Abstract
Purpose
Chronic kidney disease of uncertain etiology (CKDu) is an environmental nephropathy in which the etiological factors are yet uncertain. Leptospirosis, a spirochetal infection that is common among agricultural communities, has been identified as a potential etiology for CKDu beyond environmental nephropathy. Although CKDu is a chronic kidney disease, in endemic regions, an increasing number of cases are reported with features suggestive of acute interstitial nephritis without any known reason (AINu), with or without background CKD. The study hypothesizes that exposure to pathogenic leptospires is one of the causative factors for the occurrence of AINu.
Method
This study was carried out using 59 clinically diagnosed AINu patients, 72 healthy controls from CKDu endemic region (endemic controls [ECs]), and 71 healthy controls from CKDu non-endemic region (non-endemic controls [NECs]).
Results
The seroprevalence of 18.6, 6.9, and 7.0% was observed in the AIN (or AINu), EC, and NEC groups, respectively, from the rapid IgM test. Among 19 serovars tested, the highest seroprevalence was observed at 72.9, 38.9, and 21.1% in the AIN (AINu), EC, and NEC groups, respectively, by microscopic agglutination test (MAT), particularly for serovar Leptospira santarosai serovar Shermani. This emphasizes the presence of infection in AINu patients, and this also suggests that Leptospira exposure might play an important role in AINu.
Conclusion
These data suggest that exposure to Leptospira infection could be one of the possible causative factors for the occurrence of AINu, which may lead to CKDu in Sri Lanka.
Keywords: Chronic kidney disease of uncertain etiology, Acute interstitial nephritis, Leptospirosis, Infectious diseases
Introduction
Chronic kidney disease of uncertain etiology (CKDu) is environmental nephropathy reported in Mesoamerican countries (e.g., El Salvador, Guatemala, Nicaragua, and Costa Rica), Mexico, Egypt, India, and Sri Lanka [1]. As the name implies, the etiology of this disease remains uncertain. Diverse risk factors, both environmental and behavioral, such as contaminated drinking water, illicit alcohol consumption, smoking, betel chewing, family history, snake bites, and frequent usage of medication were proposed for the etiology of CKDu. In addition, frequent use of agrochemicals, zoonotic diseases, and the consumption of heavy metal-contaminated foods or water were also suggested [2]. Rodent-borne viral infections, Leptospira and Hanta, are common in agricultural communities and have been hypothesized as possible associates with CKDu [3]. Leptospirosis is a life-threatening zoonosis with a broad clinical spectrum, starting from asymptomatic carriers to multisystem involvement with interstitial nephritis. Interestingly, in animals, subclinical chronic interstitial nephritis which is similar to CKDu has been described for some species of Leptospira.
Leptospirosis is caused by Leptospira spp., a group belonging to helical spirochetes [4, 5, 6]. The genus Leptospira was traditionally divided into two groups, saprophytes and pathogens based on their virulence [7, 8]. More recent classification heavily relies on 16S rRNA phylogeny, DNA-DNA hybridization, pathogenicity, virulence, and in vitro growth characteristics of the genus Leptospira [9]. This classification arranges three subgroups of species, namely, infection-causing pathogens, noninfectious environmental saprophytes, and intermediate group, which elicits moderate pathogenicity [9].
In Sri Lanka, leptospirosis was first identified in 1953 among rodents in the Colombo area. The first confirmed human cases were reported in 1959 from Colombo and Batticaloa, where two cases were positive for Leptospira Icterohaemorrhagiae at a titer of 1/400, and the other at 1/50 to 1/100 for paired [10]. Seroprevalence of leptospirosis from human cases in Sri Lanka was first carried out in 1962, and up to now, 10 serogroups have been identified. A combination of published serovars and serogroups showed that serovars belonging to at least 10 serogroups are circulating in Sri Lanka [11].
Most Leptospira-infected subjects are asymptomatic at the onset or present with mild symptoms. The course of the disease may be short as 2 days or extend up to 4 weeks or longer in asymptomatic carriers. Sudden-onset fever, severe headache, nausea, diarrhea, vomiting, myalgia, and chills are common symptoms of acute leptospirosis, which are common to other tropical infections, such as dengue and malaria [12]. Since the clinical presentation of leptospirosis is nonspecific, and in the absence of confirmatory tests, misdiagnosis may happen in many instances, leading to underreporting [12]. Subclinical infection, and even anicteric febrile disease, are the most common presentation of leptospirosis which are self-limiting forms, and thus carry an excellent short-term prognosis [13]. Diagnosis and early intervention are difficult in most leptospiral infections that are usually presented as subclinical symptoms, leading to a possible chronic carrier state and increased risk for renal fibrosis [14].
