Abstract
2′,3′-Dideoxy-2′,3′-didehydro-β-l(−)-5-fluorocytidine [l(−)Fd4C] has been reported to be a potent inhibitor of the human immunodeficiency virus (HIV) in cell culture. In the present study the antiviral activity of this compound in two-drug combinations and its intracellular metabolism are addressed. The two-drug combination of l(−)Fd4C plus 2′,3′-didehydro-2′,3′-dideoxythymidine (D4T, or stavudine) or 3′-azido-3′-deoxythymidine (AZT, or zidovudine) synergistically inhibited replication of HIV in vitro. Additive antiviral activity was observed with l(−)Fd4C in combination with 2′,3′-dideoxycytidine (ddC, or zalcitabine) or 2′,3′-dideoxyinosine (ddI, or didanosine). This β-l(−) nucleoside analog has no activity against mitochondrial DNA synthesis at concentrations up to 10 μM. As we previously reported for other β-l(−) nucleoside analogs, l(−)Fd4C could protect against mitochondrial toxicity associated with D4T, ddC, and ddI. Metabolism studies showed that this drug is converted intracellularly to its mono-, di-, and triphosphate metabolites. The enzyme responsible for monophosphate formation was identified as cytoplasmic deoxycytidine kinase, and the Km is 100 μM. l(−)Fd4C was not recognized in vitro by human mitochondrial deoxypyrimidine nucleoside kinase. Also, l(−)Fd4C was not a substrate for deoxycytidine deaminase. l(−)Fd4C 5′-triphosphate served as an alternative substrate to dCTP for incorporation into DNA by HIV reverse transcriptase. The favorable anti-HIV activity and protection from mitochondrial toxicity by l(−)Fd4C in two-drug combinations favors the further development of l(−)Fd4C as an anti-HIV agent.
The emergence of viral resistance during antiviral therapy represents a major challenge requiring the development of new drugs for the control of human immunodeficiency virus (HIV) infection. Results of clinical trials are showing the increased benefit of combination antiviral drug therapy over monotherapy in the management of HIV infection (9–11, 18, 38, 39). Studies of favorable drug combinations both in vitro and in vivo have shown greater antiviral efficacy that is sustained for longer periods, compared with single drugs (12, 19, 31). These types of studies also illustrate that combination therapy for HIV infection has important potential for antiviral synergy and reduced drug toxicity.
There is a continued need for new anti-HIV agents with greater efficacy, lower toxicity, and improved resistance profiles. A proven target for HIV therapy is the virally encoded reverse transcriptase (HIV-RT). There are currently two major classes of HIV-RT inhibitors, the nucleoside analogs and the structurally unrelated nonnucleoside inhibitors. Additionally, nucleoside analogs can be differentiated by stereochemistry. The anti-HIV drug β-l(−)-2′,3′-dideoxy-3′-thiacytidine [l(−)SddC; also called 3TC, or lamivudine] is the first drug approved from the group of enantiomeric nucleoside analogs with the unnatural β-l(−) configuration that have been shown to exhibit potent antiviral activity (Fig. 1) (8). Following the discovery and approval for clinical use of l(−)SddC (3TC), the synthesis and biological evaluation of nucleoside analogs with the unnatural β-l(−) configuration have been the subject of intense investigation (12, 16, 20, 23, 26, 41).
FIG. 1.
Chemical structures of anti-HIV nucleoside analogs.
β-l(−)-2′,3′-Dideoxy-5-fluoro-3′-thiacytidine [l(−)FTC] and β-l(−)-2′,3′-dideoxy-5-fluorocytidine [l(−)FddC] are β-l(−) nucleoside analogs with potent and selective activity against HIV (25, 34). We previously reported the synergistic interaction of these β-l(−) nucleoside analogs in vitro in two-drug combinations with 3′-azido-3′-deoxythymidine (AZT, or zidovudine) and 2′,3′-didehydro-2′,3′-dideoxythymidine (D4T, or stavudine) (1). In that study none of the β-l(−) nucleoside analogs in two-drug combinations had additive toxicity in cell culture, and they could protect against the mitochondrial toxicity associated with AZT, D4T, 2′,3′-dideoxycytidine (ddC, or zalcitabine), and 2′,3′-dideoxyinosine (ddI, or didanosine). Our previous studies suggest that the ability of 5′-triphosphates of nucleoside analogs to be transported from the cytosol into mitochondria may be a major determinant in the inhibition of mitochondrial DNA (mtDNA) replication, resulting in the delayed toxicities of antiviral nucleoside analogs (4, 6). Evidence also suggests that β-l(−) nucleoside analogs can prevent the antimitochondrial effects of β-d(+) nucleoside analogs, possibly by interfering with their uptake into mitochondria (1). In the search for agents with improved pharmacological profiles, we recently reported a new compound, 2′,3′-dideoxy-2′,3′-didehydro-β-l(−)-5-fluorocytidine [l(−)Fd4C], which demonstrated exceptionally potent activity against hepatitis B virus (HBV) and HIV (28). The activity of l(−)Fd4C against HIV makes it an attractive candidate for clinical trials; therefore, it is important to study its metabolism in human cells. We report here the biological activity of l(−)Fd4C against HIV type 1 (HIV-1) when it is used in combination with either AZT, D4T, ddC, or ddI.
MATERIALS AND METHODS
Compounds.
l(−)Fd4C was synthesized in the laboratory of the late Tai-Shun Lin at Yale University (28). [3H]Deoxycytidine, [3H]5-fluorocytosine, [3H]l(−)Fd4C, and [3H]l(−)SddC ([3H]3TC) were purchased from Moravek Biochemicals (Brea, Calif.) [3H]l(−)FddC was synthesized in the laboratory of Tai-Shun Lin as previously described (27) by using [3H]5-fluorocytosine (5 Ci/mmol). ddC and AZT were purchased from ICN Pharmaceuticals, Inc. (Costa Mesa, Calif.) and Sigma (St. Louis, Mo.), respectively. D4T and ddI were purchased from Bristol-Myers Squibb (Wallingford, Conn.). l(−)Fd4C 5′-monophosphate and l(−)Fd4C 5′-triphosphate were gifts from Vion Pharmaceuticals (New Haven, Conn.). All other chemicals were of the highest grade available. All other nucleoside analog 5′-triphosphates were synthesized as previously described (3). HIV-RT was purchased from Worthington Biochemical Co. (Freehold, N.J.).
