Abstract
BACKGROUND
Transfemoral aortic valve replacement (TAVR) is the standard treatment for elderly patients with aortic valve stenosis. Although safe and well-established, there is a risk of intraprocedural hemodynamic instability and silent cerebral embolism, which can lead to a decline in neurocognitive function and dementia. In clinical practice, comprehensive cognitive testing is difficult to perform. AI-assisted digital applications may help to optimize diagnosis and monitoring.
METHODS
Neurocognitive function was assessed by validated psychometric tests using “∆elta -App”, which uses artificial intelligence and computational linguistic methods for extraction and analysis. Memory function was assessed using the ‘Consortium to Establish a Registry for Alzheimer’s Disease’ (CERAD) word list and digit span task (DST) before TAVR and before hospital discharge. The study is registered in the German Register of Clinical Trials (https://drks.de/search/de/trial/DRKS00020813).
RESULTS
From October 2020 until March 2022, 141 patients were enrolled at University Hospital Heart Centre Brandenburg. Mean age was 81 ± 6 years, 42.6% were women. Time between the pre- and post-interventional test was on average 6 ± 3 days. Memory function before TAVR was found to be below average in relation to age and educational level. The pre-post TAVR comparison showed significant improvements in the wordlist repeat, P < 0.001 and wordlist recall test of CERAD, P < 0.001. There were no changes in the digital span test.
CONCLUSIONS
Despite impaired preoperative memory function before TAVR, no global negative effect on memory function after TVAR was detected. The improvements shown in the word list test should be interpreted as usual learning effects in this task.
Cognitive performance is usually conceptualized in terms of functional domains such as memory, attention, language, or executive functions.[1] Cognitive impairments in the domains of attention and memory have been documented in patients with heart failure (HF).[2,3] Heart failure is caused by increased afterload and myocardial remodeling and is a multifactorial consequence in patients with severe AS.[4] Because of the considerable risk of open-heart surgery, transfemoral aortic valve replacement (TAVR) is the standard therapy in elderly patients at medium or high risk. In these patients, functional aspects, especially cognitive function, are of immense importance.[5] Several factors could affect cognition in TAVR patients. One key factor contributing to cognitive decline is cerebral hypoperfusion, which may be exacerbated by low cardiac output.[6] Studies show that low cardiac output is also associated with more rapid cognitive decline in patients with cardiovascular disease.[7,8] Moreover, intraprocedural hemodynamic instability during TAVR could lead to cerebral blood flow restriction, with the risk of organ-specific ischemia, micro-embolism or stroke, which are typical complications after TAVR.[9,10] On the other hand, improved cardiac output is associated with increased cerebral blood flow, particularly in the hippocampus,[11] and was associated with improved cognitive function after TAVR.[12] A recent meta-analysis showed that preexisting cognitive impairment was a significant risk factor for worse outcomes after TAVR.[13] Cognitive function appears to improve or stabilize after TAVR, particularly in patients with preexisting cognitive impairment.[14,15]
Digital neuropsychological testing may offer advantages over traditional paper-pencil testing because comprehensive cognitive testing is difficult to perform in clinical practice. Artificial Intelligence-powered digital applications may help optimizing diagnosis and monitoring. The aim of this study was to assess the cognitive functions of TAVR patients with a focus on changes in memory functions using a digital application (∆elta-app, KI-Elements, Germany) before and after TAVR.
METHODS
Patients
This prospective cohort study enrolled adult patients undergoing elective TAVR at the University Hospital Heart Center Brandenburg between October 2020 and March 2022. Inclusion criteria comprised patients with severe symptomatic aortic stenosis who were scheduled for TAVR and were classified as high surgical risk patients according to the European Society of Cardiology guidelines.[16] Exclusion criteria were emergency surgery, chronic dialysis, and lack of written informed consent for study participation.