Jeffree et al. [15] have noted that about 40% of the urban sanitation laborers who work in a high-risk environment were seropositive, from which one-third of the population were PCR positive for Leptospira, but none complained of illness, thereby indicating that they were asymptomatic [15]. Since Leptospira may colonize in the proximal tubule, resulting in chronic tubulointerstitial nephritis and fibrosis, asymptomatic exposure to Leptospira may emerge the risk for CKD. Even the severe form, though uncommon, usually manifests with acute respiratory distress syndrome (ARDS) and acute kidney injury (AKI) typically requiring dialysis [16, 17]. A study carried out in Sri Lanka showed that 9% of leptospirosis patients with overt AKI developed an early stage of CKD [18]. Gamage and Sarathkumara [3] have proposed a hypothesis that pathogenic Leptospira is a possible causative agent of acute kidney damage which eventually progresses to CKD in Sri Lanka [3]. Anti-Leptospira seropositivity is also associated with a lower estimated glomerular filtration rate and higher prevalence of CKD in CKDu endemic areas [14].
CKDu is first described as an asymptomatic disease, demonstrating predominant chronic interstitial changes in renal biopsies. In CKDu regions, an increasing number of cases of acute interstitial nephritis without any known reason (AINu) were reported [2]. Subsequently, an acute symptomatic form of the same illness has been described in both Sri Lanka and Nicaragua. Patients with AIN typically present with acute or subacute onset of nonspecific symptoms, including dysuria, fever or feverish feeling, back pain, malaise, anorexia or nausea, vomiting, and generalized ill-health. CKDu is a minimally symptomatic disease until it reaches end-stage renal failure, hence usually detected through community screening programs. Their biopsies are typified by irreversible changes such as glomerular sclerosis, periglomerular fibrosis, tubular atrophy, and interstitial fibrosis. Whereas, AINu was reported in patients with mild renal impairment who presented with previously described symptoms. In addition, histological lesions of acute tubular interstitial injury such as tubulitis, interstitial cell infiltration, and interstitial edema have been described in AINu. Characteristically, in these patients, histological changes are comparable with AIN despite significant background evidence of chronicity. Direct toxicity at the renal tubules exerted by Leptospira can induce similar lesions with local inflammatory reaction. Once the infection begins, due to the activation of monocytes and macrophages, a cascade of reactions occurs with the activation of toll-like receptor ligand and interferon gamma. If the infection is not well treated, persistent active inflammation remains for a prolonged period which induces irreversible renal damage [5, 19]. The study hypothesizes that exposure to pathogenic leptospires is one of the causative factors for the occurrence of AINu which is often reversible in the form of AKI. Nevertheless, the most critical question is whether to rule out the transition of AINu to CKDu. We propose that AINu is having a protracted course similar to transplant rejections, leading to irreversible damage. Meanwhile, we hypothesize recurrent episodes of AIN could lead to irreversible damage and CKDu. Hence, CKDu is a sequel of AINu, and subclinical leptospirosis is a competitive candidate to be the etiology of CKDu [20].
Materials and Methods
A case-control study was carried out for 2 years (from May 2017 to April 2019) on clinically suspected AINu patients, whom identified through a surveillance program in two CKDu endemic regions of Girandurukotte (7.4629° N, 81.0175° E) and Wilgamuwa (7.5331° N, 80.9152° E). All patients who fulfilled the inclusion criteria were enrolled after obtaining written consent. Age-, sex-, and occupation-matched endemic healthy controls (ECs) were enrolled from Wilgamuwa, and the non-endemic healthy controls (NECs) were from Maturata (7.0784° N, 80.8032° E), another farming area in Sri Lanka. The inclusion criteria of subjects, determination of disease activity index (AI), and chronicity index (CI) were described elsewhere in detail [2]. 3 mL of blood samples were collected from each patient at the time of the biopsy and kept for 20 min, and the centrifuges were under 3,000 rpm for 3 min to obtain the serum. Separated serum samples were stored in the minus 80°C refrigerator until analyses were performed. 59 serum samples from AINu cases, 72 serum samples from healthy controls recruited from CKDu endemic region (ECs), and 71 serum samples from healthy controls recruited from CKDu non-endemic region (NECs) were sent to Chang Gung University, Taiwan, for serovar analysis of leptospirosis. Control groups were not matched for age, gender, and other features with the AINu cases. We identified this as a limitation in our study. However, a randomly selected representative group from an endemic area and a non-endemic area were selected as control.
