Abstract
Introduction
Elevated circulatory concentrations of YKL-40 have been reported in patients with ischemic stroke. This study further investigated the association of plasma YKL-40 concentrations at admission and short, long-term prognosis after ischemic stroke.
Methods
Based on a prospective, nationwide multicenter registry focusing consecutive patients of ischemic stroke and transient ischemic attack, plasma YKL-40 levels were detected by enzyme-linked immunosorbent assay at admission, and patients were stratified into percentile according to the plasma YKL-40 concentrations. The multivariate Cox or logistic regression model was used to investigate the association of YKL-40 concentration with death and functional outcomes at 3 months, 6 months, and 12 months after ischemic stroke, with potential confounders adjusted.
Results
A total of 8,006 first-ever ischemic stroke patients, with the age of 61.7 ± 11.5, were included in this study. The mortality of 0–33%, 34–66%, 67–90%, and 91–100% groups at 12 months follow-up was 0.9%, 2.2%, 4.4%, and 9.4%, respectively (p < 0.0001), and the modified Rankin Scale 3–6 ratio was 6.8%, 10.5%, 15.7%, and 24.0%, respectively (p < 0.0001). In the multivariate regression, after adjusting for potential confounders, 91–100% group had higher risk of death (hazard ratio 2.99, 95% confidence interval 1.75–5.11)and modified Rankin Scale 3–6 (odds ratio 1.42, 95% confidence interval 1.08–1.88) at 12 months since onset of ischemic stroke compared to the 0–33% group.
Conclusions
The elevated YKL-40 at admission can potentially help predict death, functional prognosis after ischemic stroke, which may help further studies to explore the potential physiological and pathological mechanism including the effects of vulnerable plaque and collateral circulation.
Keywords: Ischemic stroke, YKL-40, Biomarker, Outcomes, Mortality
Introduction
Stroke is a major cause of death and disability worldwide. Approximately, three-quarters of the global burden of stroke deaths (approximately 6.5 million per year) and associated disability-adjusted life years (approximately 113 million) occurred in low- and middle-income countries [1, 2]. Being the most common types of cerebrovascular events in China, ischemic stroke accounts for about 70% of all strokes [3]. Accurate prognosis prediction can be used to optimize stroke care and allocation of health-care resources. Blood markers may increase the predictive accuracy of scores while keeping them quite simple and objective [4].
YKL-40, also called chitinase-3-like protein 1 or human cartilage glycoprotein-39, is a 40-kDa heparin and chitin-binding glycoprotein, a member of the mammalian chitinase-like protein family. Diverse inflammatory and atherosclerosis developments are associated with elevated YKL-40 expression levels in infiltrating macrophages [5, 6, 7]. Interleukin-1b and tumor necrosis factor-α, produced from macrophage in neuroinflammatory conditions, can upregulate YKL-40 expression in reactive macrophages [8]. Besides, the abundant expression of YKL-40 could also be detected in astrocytes in brain tissue from patients with neurological diseases [9]. YKL-40 can promote the formation of macrophages in the vascular wall and convert them into lipid-laden foam cells, which play a potential role in the formation of atherosclerotic plaques and development of stroke [10]. Despite the increased recognition of an association between elevated YKL-40 and arteriosclerosis development and cardiovascular events, including stroke [11, 12], up to now, it remains unclear whether increased YKL-40 is a potential biomarker of poor prognosis of ischemic stroke.
To illuminate this issue, based on the data from the China National Stroke Registry (CNSR-III) involving more than 14,000 imaging-confirmed ischemic strokes from 201 study sites of 22 provinces and 4 municipalities across China, we aimed to assess the concentrations of plasma YKL-40 in patients with ischemic stroke and investigate its potential role as a predictor of poor outcomes (modified Rankin Scale [mRS] 3–6) and mortality.
Materials and Methods
Study Design and Participants
The design of CNSR-III has been described in detail previously [13]. In brief, the CNSR-III was a nationwide prospective registry for patients with acute ischemic stroke and transient ischemic attack (TIA) presented to hospitals between August 2015 and March 2018 in China. A total of 201 study sites were included from 22 provinces and 4 municipalities. In the prespecified biomarker substudy of the CNSR-III, fasting blood samples were collected from 171 voluntary study sites within 24 h of admission. Participants were consecutively recruited if meeting the following criteria: (1) age>18 years; (2) ischemic stroke and TIA; (3) within 7 days from onset of symptoms to enrolment; (4) informed consent from participant or legally authorized representative. In this prespecified biomarker substudy analysis of the CNSR-III, only patients with first-ever ischemic stroke and blood YKL-40 available were included. The CNSR-III was approved by Ethics Committee at Beijing Tiantan Hospital and all participating centers.
Baseline Data Collection
Professional research coordinators at each site collected baseline data through face-to-face interview or medical records, including age, sex, health insurance, cigarette smoking, alcohol, body mass index (BMI, the weight in kilograms divided by the square of the height in meters), national institutes of health stroke scale (NIHSS), medical history of hypertension, diabetes, hypercholesterolemia, ischemic stroke, and coronary heart disease, in-hospital medication including antithrombotics, antihypertensive medicines, diabetes medicines and lipid-lowering medicines, blood tests such as blood cell count, glucose and lipid, and C-reactive protein (CRP), in-hospital complications comprising respiratory disease, liver disease, urinary system diseases, and bleeding recorded in the medical records.
Sample Collection and Measurements of YKL-40
The median time of sampling was 55 h (interquartile range: 27–96 h) after index event onset. Plasma specimens were extracted, aliquoted, and transported through cold chain to the core laboratory in Beijing Tiantan Hospital. All specimens were stored at −80°C until assays were performed centrally and blindly. The concentrations of YKL-40 were determined using enzyme-linked immunosorbent assay kits (R&D Systems, Inc, Minneapolis, MN, USA). The detection limit was 20 ng/mL, while the intra-assay and inter-assay coefficients of variation were both less than 6%.
