Abstract
This study aimed to assess the effects of a Lactobacillus helveticus ATCC 15009-derived postbiotic in mitigating experimental Salmonella Gallinarum infection. For this purpose, a sample of Lactobacillus sp. was inoculated in 2 different media, each containing different postbiotics (sensitized and nonsensitized). Both inocula had their antagonistic effect over S. Gallinarum tested through the spot-on-the-lawn method. It revealed that the sensitized postbiotic had a higher action potential over Lactobacillus sp. than the nonsensitized one (P < 0.05). Then, 48 day of hatch chicks were divided into 4 groups: A = Lactobacillus sp. (109 CFU/mL) inoculum on the 18th day; B = Lactobacillus sp. (109 CFU/mL) inoculum on the 18th day and postbiotic inoculum on the 19th day; C = postbiotic inoculum on the 19th day; and D = sterile saline inoculum on 18th and 19th days. On the 21st day, all chicks were infected with S. Gallinarum (109 CFU/mL). On the 23rd day, the animals were euthanized by cervical dislocation, and the ceca and liver were aseptically removed. Bacterial count of S. Gallinarum with serial decimal dilution was performed with these organs. It revealed that the prophylactic treatment with the postbiotic that modulates the intestinal microbiota was as efficient as the probiotic administration in reducing S. Gallinarum in the cecum and liver of chicks (P < 0.05). These data point to a new range of alternatives for preventing S. Gallinarum, which might help the poultry industry produce safer food for human consumption.
Key words: Lactobacillus, laying hen, probiotic, salmonellosis
INTRODUCTION
Fowl typhoid (FT) is a severe septicemic poultry disease caused by Salmonella enterica subsp. enterica serovar Gallinarum biovar Gallinarum. It mainly affects broiler breeder farms and commercial egg-laying hens and requires immediate notification and control, usually through the combination of biosecurity measures and antibiotic administration (Lourenço and Berchieri, 2015). The use of antibiotics as either a prevention or control measure has been criticized because S. Gallinarum tends to remain permanently in poultry, and also the use of such substances may influence the microbiological monitoring and epidemiology of the region (Lourenço and Berchieri, 2015). The misuse of antibiotics might also result in serious collateral effects, such as dysbiosis, selection of antimicrobial drug resistance genes, residual antibiotics in animal products, and environmental contamination, which are relevant public health issues (Ewbank et al., 2021).
Thus, new products have been developed as alternatives to antibiotics for poultry, such as bacteriophages, bacteriocins, organic acids, essential oils, prebiotics, probiotics, symbiotics, and, more recently, postbiotics (Humam et al., 2021).
Metabolites produced by Lactobacillus, a lactic acid bacteria (LAB), and used as feed additives are as effective as antibiotics when it comes to enhancing the growth performance of broilers (Humam et al., 2021). These metabolites, also known as postbiotics, are defined by Martín and Langella (2019) as “nonviable bacterial products or metabolic products from microorganisms that have biologic activity in the host.”
Postbiotics are known for their salutary action on the poultry microbiome, from improving the zootechnical performance of broilers to preventing and mitigating several pathogens in the gut microbiome of poultry (Abd El-Ghany et al., 2022).
The composition of postbiotics is based on organic acids and mainly on peptides, which are responsible for promoting bacterial communication or quorum sensing (QS) between several bacteria genera, such as Lactobacillus. QS is one of the mechanisms used by LAB to ensure their survival through gene regulation (Okamoto et al., 2018). QS might be triggered by intra- and interspecies of bacteria and differ between gram-negative and gram-positive microorganisms. When a minimum bacterial density is reached, oligopeptides are produced, secreted, and recognized, thus regulating gene expression and collectively modulating the microbiota, guaranteeing the survival of salutary microorganisms (Abisado et al., 2018; Okamoto et al., 2018). Thus, postbiotics may stabilize the bird's intestinal microbiome through the competitive exclusion of pathogenic bacteria and serves as a stimulus for the host's specific and unspecific immune system (Abd El-Ghany et al., 2022).
