Abstract
Background
Neoadjuvant chemotherapy (NACT) is increasingly becoming the recommended treatment for locally advanced gastric cancer (LAGC) with promising results. According to previous reports, few studies have evaluated the benefits of laparoscopic gastrectomy (LG) after NACT.
Methods
135 patients from our center who underwent gastrectomy with NACT were available, including 41 patients of LG and 94 OG between July 2018 and July 2022. To reduce selection bias, we used the nearest neighbor method and set caliper = 0.2 for 3:1 matching between LG and OG groups for propensity score matching method (PSM). After PSM, the matched 41 patients with LG and 80 patients with OG formed the cohort, respectively. Univariate and multivariate Cox analyses were performed on all variables to determine independent risk factors associated with survival.
Results
LG had a longer operating time compared to OG [260.00 min (220.00 min, 300.00 min) vs. 200.00 min (160.00 min, 260 min), P < 0.001]. The estimated blood loss, metastatic lymph nodes (LN), total LN examined, postoperative hospital stays, blood transfusion (P>0.05) and the incidence of postoperative complications did not show statistical differences from the OG group (P = 0.084). The type of surgery (LG vs. OG) did not show a significant risk propensity in the univariate and multivariate Cox analysis (HR = 0.69, P = 0.36, 95 % CI: 0.31–1.53). Through the Kaplan-Meier curves, a certain trend showed that the LG group had a better long-term survival outcomes than the OG group, although there was no statistical difference between two groups (P>0.05).
Conclusion
LG is a promising treatment option for LAGC patients receiving NACT and had an acceptable safety and efficacy compared to OG.
Keywords: Stomach neoplasm, Neoadjuvant chemotherapy, Laparoscopic gastrectomy, Open gastrectomy
Highlights
-
•
Neoadjuvant chemotherapy (NACT) is becoming the recommended treatment for locally advanced gastric cancer (LAGC).
-
•
Few studies have evaluated the benefits of laparoscopic gastrectomy (LG) after NACT.
-
•
LG is a promising treatment for LAGC patients receiving NACT and had an acceptable safety and efficacy compared to OG.
Recently, the incidence and mortality of gastric cancer (GC) have decreased globally, but GC remains the third leading cause of cancer death [1]. Surgical procedures are the main treatment for GC. The results of a study conducted by J. Yu et al. showed that laparoscopic gastrectomy (LG) for locally advanced gastric cancer (LAGC) is a safe and feasible minimally invasive technique, which is comparable to open gastrectomy (OG) in terms of oncologic outcomes [2]. It provided the same lymph node harvesting, reduced intraoperative bleeding, reduced risk of surgical site infection, accelerated early recovery of bowel function and shortened hospital stay among other short-term as well as long-term quality indicators [3].
Neoadjuvant chemotherapy (NACT) is increasingly becoming the recommended treatment for LAGC with positive results [4,5,6,7]. The PRODGY and RESOLVE studies are promising NACT regimens that demonstrated the safety and short-term effectiveness of perioperative NACT. It can reduce tumor pathological stage, improve long-term survival and quality of life [8]. However, this is a new challenge for LG due to the vascular, tissue edema or fibrotic response after NACT. In particular, lymph node dissection and anastomosis of the residual bowel is a challenge and therefore requires extensive laparoscopic experience. Therefore, it is necessary to clarify the safety as well as the effectiveness of LG after NACT [4].
According to previous reports, few studies have evaluated the benefits of LG after NACT. Therefore, it is valuable to compare the short- and long-term outcomes of LG and OG in LAGC patients after NACT.
Methods
Patient selection
This study was a retrospective analysis. The inclusion criteria for this study were as follows: (a) clinicopathological diagnosis of GC; (b) patients received at least 1 cycle of NACT; (c) no other treatment (radiation therapy or long-term hormone therapy) other than NACT and gastrectomy. The exclusion criteria were as follows: (a) patients combined with malignancies at other sites; (b) R1/2 resection; (c) patients with incomplete clinical data or follow-up information. Among them, 15 patients with tumors at other sites, 3 patients with long-term hormone use, 3 patients without follow-up information and 25 patients with incomplete baseline information were excluded. Thus, 135 patients from our center who underwent gastrectomy after NACT were available for propensity score matching method (PSM), including 41 patients of LG and 94 OG between July 2018 and July 2022.
Ethics Committee of Xijing Hospital approved the study (No. XJLL-KY-20232159) and waived the requirement for informed consent for the anonymous data.
PSM
To reduce selection bias, we used the nearest neighbor method and set caliper = 0.2 for 3:1 matching between LG and OG groups based on R software (MatchIt package). The main baseline indicators and confounding factors were set as covariates, including sex, age, longest specimen diameter, chemotherapy regimen and The Nutritional Risk Screening (NRS) 2002 score. The flow chart of screening patients is shown in Fig. 1.
Fig. 1.
The flow chart presents the inclusion and exclusion criteria.
After PSM, the matched 41 patients in LG groups and 80 patients in OG groups formed the cohort, and the distribution of propensity scores for the two groups are shown in Fig. 2, respectively.
Fig. 2.
Histogram and jitter plot of the propensity score distribution.