Follow-Up and Outcomes
The detailed follow-up procedure of the CNSR-III has been previously described [13]. In brief, participants were followed up by face-to-face or telephone interview at 3 months, 6 months, and 1 year by professional research personnel, collecting information of any death and assessing patient’s mRS score ranging from 0 (no symptoms) to 6 (death) during the follow-up period. Fatality was either confirmed on a death certificate from the attended hospital or the local citizen registry. The endpoints in our study were death and mRS 3–6.
Statistical Analysis
Categorical variables were represented by the number of cases and percentage (%), continuous variables with normal distribution were represented by the number of cases and mean ± standard deviation, and continuous variables with non-normal distribution were represented by the number of cases, median, and interquartile range. Baseline characteristics were analyzed by χ2 statistics for the categorical variables and Kruskal-Wallis test for the continuous variables. YKL-40 level was mainly categorized into 4 groups (0–33%, 34–66%, 67–90%, 91–100%) [14], as well categorized by quartiles. The association of YKL-40 with death and mRS 3–6 were analyzed using multivariable Cox regression or logistic regression with adjusting for potential confounders including age, sex, smoking, drinking, hypertension, diabetes mellitus, low-density lipoprotein (LDL), CRP, NIHSS, medicine at discharge (antiplatelet, anticoagulant, lipid-lowering, hypoglycemic, antihypertensive medicines), complications (respiratory, liver, urinary, and bleeding diseases). The unadjusted and adjusted hazard ratios (HRs) and their 95% confidence intervals (CIs) were calculated for death, along with odds ratios and 95% CIs for mRS 3–6. A 2-sided p value of <0.05 was considered to indicate statistical significance. All data were analyzed by SAS version 9.4 statistical software (SAS Institute Inc, Cary, NC, USA).
Results
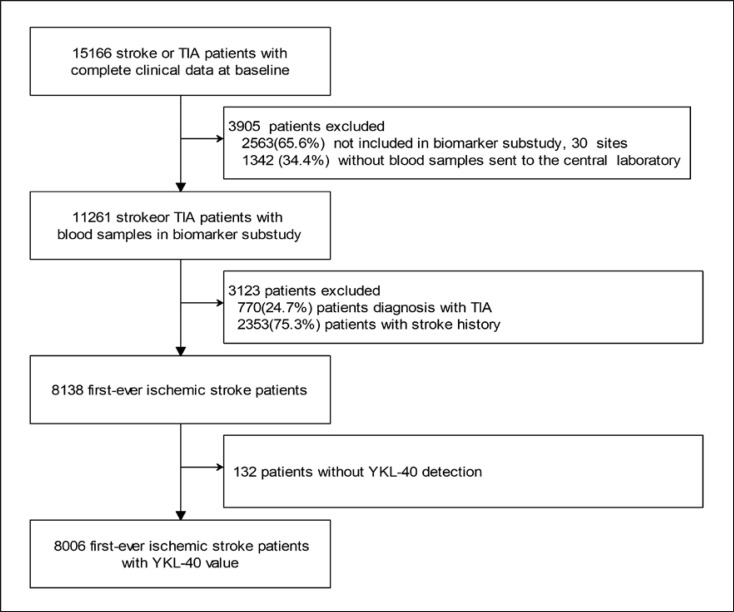
A total of 15,166 patients with complete clinical data at baseline were enrolled in CNSR-III. First, 3,905 patients were excluded because they were not included in biomarker substudy or blood samples were not sent to the central laboratory. Second, 3,123 patients were excluded because of the diagnosis of TIA or stroke history. Finally, 132 patients were excluded due to lack of adequate blood volume for YKL-40 measurements. Therefore, 8,006 patients with first-ever ischemic stroke were included in this study for analysis (shown in Fig. 1). Baseline characteristics of patients with or without YKL-40 are comparable (online suppl. Table. S1; for all online suppl. material, see www.karger.com/doi/10.1159/000527519).
Fig. 1.
Flowchart for the enrollment process of the potentially eligible first-ever ischemic stroke patients with plasma YKL-40 measured. A total of 15,166 patients with complete clinical data at baseline were enrolled in CNSR-III. First, 3,905 patients were excluded because they were not included in biomarker substudy or blood samples were not sent to the central laboratory. Second, 3,123 patients were excluded because of the diagnosis of TIA or stroke history. Finally, 132 patients were excluded because of missing YKL-40 measurement. Therefore, 8,006 patients with first-ever ischemic stroke were included in this study for analysis. CNSR-III, the Third China National Stroke Registry; TIA, transient ischemic attack.
Baseline characteristics of patients by YKL-40 categories are shown in Table 1. Patients with high concentrations of YKL-40 were older, more likely to be female, less likely to smoke or consume alcohol. Patients with high concentration of YKL-40 had higher BMI, higher concentration of blood glucose, triglyceride, cholesterol total, high-density lipoprotein, apolipoprotein A1, apolipoprotein B, and CRP at admission (Table 1). However, there was little difference in the medical history of diabetes mellitus, hypercolesterolemia, and TIA (Table 1).
Table 1.