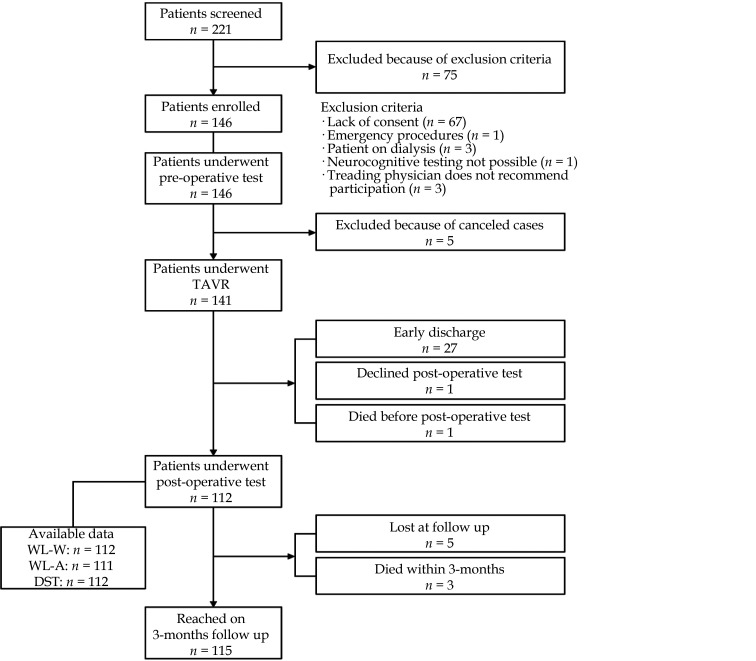
A multidisciplinary valve team that included interventional cardiologists, cardiothoracic surgeons, and cardiovascular anesthesiologists was involved in the allocation of surgical or nonsurgical treatment for all patients. Three months after discharge, patients and general practitioners were followed up by telephone to collect quantitative data on subjective well-being, outcome, and outpatient laboratory values. Patient flow is shown in Figure 1.
Figure 1.
Patient flow through the study.
TAVR: transfemoral aortic valve replacement.
The study was approved by local ethics committee (E-01-20191006) and is registered in the German Register of Clinical Trials (DRKS00020813).
TAVR procedure
The majority of patients were admitted on the day before the procedure. TAVR was performed in the hybrid catheterization laboratory under fluoroscopy guidance with the use of contrast media. The TAVR device was delivered through femoral approach in all patients. Procedures were performed under local anesthesia with conscious sedation or general anesthesia with endotracheal intubation. The prosthesis size was determined using preprocedural echocardiographic and multi-slice computed tomography angiogram findings. During TAVR, old valve is probed from the inside with a catheter. The calcified valve leaflets are pushed open while the artificial valve develops and replaces it.[17]
Neurocognitive Function Assessment
Neurocognitive function was assessed with validated tests (CERAD-WL and Digit Span Task, DST) using the “∆elta -App”. ∆elta is a certified medical product that uses artificial intelligence (AI) and computational linguistic methods for extraction and language recognition. For human validation of the AI-assisted automated scoring, all test results were manually cross-checked by a trained psychologist. A third generation iPad Air from Apple with the operating system iPadOS 14.4 was used to conduct the test.
CERAD-Wordlist (WL-W/WL-A):
The CERAD Word list is a cognitive assessment tool that measures immediate (WL-W) and delayed (WL-A) memory for new and non-associated verbal information. It assesses the ability to learn and retain new words, which falls within the sub-domain of memory. In WL-W, ten words are presented acoustically one after the other in three rounds. The order of the words is changed in each round. After each presentation, patients were asked to repeat as many words as they remember, regardless of order. At the end of our cognitive test battery, patients were asked to name the ten words presented in WL-W without being preceded by another acoustic presentation (WL-A).
Digit-Span-Task
The Digit Span Test (DST) is a neuropsychological assessment tool, that measures short-term memory, verbal attention and working memory.[18]
In this test, patients repeated from memory an increasing number of digits in the given order. The first two rounds start with a digit span of two. If the sequence of digits is reproduced correctly in at least one of the two rounds, two more rounds follow in which the digit span is increased by one more digit. In the planned sixteen rounds, the digit range will be increased to nine. If the digit range was repeated incorrectly or not at all in both rounds, the last correct digit range was taken as the maximum digit range that this subject could remember correctly, and the test was over. Alternatively, the subject may repeat the digit sequence correctly in all rounds and reach a maximum digit range of nine.
Data Collection
Medical records were reviewed until hospital discharge. The following information was obtained: demographics, comorbidities, procedural characteristics including valve type and size, intra- and post-procedural complications during the index hospital stay (cardiac decompensation, need for packed red blood cells, sepsis, or septic shock), laboratory parameters, length of stay in hospital after TAVR, in-hospital mortality as well as discharge status. Rehospitalization within 90 days after discharge, 90-day mortality and major adverse cardiac events (MACE) were assessed by a questionnaire sent to the treating primary care physician. In addition, patients were interviewed 90 days after hospital discharge using a structured telephone interview (Appendix).
Statistical Analysis
Patients were divided into one of three groups according to age following CERAD norm data. For statistical analysis, a paired t-test and a two-way ANOVA as well as chi square test for pre- and postoperative changes according to age group was used. The basis for assigning the level of cognitive impairment followed the recommendation according to CERAD.[19] To estimate the cognitive status of the patients before TAVR, test scores were transformed into age-, sex- and education-corrected standardized scores as implemented in delta-app. Postoperative cognitive decline was defined as post-TAVR performance decrease of more than 1 SD compared with the score before (i.e., individual delta score post-TAVR minus pre-TAVR of > 1 SD) A P value of less than 0.05 was considered statistically significant. SPSS 29 (IBM, Armonk, NY, USA) was used.