The reported cases of leptospirosis from Badulla and Matale in 2017 and 2018 were plotted against the rainfall in the same area to identify the pattern of leptospirosis cases, with the rainfall during the monsoon and inter-monsoon periods. The reported cases of AINu with the mean rainfall data in Girandurukotte and Wilgamuwa were also plotted to identify any pattern. The raw rainfall data were obtained from the nearest meteorological stations of the study regions for the entire study period, and the reported cases of leptospirosis were obtained from the official website of the Epidemiological Unit, Sri Lanka (https://www.epid.gov.lk/, assessed on August 20, 2021). The ethical clearance for the study was obtained from the Ethical Review Committee of the Faculty of Medicine, University of Peradeniya, Sri Lanka (2015/EC/32).
Anti-Leptospira IgG/IgM Detection
A diagnostic semiquantitative rapid test (ImmuneMed Leptospira IgM Duo Rapid, ImmuneMed Inc., Chuncheon-si, Korea) to detect IgM and IgG specific to Leptospira existing in human serum was performed according to manufactural protocols.
Microscopic Agglutination Test
Microscopic agglutination test (MAT) was performed as per OIE procedures, and sera were tested to detect the presence of anti-leptospiral antibodies and to identify infecting serovar and serogroup. A panel consisting of 19 serovars was used; Shermani/Shermani, Shermani/ATCC, Australis/Australis, Australis/Bratislava, Pomona/Kennewicki, Semaranga/Patoc, Autumnalis/Autumnalis, Lyme/Lyme, Pyogene/Manilae, Javanica/Javanica, Panama/Panama, Bataviae/Bataviae, Autumnalis/Djasiman, Pyrogenes/Pyrogenes, Copenhagini-ATCC, Canicola/Canicola, Icterohaemorrhagiae/Icterohaemorrhagiae, Inadai, Icterohaemorrhagiae/Copenhagini, and Javanica/Poi. Live leptospires were cultured and maintained in Ellinghausen-McCullough-Jonson-Harris (EMJH) medium at 30°C; 5–7-day-old cultures at a concentration of 1-2 × 108 organisms/mL were used as live antigens for MAT. Serum dilutions were prepared into 96-well microtiter plates, and live antigens were added, forming a final dilution series from 1:50 to 1:6,400. Agglutination was observed at the magnification of ×20 using a dark-field microscope. A titer value of >1:50 was considered positive, while the reporting titer was the highest dilution at which 50% agglutination was observed.
Statistical Analysis
Univariate analysis was performed to evaluate any possible association between demographic or clinical factors and Leptospira seropositivity. Baseline serum creatinine values, CI and AI indexes, age, gender, and exposure to farming were risk factors that were considered for the AIN group. For the control groups, age, gender, and baseline serum creatinine levels were considered as possible risk factors for Leptospira seroprevalence. The odds ratio was calculated using the online tool MedCalc to evaluate the statistical significance of the difference in Leptospira seropositivity among the AIN and control groups. Kappa’s statistic was also performed on SPSS for Windows to assess agreeability between rapid test and MAT.
Results
Demographic and clinical data of 59 clinically suspected AINu cases were subjected to percentage calculation. 40 available histopathological findings were used for AI and CI scoring (Table 1). Histopathological data were not available in others due to insufficient biopsy samples (n = 5); the biopsy procedure was abandoned due to small-sized kidneys (n = 4) or due to higher risk for complications (n = 5) like pre-procedure inappropriately high blood pressure (>180/100 mm Hg) and some missing data (n = 5). Over 80% of AINu patients were males and young active farmers. The age distribution ranges from 26 to 60 years, with a mean age of 43 years. 73% of them were engaged in full-time or part-time farming. The highest number of patients (49%) experienced an acute or subacute febrile illness before presenting to the renal clinic. 92% of patients had experienced at least one of the typical clinical symptoms of AINu. Only 3% were aware of a past medical history of leptospirosis.
Table 1.