[3H]l(−)Fd4C and [3H]l(−)SddC ([3H]3TC) were further purified by high-performance liquid chromatography (HPLC) by using a chiral column (Cyclobond I 2000, 500 by 10 cm; Advanced Separation Technologies, Inc., Whippany, N.J.) with water as the mobile phase at a flow rate of 1 ml/min. The UV profile was observed at 270 nm, and the radioactivity was measured in tandem with a 150TR Radiomatic Flow Scintillation Analyzer (Packard, Downers Grove, Ill.). The purified peak was collected and used in all isotopic studies. The enantiomeric purity was greater than 99% for all compounds.
Determination of antiviral activity in HIV-1-infected MT-2 cells.
Drugs were tested by using MT-2 cells infected with HIV-1 strain IIIB as described previously (30). Briefly, triplicate wells of 96-well plates containing 104 MT-2 cells were infected with HIV at a multiplicity of infection of 0.1 tissue culture infective dose per cell. Serial dilutions of drug were added after infection. Cell viability was quantitated by the tetrazolium dye reduction method (23) 5 days after infection. The percentage of protection was calculated by the equation (a − b/c − b) × 100, where a is the A595 of drug-treated, virus-infected wells, b is the A595 of untreated infected wells, and c is the A595 of untreated uninfected wells. The 50% effective concentration (EC50) for each drug was calculated from linear log10 plots of the percentage of protection versus drug concentration. Isobolograms are defined as the percent change in the EC50 of the first agent when it is used in combination with the second agent plotted against the percent change in the EC50 of the second agent when it is used in combination with the first agent.
Measurement of mtDNA.
The effects of nucleoside analogs on mtDNA content were assessed as previously described (1). Briefly, CEM cells were seeded at 2 × 105/ml in RPMI 1640 medium supplemented with 10% dialyzed fetal bovine serum. Cells were treated with various single agents and two-drug combinations for 8 days, with medium changes on days 4 and 6. Cells (105) were harvested on day 8, lysed, treated with proteinase K and DNase-free RNase, and applied to nylon membranes for hybridization with an mtDNA probe. All blots were normalized for loading by using an Alu probe and were quantitated by using a Molecular Dynamics Personal Densitometer SI with ImageQuaNT image analysis software.
Assay for the analysis of intracellular metabolites.
To evaluate the intracellular drug metabolites, CEM cells or cytoplasmic deoxycytidine kinase-deficient CEM cells (4) (5 × 105/ml) were incubated with 2 μM [3H]l(−)Fd4C (20 mCi/mmol) for 24 h. Cells were harvested by centrifugation, washed twice with ice-cold phosphate-buffered saline, and extracted with 60% methanol on ice for 15 min. The methanol-soluble fraction was evaporated to dryness, resuspended in water, and analyzed by ion-exchange HPLC using a Whatman Partisil-SAX column at a flow rate of 1 ml/min as previously described (3). Intracellular metabolites were identified by a combination of authentic cold standards and enzyme digestion of methanol-soluble extracts. Methanol-soluble extracts were digested by alkaline phosphatase as previously described (40). Radioactivity was measured in tandem with a 150TR Radiomatic Flow Scintillation Analyzer.
Assay for the activity of deoxycytidine kinase.
Purified deoxycytidine kinase from BL21(DE3) bacteria containing the PET-3d expression vector, which has the cDNA of human deoxycytidine kinase from KB cells, was used to examine the phosphorylation of l(−)Fd4C to the 5′-monophosphate derivative. Deoxycytidine kinase was purified as previously described (7). Mitochondrial deoxypyrimidine nucleoside kinase was purified by affinity chromatography with a thymidine analog ligand as described elsewhere by using chronic lymphocytic leukemia cells harvested from patients by leukophoresis (24). The kinase assays were performed as described elsewhere (44) except that the substrate conditions were 0.625 to 10 μM deoxycytidine (64 mCi/mmol), 6.5 to 162 μM [3H]l(−)Fd4C (8 mCi/mmol), 4 to 100 μM [3H]l(−)FddC (8 mCi/mmol), and 1.25 to 20 μM [3H]l(−)SddC ([3H]3TC) (27 mCi/mmol). Briefly, enzyme (0.01 U of deoxycytidine kinase or 0.0006 U of mitochondrial deoxypyrimidine nucleoside kinase; a unit is defined as the conversion of 1 nmol of substrate per min) was incubated with the kinase mixture containing 140 mM Tris (pH 7.5), 1.7 mM dithiothreitol, 8 mM NaF, and 2 mM ATP-MgCl2 for 2 h at 37°C. Reaction mixtures were applied to DE-81 discs (Whatman), washed three times in 1 mM ammonium formate followed by one wash in ethanol, and dried, and radioactivity was eluted from the discs with a solution of 0.2 N HCl and 2 M NaCl, followed by scintillation counting.
Assay for the activity of deoxycytidine deaminase.
Human liver deoxycytidine deaminase was partially purified as previously described (17). The deaminase assay was performed essentially as described elsewhere (3). Briefly, the enzyme was incubated at 37°C with 0.5 mM l(−)Fd4C or deoxycytidine in 25 mM Tris buffer (pH 7.5) for 22 h. The reaction was stopped, and the sample was prepared by methanol extraction and analyzed by reverse-phase HPLC as described above.
Chain termination assays.