RESULTS
From October 2020 to March 2022, 146 patients were enrolled, of whom 141 underwent TAVR. Mean age was 81 ± 6 years with a mean Euro-Score II of 10.41% ± 7.10%. Baseline characteristics are shown in Table 1. The mean time between preoperative cognitive assessment and TAVR was 2.0 ± 5.2 days; between TAVR and postoperative assessment 5.0 ± 3.1 days. The mean time between preoperative and postoperative cognitive assessment was 6 ± 3 days with minimum of 3 days and maximum of 31 days. A postoperative stroke was diagnosed in 2.8% cases, 3.5% suffered postoperative delirium and 14.9% required a pacemaker implantation after TAVR. The mean duration of hospital stay was 11 ± 6 days, 22.3% of patients were rehospitalized within 90 days. Overall, 3 patients died within 90 days, 5 patients within 180 days (Table 2).
Table 1. Baseline characteristics.
| Patient characteristics | ≤ 69 years | 70–79 years | ≥ 80 years | overall | P-value |
| CABG: coronary artery bypass grafting. Values are expressed as means ± SD or n (%). | |||||
| Age, yrs | 65 ± 4 | 76 ± 3 | 84 ± 3 | 81 ± 6 | < 0.001 |
| Female | 2/8 (25%) | 17/38 (44.7%) | 41/95 (43.2%) | 60/141 (42.6%) | 0.578 |
| Body mass index, kg/m2 | 30 ± 6 | 29 ± 6 | 27 ± 5 | 28 ± 5 | 0.11 |
| Smoker | 3/7 (42.9%) | 3/33 (9.1%) | 10/95 (10.5%) | 16/135 (11.9%) | 0.03 |
| EuroScore, % | 7.32 ± 3.76 | 8.69 ± 6.12 | 11.43 ± 7.52 | 10.41 ± 7.10 | 0.06 |
| Formal education, years | 14 ± 2 | 10 ± 3 | 11 ± 4 | 11 ± 4 | 0.069 |
| Comorbidities | |||||
| Arterial hypertension | 6/8 (75%) | 31/38 (81.6%) | 77/95 (81.1%) | 114/141 (80,9%) | 0.908 |
| Coronary heart disease | 5/8 (62.5%) | 25/38 (65.8%) | 61/95 (64.2%) | 91/141 (64.5%) | 0.978 |
| Atrial fibrillation | 0/8 (0%) | 7/38 (18.4%) | 41/95 (43.2%) | 48/141 (34.0%) | 0.003 |
| Peripheral vascular disease | 2/8 (25%) | 3/38 (7.9%) | 6/95 (6.3%) | 11/141 (7.8%) | 0.167 |
| Acute decompensated heart failure | 1/8 (12.5%) | 0/38 (0%) | 4/95 (4.2%) | 5/141 (3.5%) | 0.183 |
| NYHA-Classification | 3 ± 1 | 3 ± 0 | 3 ± 1 | 3 ± 1 | 0.915 |
| NYHA-Class I | 0/8 (0%) | 1/34 (2.9%) | 1/90 (1.1%) | 2/132 (1.5%) | |
| NYHA-Class II | 2/8 (25.0%) | 8/34 (23.5%) | 18/90 (20%) | 28/132 (21%) | |
| NYHA-Class III | 5/8 (62.5%) | 25/34 (73.6%) | 65/90 (72.2%) | 95/132 (71.3%) | |
| NYHA-Class IV | 1/8 (12.5%) | 0/34 (0%) | 6/90 (6.7%) | 7/132 (5.3%) | |
| Previous myocardial infarction | 1/8 (12.5%) | 3/38 (7.9%) | 9/95 (9.5%) | 13/141 (9.2%) | 0.909 |
| Stroke | 0/8 (0%) | 2/38 (5.3%) | 7/95 (7.4%) | 9/141 (6.4%) | 0.677 |
| Chronic kidney disease | 3/8 (37.5%) | 11/36 (30.6%) | 40/95 (42.1%) | 54/139 (38.8%) | 0.479 |
| Hypertensive heart disease | 0/8 (0%) | 8/38 (21.1%) | 10/95 (10.5%) | 18/131 (12.8%) | 0.139 |
| Hyperlipoproteinemia | 4/8 (50%) | 20/38 (52.6%) | 39/95 (41.1%) | 63/141 (44.7%) | 0.456 |
| Diabetes type II (insulin) | 4/8 (50%) | 11/38 (28.9%) | 18/95 (18.9%) | 33/141 (23.4%) | 0.088 |
| Chronic obstructive pulmonary disease | 0/8 (0%) | 5/38 (13.2%) | 9/95 (9.5%) | 14/141 (9.9%) | 0.510 |