Surgical technique
All patients had received at least one cycle of preoperative NACT and were analyzed in strata according to the different regimens. Gastrectomy and D2 lymph node dissection were performed at intervals of 3–8 weeks after NACT was received. Depending on the location of the tumor and its extension, the surgeon will choose the appropriate type of gastrectomy (total, proximal or distal gastrectomy) and anastomosis. The LG group takes the conventional 5 trocars, the location of which is based on operational habits. The approach of abdomen is usually adopted by the median abdominal laparotomy in the OG group. For patients in combinations with other organ resection, LG or OG is decided according to the clinical routine of each medical center. During the procedure, a laparoscopic-assisted incision of >10 cm is considered an intermediate open laparotomy [9]. Abdominal exploration was carried out to determine whether peritoneal implantion metastasis was present. During the abdominal exploration phase, abdominal exfoliative cytology specimens would be gathered for analysis. According to the guidelines for LAGC, LG or OG with D2 lymphadenectomy include No.1–7, No.8a, No.9, No.11d and No.12a [10]. To ensure incisal margin safety, frozen biopsy was routinely performed to determine the resection range. The LG or OG procedure will be performed by experienced surgeons. All histological specimens are evaluated by two pathologists and classified according to the Mandard Tumour Regression Grading (TRG) system.
Data collection and outcome assessment
All information for this study, including baseline data, blood tests, surgical and pathological outcomes, was obtained from the electronic medical records and follow-up results.
NACT regimen includes two-drug combinations (SOX, XELOX/CapOx), three-drug combinations (FLOT, DOS, FOLFOX) and molecularly targeted drugs (PD-1/PD-L1 inhibitors). Postoperative complications were determined according to the Clavien-Dindo classification system and only grade 2 or higher grade were recorded. OS was defined as the time interval between the start of NACT and the last follow-up visit or death from any cause.
NRS2002 is used to evaluate the preoperative nutritional status of patients. According to previous research results, if the NRS2002 score is >2 points, the patient is at risk of malnutrition and requires nutritional support treatment. If the total score of NRS2002 is ≤2 points, their nutritional status will be reassessed weekly [11].
Postoperative complications were defined as problems affecting the patient within 30 days after surgery, including anastomotic leak, abdominal infection, pulmonary infection and bleeding. All patients were managed according to the enhanced recovery after surgery in the perioperative period.
Statistical analysis
Continuous variables were expressed as medians with interquartile distances. Categorical variables were expressed as numbers and percentages (%). Nonparametric tests, including the x2 test or the Mann-Whitney U test, were used for group comparisons between categorical variables and ordered categorical data. Univariate and multivariate Cox analyses were performed on all variables to determine independent risk factors associated with survival.
The Restricted Mean Survival Time (RMST) is particularly useful when the proportional hazards assumption cannot be made or when the event rate is low. RMST provides an absolute measure of survival time and can be used as an alternative to hazard ratios. The baseline data analysis before propensity score matching is included in supplementary files.
This study used R software (MatchIt package) to conduct the procedure of PSM. the Tableone R package (version 4.2.2, https://cran.r-project.org) was used for data analysis. We plotted survival curves and performed COX regression analysis with the “survival” R package. P < 0.05 was considered statistically significant, and all P values were bilateral.
Results
Demographic and clinicopathologic characteristics
In the retrospective data of 135 patients, we found that some baseline indicators were imbalanced between the two groups, as shown in Supplementary Tables 1–4. Therefore, in order to reduce potential data bias and confounding variables between groups, we conducted propensity score matching.
The matched 121 patients form the cohort and baseline data are summarized in Table 1. Fig. 2 shows the histogram and jitter plot of the propensity score distribution. The matched experimental group is similar to the control group with comparability.
Table 1.
The characteristics of 121 patients after propensity score matching.
| Overall | Open gastrectomy | Laparoscopic gastrectomy | P | |
|---|---|---|---|---|
| Gender | ||||
| Female | 14 (11.6) | 10 (12.5) | 4 (9.8) | 0.77 |
| Male | 107 (88.4) | 70 (87.5) | 37 (90.2) | |
| Age (years) | 59.6 (9.8) | 59.45 (10.02) | 59.76 (9.61) | 0.87 |
| Age-adjusted Charlson Comorbidity Index | ||||
| ≤3 | 40 (33.1) | 25 (31.2) | 15 (36.6) | 0.70 |
| >3 | 81 (66.9) | 55 (68.8) | 26 (63.4) | |
| NRS2002 | ||||
| ≤2 | 87 (71.9) | 54 (67.5) | 33 (80.5) | 0.20 |
| >2 | 34 (28.1) | 26 (32.5) | 8 (19.5) | |
| BMI | 22.7 [20.9, 24.5] | 22.50 [21.05, 24.60] | 22.90 [20.30, 24.20] | 0.9 |
| Diabetes | ||||
| No | 103 (85.1) | 66 (82.5) | 37 (90.2) | 0.30 |
| Yes | 18 (14.9) | 14 (17.5) | 4 (9.8) | |
| Hypertension | ||||
| No | 82 (67.8) | 58 (72.5) | 24 (58.5) | 0.18 |
| Yes | 39 (32.2) | 22 (27.5) | 17 (41.5) | |
| Coronary disease | ||||
| No | 103 (85.1) | 68 (85.0) | 35 (85.4) | 1 |
| Yes | 18 (14.9) | 12 (15.0) | 6 (14.6) | |
| Drinking | ||||
| No | 47 (38.8) | 33 (41.2) | 14 (34.1) | 0.57 |
| Yes | 74 (61.2) | 47 (58.8) | 27 (65.9) | |
| Smoking | ||||
| No | 89 (73.6) | 62 (77.5) | 27 (65.9) | 0.25 |
| Yes | 32 (26.4) | 18 (22.5) | 14 (34.1) | |
| Status | ||||
| Alive | 38 (31.4) | 30 (37.5) | 8 (19.5) | 0.07 |
| Death | 83 (68.6) | 50 (62.5) | 33 (80.5) | |
| Hospitalization expenses (¥) | 77,304.0 [66,998.2, 87,098.0] | 72,966.89 [63,400.24, 84,312.64] | 84,052.35 [76,582.39, 93,799.61] | <0.001 |
The bold representation in the table has statistical significance.