Baseline characteristics of patients according to plasma YKL-40 percentile category
| Total | Plasma YKL-40 percentile categories |
p value | ||||
|---|---|---|---|---|---|---|
| 0–33% | 34–66% | 67–90% | 91–100% | |||
| YKL-40, mg/L | 30.2±8.5 | 64.1 ±13.2 | 136.1 ±30.9 | 228.4±21.9 | ||
| Age, n (mean ± SD), years | 8,006 (61.7± 11.5) | 2,642 (56.1 ±10.8) | 2,642 (61.9±10.4) | 1,922 (66.1 ±10.8) | 800 (69.4±9.9) | <0.0001 |
| Female, n (%) | 2,506 (31.3) | 641 (24.2) | 809 (30.6) | 717(37.3) | 339 (42.4) | 0.0001 |
| Health insurance, n (%) | 7,437 (92.9) | 2,422 (91.7) | 2,481 (93.9) | 1,789(93.1) | 745 (93.1) | 0.0167 |
| Smoking, n (%) | 2,682 (33.5) | 1,063 (40.2) | 908 (34.4) | 527 (27.4) | 184 (23.0) | <0.0001 |
| Alcohol, n (%) | 1,231 (15.4) | 476(18.0) | 411 (15.6) | 232(12.1) | 112(14.0) | <0.0001 |
| BMI, kg/m2, n (mean ± SD) | 8,006 (24.7±3.3) | 2,642 (25.1 ±3.1) | 2,642 (24.7±3.4) | 1,922 (24.3±3.4) | 800 (24.1 ±3.6) | <0.0001 |
| Prior disease history, n (%) | ||||||
| Hypertension | 4,737 (59.2) | 1,475 (55.8) | 1,580(59.8) | 1,152(59.9) | 530 (66.3) | <0.0001 |
| Diabetes Mellitus | 1,798(22.5) | 565 (21.4) | 610(23.1) | 434 (22.6) | 189 (23.6) | 0.3930 |
| Hyperlipidemia | 573 (7.2) | 182 (6.9) | 199(7.5) | 133(6.9) | 59 (7.4) | 0.7854 |
| TIA | 183 (2.3) | 62 (2.4) | 61 (2.3) | 42 (2.2) | 18 (2.3) | 0.9964 |
| Cardiovascular diseases | 1,267 (15.8) | 290(11.0) | 395 (15.0) | 380 (19.8) | 202 (25.3) | <0.0001 |
| Medication during hospital, n (%) | ||||||
| Antithrombotics | 7,836 (98.7) | 2,573 (98.6) | 2,597 (99.1) | 1,884 (98.5) | 782 (98.2) | 0.1191 |
| Antihypertensive medicines | 3,621 (45.6) | 1,145 (43.9) | 1,188(45.3) | 903 (47.2) | 385 (48.4) | 0.0508 |
| Diabetes medicines | 2,018(25.4) | 622 (23.8) | 702 (26.8) | 483 (25.3) | 211 (26.5) | 0.0841 |
| Lipid-lowering medicines | 7,701 (97.0) | 2,539 (97.2) | 2,540 (97.0) | 1,852 (96.9) | 770 (96.3) | 0.8320 |
| BWC, ×109/L,a n (median, IQR) | 7,894 (6.9,5.7–8.5) | 2,600 (7.0, 5.8–8.4) | 2,610 (6.9, 57–8.4) | 1,894(6.9, 5.7–8.4) | 790 (7.1, 5.8–8.8) | 0.0740 |
| Blood glucose, n (median, IQR) | 6,343 (5.6, 4.9–7.0) | 2,121 (5.4, 4.9–6.7) | 2,114(5.5, 4.9–7.0) | 1,498(5.6, 4.9–7.1) | 610(5.8, 5.0–7.3) | 0.0012 |
| Blood lipid, n (%) | ||||||
| TG | 7,959 (1.6±1.0) | 2,629 (1.6±0.9) | 2,628 (1.6±0.9) | 1,909 (1 7±1.2) | 793 (1.7±1.0) | 0.0012 |
| CHO | 7,963 (4.2±1.2) | 2,631 (4.1 ±1.2) | 2,628 (4.2±1.2) | 1,911 (4.2±1.2) | 793 (4.3±1.2) | 0.0114 |
| HDL | 7,956 (1.1 ±0.5) | 2,629 (1.1 ±0.7) | 2,626 (1.1 ±0.3) | 1,909 (1.1 ±0.3) | 792 (1.1 ±0.3) | <0.0001 |
| LDL | 7,957 (2.5±1.1) | 2,629 (2.5±1.1) | 2,627 (2.5±1.1) | 1,909 (2.5±1.0) | 792 (2.5±1.1) | 0.2820 |
| APOA1 | 4,234 (1.2±0.4) | 1,374 (1.2±0.3) | 1,379 (1.2±0.4) | 1,047 (1.3±0.4) | 434 (1.2±0.3) | <0.0001 |
| APOB | 4,947 (1.0±1.1) | 1,630 (1.0±0.8) | 1,609 (1.0±1.7) | 1,213 (1.0±0.3) | 496 (0.9±0.3) | 0.0057 |
| CRP, mg/L,a n (median, IQR) | 7,755 (1.8, 0.8–4.6) | 2,555(1.2, 0.7–2.8) | 2,554(1.7, 0.8–4.2) | 1,871 (2.5, 1.0–6.4) | 775 (4.2, 1.6–13.7) | <0.0001 |
| NIHSS at admission,3 n (median, IQR) | 8,006 (3.0, 2.0–6.0) | 2,642 (3.0, 1.0–6.0) | 2,642 (3.0, 2.0–6.0) | 1,922(3.0, 2.0–6.0) | 800 (4.0, 2.0–7.0) | <0.0001 |
| In-hospital complications, n (%) | ||||||
| Respiratory disease | 425 (5.3) | 59 (2.2) | 114 (4.3) | 140(7.3) | 112(14.0) | <0.0001 |
| Liver disease | 569 (7.1) | 165 (6.3) | 183 (6.9) | 170(8.8) | 51 (6.4) | 0.0057 |
| Urinary system diseases | 208 (2.6) | 39(1.5) | 58 (2.2) | 68 (3.5) | 43 (5.4) | <0.0001 |
| Bleeding | 128 (1.6) | 29(1.1) | 36 (1.4) | 42 (2.2) | 21 (2.6) | 0.0021 |
APOA1, apolipoprotein A1; APOB, apolipoprotein B; BMI, body mass index; BWC, Blood white cell; CHO, cholesterol; CRP, C-reactive protein; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; SD, standard deviation; TG, triglyceride.
Non-normal distribution variables.