Baseline demographic and clinical characteristics of AINu patients
| Percentage (%) (n = 59) |
|
|---|---|
| Demographic characteristics | |
| Gender | |
| Male | 80 |
| Female | 20 |
| Age | |
| >40 | 48 |
| <40 | 52 |
| Farming | |
| Yes | 73 |
| No | 27 |
| Known history of leptospirosis | |
| Yes | 3 |
|
| |
| Baseline clinical characteristics | |
| Clinical symptoms | |
| Fever | 49 |
| Back/abdominal/loin pain | 58 |
| Arthralgia | 61 |
| Mean serum creatinine, Scr/µmol/L | 167 |
| Urine full report | |
| U. protein | |
| Trace | 62 |
| + | 12 |
| + + | 1 |
| Nil | 25 |
| U. pus cells | |
| 0–49 | 88 |
| 50–99 | 11 |
| 100–149 | 0 |
| >150 | 1 |
| U. red cells | |
| Nil | 91 |
| Histopathology scoring | |
| AI | |
| 0, 1 | 60 |
| >2 | 20 |
| CI | |
| 0, 1 | 51 |
| 2, 3 | 17 |
| Full blood count (mean values) | |
| Hemoglobin, g/dL | 12.6 |
| WBC, mm3 | 9,108 |
| Lymphocytes, % | 26.0 |
| Neutrophils, % | 62.4 |
| Monocytes, % | 4.5 |
| US scan (R), cm | 10.3 |
| US scan (L), cm | 9.6 |
Rapid IgG and IgM Tests
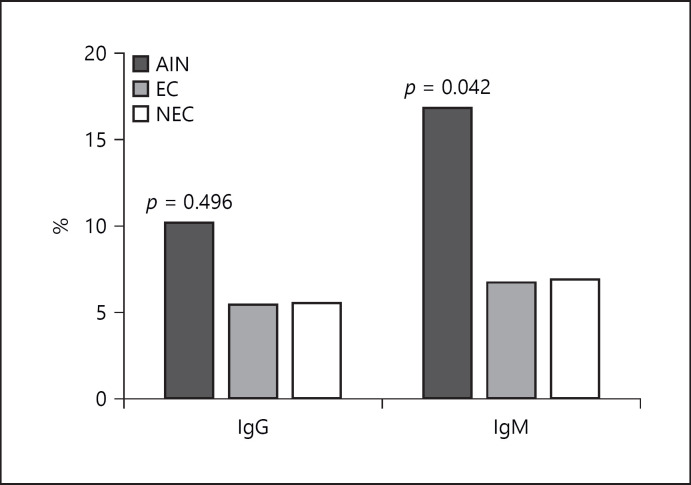
A seroprevalence of 19%, 7%, and 7% was observed in the AIN (or AINu), EC, and NEC groups, respectively, for the rapid IgM test which gave lower seroprevalence rates of 10%, 6%, and 6% for the AIN, EC, and NEC groups for IgG, respectively (Fig. 1). The odds ratio obtained (3.0708, 95% CI: 1.0017 to 9.4139, p value = 0.0496) showed that the increase in seroprevalence in the AIN group was statistically significant compared to the EC and NEC groups for rapid IgM. A χ2 test confirmed this statistically significant association with a χ2 value of 4.141 (p value = 0.042). Further, we calculated the odds ratio and adjusted for age and gender in all three groups.
Fig. 1.
Seroprevalence of three groups against IgG and IgM in rapid test (χ2 test for statistical analysis).
Acute Interstitial Nephritis without Any Known Reason
AIN patients’ gender has a 0.46 (p = 0.443) higher effect on getting IgM results positive than negative (lower impact). Age has a 0.64 (p = 0.759) lower effect on getting IgG results positive than negative. On contrary, their age has a 3.33 times higher (p = 0.135) effect on getting IgM results positive than negative. AIN patients’ gender and age have a 1.39 (p = 0.727) and 4.92 (p = 0.006) times higher effect on getting MAT results positive than negative.
Non-Endemic Control
Non-endemic healthy individuals’ gender has 0.97 (p = 0.984) times lower effect on getting IgG results positive than negative but has 1.413 (p = 0.497) times higher effect on getting IgM results positive than negative. Age of NEC has 0.40 (p = 0.539) and 0.28 (p = 0.022) times higher effect on getting IgG and IgM results positive than negative. Interestingly, gender and age of NEC individuals have a 2.30 (p = 0.16) times higher effect and 0.54 (0.329) times lower effect, respectively, to get MAT results positive than negative.
Endemic Control
Gender of endemic healthy individuals has 1.13 (p = 0.833) higher effect on getting IgG positive results than negative. Age has a 0.77 (p = 0.63) lower effect on getting IgM positive than negative results. Interestingly, both gender and age have 1.53 (p = 0.44) and 1.14 (p = 0.787) times higher effects, respectively, on getting MAT results positive than negative.