Lactobacillus is a gram-positive microorganism that expresses QS through oligopeptides (Abisado et al., 2018) and might undergo biotic and abiotic stresses, which may trigger such behavior (Papadimitriou et al., 2016).
An in vitro experiment was carried out to verify 2 hypotheses. The first hypothesis is that a postbiotic produced by a specific Lactobacillus helveticus ATCC 15009 may meliorate the inhibition of S. Gallinarum by another Lactobacillus sp. The other hypothesis is that a biotic stress induced in L. helveticus ATCC 15009 by S. Gallinarum may lead to the production of a more efficient postbiotic, further improving the inhibition potential of Lactobacillus sp. The in vivo experiment aimed at evaluating the postbiotic with the best results in the in vitro section, in mitigating S. Gallinarum infection in layer chicks and comparing it to the administration of probiotics.
MATERIALS AND METHODS
Ethics Committee
This research was approved by the Ethics Committee on Animal Use (CEUA, FMVZ, UNESP), under protocol number 0017/2020.
Bacterial Samples
Salmonella Gallinarum
The sample of S. Gallinarum resistant to rifampicin (Rif) and nalidixic acid (Nal) used in this study belongs to the Avian Pathology Service's Bacteria Collection at the São Paulo State University (UNESP), in Botucatu, São Paulo (SP), Brazil. It was collected from commercial egg-laying hens with clinical signs of salmonellosis.
Lactobacillus Sp. and Lactobacillus Helveticus ATCC 15009
The sample of Lactobacillus sp. used as a probiotic in this study also belongs to the Avian Pathology Service's Bacteria Collection at UNESP, in Botucatu, SP, Brazil. It was collected from cloacal swabs of healthy broiler chickens. The sample of L. helveticus ATCC 15009 used as a donor for the postbiotic production was donated by the Oswaldo Cruz Foundation (FIOCRUZ), in Rio de Janeiro, RJ, Brazil.
In Vitro Experiment
Experimental Design
Three experimental groups were submitted to a spot-on-the-lawn (Okamoto et al., 2018) antagonism plate inhibition test: Lactobacillus sp. with no postbiotic, Lactobacillus sp. added to a sensitized postbiotic, and Lactobacillus sp. added to a nonsensitized postbiotic. A total of 3 replicates and 3 inhibition halos in each replicate were performed.
Postbiotic Production
Two different postbiotics were produced. To produce the sensitized postbiotic (Postbiotic A), 5 colony-forming units (CFU) of L. helveticus ATCC 15009 and 5 CFU of S. Gallinarum were both inoculated in the same 10 mL of sterile media containing ½ De Man, Rogosa, and Sharpe (MRS; Acumedia, Neogen, Lansing, MI) broth and ½ brain heart infusion (BHI; Acumedia, Neogen, Lansing, MI), followed by incubation at 38°C for 24 h. To eliminate metabolites possibly produced by S. Gallinarum, 1 mL of this culture was inoculated in 9 mL of MRS broth and incubated under the same conditions. Finally, the culture of sensitized L. helveticus ATCC 15009 was submitted to centrifugation (10,000 × g, 10 min, 4°C) and the supernatant was sterilized with a 0.22-μm cellulose membrane filter.
The nonsensitized postbiotic (Postbiotic B) was produced by inoculating 5 CFU of L. helveticus ATCC 15009 in 10 mL of MRS broth, followed by incubation at 38°C for 24 h. The culture was then submitted to centrifugation (10,000 × g, 10 min, 4°C) and the supernatant was sterilized with a 0.22-μm cellulose membrane filter. Prior to use, both postbiotics were inoculated in dishes containing nutrient agar to verify absence of contaminant microorganisms.
Plate Inhibition Test
To evaluate the postbiotics’ potential for enhancing the inhibition of S. Gallinarum by Lactobacillus sp., 200 µL of each postbiotic sample was separately added to 120 µL of a Lactobacillus sp. strain previously cultivated in MRS broth (0.5 tube on the MacFarland scale) and 200 µL of a sterile MRS broth and then incubated at 38°C for 24 h. Both inocula were submitted to the spot-on-the-lawn antagonism plate test as described by Okamoto et al. (2018). The pure Lactobacillus sp. culture was also submitted to this test to verify its natural inhibition against S. Gallinarum.