Human mitochondrial DNA polymerase γ (pol γ) was purified as described elsewhere (4). Triphosphates of ddC, l(−)Fd4C, and l(−)SddC (3TC) were analyzed for their chain elongation activities by using M13mp19 phage DNA hybridized with a 5′-32P-22-mer oligonucleotide primer as described elsewhere (21). Incorporation of one template complementary nucleotide into the 3′ terminus of the primer was carried out in an 8-μl mixture containing 1 U of HIV-RT or pol γ (1 Unit is defined as the incorporation of 1 nmol of dTTP into activated DNA in 1 h), 50 mM Tris, 60 mM KCl, 1 mM dithiothreitol, 10 mM MgCl2, 0.05 μM 22-mer oligonucleotide (5′-GTAAAACGACGGCCAGTGAATT-3′) annealed to M13mp19 phage DNA (3′-CATTTTGCTGCCGGTCACTTAAGCTCGA-5′), and 0.5 μM nucleoside analog 5′-triphosphate. After incubation for 30 min at 37°C, the reaction was stopped by the addition of EDTA and formamide-containing dyes. The reaction products were analyzed by autoradiography on a 15% denaturing acrylamide gel.
RESULTS
Antiviral effect of l(−)Fd4C on HIV replication in MT-2 cells.
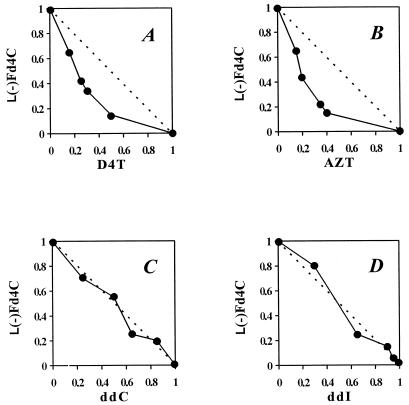
Experiments were performed with various two-drug combinations containing l(−)Fd4C and the data used to plot isobolograms. Antiviral activity for a two-drug combination resulting in a curve below the line between the EC50s of the drugs as single agents indicates synergistic antiviral activity. The combination of l(−)Fd4C with either AZT or D4T demonstrated synergistic activity at blocking HIV-induced cell killing (Fig. 2A and B). Additive antiviral activity was noted with the combination of l(−)Fd4C with either ddC or ddI (Fig. 2C and D).
FIG. 2.
Antiviral isobolograms of drug combination data obtained in MT-2 cells with l(−)Fd4C plus D4T (A), l(−)Fd4C plus AZT (B), l(−)Fd4C plus ddC (C), and l(−)Fd4C plus ddI (D). Numbers along each axis are proportions of the EC50 (taken as 1) for the drug indicated as a single agent. [EC50s for single agents are 0.036 μM AZT, 1.8 μM D4T, 0.6 μM ddC, 12 μM ddI, and 0.5 μM l(−)Fd4C.] Each datum point represents a combination that produces an effect equivalent to that of the EC50 for either drug used alone.
Table 1 represents a typical experiment demonstrating nucleoside analog-induced mitochondrial toxicity. The anti-HIV β-d(+) nucleoside analogs D4T, ddC, and ddI decrease mtDNA levels in treated cells, whereas l(−)Fd4C had minimal effects on mtDNA content. In agreement with what we previously reported for l(−)SddC (3TC), l(−)FddC, and l(−)FTC, combination with l(−)Fd4C could protect cells against drug-induced decreases in mtDNA content (1). In addition, we observed that 4 days of exposure to l(−)Fd4C in combination with either AZT, D4T, ddC, or ddI did not demonstrate more than additive toxicity towards CEM cells (data not shown). We previously reported the concentration that inhibited CEM cell growth by 50% to be 7 μM (28).
TABLE 1.
Effects of l(−)Fd4C in combination with β-d(+) nucleoside analogs on mtDNA contenta
| Drug (concn) | mtDNA content (% of control)
|
|
|---|---|---|
| With drug alone | With drug plus 0.5 μM l(−)Fd4C | |
| ddC (0.1 μM) | 38 ± 9 | 74 ± 12 |
| ddI (50 μM) | 47 ± 8 | 67 ± 10 |
| D4T (30 μM) | 27 ± 4 | 82 ± 7 |
| l(−)Fd4C (1 μM) | 93 ± 12 | |
Treatment of CEM cells with drugs was performed as described in Materials and Methods. The CEM cells were harvested after 8 days of drug exposure to assess mtDNA content. Values are means ± standard deviations from three independent experiments with each data point in duplicate.
Phosphorylation of l(−)Fd4C by cytoplasmic deoxycytidine kinase.
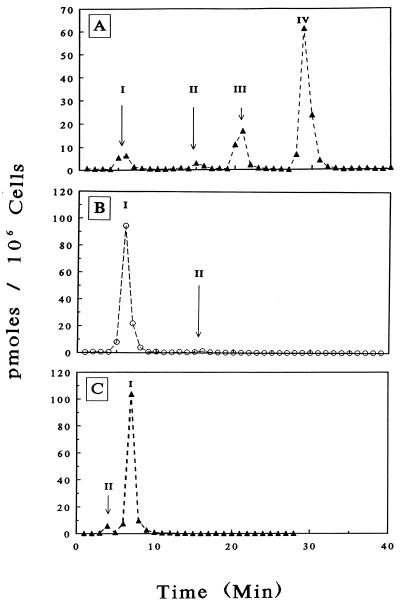
Because l(−)Fd4C is a nucleoside analog and must be converted to the 5′-triphosphate metabolite in order to be substrate for the viral DNA polymerase, it is important to determine the intracellular phosphorylation pattern of this compound in cell culture. The HPLC nucleotide profile from human CEM cells after 24 h of exposure to 2 μM [3H]l(−)Fd4C (20 mCi/mmol) revealed the presence of phosphorylated nucleoside metabolites as confirmed by enzymatic digestion (Fig. 3) and authentic standards. These data suggest that l(−)Fd4C can enter cells and undergo activation despite its unnatural β-l(−) configuration.
FIG. 3.