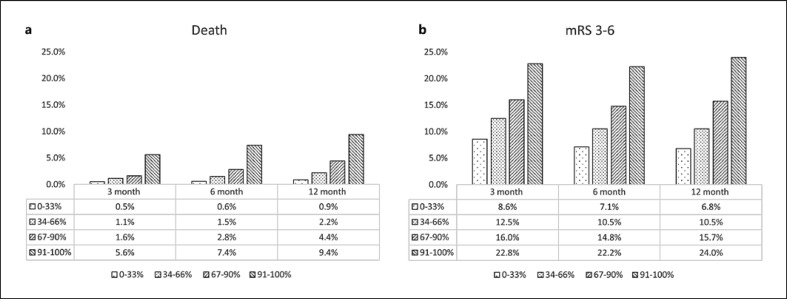
The mortality was lower in 0–33% group than those in 34–66% group or 67–90% group or 91–100% group at 3 months follow-up (0.5% vs. 1.1% vs. 1.6% vs. 5.6%, p < 0.0001), 6 months follow-up (0.6% vs. 1.5% vs. 2.8% vs. 7.4%, p < 0.0001), and 12 months follow-up (0.9% vs. 2.2% vs. 4.4% vs. 9.4%, p < 0.0001). The mRS 3–6 ratio was lower in 0–33% group than those in 34–66% group or 67–90% group or 91–100% group at 3 months follow-up (8.6% vs. 12.5% vs. 16.0% vs. 22.8, p < 0.0001), 6 months follow-up (7.1% vs. 10.5% vs. 14.8% vs. 22.2%, p < 0.0001), and 12 months follow-up (6.8% vs. 10.5% vs. 15.7% vs. 24.0%, p < 0.0001) (shown in Fig. 2).
Fig. 2.
a, b Comparison of outcome in ischemic stroke patients categorized by plasma YKL-40 percentiles. mRS, modified Rankin Scale.
The unadjusted and adjusted HRs of death and odd ratios of mRS 3–6 in patients with first-ever ischemic stroke by YKL-40 percentiles at 3 months, 6 months, and 12 months are shown in Tables 2 and 3, respectively. After adjusting for potential confounders, the multivariable Cox regression analyses showed that 91–100% group of YKL-40 was an independent factor for death (Table 2) at 3 months, 6 months, and 12 months follow-up, and the multivariable logistic regression analyses showed that 91–100% group of YKL-40 was an independent factor for mRS 3–6 (Table 3) at 12-month follow-up.
Table 2.
Unadjusted and adjusted HRs of death in patients categorized by plasma YKL-40 percentiles
| 3 months |
6 months |
12 months |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| patients, n (%) | HR (unadjusted) | HR (adjusted3) | patients, n (%) | HR (unadjusted) | HR (adjusteda) | patients, n (%) | HR (unadjusted) | HR (adjusted3) | |
| 0–33% | 13 (0.5) | Reference | 15 (0.6) | Reference | 23 (0.9) | Reference | |||
| 34–66% | 29(1.1) | 2.24 (1.16–4.30) | 1.41 (0.68–2.93) | 40(1.5) | 2.67 (1.48–4. 83) | 1.71 (0.90–3.27) | 59 (2.2) | 2.57(1.59–4.17) | 1.63 (0.98–2.72) |
| 67–90% | 30(1.6) | 3.19(1.67–6.11) | 1.37 (0.65–2.89) | 54 (2.8) | 4.99 (2.82–8.84) | 2.16(1.14–4.10) | 84 (4.4) | 5.10(3.21–8.09) | 2.23 (1.35–3.69) |
| 91–100% | 45 (5.6) | 11.8(6.36–21.84) | 2.72(1.27–5.84) | 59 (7.4) | 13.53(7.68–23.84) | 3.46(1.77–6.76) | 75 (9.4) | 11.35 (7.11–18.11) | 2.99(1.75–5.11) |
HR, hazard ratios.
Adjusted for: age, sex, smoking, drinking, hypertension, diabetes mellitus, LDL, C-reactive protein, NIHSS, medicine at discharge (antiplatelet, anticoagulant, lipid-lowering, hypoglycemic, antihypertensive medicines), complications (respiratory, liver, urinary, and bleeding diseases).
Table 3.
Unadjusted and adjusted odds ratios of mRS 3–6 in patients categorized by plasma YKL-40 percentiles
| 3 months |
6 months |
12 months |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| patients, n (%) | OR (unadjusted) | OR (adjusteda) | patients, n (%) | OR (unadjusted) | OR (adjusteda) | patients, n (%) | OR (unadjusted) | OR (adjusteda) | |
| 0–33% | 226 (8.6) | Reference | 184 (7.1) | Reference | 174 (6.8) | Reference | |||
| 34–66% | 328(12.5) | 1.51 (1.27–1.81) | 1.16(0.95–1.42) | 274(10.5) | 1.54 (1.27–1.87) | 1.09 (0.88–1.35) | 271 (10.5) | 1.61 (1.32–1.96) | 1.11 (0.89–1.38) |
| 67–90% | 303 (16.0) | 2.01 (1.67–2.41) | 1.18(0.95–1.47) | 280(14.8) | 2.28 (1.87–2.78) | 1.21 (0.96–1.52) | 293(15.7) | 2.57 (2.11–3.14) | 1.30(1.03–1.63) |
| 91–100% | 179(22.8) | 3.13(2.52–3.88) | 1.15 (0.88–1.52) | 174 (22.2) | 3.75 (2.99–4.70) | 1.26 (0.95–1.67) | 187 (24.0) | 4.36 (3.48–5.47) | 1.42(1.08–1.88) |
OR, odds ratios.
Adjusted for: age, sex, smoking, drinking, hypertension, diabetes mellitus, LDL, C-reactive protein, NIHSS, medicine at discharge (antiplatelet, anticoagulant, lipid-lowering, hypoglycemic, antihypertensive medicines), complications (respiratory, liver, urinary, and bleeding diseases).
We classified ischemic stroke according to the Toast classification. In each subtype of ischemic stroke, 12-month mortality (online supp. Table S2) and mRS 3–6 ratio (online suppl. Table S3) were significantly increased with increasing YKL-40 concentration.