| Previous PTCA | 2/8 (25%) | 13/38 (34.2%) | 38/95 (40.0%) | 53/141 (37.6%) | 0.619 |
| Previous CABG | 2/8 (25%) | 6/38 (15.8%) | 6/95 (6.3%) | 14/141 (9.9%) | 0.087 |
| Cardiac device | 0/8 (0%) | 1/38 (2.6%) | 15/95 (15.8%) | 16/141 (11.3%) | 0.056 |
| Laboratory parameters | |||||
| Serum creatinine, µmol/L | 100 ± 26 | 93 ± 46 | 99 ± 33 | 97 ± 36 | 0.703 |
| eGFR, ml/min | 65 ± 27 | 69 ± 21 | 59 ± 19 | 62 ± 20 | 0.041 |
| NT-proBNP, pg/mL | 7398 ± 9023 | 2213 ± 3829 | 4274 ± 7083 | 39002 ± 6580 | 0.101 |
| Hemoglobin, mmol/L | 8.3 ± 1.0 | 8.1 ± 1.1 | 7.9 ± 1.0 | 8.0 ± 1.0 | 0.406 |
| Echocardiographic parameters | |||||
| LVEF (%) | 47 ± 14 | 50 ± 12 | 49 ±15 | 50 ±14 | 0.867 |
| TAPSE (mm) | 21 ± 5 | 20 ± 6 | 20 ± 5 | 20 ± 5 | 0.986 |
| Procedural characteristics | |||||
| Valve type | 0.011 | ||||
| Evolut R | 4/8(50%) | 14/36 (38.9%) | 65/95 (68.4%) | 83/139 (59.7%) | |
| Evolut Pro | 0/8 (0%) | 9/36 (35%) | 16/95 (16.8%) | 25/139 (18.0%) | |
| Sapien 3** | 4/8 (50%) | 13/36 (30.6) | 13/95 (14.8%) | 30/139 (21.6%) | |
| Balloon-expanding valve | 4/8 (50%) | 12/37 (32.4%) | 13/95 (13,7%) | 29/140 (20.7%) | 0.006 |
| Self-expanding valve | 4/8 (50%) | 25/37 (67.6%) | 82/95 (86.3%) | 111/140 (79.3%) | 0.006 |
| Pre-dilatation | 7/8 (87.5%) | 25/37 (67.6%) | 64/95 (67.4%) | 96/140 (68.6%) | 0.494 |
| Post-dilation | 2/8 (25%) | 7/37 (18.9%) | 20/95 (21.1%) | 29/140 (20.7%) | 0.919 |
| Valve-in-valve | 1/8 (12.5%) | 1/37 (2.7%) | 4/95 (4.7%) | 6/140 (4.3%) | 0.462 |
Table 2. Process parameter and outcome grouped according to age.
| Patient characteristics | ≤ 69 years | 70 – 79 years | ≥ 80 years | Overall | P-value |
| Values are expressed as means ± SD or n (%). AKI: acute kidney injury; MACE: major adverse cardiac events; TAVR: transfemoral aortic valve replacement. | |||||
| Process parameter | |||||
| Time between (days) | |||||
| preoperative cognitive test an TAVR | 1.9 ± 1.6 | 1.7 ± 1.5 | 2.0 ± 6.2 | 2.0 ± 5.2 | 0.677 |
| TAVR and postoperative cognitive test | 4.2 ± 1.3 | 5.1 ± 4.9 | 5.8 ± 1.3 | 4.5 ± 3.1 | 0.395 |
| preoperative tests and tests post TAVR | 5.8. ± 1.3 | 6.9 ± 5.1 | 5.8 ± 2.0 | 6.1 ± 3.3 | 0.211 |
| Outcome | |||||
| Died within procedure | 0/8 (0%) | 0/38 (0%) | 0/95 (0)% | 0/141 (0%) | |
| Admission to intensive care | 3/7 (42.9%) | 3/35 (8.6%) | 1/92 (1.1%) | 7/134 (5.2%) | < 0.001 |
| AKI | 3/5 (60%) | 2/24 (8.3%) | 13/66 (19.7%) | 18/95 (18.9) | 0.026 |
| Hemodynamic relevant pericardial effusion | 0/8 (0%) | 1/38 (2.6%) | 0/95 (0%) | 1/141 (0.7%) | < 0.001 |
| Delir | 1/8 (12.5%) | 1/38 (2.6%) | 3/95 (3.2%) | 5/141 (3.5%) | 0.366 |
| Stroke | 0/8 (0%) | 1/38 (2.6%) | 3/95 (3.2%) | 4/141 (2.8%) | 0.872 |
| Pacemaker implantation | 2/8 (25%) | 3/38 (7.9%) | 16/95 (16.8%) | 21/141 (14.9%) | 0.302 |
| Bleeding requiring transfusion | 1/8 (12.5%) | 5/36 (13.9%) | 4/95 (4.2%) | 10/141 (7.1%) | 0.134 |
| Length of stay in hospital, days | 16.5 ± 11.8 | 11.7 ± 5.7 | 10.8 ± 5.0 | 11.0 ± 6.0 | 0.026 |