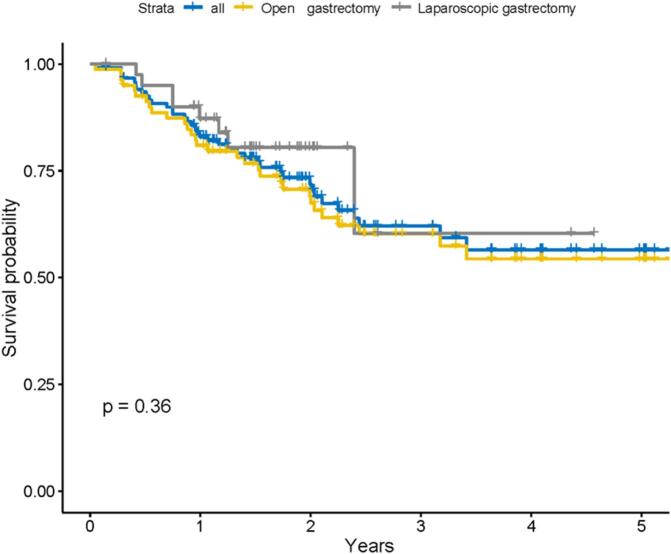
There were 4 females and 37 males in the LG group with a mean age of 59.76 (9.61) years. The mean BMI was 22.90 [20.30, 24.20] kg/m2. The hospitalization expenses were more expensive in the LG group compared to the OG group (P < 0.001). Survival outcomes were analyzed by Kaplan-Meier curves, and the LG group had a trend of better long-term survival than the OG group, but did not show statistical differences (P > 0.05) (Fig. 3).
Fig. 3.
Kaplan–Meier survival curves of GC patients undergoing laparoscopic gastrectomy (LG) versus open gastrectomy (OG).
Neoadjuvant chemotherapy regimen and pathological results
In the LG group, 14 patients (34.1 %) received two-drug combinations, 21 patients (51.2 %) received three-drug combinations and 6 patients (14.6 %) received molecularly targeted drugs. According to TNM classification, postoperative pathological examination showed 8 patients (19.5 %) were at stage I, 7 patients (17.1 %) were at stage II, 25 patients (61.0 %) were at stage III and 1 patient (2.4 %) were at stage IV.
Therefore, combining the results of the study conducted by S. Derieux, we divided the patients into two subgroups: pathological responders (TRG 1 to TRG 3) and pathological nonresponders (TRG 4 to TRG 5) [12,13,14]. In the LG group, pathological responders (TRG 1 to TRG 3) were present in 21 patients (51.2 %) and pathological non-responders (TRG 4 to TRG 5) in 20 (48.8 %). The indicators were balanced and comparable between the two groups (Table 2).
Table 2.
Neoadjuvant chemotherapy regimens and histopathology.
| Overall | Open gastrectomy | Laparoscopic gastrectomy | P | |
|---|---|---|---|---|
| Regimen for NACT | ||||
| Single-drug combinations | 2 | 2 | 0 | <0.001 |
| Two-drug combinations | ||||
| SOX (%) | 31 | 18 | 13 | |
| XELOX (%) | 8 | 7 | 1 | |
| Three-drug combinations | ||||
| FLOT (%) | 22 | 8 | 14 | |
| DOS (%) | 34 | 32 | 2 | |
| FOLFOX (%) | 9 | 4 | 5 | |
| Molecularly targeted drugs | 15 | 9 | 6 | |
| Chemotherapy cycle | ||||
| 1 | 24 (19.8) | 19 (23.8) | 5 (12.2) | 0.33 |
| 2 | 24 (19.8) | 17 (21.2) | 7 (17.1) | |
| 3 | 35 (28.9) | 20 (25.0) | 15 (36.6) | |
| ≥4 | 38 (31.4) | 24 (30.0) | 14 (34.1) | |
| TRG (%) | ||||
| 1 | 2 (1.7) | 1 (1.2) | 1 (2.4) | 0.66 |
| 2 | 24 (19.8) | 14 (17.5) | 10 (24.4) | |
| 3 | 30 (24.8) | 20 (25.0) | 10 (24.4) | |
| 4 | 50 (41.3) | 33 (41.2) | 17 (41.5) | |
| 5 | 15 (12.4) | 12 (15.0) | 3 (7.3) | |
| p/ypT stage | ||||
| 1 | 15 (12.4) | 12 (15.0) | 3 (7.3) | 0.43 |
| 2 | 14 (11.6) | 7 (8.8) | 7 (17.1) | |
| 3 | 47 (38.8) | 31 (38.8) | 16 (39.0) | |
| 4 | 45 (37.2) | 30 (37.5) | 15 (36.6) | |
| p/ypN stage | ||||
| 0 | 40 (33.1) | 27 (33.8) | 13 (31.7) | 0.78 |
| 1 | 54 (44.6) | 34 (42.5) | 20 (48.8) | |
| 2 | 27 (22.3) | 19 (23.8) | 8 (19.5) | |
| p/yp TNM stage | ||||
| 1 | 21 (17.4) | 13 (16.2) | 8 (19.5) | 0.53 |
| 2 | 28 (23.1) | 21 (26.2) | 7 (17.1) | |
| 3 | 66 (54.5) | 41 (51.2) | 25 (61.0) | |
| 4 | 6 (5.0) | 5 (6.2) | 1 (2.4) | |
| Differentiation | ||||
| Poor | 75 (62.0) | 47 (58.8) | 28 (68.3) | 0.56 |
| Moderately | 35 (28.9) | 24 (30.0) | 11 (26.8) | |
| Well | 4 (3.3) | 4 (5.0) | 0 (0.0) | |
| Unknown | 7 (5.8) | 5 (6.2) | 2 (4.9) | |
| Perineural invasion | ||||
| No | 29 (24.0) | 20 (25.0) | 9 (22.0) | 0.88 |
| Yes | 92 (76.0) | 60 (75.0) | 32 (78.0) | |
| Vascular invasion | ||||
| No | 43 (35.5) | 31 (38.8) | 12 (29.3) | 0.41 |
| Yes | 78 (64.5) | 49 (61.3) | 29 (70.7) | |
| Lauren type | ||||
| Unknown | 21 (17.4) | 16 (20.0) | 5 (12.2) | 0.66 |
| Intestinal | 29 (24.0) | 20 (25.0) | 9 (22.0) | |
| Diffuse | 21 (17.4) | 13 (16.2) | 8 (19.5) | |