The unadjusted and adjusted HRs of death and odd ratios of mRS 3–6 by YKL-40 quartile at 3 months, 6 months, and 12 months are shown in online supplementary Tables S4 and S5, respectively. After adjusting for potential confounders, 76–100% group of YKL-40 was an independent factor for death (online suppl. Table S4) at 6 months and 12 months follow-up, and the multivariable logistic regression analyses showed that 76–100% group of YKL-40 was an independent factor for mRS 3–6 (online suppl. Table S5) at 12 months follow-up.
Receiver operating characteristic analysis indicated that the optimal cut-off values for YKL-40 at admission predicting the risk of death and mRS 3–6 at 12 months follow-up were 135.7 mg/L and 42.5 mg/L, respectively.
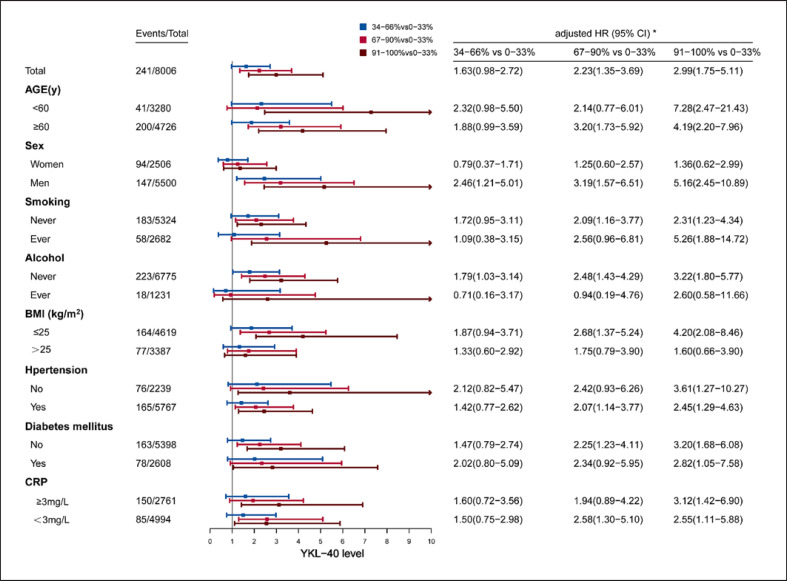
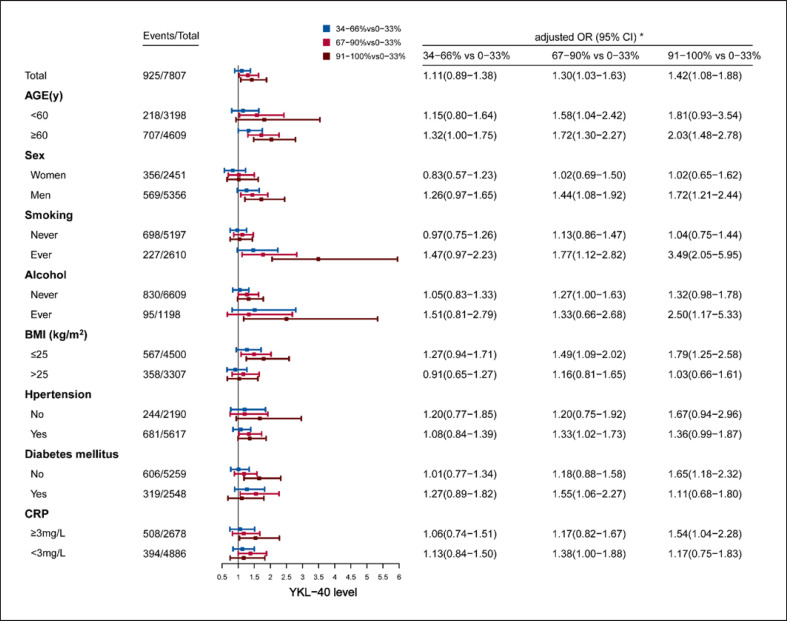
In the subgroup analysis, the associations of YKL-40 percentile categories and death (shown in Fig. 3) as well as mRS 3–6 (shown in Fig. 4) were investigated according to age, sex, smoking, alcohol, BMI, hypertension, diabetes mellitus, and CRP. Higher YKL-40 levels showed significantly higher risk of death as well as mRS 3–6 at 12 months in most subgroups, whereas in some subgroups, only trend instead of statistical difference was found. The associations of YKL-40 quartile categories and death (shown in online suppl. Fig. 1) as well as mRS 3–6 (shown in online suppl. Fig. 2) were also investigated according to above subgroup methods, higher YKL-40 levels also showed similar higher risk of death as well as mRS 3–6.
Fig. 3.
Subgroup analysis of death at 12 months by plasma YKL-40 percentile categories. *HR had been adjusted for potential confounders including age, sex, smoking, drinking, hypertension, DM, LDL, CRP, NIHSS, medicine at discharge (antiplatelet, anticoagulant, lipid-lowering, hypoglycemic, antihypertensive medicines), complications (respiratory, liver, urinary and bleeding diseases). BMI, body mass index; CI, confidence intervals; CRP, C-reactive protein; HR, hazard ratios.
Fig. 4.
Subgroup analysis of mRS 3–6 at 12 months by plasma YKL-40 percentile categories. *OR had been adjusted for potential confounders including age, sex, smoking, drinking, hypertension, DM, LDL, CRP, NIHSS, medicine at discharge (antiplatelet, anticoagulant, lipid-lowering, hypoglycemic, antihypertensive medicines), complications (respiratory, liver, urinary and bleeding diseases). BMI, body mass index; CI, confidence intervals; CRP, C-reactive protein; OR, odds ratios.