HPLC analysis of l(−)Fd4C metabolites in CEM cells. (A) Methanol extracts prepared from CEM cells treated for 24 h with 2 μM [3H]L(−)Fd4C (20 mCi/mmol) were applied to a Partisil-SAX ion-exchange column as described in Materials and Methods. (B and C) Ion-exchange and reverse-phase chromatograms, respectively, of methanol-soluble extracts described above and digested with 25 U of alkaline phosphatase. Metabolites: I, l(−)Fd4C; II, l(−)Fd4C 5′-monophosphate; III, l(−)Fd4C 5′-diphosphate; IV, l(−)Fd4C 5′-triphosphate.
The fact that l(−)Fd4C is an analog of deoxycytidine suggests that cytoplasmic deoxycytidine kinase might catalyze the phosphorylation of this compound to the 5′-monophosphate metabolite. To further investigate the phosphorylation of l(−)Fd4C intracellularly, CEM cells were exposed to 2 μM [3H]l(−)Fd4C (20 mCi/mmol), and the methanol-soluble metabolites were analyzed by HPLC. These CEM cells had approximately 140 pmol of l(−)Fd4C phosphorylated metabolites/106 cells. However, when cytoplasmic deoxycytidine kinase-deficient CEM cells were used under identical conditions, l(−)Fd4C phosphorylated metabolite levels decreased more than 70-fold, to less than 2 pmol/106 cells. These results suggest a major role for cytoplasmic deoxycytidine kinase in the monophosphorylation of l(−)Fd4C. Indeed, when l(−)Fd4C was incubated with human deoxycytidine kinase, it served as a substrate with an apparent Km of 100 μM, compared to 7 μM for deoxycytidine (Table 2). The relative Vmax of l(−)Fd4C was 3.2-fold greater than that of deoxycytidine. By comparison, l(−)FddC is similar to l(−)Fd4C, with a higher Km and a higher Vmax than deoxycytidine, whereas l(−)SddC (3TC) has a Km similar to that of deoxycytidine but a much lower relative Vmax.
TABLE 2.
Substrate properties of β-l(−) nucleoside analogs with human dCyd kinasesa
| Substrate | Result with cytoplasmic dCyd kinase
|
Relative Vmaxc with mitochondrial deoxypyrimidine kinase | |
|---|---|---|---|
| Kmb (μM) | Relative Vmaxc | ||
| dCyd | 7 ± 0.1 | 1 | 1 |
| l(−)Fd4C | 100 ± 1 | 3.2 | <0.01 |
| l(−)FddC | 45 ± 5 | 6.4 | <0.01 |
| l(−)SddC (3TC)d | 8 ± 1 | 0.033 | <0.01 |
dCyd, deoxycytidine.
Values are means ± standard deviations from three independent experiments with each data point in triplicate.
Vmax values are relative to those for deoxycytidine.
Values are taken from reference 4
Another deoxynucleoside kinase capable of using deoxycytidine as a substrate is the mitochondria-associated deoxypyrimidine nucleoside kinase. When l(−)Fd4C was incubated with human mitochondrial deoxypyrimidine nucleoside kinase, no phosphorylation of l(−)Fd4C could be detected.
Susceptibility of l(−)Fd4C to deamination.
An important enzyme in deoxycytidine catabolism is deoxycytidine deaminase. This enzyme is also important because a deoxycytidine analog catabolized to a deoxyuridine analog by deoxycytidine deaminase may have dramatically reduced antiviral activity. Because deoxycytidine deaminase is critical for the efficacy of deoxycytidine analogs, its activity toward l(−)Fd4C was determined. Following a 22-h exposure of either deoxycytidine or l(−)Fd4C to partially purified human deoxycytidine deaminase, no detectable l(−)Fd4U was present. In contrast, deoxyuridine was the only nucleoside detectable after the incubation of deoxycytidine deaminase with deoxycytidine.
Chain termination of DNA synthesis by HIV-RT and mitochondrial DNA pol γ.
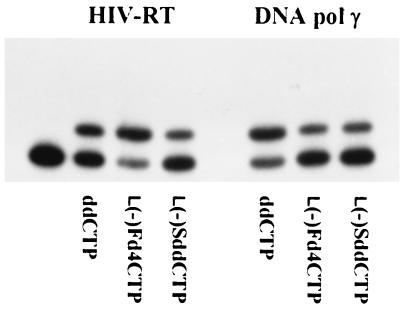
The ability of HIV-RT to utilize l(−)Fd4C 5′-triphosphate as an alternative substrate for the incorporation of l(−)Fd4C 5′-monophosphate into DNA was assessed by an incorporation assay. Because l(−)Fd4C is a dideoxynucleoside analog lacking a 3′ OH group, addition of l(−)Fd4C 5′-monophosphate onto DNA 3′ termini will result in termination of DNA chain elongation. To measure nucleotide analog incorporation, a DNA primer was radiolabeled on the 5′ termini and annealed to a DNA template. The primer-template sequence was chosen such that the first base incorporated during DNA synthesis would be dCMP or a deoxycytidine analog, either ddC 5′-monophosphate, l(−)SddC (3TC) 5′-monophosphate, or l(−)Fd4C 5′-monophosphate. As Fig. 4 illustrates, the 5′-triphosphate of l(−)Fd4C, like those of ddC and l(−)SddC (3TC), was a substrate for incorporation by HIV-RT.
FIG. 4.
β-l(−) Deoxycytidine analogs as substrates of HIV-RT and mitochondrial DNA pol γ. Shown is an autoradiograph of chain elongation assessed by using a 32P-22-mer oligonucleotide primer annealed to M13mp19 phage DNA in the presence of enzyme and 0.5 μM nucleoside analog 5′-triphospate. The left lane shows the position of the primer in the incubation mixture without substrate. Reaction conditions were as described in Materials and Methods.