Microscopic Agglutination Test
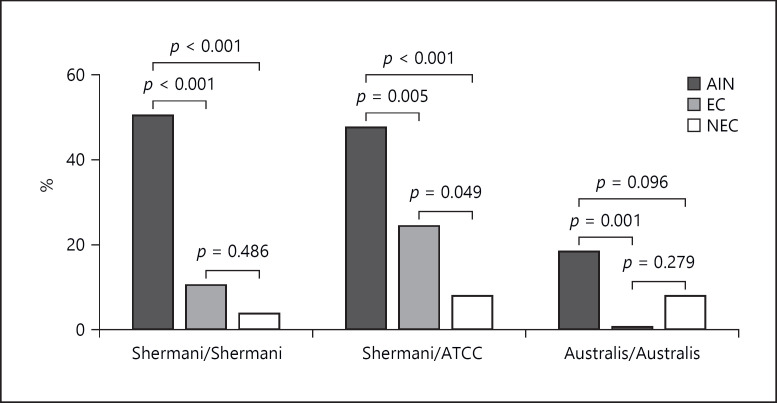
The highest rate of seroprevalence, 73% for MAT, was observed in the AIN group (Table 2). The odds ratio of 4.2232 (95% CI: 2.0064 to 8.8895, p value = 0.0001) showed that the AIN group had a statistically higher seroprevalence of Leptospira compared with the EC group. A χ2 test confirmed statistically significant associations with a value of 15.094 (p value < 0.001). Predominantly affecting serovars are described further in MAT analysis for all three groups. The AIN group has a statistically significant association in the χ2 test with a p value of < 0.0001 for Shermani/ATC and Shermani serovars (Fig. 2). The predominantly infecting serovars were Shermani/ATCC, Shermani/Shermani, and Australis/Australis with 52, 41, and 21 positive reactions, respectively. Other serovars that yielded positive reactions include Australis/Bratislava (n = 17), Semaranga/Patoc (n = 16), Pyogenes/Manilae (n = 1), Javanica/Javanica (n = 6), and Pyrogenes/Pyrogenes (n = 1) (Table 3).
Table 2.
Results of rapid IgG, IgM, and MAT
| Rapid IgG |
Rapid IgM |
MAT |
||||
|---|---|---|---|---|---|---|
| N | % | n | % | N | % | |
| AIN (n = 59) | ||||||
| Positive | 6 | 10 | 11 | 19 | 43 | 73 |
| Negative | 53 | 90 | 48 | 81 | 16 | 27 |
| EC (n = 72) | ||||||
| Positive | 4 | 6 | 5 | 7 | 28 | 39 |
| Negative | 68 | 94 | 67 | 93 | 44 | 61 |
| NEC (n = 71) | ||||||
| Positive | 4 | 6 | 5 | 7 | 15 | 21 |
| Negative | 67 | 94 | 66 | 93 | 56 | 79 |
Fig. 2.
MAT positive rate for predominantly affecting serovars (ordinary one-way ANOVA for statistical analysis).
Table 3.
Vectors reported in Sri Lanka and pathogenic to humans or not for each serotype
| AIN |
EC |
NEC |
Total | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 01:50 | 1:100 | 1:200 | 1:400 | 1:800 | 1:1,600 | 1:6,400 | 01:50 | 1:100 | 1:200 | 1:400 | 1:800 | 1:1,600 | 01:50 | 1:100 | 1:200 | 1:400 | 1:800 | 1:1,600 | ||
| SHE | 7 | 10 | 8 | 3 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 2 | 1 | 41 | |||||
| ATC | 4 | 13 | 6 | 4 | 1 | 1 | 1 | 5 | 9 | 1 | 1 | 1 | 2 | 1 | 2 | 52 | ||||
| AUS | 4 | 5 | 5 | 1 | 4 | 1 | 1 | 21 | ||||||||||||
| BRA | 3 | 2 | 3 | 1 | 1 | 4 | 1 | 1 | 1 | 17 | ||||||||||
| KEN | 2 | 2 | ||||||||||||||||||
| PAT | 2 | 1 | 2 | 2 | 2 | 1 | 5 | 1 | 16 | |||||||||||
| AUT | ||||||||||||||||||||
| LYM | ||||||||||||||||||||
| MAN | 1 | 1 | ||||||||||||||||||
| JAV | 1 | 3 | 1 | 1 | 6 | |||||||||||||||
| PAN | ||||||||||||||||||||
| BAT | ||||||||||||||||||||
| DJA | 4 | 4 | ||||||||||||||||||
| PYR | 1 | 1 | ||||||||||||||||||
| CPA | ||||||||||||||||||||
| CAN | ||||||||||||||||||||
| ICT | 1 | 1 | ||||||||||||||||||
| INA | ||||||||||||||||||||
| CPI | ||||||||||||||||||||
| POI | 1 | 1 | ||||||||||||||||||
SHE, Shermani; ATC, Shermani-ATCC; AUS, Australis; BRA, Bratislava; KEN, Kennewicki; PAT, Patoc; AUT, Autumnalis; LYM, Lyme; MAN, Manilae; JAV, Javanica; PAN, Panama; BAT, Bataviae; DJA, Djasiman; PYR, Pyrogenes; CPA, Copenhagini-ATCC; CAN, Canicola; ICT, Icterohaemorrhagiae; INA, Inadai; CPI, Icterohaemorrhagiae/Copenhagini; POI, Poi. Shermani/Shermani, Shermani/ATCC were the same serovar from ATCC with different culture batches maintained.