Discussion
In the large scale, nationwide stroke registry in China, we found that ischemic stroke patients with higher plasma YKL-40 level had worse prognosis, and higher plasma YKL-40 level was an independent risk factor for mortality, disability of short and long term. It provided strong evidence that YKL-40 should be further investigated as a promising marker of ischemic stroke prognosis, which may improve the ability to predict the prognosis after ischemic stroke.
Previous two small-scale studies, which just included a little more than 100 patients with ischemic stroke, have reported that elevated YKL-40 was associated with higher mortality and poor functional prognosis [15, 16]. In the multicenter stroke registry study which included more than 8,000 patients with first-ever ischemic stroke, we confirmed the previous findings after adjusting for potential confounders such as age, sex, NIHSS, CRP, LDL at admission, in-hospital complications, and medication at discharge, which suggested that blood YKL-40 at admission was independently associated with the mortality and functional prognosis of ischemic stroke.
The mechanism of the relationship between YKL-40 and prognosis of ischemic stroke is mainly referred to the endothelial dysfunction and atherosclerosis induced by YKL-40. YKL-40 is thought to be important in the activation of monocytes and their formation into macrophages, and to facilitate their transformation into lipid-laden foam cells, thereby initiating and accelerating the step of plaque formation [10]. YKL-40 also inhibits macrophage apoptosis by upregulating the apoptosis inhibitor to suppress the activation of caspase-9, which may impede normal programmed cell removal and promote substantial accumulation in early-stage plaques, thereby leading to the progression of atherosclerosis [17].
The immune response to acute ischemic stroke play an important role in stroke pathobiology and affects the outcome [18]. Some inflammatory factors, like circulatory CRP is mainly released from hepatocytes in the liver in response to any systemic inflammation [19]. Thus, the CRP is nonspecific and insensitive to ischemic stroke. In contrast, circulatory YKL-40 in patient with ischemic stroke is mainly released from brain astrocytes and astrocyte and macrophage in response to local neuroinflammation [9]. So circulatory YKL-40 level rapidly, specifically, and directly reflects neuroinflammation without the need of a secondary mediator.
Some population-based studies have shown that elevated YKL-40 was associated with increased risk of ischemic stroke [11, 20]. These results might suggest that YKL-40 reflects atherosclerosis development primarily elicited by vascular risk factors, which was consistent with our findings that most vascular risk factors at baseline were more common in the patients with higher YKL-40 concentrations. This, at least in part, explains the good predictive value of YKL-40 across different ischemic stroke subtypes in this study, since even among patients with undetermined stroke, there is a higher proportion of vascular risk factors [21]. However, the correlation of between YKL-40 and risk factors is complex [22]. We also find the negative correlation between consumption of smoking, alcohol, and YKL-40. The definite causes should be further investigated after adjusting for more potential confounders, including but not limited to race, sex, and other risk factors.
Elevated YKL-40 levels were also detected in several neurological disorders such as stroke and Alzheimer’s disease but not in dementia with Lewy bodies [23, 24]. The exact mechanism is unknown, and one possible mechanism is that YKL-40 expression is affected by reactive astrocytes and astrocytes are highly heterogeneous, both morphologically and functionally, in different neurological diseases [25].
Previous studies have reported that elevated YKL-40 reflected a greater burden of infarct volume and stroke severity [15]. Furthermore, patients with higher blood YKL-40 concentrations were more likely to have higher NIHSS at admission [15], which is similar to our finding that NIHSS in the 90–100% group was much higher than the other groups. However, in our study, after adjusting for NIHSS, YKL-40 was still positively associated with worse prognosis, which indicates that YKL-40 may independently contribute to the worse prognosis through some other mechanism. There are several studies reported that upregulated blood YKL-40 levels were related with poor collateral circulation [26] which were strongly associated with poor stroke outcomes. A meta-analysis reported that high collateralization had a 36% reduced mortality risk compared to low collateralization in coronary artery disease patients [27], and Lee W.J. also found that poor collateral was independently associated with a poor mRS score (odds ratio: 0.21, 95% CI: 0.08–0.58) at 3 months [28]. Blood YKL-40 in patient with ischemic stroke can be released from astrocytes in brain parenchyma in response to local neuroinflammation [29]. Inflammation, angiogenesis, and arteriogenesis are the leading key factors in the development of collaterals. Above-mentioned findings warrant further studies on the role of YKL-40 affecting collateral formation, which may help build up new strategies to enhance collateral formation, thus improve the prognosis of stroke patients.
There are many clinical indicators that can help assess the prognosis of ischemic stroke. For example, NIHSS is a standardized indicator of stroke severity and often used to predict ischemic stroke outcome [30, 31, 32], but the NIHSS evaluation requires special training and NIHSS is less reliable in patients with posterior circulation syndromes compared to anterior ones [30], and left hemispheric stroke syndromes tend to have higher NIHSS scores than right ones [33, 34]. Therefore, some readily measurable biomarkers which are independently associated with the prognosis of stroke, such as YKL-40, may further improve the predictive ability with simplicity.
There are some limitations in this study. First, plasma YKL-40 was only tested once at admission, incapacitating to illuminate the YKL-40 change over time after ischemic stroke. In addition, this study did not analyze the association between YKL-40 gene polymorphism and prognosis of ischemic stroke, and the Mendelian randomization studies are needed in the future to explore causative role of YKL-40 in stroke progression. Despite of these limitations, based on the CNSR-III, which was a nationwide, prospective, multicenter, and comprehensive registry designed to observe the characteristics and outcomes of stroke patients, our study contributes to a better comprehension of the circulatory YKL-40 and stroke prognosis. Further studies should focus on finding potential mechanisms, including vulnerable plaque and collateral circulations, and figure out the effect of blood YKL-40 on different classification of ischemic stroke.
Conclusion
Higher YKL-40 is an independent factor of death, poor functional outcomes at 12 months follow-up after ischemic stroke, and may be useful to identify severe patients and patients with higher risk of poor prognosis, in whom more medical resources should be given. Further studies are required to investigate the determinants of outcomes in patients with higher YKL-40 to provide optimal stroke treatment.