The interaction of l(−)Fd4C 5′-triphosphate with mitochondrial DNA pol γ was also investigated in order to assess the selectivity of l(−)Fd4C 5′-triphosphate. Mitochondrial DNA pol γ is a key enzyme required for mtDNA synthesis, and inhibition of mtDNA synthesis by the 5′-triphosphates of nucleoside analogs has been proposed to play a major role in their toxicity (32). When human mitochondrial DNA pol γ was used, incorporation of the 5′-monophosphates of ddC, l(−)SddC (3TC), and l(−)Fd4C was evident (Fig. 4). Further studies on the interaction of l(−)Fd4C 5′-triphosphate with HIV-RT and human DNA polymerases will be presented elsewhere (22).
DISCUSSION
The discovery of β-l(−) nucleoside analogs as antiviral agents has generated tremendous interest in their development. We recently reported the anti-HIV and anti-HBV activities of a new β-l(−) nucleoside analog, l(−)Fd4C (28). The antiretroviral activity of a nucleoside analog is dependent upon many factors, including intracellular phosphorylation and the interaction of its anabolites with HIV-RT. We have demonstrated the intracellular phosphorylation of l(−)Fd4C to the 5′-triphosphate metabolite and the role of cytoplasmic deoxycytidine kinase in the first step of this process. However, the mitochondrial deoxypyrimidine nucleoside kinase could not utilize l(−)Fd4C as a substrate. The behavior of l(−)Fd4C towards cytoplasmic deoxycytidine kinase is different from that of l(−)SddC (3TC). l(−)Fd4C has a higher Km and a higher relative Vmax than deoxycytidine, whereas l(−)SddC (3TC) has a Km similar to that of deoxycytidine but a much lower Vmax. Thus, increasing dosages of l(−)SddC (3TC) beyond the maximum rate of l(−)SddC (3TC) 5′-monophosphate formation will have little effect on the total level of intracellular phosphorylated metabolites, whereas higher doses of l(−)Fd4C may be given to increase active metabolite levels. This difference in enzyme kinetics also suggests that the degree of phosphorylation of l(−)Fd4C may have an impact on the scheduling of treatment. Indeed, further intracellular studies in which the intracellular half-life of phosphorylated l(−)Fd4C metabolites was significantly greater than that of phosphorylated l(−)SddC (3TC) metabolites support this observation (43). Thus, less-frequent dosing and escalation of dosage to overcome viral resistance may be achieved with l(−)Fd4C compared with l(−)SddC (3TC). These results show important differences between β-l(−) nucleoside analogs and demonstrate that each nucleoside analog must be evaluated as a unique compound (for a review, see reference 37). Another aspect of deoxycytidine analog metabolism is the possibility of catabolism to the uridine derivative by deoxycytidine deaminase activity, resulting in a drug with decreased antiviral activity. To assess if l(−)Fd4C can be deaminated by deoxycytidine deaminase activity, we tested the activity of human deoxycytidine deaminase against l(−)Fd4C. Consistent with what has been reported for other β-l(−) deoxycytidine analogs (3, 14, 17, 29), deamination of l(−)Fd4C could not be detected.
The delayed toxicity of a number of clinically approved anti-HIV nucleoside analogs has been suggested to be the result of an inhibition of mtDNA synthesis subsequent to their incorporation into mtDNA by mitochondrial DNA pol γ (4–6). However, inhibition of mitochondrial DNA pol γ by nucleoside analogs in vitro is not indicative of their impact on mtDNA, as in the case of l(−)SddC (3TC), where the 5′-triphosphate has a Ki of 0.01 μM for mitochondrial DNA pol γ, but in CEM cell culture the concentration required to decrease mtDNA content by 50% is 50 μM (3, 4). Therefore, it is important to assess the effect on multiple mitochondrial factors in addition to mitochondrial DNA pol γ inhibition. In the present study we assessed the drug’s effect on mitochondrial DNA pol γ and mtDNA content in whole cells. Where incorporation of l(−)Fd4C into mtDNA by mitochondrial DNA pol γ was observed, use of l(−)Fd4C did not result in a decrease in mtDNA content in CEM cells after 8 days of culture. Furthermore, l(−)Fd4C has no greater than additive cytotoxicity in two-drug combinations and, at least in part, protects cells from mitochondrial toxicity induced by D4T, ddC, and ddI. Further studies will address whether an alteration of either natural deoxynucleoside triphosphate (dNTP) pool sizes or dideoxynucleoside analog metabolism plays any role in the protection of mtDNA by l(−)Fd4C. However, previous studies suggest that the mitochondrial uptake of nucleoside analog 5′-triphosphates from the cytoplasm may play a role in the inhibition of mtDNA synthesis (4, 6). Indeed, we recently reported a mitochondrial carrier activity capable of transporting dNTPs (2). The role of this mitochondrial dNTP transport activity in the antimitochondrial effects of β-d(+) nucleoside analogs and their reversal by β-l(−) nucleoside analogs is under investigation.
These in vitro studies have shown that some two-drug combinations with l(−)Fd4C are synergistic against HIV. Although several biochemical mechanisms could account for this antiviral synergy, the alteration of either nucleoside analog metabolism or natural dNTP pool size and the synergistic inhibition of HIV-RT appear not to be related to the synergy reported for other nucleoside analog combinations (1, 33, 42). The role of these factors in the synergy of l(−)Fd4C two-drug combinations requires further investigation. We recently reported a cytosolic exonuclease activity capable of removing nucleoside analogs from DNA and its inhibition by free nucleoside analog 5′-monophosphates (36). The role of this cytosolic exonuclease in determining nucleoside analog antiviral synergy is under investigation.