In Vivo Experiment
This section aims to evaluate the postbiotic with the best results in the in vitro section in modulating the gut microbiome of laying chicks to inhibit S. Gallinarum. For this reason, the Postbiotic A was chosen for this section.
Experimental Design
A total of 48 d of hatch laying chicks (Hy-Line W80) were randomly distributed into 4 experimental groups (A, B, C, and D) with 12 chicks each. The chicks were placed in 4 cages (0.5 × 0.8 m) within an acclimated environment. The animals were provided ad libitum, the same water and feed, specifically formulated solely to fulfill their macro and micronutrient requirements, as outlined by Rostagno et al. (2017). Each chick was considered an experimental unit, and each group was considered the experimental group.
The Lactobacillus sp. inoculum was prepared a day in advance, by adding 5 CFU of Lactobacillus sp. to a sterile Erlenmeyer flask containing 50 mL of sterile MRS broth. The mixture was then incubated at 37°C for 24 h.
The experiment started with the arrival of the chicks at day of hatch and ended on 23rd day of age of the birds. On the 18th day, Groups A and B received Lactobacillus sp. inoculum at a concentration of 1.6 × 109 CFU/mL, while groups C and D received sterile saline inoculum. On the 19th day, Groups A and C received Postbiotic A inoculum, and Groups B and D received sterile saline inoculum. On the 21st day, all Groups (A, B, C, and D) were challenged with S. Gallinarum at a concentration of 7.0 × 109 CFU/mL. On the 23rd day, all groups were submitted to euthanasia and had their liver and ceca aseptically removed for assessment of S. Gallinarum quantification.
All inocula of bacteria, sterile saline, and postbiotic were made intraesophageally, through oral gavage needle at a dose of 1 mL per chick.
Bacterial quantifications of the inocula were made through serial decimal dilutions with phosphate-buffered saline (PBS), from 10−1 to 10−8. Each dilution was poured on petri dishes containing MRS agar for Lactobacillus sp. and Brilliant Green Agar (BGA; Acumedia, Neogen, Lansing, MI) for S. Gallinarum, followed incubation at 38°C for 24 h.
Bacterial Enumeration of S. Gallinarum in Ceca and Liver
The assessment of the treatments was performed by bacterial quantification of S. Gallinarum in the liver and ceca of the chicks. After collection, each organ was separately weighed and diluted (1:10) in PBS, and serial decimal dilutions were performed as previously described. Then each dilution was poured on BGA supplemented with Nal (100 µg/mL) and Rif (100 µg/mL) and incubated at 38°C for 24 h. After this period, CFU were counted, and the results are expressed in CFU/g of organ.
Statistical Analysis
This research underwent a randomized design. GraphPad's Prism (8.0.1) and SAS (9.04.01) were used for the data analysis. The assumptions of normally distributed data from both experiments were graphically analyzed through histograms and QQ plots and were numerically analyzed through a Shapiro-Wilk test. The data were logarithmically transformed, but no normal distribution could be reached. The variables with abnormal distribution were evaluated through the Kruskal-Wallis test with Dunn's multiple comparison adjustment. The data are displayed as medians (Q1–Q3). For all results, a statistical difference was considered significant when P < 0.05.