| Mixed | 50 (41.3) | 31 (38.8) | 19 (46.3) | |
| The length and diameter of the specimen | 15.0 [12.0, 17.0] | 15.00 [12.00, 17.00] | 14.00 [11.00, 17.00] | 0.54 |
| The short diameter of the specimen | 3.5 [3.0, 4.0] | 3.50 [3.00, 4.12] | 3.50 [3.00, 4.00] | 0.51 |
Surgery and postoperative complications
According to the tumor site, 5 patients (12.2 %) were laparoscopic proximal gastrectomy, 14 patients (34.1 %) were laparoscopic distal gastrectomy and 22 patients (53.7 %) were laparoscopic total gastrectomy. LG had a longer operating time compared to OG [260.00 min (220.00 min, 300.00 min) vs. 200.00 min (160.00 min, 260.00 min), P < 0.001].
There was no significant difference in terms of estimated blood loss, metastatic LN, total LN examined, postoperative hospital stays and blood transfusion, which indicates that the LG group has the same surgical effectiveness and safety as the OG. In the LG group, 15 patients had potential Clavien-Dindo grade 2 complications, including 4 patients with hypoproteinemia, 2 patients with anemia, 13 patients with ascites, 2 patients with post-surgical wound complications and 2 patients with pulmonary infection. Two patients had intraperitoneal hemorrhage of grade Clavien-Dindo 3b. The incidence of postoperative complications did not show statistical differences between the OG and LG group (P = 0.084) (Table 3).
Table 3.
Surgical data and postoperative complications.
| Overall | Open gastrectomy | Laparoscopic gastrectomy | P | |
|---|---|---|---|---|
| Type of gastrectomy | ||||
| Proximal gastrectomy | 25 (20.7) | 20 (25.0) | 5 (12.2) | 0.14 |
| Distal gastrectomy | 31 (25.6) | 17 (21.2) | 14 (34.1) | |
| Total gastrectomy | 65 (53.7) | 43 (53.8) | 22 (53.7) | |
| Operating time (min) | 235.0 [180.0, 270.0] | 200.00 [160.00, 260.00] | 260.00 [220.00, 300.00] | <0.001 |
| Estimated blood loss (ml) | 150.0 [100.0, 200.0] | 150.00 [100.00, 200.00] | 100.00 [50.00, 300.00] | 0.24 |
| Metastatic LN | 1.0 [0.0, 5.0] | 1.50 [0.00, 5.00] | 1.00 [0.00, 5.00] | 0.90 |
| Total LN examined | 21.0 [18.0, 26.0] | 21.00 [18.00, 25.25] | 22.00 [20.00, 27.00] | 0.07 |
| Postoperative hospital stay (days) | 7.9 [6.6, 9.8] | 7.90 [6.88, 8.94] | 7.93 [6.01, 9.93] | 0.97 |
| Anastomosis | ||||
| Roux-en-Y | 77 (63.6) | 53 (66.2) | 24 (58.5) | 0.02 |
| Esophagogastric tubular anastomosis | 24 (19.8) | 19 (23.8) | 5 (12.2) | |
| Delta anastomosis (Billroth I) | 2 (1.7) | 2 (2.5) | 0 (0.0) | |
| Billroth II | 3 (2.5) | 1 (1.2) | 2 (4.9) | |
| Billroth II with Braun anastomosis | 15 (12.4) | 5 (6.2) | 10 (24.4) | |
| Blood transfusion | ||||
| No | 106 (87.6) | 69 (86.2) | 37 (90.2) | 0.77 |
| Yes | 15 (12.4) | 11 (13.8) | 4 (9.8) | |
| Complication | ||||
| No | 89 (73.6) | 63 (78.8) | 26 (63.4) | 0.08 |
| Yes | 32 (26.4) | 17 (21.2) | 15 (36.6) | |
| G2 (%) | 32 | 17 | 15 | |
| Hypoproteinemiaa (%) | 3 | 4 | ||
| Anemia (%) | 3 | 2 | ||
| Ascites (%) | 13 | 13 | ||
| Pelvic effusion (%) | 1 | 0 | ||
| Post-surgical wound complications (%) | 3 | 2 | ||
| Deep venous thrombosis (%) | 1 | 0 | ||
| Pulmonary infection (%) | 3 | 2 | ||
| G3b (%) | 4 | 2 | 2 | |
| Intraperitoneal hemorrhage (%) | 0 | 2 | ||
| Anastomotic leakage (%) | 2 | 1 |
Univariate and multivariate analysis affecting prognosis
We used Cox regression analysis to analyze the risk factors for prognosis of LAGC patients. The results of univariate Cox analysis showed that p/yp TNM stage (3 + 4 vs. 1 + 2), metastatic LN (>3 vs. ≤3), perineural invasion (yes vs. no), vascular invasion (yes vs. no), BMI (>23.8 vs. ≤23.8), transfusion (yes vs. no), TRG (4/5 vs. 1/2/3) were potential risk factors affecting the prognosis of GC patients.