More studies are required to identify any changes in HIV-RT that are associated with the development of resistance to l(−)Fd4C and the cross-resistance profile with other anti-HIV nucleoside analogs. Development of HIV resistance to l(−)SddC (3TC), l(−)FddC, and l(−)FTC has been observed (13, 15, 35), and this should be expected with l(−)Fd4C. Reverse transcriptases from these resistant viruses were consistent with a mutation at codon 184. An l(−)Fd4C-associated mutation at codon 184, similar to mutations associated with other β-l(−) nucleoside analogs, may lead to a phenotypic suppressive effect such as that suggested for an increase in AZT sensitivity by the l(−)SddC (3TC)-associated mutation of codon 184 in AZT-resistant virus. The codon 184 mutation has not been observed to suppress a D4T-resistant phenotype, but its combination with l(−)Fd4C should be considered because of antiviral synergistic activity. Combinations of ddI and ddC were only additive in combination with l(−)Fd4C, but considering the protection from mtDNA perturbations, these drug combinations may decrease the delayed toxicity of ddI and ddC.
l(−)Fd4C has potent in vitro activity against HIV. The difference in the effective concentration of l(−)Fd4C required to inhibit HIV replication at submicromolar levels and the concentrations required to inhibit cell growth make l(−)Fd4C at least as effective as l(−)SddC (3TC). We have also observed that the intracellular half-life of l(−)Fd4C phosphorylated metabolites is approximately 5 times longer than that of l(−)SddC (3TC) phosphorylated metabolites (43). Given similar pharmacokinetics, it is likely that the frequency of l(−)Fd4C doses required to suppress HIV replication will be lower than that for l(−)SddC (3TC). In conclusion, the synergistic effect of l(−)Fd4C with AZT and D4T, concomitant with its reversal of mitochondrial toxicity, supports the further evaluation of l(−)Fd4C for the treatment of HIV infection.
ACKNOWLEDGMENT
This work was supported by Public Health Service grant AI38204 from the National Institutes of Health.
REFERENCES
- 1.Bridges E G, Dutschman G E, Gullen E A, Cheng Y-C. Favorable interaction of β-l(−) nucleoside analogues with clinically approved anti-HIV nucleoside analogues for the treatment of human immunodeficiency virus. Biochem Pharmacol. 1996;51:731–736. doi: 10.1016/0006-2952(96)00056-1. [DOI] [PubMed] [Google Scholar]
- 2.Bridges E G, Jiang Z L, Cheng Y C. Identification of a novel mitochondrial dNTP carrier and its interaction with anti-HIV nucleoside analogs. Proc Am Assoc Cancer Res. 1997;38:414. [Google Scholar]
- 3.Chang C-N, Doong S-L, Zhou J H, Beach J W, Jeong L S, Chu C K, Tsai C-H, Liotta D C, Schinazi R F, Cheng Y-C. Deoxycytidine deaminase-resistant stereoisomer is the active form of (±)-2′,3′-dideoxy-3′-thiacytidine in the inhibition of hepatitis B virus replication. J Biol Chem. 1992;267:13938–13942. [PubMed] [Google Scholar]
- 4.Chang C N, Skalski V, Zhou J H, Cheng Y-C. Biochemical pharmacology of (+) and (−)-2′,3′-dideoxy-3′-thiacytidine as anti-hepatitis B virus agents. J Biol Chem. 1992;267:22414–22420. [PubMed] [Google Scholar]
- 5.Chen C-H, Vazquez-Padua M, Cheng Y-C. The effect of anti-HIV nucleoside analogs on mitochondrial DNA and its implication for delayed toxicity. Mol Pharmacol. 1991;39:625–628. [PubMed] [Google Scholar]
- 6.Chen C H, Cheng Y-C. The role of cytoplasmic deoxycytidine kinase in the mitochondrial effects of the anti-human immunodeficiency virus compound, 2′,3′-dideoxycytidine. J Biol Chem. 1992;267:2856–2859. [PubMed] [Google Scholar]
- 7.Cheng Y-C, Domin B, Lee L-S. Human deoxycytidine kinase. Purification and characterization of the cytoplasmic and mitochondrial isozymes derived from blast cells of acute myelocytic leukemia patients. Biochim Biophys Acta. 1977;481:481–492. doi: 10.1016/0005-2744(77)90281-9. [DOI] [PubMed] [Google Scholar]
- 8.Coates J A V, Cammack N, Jenkinson H J, Mutton I M, Pearson B A, Storer R, Cameron J M, Penn C R. The separated enantiomers of 2′-deoxy-3′-thiacytidine (BCH 189) both inhibit human immunodeficiency virus replication in vitro. Antimicrob Agents Chemother. 1992;36:202–205. doi: 10.1128/aac.36.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collier A C, Coombs R W, Schoenfeld D A, Bassett R L, Timpone J, Baruch A, Jones M, Facey K, Whitacre C, McAuliffe V J, Friedman H M, Merigan T C, Reichman R C, Hooper C, Corey L. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine and zalcitabine. N Engl J Med. 1996;334:1011–1017. doi: 10.1056/NEJM199604183341602. [DOI] [PubMed] [Google Scholar]
- 10.Delta Coordinating Committee. Delta: a randomized double-blind controlled trial comparing combinations of zidovudine plus didanosine or zalcitabine with zidovudine alone in HIV-infected individuals. Lancet. 1996;348:283–291. [PubMed] [Google Scholar]
- 11.Eron J J, Benoit S L, Jemsek J, MacArthur R D, Santana J, Quinn J B, Kuritzkes D R, Fallon M A, Rubin M. Treatment with lamivudine, zidovudine, or both in HIV-positive patients with 200 to 500 CD4+ cells per cubic millimeter. N Engl J Med. 1995;333:1662–1669. doi: 10.1056/NEJM199512213332502. [DOI] [PubMed] [Google Scholar]