| Discharge status | |||||
| Home | 8/8 (100%) | 33/38 86.8%) | 84/95 (88.4%) | 125/141 (88.7%) | 0.562 |
| Rehabilitation | 0/8 (0%) | 2/38 (5.3% | 3/95 (3.2%) | 5/141 (3.5%) | 0.718 |
| Nursing-Home | 0/8 (0%) | 0/38 (0%) | 1/95 (1.1%) | 1/141(0.7%) | 0.784 |
| Other hospital | 0/8 (0%) | 3/38 (7.9%) | 6/95 (6.3%) | 9/141 (6.4%) | 0.708 |
| Died in hospital | 0/8 (0%) | 0/38 (0%) | 1/95 (1.1%) | 1/141 (0.7%) | 0.784 |
| Follow-up | |||||
| Died within 3 months | 0/7 (0%) | 0/36 (0%) | 3/90 (3.3%) | 3/133 (2.3%) | 0.480 |
| Rehospitalization within 3 months | 1/6 (16.7%) | 6/25 (24.0%) | 16/72 (22.2%) | 23/103 (22.3%) | 0.927 |
| MACE within 3 months | 0/5 (0%) | 2/19 (10.5) | 2/58 (3.4%) | 4/82 (4.9%) | 0.403 |
| Died within 6 months | 1/7 (14.3%) | 0/27 (0%) | 4/60 (6.7%) | 5/94 (5.3%) | 0.222 |
| Rehospitalization within 6 months | 2/6 (33.3%) | 5/34 (14.7% | 10/81 (12.5%) | 17/121 (14.1%) | 0.281 |
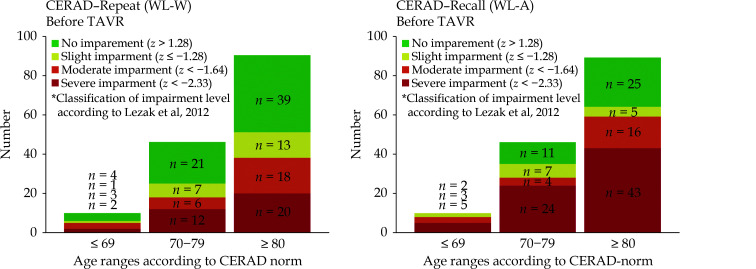
Preoperative CERAD-Wordlist WL-W and WL-A in comparison to CERAD-comparison group
There was a significant decrease with age in memory function (Table 3). Regardless of the age group, significant pre-intervention impairment in memory function was evident in both WL-W and WL-A to the standard comparison group defined by Luck, et al.[20] Before implantation, adjusted for age and education level, TAVR patients had a significant lower memory function, compared to reported comparison group of healthy individuals (Z-value MW (SD), Table 3, Figure 2).
Table 3. Memory function depending on age group.
| Age | ≤ 69 years | 70–79 years | ≥ 80 years | P-value |
| Values are expressed as means ± SD for absolute data and as % and SD for relative values. DST: digit span test; WL-A: wordlist repeat; WL-W: wordlist recall. | ||||
| Preoperative memory function | ||||
| WL-W-task, n = 146 | n = 10 | n = 46 | n = 90 | |
| Repeated words | 16.10 ± 2.38 | 15.35 ± 3.50 | 13.17 ± 3.75 | < 0.001 |
| Repeated words z-value | –1.73 ± 1.10 | –1.48 ± 1.15 | –1.46 ± 1.11 | 0.769 |
| WL-A-task, n = 145 | n = 10 | n = 46 | n = 89 | |
| Repeated words | 3.20 ± 1.48 | 2.65 ± 2.30 | 1.79 ± 2.06 | 0.023 |
| Repeated words z-value | -2.48 ± 1.01 | -2.20 ± 1.21 | -2.00 ± 1.09 | 0.351 |
| DST, n = 146 | n = 10 | n = 46 | n = 90 | |
| Digit span | 6.00 ± 1.25 | 5.39 ± 1.06 | 5.40 ± 0.95 | 0.191 |
| Pre-post-operative comparison | ||||
| WL-W, n = 112 | n = 5 | n = 39 | n = 68 | |
| Change of mean repeated words (t1-t0) | 2.00 ± 3.08 | 2.56 ± 2.81 | 1.90 ± 3.46 | 0.590 |
| Changes in % | 13.47% ± 19.59% | 16.79% ± 19.67% | 19.26% ± 32.82% | 0.849 |
| WL-A, n = 111 | n = 5 | n = 39 | n = 67 | |
| Change of mean repeated words (t1-t0) | 0.40 ± 2.30 | 0.74 ± 1.67 | 0.94 ± 1.99 | 0.758 |
| Changes in % | 70% ± 186.58% | 50.62% ± 121.83% | 83.63% ± 166.09% | 0.565 |
| DST, n = 111 | n = 5 | n = 40 | n = 68 | |
| Change of mean repeated numbers (t1-t0) | –0.20 | –0.05 | 0.06 | 0.748 |
| Changes in % | –2.5 ± 13.4 | 0.46 ± 18.37 | 1.51 ± 15.46 | 0.846 |
Figure 2.