RESULTS AND DISCUSSION
In Vitro Experiment
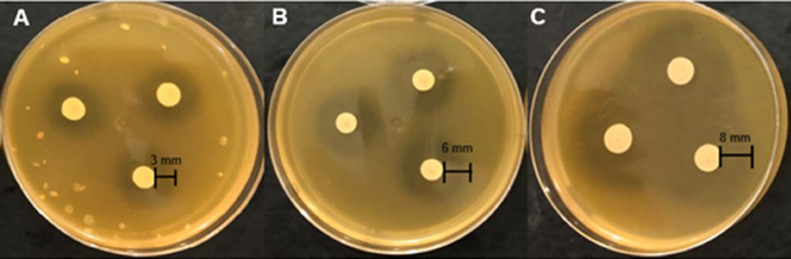
The Lactobacillus sp. possesses an intrinsic inhibition potential against S. Gallinarum, considering its median halo formed when confronted with S. Gallinarum (2.25 [1.62–3]) in the spot-on-the-lawn antagonism plate test (Table 1 and Figure 1A). Lactobacillus spp. is widely used in the animal industry as an alternative to antibiotics, given its innate antagonism against pathogenic bacteria, such as Shigella spp., Clostridium spp., Campylobacter spp., Escherichia coli, Staphylococcus aureus, Yersinia spp., and Salmonella spp. (Vuyst, 1994).
Table 1.
Median (Q1–Q3) (in mm) of the inhibition halos of Salmonella Gallinarum by Lactobacillus sp. in the spot-on-the-lawn antagonism test.
| Inoculum | Median (Q1–Q3) mm |
|---|---|
| Lactobacillus sp. + Postbiotic A | 7 (6.5–8)a |
| Lactobacillus sp. + Postbiotic B | 5 (4.2–6)b |
| Lactobacillus sp. | 2.25 (1.62–3)c |
Different lowercase letters indicate a statistically significant difference (P < 0.05) in the Kruskal-Wallis test adjusted by Dunn's multiple comparison test.
Figure 1.
Antagonistic effect of Lactobacillus sp. against Salmonella Heidelberg on the spot-on-the-lawn method. Diameters of the inhibition halos formed by: (A) pure Lactobacillus sp.; (B) Lactobacillus sp. + Postbiotic B; (C) Lactobacillus sp. + Postbiotic A.
The inhibition potential of the Lactobacillus sp. strain more than doubled after the addition of Postbiotic B when compared the medians from both Lactobacillus sp. inocula with and without Postbiotic B (5 vs. 2.25, Table 1 and Figure 1A vs. B). These data suggest the presence of substances produced by L. helveticus ATCC 15009 that induce bacterial communication and therefore enhance the antagonism of Lactobacillus sp. over S. Gallinarum. In the microenvironment, gram-positive bacteria regularly secrete QS-promoting molecules (oligopeptides), which trigger changes in bacterial gene expression when a threshold is reached. This behavior modulates the virulence, bacteriocin production, proliferation, and biochemical behavior of the microbiome (Abisado et al., 2018; Tonkin et al., 2021) promoting host health.
Furthermore, greater halos were found when Postbiotic A was added to Lactobacillus sp. (P < 0.05, Table 1 and Figure 1C), due to the significant increase in the inhibition halo medians, which were 3 times higher (2.33 vs. 7; Table 1). These findings confirm the assumption that previous biotic stress caused by S. Gallinarum in L. helveticus ATCC15009 could enhance or even increase the production of QS-inducing substances. The biotic stress is a natural process in which LAB such as Lactobacillus spp. suffer when it settles in the host microbiome or in complex communities where other microorganisms are present (Papadimitriou et al., 2016).
In Vivo Experiment
The control group of chicks presented higher median of CFU of S. Gallinarum per gram of cecum and liver (P < 0.05) compared with the other groups (Table 2). This finding confirms the assumption that birds with no treatment prior to S. Gallinarum infection are susceptible to higher concentrations of the pathogen in their organism. They are subject to higher morbidity, lower zootechnical performance, and greater environmental excretion of the pathogen, leading to the perpetuation of the disease in the flock (Milbradt et al., 2017; Gut et al., 2018).
Table 2.