Comparison between MAT and Rapid Test Results
Out of 80 samples that were positive for MAT in the study population, 18 were positive for the rapid test as well (Table 4). To assess agreeability between MAT and rapid test assays, Cohen’s Kappa was calculated. χ2 value of 0.154 (p value = 0.03) was obtained for rapid and MAT assays. This value infers low agreeability between the assays.
Table 4.
Comparison of MAT and rapid test results
| Rapid positive | Rapid negative | |
|---|---|---|
| AIN (n = 59) | ||
| MAT positive | 12 | 31 |
| MAT negative | 1 | 15 |
| EC (n = 72) | ||
| MAT positive | 5 | 23 |
| MAT negative | 2 | 42 |
| NEC (n = 71) | ||
| MAT positive | 1 | 14 |
| MAT negative | 5 | 51 |
| Total (n = 202) | ||
| MAT positive | 18 | 68 |
| MAT negative | 8 | 108 |
Univariate Analysis of Clinical and Demographic Data
Demographic data such as age and gender along with serum creatinine levels, AI, CI, and other clinical data collected from the AIN group were subjected to a univariate analysis. In this group, it was shown that seropositivity through MAT was statistically higher in individuals above the age of 40 years with serum creatinine levels higher than 120 μmol/L. The seropositivity through MAT was statistically higher in patients who have AI ≤1. Demographic data collected from the control groups EC and NEC were also subjected to univariate analysis. However, there was no statistical significance between Leptospira seropositivity and factors such as age or gender and no statistical difference between seropositivity in the 2 control groups EC and NEC. Further, the point biserial correlation has not revealed a relationship between the duration between the onset of the symptoms and the time of biopsy and IgG, IgM in rapid test, and MAT positivity.
Rainfall Pattern and Presentation of AINu Patient
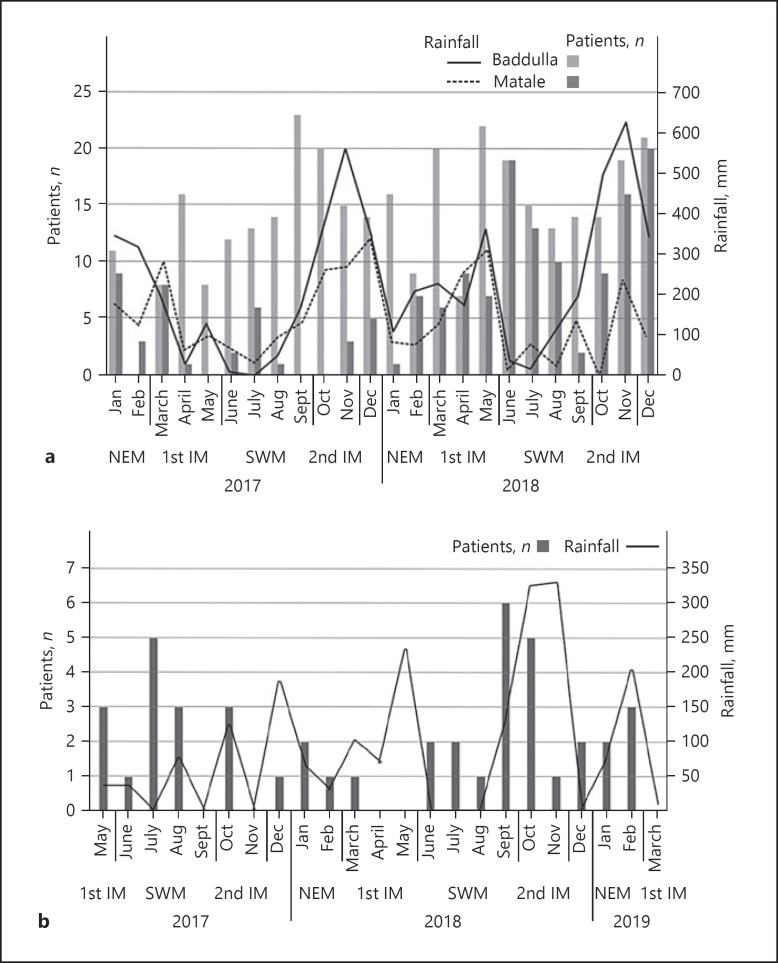
Further, we compared the incidence of AINu cases with the rainfall data based on the assumption that the leptospirosis outbreaks may occur after seasonal rainfall. The number of AINu patient presentations was calculated based on the amount of rainfall. Accordingly, 42% of AINu patients were reported in the southwest monsoon period, while 23% were reported in both the northeast and 2nd inter-monsoon periods. However, only 13% of patients were reported in the 1st inter-monsoon period. When considering the amount of rainfall, the highest rainfall (>300 mm) was noted during the 2nd inter-monsoon period in the study area. On the contrary, none of the patients were observed in the 1st inter-monsoon where the >200 mm rainfall had taken place, and few AINu patients were reported in February when rainfall is lower (<30 mm) (Fig. 3b). According to Figure 3a, leptospirosis reported cases in Badulla have become high with rainfall in the same area when compared with Matale leptospirosis reported cases and the rainfall.