- 12.Eron J J, Jr, Johnson V A, Merrill D P, Chou T-C, Hirsch M S. Synergistic inhibition of replication of human immunodeficiency virus type 1, including that of a zidovudine-resistant isolate, by zidovudine and 2′,3′-dideoxycitidine in vitro. Antimicrob Agents Chemother. 1992;36:1559–1562. doi: 10.1128/aac.36.7.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faraj A, Agrofoglio L A, Wakefield J K, McPherson S, Morrow C D, Gosselin G, Mathes C, Imbach J-L, Schinazi R F, Sommadossi J P. Inhibition of human immunodeficiency virus type 1 reverse transcriptase by the 5′-triphosphate β enantiomers of cytidine analogs. Antimicrob Agents Chemother. 1994;38:2300–2305. doi: 10.1128/aac.38.10.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furman P A, Davis M, Liotta D C, Paff M, Frick L W, Nelson D J, Dornsife R E, Wurster J A, Wilson L J, Fyfe J A, Tuttle J W, Miller W H, Condreay L, Averett D R, Schinazi R F, Painter G R. The anti-hepatitis B virus activities, cytotoxicities, and anabolic profiles of the (−) and (+) enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob Agents Chemother. 1992;36:2686–2692. doi: 10.1128/aac.36.12.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao Q, Gu Z, Parniak M A, Cameron J, Cammack N, Boucher C, Wainberg M A. The same mutation that encodes low-level human immunodeficiency virus type 1 resistance to 2′,3′-dideoxyinosine and 2′,3′-dideoxycytidine confers high-level resistance to the (−) enantiomer of 2′,3′-dideoxy-3′-thiacytidine. Antimicrob Agents Chemother. 1993;37:1390–1392. doi: 10.1128/aac.37.6.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gosselin G, Schinazi R F, Sommadossi J-P, Mathé C, Bergogne M-C, Aubertin A-M, Kirn A, Imbach J-L. Anti-human immunodeficiency virus activities of the β-l enantiomer of 2′,3′-dideoxycytidine and its 5-fluoro derivative in vitro. Antimicrob Agents Chemother. 1994;38:1292–1297. doi: 10.1128/aac.38.6.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grove K L, Guo X, Liu S-H, Chu C-K, Cheng Y-C. Anticancer activity of β-l-dioxolane-cytidine, a novel nucleoside analogue with the unnatural l-configuration. Cancer Res. 1995;55:3008–3011. [PubMed] [Google Scholar]
- 18.Hammer S M, Katzenstein D A, Hughes M D, Gundacker H, Schooley R T, Haubrich R H, Henry W K, Lederman M M, Phair J P, Niu M, Hirsch M S, Merigan T C. A trial comparing nucleoside monotherapy with combination therapy in HIV-infected adults with CD4 cell counts from 200 to 500 per cubic millimeter. AIDS Clinical Trials Group Study 175 Study Team. N Engl J Med. 1996;335:1081–1090. doi: 10.1056/NEJM199610103351501. [DOI] [PubMed] [Google Scholar]
- 19.Johnson V A, Merrill D P, Videler J A, Chou T-C, Byington R E, Eron J J, D’Aquila R T, Hirsch M S. Two-drug combinations of zidovudine, didanosine, and recombinant interferon-α A inhibit replication of zidovudine-resistant human immunodeficiency virus type I synergistically in vitro. J Infect Dis. 1991;164:646–655. doi: 10.1093/infdis/164.4.646. [DOI] [PubMed] [Google Scholar]
- 20.Kim K O, Schinazi R F, Shanmuganathan K, Jeong L S, Beach J W, Nampalli S, Cannon D L, Chu C K. l-beta-(2S,4S)- and l-alpha-(2S,4R)-dioxolanyl nucleosides as potential anti-HIV agents: asymmetric synthesis and structure-activity relationships. J Med Chem. 1993;36:519–528. doi: 10.1021/jm00057a001. [DOI] [PubMed] [Google Scholar]
- 21.Kukhanova M, Liu S-H, Mozzherin D, Lin T-S, Chu C-K, Cheng Y-C. l- and d-enantiomers of 2′,3′-dideoxycytidine 5′-triphosphate analogs as substrates for human DNA polymerases: implications for the mechanism of toxicity. J Biol Chem. 1995;270:23055–23059. doi: 10.1074/jbc.270.39.23055. [DOI] [PubMed] [Google Scholar]
- 22.Kukhanova, M., X. Li, S. H. Chen, I. King, T. Doyle, W. Prusoff, and Y. C. Cheng. Interaction of β-l-2′,3′-dideoxy-2′,3′-didehydro-5-fluorocytidine 5′-triphosphate with HIV-1 reverse transcriptase and human DNA polymerases: implications for HIV drug design. Mol. Pharmacol., in press. [PubMed]
- 23.Larder B A, Chesebro B, Richman D D. Susceptibilities of zidovudine-susceptible and -resistant human immunodeficiency virus isolates to antiviral agents determined by using a quantitative plaque reduction assay. Antimicrob Agents Chemother. 1990;34:436–441. doi: 10.1128/aac.34.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee L-S, Cheng Y-C. Human deoxythymidine kinase. I. Purification and general properties of the cytoplasmic and mitochondrial isozymes derived from blast cells of acute myelocytic leukemia. J Biol Chem. 1976;251:2600–2604. [PubMed] [Google Scholar]
- 25.Lin T S, Luo M Z, Liu M C, Pai S B, Dutschman G E, Cheng Y C. Antiviral activity of β-l(−)-2′,3′-dideoxy-5-fluorocytidine (l(−)FddC) and β-l(−)-2′,3′-dideoxycytidine (l(−)ddC) against hepatitis B virus and human immunodeficiency virus type 1 in vitro. Biochem Pharmacol. 1994;47:171–174. doi: 10.1016/0006-2952(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 26.Lin T S, Luo M Z, Liu M C, Pai S B, Dutschman G E, Cheng Y C. Synthesis and biological evaluation of 2′,3′-dideoxy-l-pyrimidine nucleosides as potential antiviral agents against human immunodeficiency virus (HIV) and hepatitis B virus (HBV) J Med Chem. 1994;37:798–803. doi: 10.1021/jm00032a013. [DOI] [PubMed] [Google Scholar]