CERAD-wordlist repeat (WL-W) and wordlist recall (WL-A) before TAVR according to age groups.
TAVR: transfemoral aortic valve replacement.
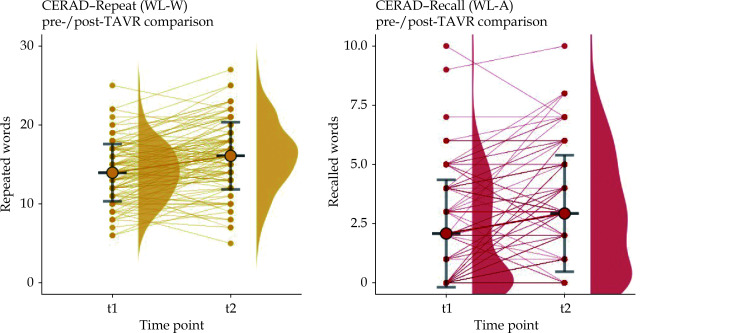
CERAD-Wordlist Pre- and Post-operative
In our study cohort there was a significant decrease in pre-interventional memory function with age, for WL-W (P < 0.001) and WL-A (P = 0.023). After TAVR-implantation, memory function increased in both tests: WL-W (P < 0.001) WL-A (P < 0.001) (Figure 3). This effect was independent of age.
Figure 3.
CERAD-wordlist repeat (WL-W) and wordlist recall (WL-A) in pre- and post-TAVR comparison.
WL-W: P < 0.001, WL-A: P < 0.001. TAVR: transfemoral aortic valve replacement.
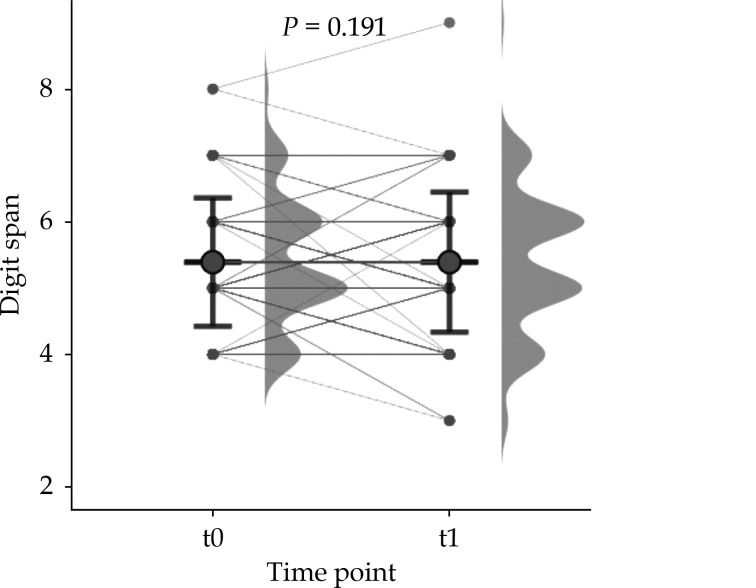
Digit Span Pre- and Post-operative
The mean digit span was 5.39 numbers. There was no age-related effect in memory function in the DST-task, F= 1.68, P = 0.191. Also, no significant changes after TAVR could be found (Figure 4).
Figure 4.
Digit-Span (DST) in pre- and post-TAVR comparison.
TAVR: transfemoral aortic valve replacement.
DISCUSSION
A major proportion of our study cohort had a severe impairment in memory function prior to TAVR implantation. Compared to other normative data with even more liberal criteria, where downward deviations are weighted less heavily,[20] our subjects perform comparatively poorly.