Statement of Ethics
The protocol of the CNSR-III study was approved by the Ethics Committee at Beijing Tiantan Hospital (IRB approval number: KY2015-001-01) and all participating centers. Written informed consent was obtained from all patients or legally authorized representatives before entering into the study.
Conflict of Interest Statement
The authors have no conflicts to declare.
Funding Sources
The study was funded by the National Natural Science Foundation of China (81870907), the National Key R&D Plan of the Ministry of Science and Technology of China (2016YFC1301604, 2017YFC1307702). The authors declare that the funding bodies did not influence the design of the study, the collection, analysis, and interpretation of data, and the writing of the manuscript.
Author Contributions
Yongjun Wang designed the study. Haiqiang Qin and Gaifen Liu conceived of this article. Haiqiang Qin and Gaifen Liu drafted the article, with further contributions from Jing Zhang, Miaoxin Yu, Runhua Zhang, Guitao Zhang, and Xingquan Zhao. Jinxi Lin, Xianhong Liang, and Li Liu determined the plasma YKL-40 levels. Yijun Zhang and Anxin Wang managed and analyzed the data. All authors interpreted data and approved the final version of the article.
Data Availability Statement
All data generated or analyzed during this study are included in this article and online supplementary materials. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary data
Acknowledgments
We thank all participating hospitals, physicians, and nurses, and the CNSR-III Steering Committee members.
Funding Statement
The study was funded by the National Natural Science Foundation of China (81870907), the National Key R&D Plan of the Ministry of Science and Technology of China (2016YFC1301604, 2017YFC1307702). The authors declare that the funding bodies did not influence the design of the study, the collection, analysis, and interpretation of data, and the writing of the manuscript.
References
- 1.Feigin VL, Krishnamurthi RV, Parmar P, Norrving B, Mensah GA, Bennett DA, et al. Update on the global burden of ischemic and hemorrhagic stroke in 1990-2013: the GBD 2013 study. Neuroepidemiology. 2015;45((3)):161–176. doi: 10.1159/000441085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, et al. Global burden of stroke and risk factors in 188 countries, during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016 Aug;15((9)):913–924. doi: 10.1016/S1474-4422(16)30073-4. [DOI] [PubMed] [Google Scholar]
- 3.Qin H, Chen Y, Liu G, Turnbull I, Zhang R, Li Z, et al. Management characteristics and prognosis after stroke in China: findings from a large nationwide stroke registry. Stroke Vasc Neurol. 2020 Jun 22;6((1)):1–9. doi: 10.1136/svn-2020-000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katan M, Elkind MS. The potential role of blood biomarkers in patients with ischemic stroke: an expert opinion. Clin Transl Neurosci. 2018;2((1)):2514183X1876805. [Google Scholar]
- 5.Michelsen AE, Rathcke CN, Skjelland M, Holm S, Ranheim T, Krohg-Sorensen K, et al. Increased YKL-40 expression in patients with carotid atherosclerosis. Atherosclerosis. 2010 Aug;211((2)):589–595. doi: 10.1016/j.atherosclerosis.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 6.Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011;73:479–501. doi: 10.1146/annurev-physiol-012110-142250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Zheng J, Xue Q, Zhao Y. YKL-40 promotes the progress of atherosclerosis independent of lipid metabolism in apolipoprotein E(−/−) mice fed a high-fat diet. Heart Vessels. 2019 Nov;34((11)):1874–1881. doi: 10.1007/s00380-019-01434-w. [DOI] [PubMed] [Google Scholar]
- 8.Clausen BH, Lambertsen KL, Babcock AA, Holm TH, Dagnaes-Hansen F, Finsen B. Interleukin-1beta and tumor necrosis factor-alpha are expressed by different subsets of microglia and macrophages after ischemic stroke in mice. J Neuroinflammation. 2008 Oct 23;5:46. doi: 10.1186/1742-2094-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonneh-Barkay D, Bissel SJ, Kofler J, Starkey A, Wang G, Wiley CA. Astrocyte and macrophage regulation of YKL-40 expression and cellular response in neuroinflammation. Brain Pathol. 2012 Jul;22((4)):530–546. doi: 10.1111/j.1750-3639.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomsen SB, Rathcke CN, Skaaby T, Linneberg A, Vestergaard H. The association between genetic variations of CHI3L1, levels of the encoded glycoprotein YKL-40 and the lipid profile in a Danish population. PLoS One. 2012;7((10)):e47094. doi: 10.1371/journal.pone.0047094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kjaergaard AD, Bojesen SE, Johansen JS, Nordestgaard BG. Elevated plasma YKL-40 levels and ischemic stroke in the general population. Ann Neurol. 2010 Nov;68((5)):672–680. doi: 10.1002/ana.22220. [DOI] [PubMed] [Google Scholar]
- 12.Vega A, Sanchez-Nino MD, Ortiz A, Abad S, Macias N, Aragoncillo I, et al. The new marker YKL-40, a molecule related to inflammation, is associated with cardiovascular events in stable haemodialysis patients. Clin Kidney J. 2020 Apr;13((2)):172–178. doi: 10.1093/ckj/sfz056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Jing J, Meng X, Pan Y, Wang Y, Zhao X, et al. The Third China National Stroke Registry (CNSR-III) for patients with acute ischaemic stroke or transient ischaemic attack: design, rationale and baseline patient characteristics. Stroke Vasc Neurol. 2019 Sep;4((3)):158–164. doi: 10.1136/svn-2019-000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kjaergaard AD, Helby J, Johansen JS, Nordestgaard BG, Bojesen SE. Elevated plasma YKL-40 and risk of infectious disease: a prospective study of 94665 individuals from the general population. Clin Microbiol Infect. 2020 Oct;26((10)):1411 e1–e9. doi: 10.1016/j.cmi.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Park HY, Jun CD, Jeon SJ, Choi SS, Kim HR, Choi DB, et al. Serum YKL-40 levels correlate with infarct volume, stroke severity, and functional outcome in acute ischemic stroke patients. PLoS One. 2012;7((12)):e51722. doi: 10.1371/journal.pone.0051722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen XL, Li Q, Huang WS, Lin YS, Xue J, Wang B, et al. Serum YKL-40, a prognostic marker in patients with large-artery atherosclerotic stroke. Acta Neurol Scand. 2017 Aug;136((2)):97–102. doi: 10.1111/ane.12688. [DOI] [PubMed] [Google Scholar]