- 27.Lin T S, Luo M Z, Liu C C, Zhu Y L, Gullen E, Dutschman G E, Cheng Y C. Synthesis of a series of purine 2′,3′-dideoxy-l-nucleoside analogues as potential antiviral agents. Nucleosides Nucleotides. 1995;14:1759–1783. [Google Scholar]
- 28.Lin T-S, Luo M-Z, Liu M-C, Zhu Y-L, Gullen E, Dutschman G E, Cheng Y-C. Design and synthesis of 2′,3′-dideoxy-2′,3′-didehydro-β-l-cytidine (β-l-d4C) and 2′,3′-dideoxy-2′,3′-didehydro-β-l-5-fluorocytidine (β-l-Fd4C), two exceptionally potent inhibitors of human hepatitis B virus (HBV) and potent inhibitors of human immunodeficiency virus (HIV) in vitro. J Med Chem. 1996;39:1757–1759. doi: 10.1021/jm950836q. [DOI] [PubMed] [Google Scholar]
- 29.Martin L T, Faraj A, Schinazi R F, Gosselin G, Mathe C, Imbach J-L, Sommadossi J-P. Effect of stereoisomerism on the cellular pharmacology of β-enantiomers of cytidine analogs in Hep-G2 cells. Biochem Pharmacol. 1997;53:75–87. doi: 10.1016/s0006-2952(96)00653-3. [DOI] [PubMed] [Google Scholar]
- 30.Mellors J W, Dutschman G E, Im G J, Tramontano E, Winkler S R, Cheng Y-C. In vitro selection and molecular characterization of human immunodeficiency virus-1 resistant to non-nucleoside inhibitors of reverse transcriptase. Mol Pharmacol. 1992;41:446–451. [PubMed] [Google Scholar]
- 31.Merrill D P, Moonis M, Hou T-C, Hirsch M S. Lamivudine or stavudine in two- and three-drug combinations against human immunodeficiency virus type 1 replication in vitro. J Infect Dis. 1996;173:355–364. doi: 10.1093/infdis/173.2.355. [DOI] [PubMed] [Google Scholar]
- 32.Parker W, Cheng Y-C. Mitochondrial toxicity of antiviral nucleoside analogs. J NIH Res. 1994;6:57–61. [Google Scholar]
- 33.Parker W B, Shaddix S C, Bowdon B J, Rose L M, Vince R, Shannon W M, Bennett L L., Jr Metabolism of carbovir, a potent inhibitor of human immunodeficiency virus type 1, and its effects of cellular metabolism. Antimicrob Agents Chemother. 1993;37:1004–1009. doi: 10.1128/aac.37.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schinazi R F, McMillan A, Cannon D, Mathis R, Lloyd R M, Peck A, Sommadossi J-P, St. Clair M, Wilson J, Furman P A, Painter G, Choi W-B, Liotta D C. Selective inhibition of human immunodeficiency viruses by racemates and enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob Agents Chemother. 1992;36:2423–2431. doi: 10.1128/aac.36.11.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schinazi R F, Lloyd R M, Jr, Nguyen M-H, Cannon D L, McMillan A, Ilksoy N, Chu C K, Liotta D C, Bazmi H Z, Mellors J W. Characterization of human immunodeficiency viruses resistant to oxathiolane-cytosine nucleosides. Antimicrob Agents Chemother. 1993;37:875–881. doi: 10.1128/aac.37.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skalski V, Liu S-H, Cheng Y-C. Removal of anti-human immunodeficiency virus 2′,3′-dideoxynucleoside monophosphates from DNA by a novel human cytosolic 3′-5′ exonuclease. Biochem Pharmacol. 1995;50:815–821. doi: 10.1016/0006-2952(95)00205-e. [DOI] [PubMed] [Google Scholar]
- 37.Sommadossi J P. Nucleoside analogs: similarities and differences. Clin Infect Dis. 1993;16:S7–S15. doi: 10.1093/clinids/16.supplement_1.s7. [DOI] [PubMed] [Google Scholar]
- 38.Staszewski S, Loveday C, Picazo J J, Dellarnonica P, Skinhoj P, Johnson M A, Danner S A, Harrigan P R, Hill A M, Verity L, McDade H. Safety and efficacy of lamivudine-zidovudine combination therapy in zidovudine-experienced patients. A randomized controlled comparison with zidovudine monotherapy. Lamivudine European HIV Working Group. JAMA. 1996;276:111–117. [PubMed] [Google Scholar]
- 39.St. Clair, M., K. N. Pennington, J. Rooney, and D. W. Barry. 1995. Rapid screening of antiretroviral combinations. J. Acquired Immune Defic. Syndr. 10(Suppl. 1):S24–S27. [PubMed]
- 40.Townsend A, LeClerc J M, Dutschman G E, Cooney D, Cheng Y C. Metabolism of 1-β-d-arabinofuranosyl-5-azacytosine and incorporation into DNA of human T-lymphoblastic cells (Molt-4) Cancer Res. 1985;45:3522–3528. [PubMed] [Google Scholar]
- 41.Van Draanen N A, Tisdale M, Parry N R, Jansen R, Dornsife R E, Tuttle J V, Averett D R, Koszalka G W. Influence of stereochemistry on antiviral activities and resistance profiles of dideoxycytidine nucleosides. Antimicrob Agents Chemother. 1994;38:868–871. doi: 10.1128/aac.38.4.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White E L, Parker W B, Ross L J, Shannon W M. Lack of synergy in the inhibition of HIV-1 reverse transcriptase by combinations of the 5′-triphosphates of various anti-HIV nucleoside analogues. Antivir Res. 1993;22:295–308. doi: 10.1016/0166-3542(93)90039-l. [DOI] [PubMed] [Google Scholar]
- 43.Zhu Y-L, Dutschman G E, Liu S-H, Bridges E G, Cheng Y-C. Anti-hepatitis B virus activity and metabolism of 2′,3′-dideoxy-2′,3′-didehydro-β-l(−)-5-fluorocytidine. Antimicrob Agents Chemother. 1998;42:1805–1810. doi: 10.1128/aac.42.7.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu Y-L, Pai S B, Liu S-H, Grove K L, Jones B C N M, Simons C, Zemlicka J, Cheng Y-C. Inhibition of replication of hepatitis B virus by cytallene in vitro. Antimicrob Agents Chemother. 1997;41:1755–1760. doi: 10.1128/aac.41.8.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]