Median (1° quartile–3° quartile) of colony-forming units of Salmonella Gallinarum per gram of cecum and liver.
| Medians of CFU (Q1–Q3) per organ |
|||
|---|---|---|---|
| Groups | Inoculum | Cecum | Liver |
| A | Lactobacillus sp. + Postbiotic A | 5 × 104 (0–105)a | 0 (0–0)a |
| B | Lactobacillus sp. | 103 (0–9 × 104)a | 0 (0–5 × 102)a |
| C | Postbiotic A | 2 × 104 (0–3 × 104)a | 0 (0–0)a |
| D | Control | 5 × 105 (1 × 105–1.5 × 106)b | 2 × 106 (9 × 105–4.5 × 106)b |
Different lowercase letters in the same column indicate a statistically significant difference (P < 0.05) in the Kruskal-Wallis test adjusted by Dunn's multiple comparison test.
Groups “A” and “C,” in which preventive treatments were inoculated (Lactobacillus sp. and/or Postbiotic A) presented the lowest medians of CFU of S. Gallinarum per gram of cecum and liver (P < 0.05) when compared with the control group (Table 2). Bacteria are unicellular microorganisms that have existed for at least 3.7 billion yr and have been in a constant evolutionary process ever since (Pearce et al., 2018; Granato et al., 2019). This bacterial process of selection and evolution, summarized by the “Game Theory,” made it possible for bacteria to develop and improve their cellular communication or QS (Granato et al., 2019).
QS is a complex system that collectively modulates microbial behavior through gene expression regulation. In LAB such as Lactobacillus, this system is related to the production and secretion of bacteriocin (Miller and Bassler, 2001), a short-chain peptide that presents both antimicrobial and signaling activity, which modulate cell behavior in a beneficial way, promoting survival of the genus and species.
The inocula of Postbiotic A, possibly containing short-chain peptides that promote QS, were probably responsible for the decrease in CFU of S. Gallinarum in the cecum and liver of chicks (P < 0.05; Table 2). The addition of postbiotic may have changed the chick's microbiota in a healthy way, modulating the bacterial behavior in favor of LAB and inhibiting S. Gallinarum, as occurred in the in vitro experiment.
The median (and quartiles) values of 0 found in CFU per gram of liver (Table 2), in groups “A” and “C” (inocula of Postbiotic A + Lactobacillus sp. and Postbiotic A, respectively) could be biologically relevant. However, it would require a characteristic group with at least 252 chicks/group (G*Power 3) (Faul et al., 2007), for a statistical difference (P < 0.05) to be detected when compared with the group inoculated only with Lactobacillus sp.
The in vitro experiment made it possible for our research group to conclude that biotic stress improves QS among LAB, whether by producing more and/or different oligopeptides. Furthermore, the in vivo experiment allowed us to conclude that the administration of postbiotics is equivalent to the administration of probiotics, which makes it a viable alternative for the prevention of S. Gallinarum. Although the literature over S. Gallinarum inhibition through microbiota modulation by postbiotic administration is scarce, results may be extended to other serovars, however more studies approaching such theme are needed.
DISCLOSURES
The authors declare no conflict of interest that is relevant to the content of this article.