- 17.Huan W, Yandong L, Chao W, Sili Z, Jun B, Mingfang L, et al. YKL-40 aggravates early-stage atherosclerosis by inhibiting macrophage apoptosis in an aven-dependent way. Front Cell Dev Biol. 2021;9:752773. doi: 10.3389/fcell.2021.752773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anrather J, Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics. 2016 Oct;13((4)):661–670. doi: 10.1007/s13311-016-0483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kjaergaard AD, Johansen JS, Bojesen SE, Nordestgaard BG. Elevated plasma YKL-40, lipids and lipoproteins, and ischemic vascular disease in the general population. Stroke. 2015 Feb;46((2)):329–335. doi: 10.1161/STROKEAHA.114.007657. [DOI] [PubMed] [Google Scholar]
- 21.Tejada Meza H, Artal Roy J, Perez Lazaro C, Bestue Cardiel M, Alberti Gonzalez O, Tejero Juste C, et al. Epidemiology and characteristics of ischaemic stroke in young adults in Aragon. Neurologia. 2022 Jul–Aug;37((6)):434–440. doi: 10.1016/j.nrleng.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Schernthaner GH, Hobaus C, Brix J. YKL-40 and its complex association with metabolic syndrome, obesity, and cardiovascular disease. Anatol J Cardiol. 2016 Dec;16((12)):959. doi: 10.14744/AnatolJCardiol.2016.22332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wennstrom M, Surova Y, Hall S, Nilsson C, Minthon L, Hansson O, et al. The inflammatory marker YKL-40 is elevated in cerebrospinal fluid from patients with Alzheimer's but not Parkinson's disease or dementia with lewy bodies. PLoS One. 2015;10((8)):e0135458. doi: 10.1371/journal.pone.0135458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Llorens F, Thune K, Tahir W, Kanata E, Diaz-Lucena D, Xanthopoulos K, et al. YKL-40 in the brain and cerebrospinal fluid of neurodegenerative dementias. Mol Neurodegener. 2017 Nov 10;12((1)):83. doi: 10.1186/s13024-017-0226-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matias I, Morgado J, Gomes FCA. Astrocyte heterogeneity: impact to brain aging and disease. Front Aging Neurosci. 2019;11:59. doi: 10.3389/fnagi.2019.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akboga MK, Yalcin R, Sahinarslan A, Yilmaz Demirtas C, Abaci A. Effect of serum YKL-40 on coronary collateral development and SYNTAX score in stable coronary artery disease. Int J Cardiol. 2016 Dec 1;224:323–327. doi: 10.1016/j.ijcard.2016.09.042. [DOI] [PubMed] [Google Scholar]
- 27.Song GY, Wang MY, Wang Y, Liu XB, Feng Y, Kong XQ, et al. [Effect of transcatheter aortic valve replacement using Venus-A valve for treating patients with severe aortic stenosis] Zhonghua Xin Xue Guan Bing Za Zhi. 2017 Oct 24;45((10)):843–847. doi: 10.3760/cma.j.issn.0253-3758.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, Held P, et al. Efficacy and safety of ticagrelor versus aspirin in acute stroke or transient ischaemic attack of atherosclerotic origin: a subgroup analysis of SOCRATES, a randomised, double-blind, controlled trial. Lancet Neurol. 2017 Apr;16((4)):301–310. doi: 10.1016/S1474-4422(17)30038-8. [DOI] [PubMed] [Google Scholar]
- 29.Bonneh-Barkay D, Wang G, Starkey A, Hamilton RL, Wiley CA. In vivo CHI3L1 (YKL-40) expression in astrocytes in acute and chronic neurological diseases. J Neuroinflammation. 2010 Jun 11;7((1)):34. doi: 10.1186/1742-2094-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato S, Toyoda K, Uehara T, Toratani N, Yokota C, Moriwaki H, et al. Baseline NIH stroke scale score predicting outcome in anterior and posterior circulation strokes. Neurology. 2008 Jun 10;70((24 Pt 2)):2371–2377. doi: 10.1212/01.wnl.0000304346.14354.0b. [DOI] [PubMed] [Google Scholar]
- 31.Rost NS, Bottle A, Lee JM, Randall M, Middleton S, Shaw L, et al. Stroke severity is a crucial predictor of outcome: an international prospective validation study. J Am Heart Assoc. 2016 Jan 21;5((1)):e002433. doi: 10.1161/JAHA.115.002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozyolkin O, Kuznietsov A, Novikova L. Prediction of the lethal outcome of acute recurrent cerebral ischemic hemispheric stroke. Medicina. 2019 Jun 25;55((6)):311. doi: 10.3390/medicina55060311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fink JN, Selim MH, Kumar S, Silver B, Linfante I, Caplan LR, et al. Is the association of National Institutes of Health Stroke Scale scores and acute magnetic resonance imaging stroke volume equal for patients with right- and left-hemisphere ischemic stroke? Stroke. 2002 Apr;33((4)):954–958. doi: 10.1161/01.str.0000013069.24300.1d. [DOI] [PubMed] [Google Scholar]
- 34.Agis D, Goggins MB, Oishi K, Oishi K, Davis C, Wright A, et al. Picturing the size and site of stroke with an expanded national institutes of health stroke scale. Stroke. 2016 Jun;47((6)):1459–1465. doi: 10.1161/STROKEAHA.115.012324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
All data generated or analyzed during this study are included in this article and online supplementary materials. Further inquiries can be directed to the corresponding author.