Fig. 3.
a Rainfall and Lepto patient presentation 2017-2018. b Rainfall and AINu patient presentation. 1st IM, first inter-monsoon; SWM, southwest monsoon; 2nd IM, second inter-monsoon; NEM, northeast monsoon.
Discussion
Statistically significant seropositivity of both MAT and rapid tests, in a subgroup of patients who presented with symptoms of AIN in two endemic areas, in comparison to two control groups was observed. These patients were clinically or histologically indistinguishable from seronegative patients, suggesting the possibility of a spectrum of the same clinical entity. Both AIN and CKDu could be counted as consequences of acute and chronic exposure to zoonotic diseases, nephrotoxic agents, and recent exposure to certain risk behaviors, such as applying agrochemicals and heavy physical work under excessive ambient temperature. In general, leptospirosis was not considered a priority cause of CKDu, but there is growing evidence, supporting the association between the two diseases. Data obtained in this study indicated that at least some serovars of Leptospira can lead to CKDu in endemic areas of leptospirosis. Leptospirosis is common among agricultural communities, indicating the association of this spirochetal infection as a risk factor for the occurrence of CKDu. Sugarcane workers in Nicaragua revealed increased anti-Leptospira seropositivity and greater urinary biomarkers for kidney injury [21].
Seropositivity by IgM antibody in the rapid test showed higher values in the AINu group compared to the other two groups (EC and NEC). In contrast, lower seropositivity showed in all three groups for IgG antibody in the same test, emphasizing the presence of acute infection in AINu patients. Kappa value of 0.154 (p = 0.03) was obtained for rapid and MAT assays, showing low agreeability between the assays. This might be due to the infecting serovars which showed positive results for both rapid tests might not be included in our MAT panel. Considering the MAT results, seropositivity was statistically higher in the AINu group compared to the EC group. This suggests that Leptospira exposure might play a major role in AINu at least by Leptospira santarosai serovar Shermani.
