atchison C, Whitaker M, et al. Increasing SARS-CoV-2 antibody prevalence in England at the start of the second wave: REACT-2 round 4 cross-sectional study in 160,000 adults. medRxiv. 2021. [DOI]
- 20.Ward H, Cooke G, Whitaker M, et al. REACT-2 round 5: increasing prevalence of SARS-CoV-2 antibodies demonstrate impact of the second wave and of vaccine roll-out in England. medRxiv. 2021. [DOI]
- 21.Ward H, Whitaker M, Flower B, et al. Population antibody responses following COVID-19 vaccination in 212,102 individuals. Nat Commun. 2022;13(1):907. doi: 10.1038/s41467-022-28527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choe PG, Kang CK, Suh HJ, et al. Waning antibody responses in asymptomatic and symptomatic SARS-CoV-2 infection. Emerg Infect Dis. 2021;27(1):327–329. doi: 10.3201/eid2701.203515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Budd J, Miller BS, Weckman NE, et al. Lateral flow test engineering and lessons learned from COVID-19. Nat Rev Bioeng. 2023;1:13–31. doi: 10.1038/s44222-022-00007-3. [DOI] [Google Scholar]