REFERENCES
- Abd El-Ghany W.A., Abdel-Latif M.A., Hosny F., Alatfeehy N.M., Noreldin A.E., Quesnell R.R., Chapman R., Sakai L., Elbestawy A.R. Comparative efficacy of postbiotic, probiotic, and antibiotic against necrotic enteritis in broiler chickens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abisado R.G., Benomar S., Klaus J.R., Dandekar A.A., Chandler J.R. Bacterial quorum sensing and microbial community interactions. Am. Soc. Microbiol. 2018;9:13. doi: 10.1128/mBio.02331-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewbank A.C., Esperón F., Sacristán C., Sacristán I., Krul R., Cavalcanti E.M., Calatayud O., Bueno I., Strefezzi R.F., Catão-Dias J.L. Seabirds as anthropization indicators in two different tropical biotopes: a one health approach to the issue of antimicrobial resistance genes pollution in oceanic Islands. Sci. Total Environ. 2021;754 doi: 10.1016/j.scitotenv.2020.142141. [DOI] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.G., Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Granato E.T., Meiller-legrand T.A., Foster K.R. Review the evolution and ecology of bacterial warfare. Curr. Biol. 2019;29:R521–R537. doi: 10.1016/j.cub.2019.04.024. [DOI] [PubMed] [Google Scholar]
- Gut A.M., Vasiljevic T., Yeager T., Donkor O.N. Salmonella infection – prevention and treatment by antibiotics and probiotic yeasts: a review. Microbiology (United Kingdom) 2018;164:1327–1344. doi: 10.1099/mic.0.000709. [DOI] [PubMed] [Google Scholar]
- Humam A.M., Loh T.C., Foo H.L., Izuddin W.I., Zulkifli I., Samsudin A.A., Mustapha N.M. Supplementation of postbiotic RI11 improves antioxidant enzyme activity, upregulated gut barrier genes, and reduced cytokine, acute phase protein, and heat shock protein 70 gene expression levels in heat-stressed broilers. Poult. Sci. 2021;100(3):100908. doi: 10.1016/j.psj.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenço P., Berchieri Â. Mundo Agro Editora Ltda.; Campinas, SP: 2015. Salmonella Gallinarum: Pesquisas, Achados e Caminhos Apontados Por Grupo de Pesquisadores. [Google Scholar]
- Martín R., Langella P. Emerging health concepts in the probiotics field: streamlining the definitions. Front. Microbiol. 2019;10:1047. doi: 10.3389/fmicb.2019.01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbradt E.L., Okamoto A.S., Padovani C.R., Fascina V.B., Silva T.M., Altarugio R., Hataka A., Schmidt E.M.S., Andreatti Filho R.L. Use of organic acids and a competitive exclusion product as growth promotes and Salmonella Enteritidis control in commercial Turkeys. Br. J. Poult. Sci. 2017;19:551–558. [Google Scholar]
- Miller M.B., Bassler B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001;55:165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- Okamoto A.S., Filho R.L.A., Milbradt E.L., Moraes A.C.I., Vellano I.H.B., Guimarães-Okamoto P.T.C. Bacterial communication between Lactobacillus spp. isolated from poultry in the inhibition of Salmonella Heidelberg—proof of concept. Poult. Sci. 2018;97:2708–2712. doi: 10.3382/ps/pey141. [DOI] [PubMed] [Google Scholar]
- Papadimitriou K., Alegría Á., Bron P.A., Angelis M.D., Gobbetti M., Kleerebezem M., Lemos J.A., Linares D.M., Ross P., Stanton C., Turroni F., Sinderen D.V., Varmanen P., Ventura M., Zuñiga M., Tsakalidou E., Kok J. Stress physiology of lactic acid bacteria. Microbiol. Mol. Rev. 2016;80:837–890. doi: 10.1128/MMBR.00076-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce B.K.D., Tupper A.S., Pudritz R.E., Higgs P.G. Constraining the time interval for the origin of life on earth. Astrobiology. 2018;18:1–22. doi: 10.1089/ast.2017.1674. [DOI] [PubMed] [Google Scholar]
- Rostagno H.S., Albino L.F.T., Hannas M.I., Donzele J.L., Sakomura N.K., Perazzo F.G., Saraiva A., Teixeira M.L., Rodrigues P.B., Oliveira R.F., Barreto S.L.T., Brito C.O. Tabelas Brasileiras Para Aves e Suínos. 4th ed. Publisher UFV(Publisher from the Federal University of Viçosa; Viçosa, Brazil): 2017. [Google Scholar]
- Tonkin M., Khan S., Wani M.Y., Ahmad A. Quorum Sensing – A Stratagem for Conquering Multi-Drug Resistant Pathogens. Curr. Pharm. Des. 2021;27(25):2835–2847. doi: 10.2174/1381612826666201210105638. [DOI] [PubMed] [Google Scholar]
- Vuyst L.D. In: Pages 1–539 in Bacteriocins of Lactic Acid Bacteria. 1st ed. Vuyst L.D., Vandamme E.J., editors. Springer; New York, NY: 1994. Bacteriocins and bacteriocin-like substances from Lactobacillus. [Google Scholar]