Particularly for high-risk and elderly patients, TAVR has become the standard of care. Recent research suggests, that TAVR is also suitable as a treatment option for patients with lower operative risk and its use is expected to increase in the future.[21,22]
After TAVR, WL-W and WL-A showed significantly better memory function. Such improvements are expected as learning effects and have been previously reported for this type of task.[23] A limitation to transferability is that, unlike the comparative psychometric validation data, our cohort underwent TAVR implantation as an additional intervention between pre- and posttest. However, the fact that there were no significant changes in DST underscores the likelihood, that the significant changes in CERAD were only learning effects. In conclusion, we can at least exclude a systematic negative effect of TAVR on deterioration of memory function as measured by WL-W/WL-A and DST with the “∆elta -App”.
These findings are consistent with previous published data in this field, that could not show a systematic effect on global cognition after TAVR.[14,15] When applied to our methodology, this underlines the feasibility of assessing cognitive function in TAVR patients using established but digitized tasks, which has only been reported with a comparable test-battery in an unselected elderly population.[24] Given the lack of conclusive evidence and the potential for underdiagnosis and underreporting of adverse cognitive outcomes after TAVR, digital cognitive assessment may provide a robust methodology for future research or routine individualized measurement in patients undergoing TAVR. AI-assisted analysis offers the opportunity to lower the inhibition threshold for cognitive function assessment and facilitate the collection of these endpoints. An individual assessment of cognition seems particularly necessary. Talbot-Hamon et al. pointed out, that “by using mean scores, the larger pool of individuals with cognitive stability or improvement is likely to dilute and mask the small but clinically relevant subset of individuals with cognitive decline after TAVR”.[25]
There are some limitations to our study. Unlike the original published version of CERAD, which uses a visual presentation of the wordlist, we used an auditory presentation of the wordlist in the “∆elta -App". Comparative studies have shown that working memory (i.e., word recall) did not show a visual superiority effect over auditory administration.[26] As a significant proportion of the age group had significant visual impairment and may have under-reported dyslexia that impaired visual comprehension–an auditory administration may be more appropriate for these older individuals. On the other hand, hearing impairments may also have had an impact on test performance. To counter this, each patient was asked before starting the word list whether auditive presentation could be understood clearly. Future research should verify advantages and disadvantages with a larger data set comparing visually and auditorily administered cognitive screening tests in high age TAVR-collectives.
In conclusion, a considerable proportion of the study population had significantly worse memory function before TAVR in contrast to the age-standardized comparison group. An overall negative effect of TAVR on memory could not be demonstrated.
DISCLOSURE
The authors express their special thanks to Saskia Klawa and David Reiners for data extraction.
Fundings
Funded by the Ministry of Science, Research and Cultural Affairs of the State of Brandenburg and the MHB publication fund supported by DFG. Jonathan Nübel declares that this work is supported by a research grant of the German Cardiac Society (DGK).
Author contributions
Conceptualization: JN, AHF; Methodology: JN, AHF, CB, MH; Formal analysis and investigation: JN, MH Writing—original draft preparation: JN, AHF; Performance of experiments or therapy: JN, CB, MH; Writing—review and editing: MH, JS, GF, CB; Funding acquisition and Resources: JN, AHF; Supervision: AHF All authors approved the final manuscript.
Financial & competing interests’ disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Ethics
This study was approved by the Ethics committee of the Brandenburg Medical School (MHB) E-01-20191006.
Acknowledgments
The authors express their special thanks to Saskia Klawa and David Reiners for data extraction.
References
- 1.Harvey PD Domains of cognition and their assessment. Dialogues Clin Neurosci. 2019;21:227–237. doi: 10.31887/DCNS.2019.21.3/pharvey. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goh FQ, Kong WKF, Wong RCC, et al. Cognitive impairment in heart failure-a review. Biology (Basel) 2022; 11.