Multivariate Cox regression revealed that perineural invasion (HR = 5.51, 95 % CI: 1.37–22.26, P = 0.02), BMI (HR = 0.01, 95 % CI: 0.01–0.17, P = 0.02), TRG (HR = 4.04, 95 % CI: 1.73–9.45, P < 0.001) and transfusion (HR = 2.41, 95 % CI: 1.05–5.48, P = 0.04) were independent risk factors. Notably, the type of surgery (LG vs. OG) did not show a significant risk propensity in the univariate analysis (HR = 0.69, 95 % CI: 0.31–1.53, P = 0.36) and was not an independent risk factor for overall survival. The data are presented in Table 4.
Table 4.
Univariate and multivariate Cox regression analyses of clinicopathological variabilities.
| Characteristics | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| HR | CI | P | HR | CI | P | |
| Gender | ||||||
| Female | ||||||
| Male | 0.55 | 0.24–1.24 | 0.15 | |||
| Age (years) | ||||||
| ≤68 | ||||||
| >68 | 1.6 | 0.7–3.66 | 0.27 | |||
| Type of gastrectomy | 1.45 | 0.93–2.25 | 0.10 | |||
| Proximal gastrectomy | ||||||
| Distal gastrectomy | 0.72 | 0.24–2.14 | 0.55 | |||
| Total gastrectomy | 1.72 | 0.74–4.00 | 0.21 | |||
| Type of surgery | ||||||
| Open gastrectomy | ||||||
| Laparoscopic gastrectomy | 0.69 | 0.31–1.53 | 0.36 | |||
| CCI | ||||||
| ≤3 | ||||||
| >3 | 1.74 | 0.84–3.60 | 0.13 | |||
| Operating time (min) | ||||||
| ≤210 | ||||||
| >210 | 0.96 | 0.51–1.83 | 0.91 | |||
| Estimated blood loss (ml) | ||||||
| ≤100 | ||||||
| >100 | 1.67 | 0.83–3.38 | 0.15 | |||
| Diabetes | ||||||
| No | ||||||
| Yes | 1.19 | 0.53–2.71 | 0.67 | |||
| Hypertension | ||||||
| No | ||||||
| Yes | 0.82 | 0.40–1.65 | 0.57 | |||
| Coronary disease | ||||||
| No | ||||||
| Yes | 0.52 | 0.16–1.69 | 0.28 | |||
| Drinking | ||||||
| No | ||||||
| Yes | 0.79 | 0.42–1.51 | 0.47 | |||
| Smoking | ||||||
| No | ||||||
| Yes | 0.93 | 0.44–1.96 | 0.84 | |||
| NRS2002 | ||||||
| ≤2 | ||||||
| >2 | 1.59 | 0.82–3.07 | 0.17 | |||
| Chemotherapy cycle | ||||||
| 1 | ||||||
| 2 | 0.96 | 0.29–3.15 | 0.95 | |||
| 3 | 1.83 | 0.68–4.90 | 0.23 | |||
| ≥4 | 2.27 | 0.88–5.91 | 0.09 | |||
| p/ypT stage | ||||||
| 1 | ||||||
| 2 | 1.38 | 0.19–9.83 | 0.75 | |||
| 3 | 2.38 | 0.53–10.78 | 0.26 | |||
| 4 | 5.12 | 1.20–21.88 | 0.03 | |||
| p/ypN stage | ||||||
| 0 | ||||||
| 1 | 2.76 | 1.02–7.50 | 0.05 | |||
| 2 | 7.05 | 2.56–19.38 | <0.001 | |||
| p/yp TNM stage | ||||||
| 1 + 2 | ||||||
| 3 + 4 | 4.05 | 1.78–9.25 | <0.001 | |||
| Metastatic LN | ||||||
| ≤3 | ||||||
| >3 | 2.22 | 1.17–4.23 | 0.02 | 1.27 | 6.32–2.54 | 0.50 |
| Total LN examined | ||||||
| ≤29 | ||||||
| >29 | 1.13 | 0.44–2.89 | 0.80 | |||
| Perineural invasion | ||||||
| No | ||||||
| Yes | 4.61 | 1.41–15.03 | 0.01 | 5.51 | 1.37–22.26 | 0.02 |
| Vascular invasion | ||||||
| No | ||||||
| Yes | 2.22 | 1.05–4.71 | 0.04 | 0.68 | 0.27–1.73 | 0.42 |
| Lauren type | ||||||
| Unknown | ||||||
| Intestinal | 0.94 | 0.32–2.70 | 0.90 | |||
| Diffuse | 3.38 | 1.31–8.75 | 0.01 | |||
| Mixed | 0.58 | 0.21–1.62 | 0.29 | |||
| Regimen for NACT | ||||||
| Single-drug | ||||||
| Two-drug combinations | 0.27 | 0.08–0.97 | 0.04 | |||
| Three-drug combinations | 0.29 | 0.09–0.99 | 0.04 | |||
| Molecularly targeted drugs | 0.25 | 0.04–1.53 | 0.13 | |||
| BMI (kg/m2) | ||||||
| ≤23.8 | ||||||
| >23.8 | 0.46 | 0.21–1.01 | 0.05 | 0.01 | 0.01–0.17 | 0.02 |
| TRG | ||||||
| 1 + 2 + 3 | ||||||
| 4 + 5 | 4.83 | 2.12–11.00 | <0.001 | 4.04 | 1.73–9.45 | <0.001 |
| Postoperative hospital stay (days) | ||||||
| ≤6.05 | ||||||
| >6.05 | 0.88 | 0.40–1.92 | 0.75 | |||
| Transfusion | ||||||
| No | ||||||
| Yes | 2.20 | 1.00–4.81 | 0.04 | 2.41 | 1.05–5.48 | 0.04 |
| The length and diameter of the specimen | ||||||
| ≤14 | ||||||
| >14 | 1.45 | 0.76–2.78 | 0.26 | |||
| The short diameter of the specimen | ||||||
| ≤3 | ||||||
| >3 | 1.66 | 0.86–3.22 | 0.13 | |||
| Complication | ||||||
| No | ||||||
| Yes | 1.23 | 0.61–2.49 | 0.56 | |||
Stratified analysis
Types of gastrectomy, TNM stage and NACT regimens are important factors affecting the prognosis of GC patients. Therefore, we performed a further subgroup stratification analysis.
Through the Kaplan-Meier curves, we found that there was a significant prognostic difference between different TNM stages (P = 0.0045) (Fig. 4). After dichotomizing stage, the long-term survival outcome of stage1/2 patients was still better than that of stage3/4 patients (p < 0.001). From the Supplementary Fig. 1, it can be found that for RMST within the 5.42 years, the difference between stage 3/4 and stage 1/2 is −1.521 years (95 % CI: −2.298-0.743, P < 0.001).
Fig. 4.
Stratification analysis of TNM stages of GC patients undergoing NACT.
Interestingly, in the analysis according to type of gastrectomy, a certain trend showed that patients with distal gastrectomy had a better prognosis than proximal gastrectomy, with total gastrectomy patients having the worst prognosis, although these differences were not statistically different (P = 0.097) (Fig. 5).
Fig. 5.
Kaplan–Meier survival curves of GC patients undergoing distal gastrectomy versus proximal gastrectomy versus total gastrectomy.
Similarly, we found no differences in different NACT regimens (p = 0.17). No differences in prognostic outcomes existed for single-drug regimen, two-drug combination regimens, three-drug combination regimens or molecularly targeted drugs (Fig. 6). From the Supplementary Fig. 2, it can be found that for RMST within the 5.42 years, the difference between molecularly targeted drugs and single/two/three-drug combinations is 0.678 years (95 % CI: −0.651-2.007, P = 0.317).
Fig. 6.
The long-term survival outcomes of the different regimens.
Discussion
Southeast Asia is a region with a high incidence of GC, and most patients are in the progressive stage at the time of first diagnosis [15]. For this type of patients, NACT has gradually become an important part of comprehensive perioperative treatment, but surgical gastrectomy is undeniably still the most effective means of treatment [2]. The combination of preoperative NACT and surgical gastrectomy, especially the widespread application of minimally invasive techniques, has led to a significant increase in long-term survival rate of GC patients [16,17].
In the present study, we found a trend toward better survival in the LG group by Kaplan-Meier survival curves, although there was no statistical difference in the postoperative survival outcomes between the two groups. It was shown by multivariate Cox analysis that the type of surgery (LG vs. OG) was not an independent risk factor for overall survival (HR = 0.69, 95 % CI: 0.31–1.53, P = 0.36), which is consistent with previous studies [18].
The report of T. Kosuga et al. found that total gastrectomy leads to inadequate caloric intake and caloric losses, which is consistent with our outcome [19]. We found that the prognosis of the various types of gastrectomy also showed some trend differences, although without statistically different. Among them, patients with distal gastrectomy had a better prognosis than proximal gastrectomy, and patients with total gastrectomy had the worst prognosis. This may be due to the fact that total gastrectomy patients, in whom no gastric function is preserved, would suffer from long-term deficiencies of trace elements such as hemoglobin or internal factors [20].