- 3.Frey A, Sell R, Homola GA, et al Cognitive deficits and related brain lesions in patients with chronic heart failure. JACC Heart Fail. 2018;6:583–592. doi: 10.1016/j.jchf.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Genereux P, Pibarot P, Redfors B, et al Staging classification of aortic stenosis based on the extent of cardiac damage. Eur Heart J. 2017;38:3351–3358. doi: 10.1093/eurheartj/ehx381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoenenberger AW, Zuber C, Moser A, et al Evolution of cognitive function after transcatheter aortic valve implantation. Circ Cardiovasc Interv. 2016;9:e003590. doi: 10.1161/CIRCINTERVENTIONS.116.003590. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Rosenberg GA Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42:3323–3328. doi: 10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okonkwo OC, Cohen RA, Gunstad J, Poppas A Cardiac output, blood pressure variability, and cognitive decline in geriatric cardiac patients. J Cardiopulm Rehabil Prev. 2011;31:290–2907. doi: 10.1097/HCR.0b013e318220a817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eggermont LH, de Boer K, Muller M, et al Cardiac disease and cognitive impairment: a systematic review. Heart. 2012;98:1334–1340. doi: 10.1136/heartjnl-2012-301682. [DOI] [PubMed] [Google Scholar]
- 9.Kahlert P, Al-Rashid F, Dottger P, et al Cerebral embolization during transcatheter aortic valve implantation: a transcranial Doppler study. Circulation. 2012;126:1245–1255. doi: 10.1161/CIRCULATIONAHA.112.092544. [DOI] [PubMed] [Google Scholar]
- 10.Fanning JP, Wesley AJ, Platts DG, et al The silent and apparent neurological injury in transcatheter aortic valve implantation study (SANITY): concept, design and rationale. BMC Cardiovasc Disord. 2014;14:45. doi: 10.1186/1471-2261-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vlastra W, van Nieuwkerk AC, Bronzwaer AGT, et al Cerebral blood flow in patients with severe aortic valve stenosis undergoing transcatheter aortic valve implantation. J Am Geriatr Soc. 2021;69:494–499. doi: 10.1111/jgs.16882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuchiya S, Matsumoto Y, Suzuki H, et al Transcatheter aortic valve implantation and cognitive function in elderly patients with severe aortic stenosis. EuroIntervention. 2020;15:e1580–e1587. doi: 10.4244/EIJ-D-19-00489. [DOI] [PubMed] [Google Scholar]
- 13.Sim JJL, Ling RR, Neo VSQ, et al The Impact of cognitive impairment on clinical outcomes after transcatheter aortic valve implantation (from a systematic review and meta-analysis) Am J Cardiol. 2022;185:63–70. doi: 10.1016/j.amjcard.2022.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Lai KS, Herrmann N, Saleem M, Lanctot KL Cognitive outcomes following transcatheter aortic valve implantation: a systematic review. Cardiovasc Psychiatry Neurol. 2015;2015:209569. doi: 10.1155/2015/209569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan MM, Herrmann N, Gallagher D, et al Cognitive outcomes after transcatheter aortic valve implantation: a meta-analysis. J Am Geriatr Soc. 2018;66:254–262. doi: 10.1111/jgs.15123. [DOI] [PubMed] [Google Scholar]
- 16.Vahanian A, Beyersdorf F, Praz F, et al 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632. doi: 10.1093/eurheartj/ehab395. [DOI] [PubMed] [Google Scholar]
- 17.Bourantas CV, Serruys PW Evolution of transcatheter aortic valve replacement. Circ Res. 2014;114:1037–1051. doi: 10.1161/CIRCRESAHA.114.302292. [DOI] [PubMed] [Google Scholar]
- 18.Humpstone HJ Memory span tests. Psychol Clin. 1919;12:196–200. [PMC free article] [PubMed] [Google Scholar]
- 19.Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological assessment: OUP USA; 2012.
- 20.Luck T, Riedel-Heller SG, Wiese B, et al [CERAD-NP battery: Age-, gender- and education-specific reference values for selected subtests. Results of the German Study on Ageing, Cognition and Dementia in Primary Care Patients (AgeCoDe)] Z Gerontol Geriatr. 2009;42:372–384. doi: 10.1007/s00391-009-0031-y. [DOI] [PubMed] [Google Scholar]
- 21.Mack MJ. Is TAVR ready for prime time in low-risk patients? J Am Coll Cardiol 2018; 72: 2106–2108.
- 22.Popma JJ, Deeb GM, Yakubov SJ, et al Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706–1715. doi: 10.1056/NEJMoa1816885. [DOI] [PubMed] [Google Scholar]
- 23.Burkhart CS, Birkner-Binder D, Gagneux A, et al Evaluation of a summary score of cognitive performance for use in trials in perioperative and critical care. Dement Geriatr Cogn Disord. 2011;31:451–459. doi: 10.1159/000329442. [DOI] [PubMed] [Google Scholar]
- 24.Bjorngrim S, van den Hurk W, Betancort M, et al Comparing traditional and digitized cognitive tests used in standard clinical evaluation—a study of the digital application minnemera. Front Psychol. 2019;10:2327. doi: 10.3389/fpsyg.2019.02327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talbot-Hamon C, Afilalo J Cognitive function after transcatheter aortic valve replacement: reassuring findings for now. J Am Geriatr Soc. 2018;66:227–278. doi: 10.1111/jgs.15185. [DOI] [PubMed] [Google Scholar]
- 26.Shen J, Sherman M, Souza PE Test administration methods and cognitive test scores in older adults with hearing loss. Gerontology. 2020;66:24–32. doi: 10.1159/000500777. [DOI] [PMC free article] [PubMed] [Google Scholar]