Notably, the LG group had a significantly longer operating time. The reason may be that most GC patients receiving NACT are in the advanced stage, which means large size and anatomical complexity of the tumor inevitably prolong the operating time, although laparoscopy facilitates lymph node dissection and delicate manipulation. Interestingly, in the Cox regression analysis, we found that operating time was not an independent factor affecting prognosis and did not increase the risk of long-term survival. Therefore, the prolonged operating time is acceptable for intraoperative procedures.
NACT causes tissue and perivascular edema and disrupts the anatomical plane, which can increase the difficulty of the procedure as well as the risk of postoperative complications. However, laparoscopic surgery can compensate for these disadvantages with the help of visual magnification effect and more delicate manipulation.
A study on preoperative induction chemotherapy reported by D. Mizrak Kaya et al. showed that in patients with gastric adenocarcinoma, the three-drug combination was safe and feasible compared to the two-drug combination, but did not show significant advantages [21]. Their results are consistent with our findings. The NACT regimens within our study were mainly two-drug combination and three-drug combination regimens, with a high overall variability. In the Kaplan-Meier curve analysis and RSMT, we did not find statistically significant differences between the regimens. It was also not found to be an independent risk factor in the Cox regression analysis.
Y. Wang et al. found that in total laparoscopic gastrectomy, the number of lymph nodes resection, intraoperative bleeding and postoperative complication rates were comparable to those in OG after NACT (P > 0.05), and the incision length was shorter with less invasive in LG (P < 0.001) [22]. Similarly, M. Fujisaki et al. found that LG group significantly reduced hospital stay and bleeding compared to the OG group [18]. In our study, the two groups did not show a statistical difference in estimated blood loss, metastatic LN, total LN examined and complication, which also indicates the acceptable safety and efficacy of laparoscopy.
TNM stage has been reported in several studies as an independent risk factor affecting the prognosis of patients with gastric cancer [23]. By the Kaplan-Meier curve analysis, we found that there were significant prognostic differences between different TNM stages. After dichotomizing stages in the RSMT, the survival outcomes of stage 1/2 patients were better than stage 3/4 patients (P<0.001) [24].
Undeniably, there are some limitations in this study. First, although the PSM can reduce selection bias in studies, hidden bias still exists because this method can only balance the observed variables. Otherwise, the population receiving NACT is limited and the effective sample size is small. Therefore, we hope to clarify the advantages of LG with larger prospective studies in the future.
Funding
This study was funded in part by grants from the Scientific and Technological Innovation Team Plan of Shaanxi Province (2021TD-43).
Ethics approval statement
Xijing Hospital's ethics review committee approved this retrospective study and waived the requirement for informed consent (XJLL-KY-20232159).
CRediT authorship contribution statement
Conceptualization, Qinchuan Yang and Xiaohua Li; Data curation, Qinchuan Yang; Formal analysis, Qinchuan Yang; Funding acquisition, Xiaohua Li; Investigation, Qinchuan Yang, Haikun Zhou, Chao Yue, Yannian Wang, Ruiqi Gao and Zhiyu Guo; Methodology, Qinchuan Yang, Bo Shan and Weidong Wang; Project administration, Gang Ji and Xiaohua Li; Resources, Qinchuan Yang; Software, Qinchuan Yang, Bo Shan, Weidong Wang and Changming Zhang; Supervision, Gang Ji and Xiaohua Li; Validation, Qinchuan Yang, Bo Shan and Changming Zhang; Visualization, Qinchuan Yang, Bo Shan and Weidong Wang; Writing, Qinchuan Yang; Review, Qinchuan Yang, Gang Ji and Xiaohua Li.
All authors approved the final version of this manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Thanks to the medical staff and patients for their invaluable help in the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sopen.2023.10.001.
Contributor Information
Gang Ji, Email: Jigang@fmmu.edu.cn.
Xiaohua Li, Email: xjyylixiaohua@163.com.
Appendix A. Supplementary data
Supplementary material
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Yu J., Huang C., Sun Y., Su X., Cao H., Hu J., et al. Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: the CLASS-01 randomized clinical trial. JAMA. 2019;321:1983–1992. doi: 10.1001/jama.2019.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Z.Y., Chen Q.Y., Zhong Q., Li P., Xie J.W., Wang J.B. Intraoperative Adverse Events, Technical Performance, and Surgical Outcomes in Laparoscopic Radical Surgery for Gastric Cancer: A Pooled Analysis From 2 Randomized Trials. Ann Surg. 2023;278(2):222–229. doi: 10.1097/SLA.0000000000005727. [DOI] [PubMed] [Google Scholar]
- 4.Coccolini F., Nardi M., Montori G., Ceresoli M., Celotti A., Cascinu S., et al. Neoadjuvant chemotherapy in advanced gastric and esophago-gastric cancer. Meta-analysis of randomized trials. Int J Surg. 2018;51:120–127. doi: 10.1016/j.ijsu.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Reddavid R., Sofia S., Chiaro P., Colli F., Trapani R., Esposito L., et al. Neoadjuvant chemotherapy for gastric cancer. Is it a must or a fake? World J Gastroenterol. 2018;24:274–289. doi: 10.3748/wjg.v24.i2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang Y.K., Yook J.H., Park Y.K., Lee J.S., Kim Y.W., Kim J.Y., et al. PRODIGY: a phase III study of neoadjuvant docetaxel, oxaliplatin, and s-1 plus surgery and adjuvant S-1 versus surgery and adjuvant S-1 for resectable advanced gastric cancer. J Clin Oncol. 2021;39:2903–2913. doi: 10.1200/JCO.20.02914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X., Liang H., Li Z., Xue Y., Wang Y., Zhou Z., et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. 2021;22:1081–1092. doi: 10.1016/S1470-2045(21)00297-7. [DOI] [PubMed] [Google Scholar]
- 8.Li Z., Shan F., Ying X., Zhang Y., E J.Y., Wang Y., et al. Assessment of laparoscopic distal gastrectomy after neoadjuvant chemotherapy for locally advanced gastric cancer: a randomized clinical trial. JAMA Surg. 2019;154:1093–1101. doi: 10.1001/jamasurg.2019.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lombardi P.M., Bernasconi D., Baiocchi G.L., Berselli M., Biondi A., Castoro C., et al. Open versus laparoscopic gastrectomy for advanced gastric cancer: a propensity score matching analysis of survival in a western population-on behalf of the Italian Research Group for Gastric Cancer. Gastric Cancer. 2022;25:1105–1116. doi: 10.1007/s10120-022-01321-w. [DOI] [PubMed] [Google Scholar]
- 10.Wang F.H., Zhang X.T., Li Y.F., Tang L., Qu X.J., Ying J.E., et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun. 2021;41:747–795. doi: 10.1002/cac2.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu C., Wang B., Gao Y., Ma X. Prevalence and relationship of malnutrition and distress in patients with Cancer using questionnaires. BMC Cancer. 2018;18:1272. doi: 10.1186/s12885-018-5176-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandard A.M., Dalibard F., Mandard J.C., Marnay J., Henry-Amar M., Petiot J.F., et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic Correlations Cancer. 1994;73:2680–2686. doi: 10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 13.Derieux S., Svrcek M., Manela S., Lagorce-Pages C., Berger A., André T., et al. Evaluation of the prognostic impact of pathologic response to preoperative chemotherapy using Mandard's Tumor Regression Grade (TRG) in gastric adenocarcinoma. Dig Liver Dis. 2020;52:107–114. doi: 10.1016/j.dld.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Gronnier C., Mariette C., Lepage C., Monterymard C., Jary M., Ferru A., et al. Perioperative cetuximab with cisplatin and 5-fluorouracil in esogastric adenocarcinoma: a phase II study. Cancers (Basel) 2023;15 doi: 10.3390/cancers15072188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allemani C., Matsuda T., Di Carlo V., Harewood R., Matz M., Nikšić M., et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu L., Wang C., Li F., Zhang X., Cheng X., Lin S., et al. The safety and efficacy of laparoscopic gastrectomy for patients with locally advanced gastric cancer following neoadjuvant chemotherapy. Sci Rep. 2022;12:10384. doi: 10.1038/s41598-022-14717-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Y., Chen J., Sun X., Lou Y., Fang M., Zhou F., et al. Survival and complications after neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy for locally advanced gastric cancer: a systematic review and meta-analysis. Front Oncol. 2023;13 doi: 10.3389/fonc.2023.1177557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujisaki M., Mitsumori N., Shinohara T., Takahashi N., Aoki H., Nyumura Y., et al. Short- and long-term outcomes of laparoscopic versus open gastrectomy for locally advanced gastric cancer following neoadjuvant chemotherapy. Surg Endosc. 2021;35:1682–1690. doi: 10.1007/s00464-020-07552-1. [DOI] [PubMed] [Google Scholar]
- 19.Kosuga T., Ichikawa D., Komatsu S., Okamoto K., Konishi H., Shiozaki A., et al. Feasibility and nutritional benefits of laparoscopic proximal gastrectomy for early gastric cancer in the upper stomach. Ann Surg Oncol. 2015;22(Suppl. 3):S929–S935. doi: 10.1245/s10434-015-4590-4. [DOI] [PubMed] [Google Scholar]
- 20.Wang H.M., Wang T.J., Huang C.S., Liang S.Y., Yu C.H., Lin T.R., et al. Nutritional status and related factors in patients with gastric cancer after gastrectomy: a cross-sectional study. Nutrients. 2022;14 doi: 10.3390/nu14132634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizrak Kaya D., Nogueras González G.M., Harada K., Blum Murphy M.A., Lee J.H., Bhutani M.S., et al. Efficacy of three-drug induction chemotherapy followed by preoperative chemoradiation in patients with localized gastric adenocarcinoma. Oncology. 2020;98:542–548. doi: 10.1159/000506519. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y., Lei X., Liu Z., Shan F., Ying X., Li Z., et al. Short-term outcomes of laparoscopic versus open total gastrectomy after neoadjuvant chemotherapy: a cohort study using the propensity score matching method. J Gastrointest Oncol. 2021;12:237–248. doi: 10.21037/jgo-20-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiaobin C., Zhaojun X., Tao L., Tianzeng D., Xuemei H., Fan Z., et al. Analysis of related risk factors and prognostic factors of gastric cancer with bone metastasis: a SEER-based study. J Immunol Res. 2022;2022 doi: 10.1155/2022/3251051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng J., Zhang R., Pan Y., Wang B., Wu L., Jiao X., et al. Comparison of the staging of regional lymph nodes using the sixth and seventh editions of the tumor-node-metastasis (TNM) classification system for the evaluation of overall survival in gastric cancer patients: findings of a case-control analysis involving a single institution in China. Surgery. 2014;156:64–74. doi: 10.1016/j.surg.2014.03.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material