A recent study on water microbiome in thirty household wells in Medawachchiya, where the CKDu prevalence is high has revealed a 0.01 abundance of Leptospira as a percentage of the total microbial count [22]. Although the levels may not be harmful to drink, when water used for bathing could lead to entering Leptospira into the body through cuts or abrasions in the skin. In Sri Lanka, the main determining factor of water quality is the climate [23, 24]. Therefore, we suggest that AINu patients may be affected by Leptospira infection after a heavy rainfall by exposing them to the urine of infected animals either from direct contact or from contact with soil or water that is contaminated by the urine. The increasing presentations of AINu cases during the rainy period provide evidence for this hypothesis. A study carried out in Thailand also showed that 57% of serologically diagnosed leptospirosis patients were observed with decreased renal function with serum creatinine levels above 1.5 mg/dL [25]. The common serotypes identified in their study were L. pyrogens and L. sejroe while other serotypes were Bratislava, Pomana, Copenhageni, Javanica, Ballico, Bangkok, Wollfi, and Akiyami. This study also found three similar serotypes (Bratislava, Javanica, and Pyrogens) in AINu patients. A prospective study of Leptospira diversity in Sri Lanka has identified six serogroups, specifically Autumnalis, Pyrogens, Icterohaemorragahiae, Grippotyphosa, Celledoni, and Bataviae [26]. However, AKI and cardiovascular involvement were only observed with L. interrogans infections in which serotypes identified in that serogroup were Pyrogens, Autumnalis, Bataviae and, Icterohaemorragahiae [26]. In addition, Leptospira santarosai serovar Shermani has also been identified in Sri Lanka. This is a serovar prevalent in Taiwan but has not been previously reported in Sri Lanka. In this study, we identified all four serotypes including higher seroprevalence of L. Shermani, emphasizing the evidence of acute renal impairment in AINu patients. Chronic Leptospira kidney infection can be a cause of progressive CKD especially if they developed recurrent episodes or protracted disease [27]. In conclusion, exposure to Leptospira infection may be one of the important causative factors for the occurrence of AINu. Since the awareness of exposure to Leptospira is low among the study groups (3%), we propose that leptospirosis may be an underdiagnosed disease in Sri Lanka, particularly in areas with high incidences of CKDu and confirmatory tests are not widely available. However, further studies and prospective studies should be carried out for the early diagnosis of leptospirosis and to identify the associations between leptospirosis and CKD/CKDu.
Statement of Ethics
This study protocol was reviewed and approved by the Faculty of Medicine, University of Peradeniya, Sri Lanka, approval number (2015/EC/32). Consent to participate in the study was taken in writing before the recruitment.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was conducted with financial support from the Ministry of Science and Technology, Taiwan (NMRPD1H0031-2), and Chang Gung Memorial Hospital, Taiwan (CMRPG3K2421), and National Science Foundation, Sri Lanka (RPHS/2016/CKDu/06).
Author Contributions
Shakila Premarathne: substantial contribution to the conception of the work, interpretation, original drafting of the work, methodology, accountability for all aspects of the work, editing, and final approval of the version. Chandika D. Gamage: substantial contribution to the conception of the work, revising the work critically for important intellectual content, methodology, resources, interpretation, accountability for all aspects of the work, supervision, reviewing, and final approval of the version. Rohana Chandrajith: substantial contribution to the design of the work, revising the work critically for important intellectual content, fund acquisition, investigation, resources, software, visualization, accountability for all aspects of the work, supervision, reviewing, editing, and final approval of the version. Neelakanthi Ratnatunge and Sulochana Wijetunge: substantial contribution to the design of the work, revising the work critically for important intellectual content, investigation, supervision, accountability for all aspects of the work, and reviewing and final approval of the version. Abdul W. Wazil: substantial contribution to the design of the work, revising the work critically for important intellectual content, investigation, accountability for all aspects of the work, and reviewing and final approval of the version. Li-Fang Chou: substantial contribution to the design of the work, original drafting of the work, data curation, visualization, accountability for all aspects of the work, and final approval of the version. Yi-Ching Ko: substantial contribution to the design of the work, formal analysis, original drafting of the work, software, accountability for all aspects of the work, and final approval of the version. Chiung-Tseng Huang and Thamalu Sonnadara: substantial contribution to the design of the work, original drafting of the work, data curation, accountability for all aspects of the work, and final approval of the version. Huang-Yu Yang: substantial contribution to the conception of the work, revising the work critically for important intellectual content, data curation, resources, accountability for all aspects of the work, and reviewing and final approval of the version. Amanda Fonseka: substantial contribution to the design of the work, original drafting of the work, analysis and interpretation, accountability for all aspects of the work, and final approval of the version. Dulanjali Herath and Pasan Hewavitharane: substantial contribution to the design of the work, original drafting of the work, data curation, analysis, accountability for all aspects of the work, and final approval of the version. Chih-Wei Yang: substantial contribution to the conception and design of the work, drafting and revising the work critically for important intellectual content, methodology, formal analysis, data curation, investigation, project administration, fund acquisition, resources, software, data interpretation, accountability for all aspects of the work, supervision, writing, reviewing, editing, and final approval of the version. Nishantha Nanayakkara: substantial contribution to the conception and design of the work, drafting and revising the work critically for important intellectual content, methodology, investigation, project administration, fund acquisition, data interpretation, accountability for all aspects of the work, supervision, writing, reviewing, editing, and final approval of the version.
Data Availability Statement
The data set used or analyzed during the current study is not publicly available due to the pending examination of the postgraduate student (SP) but is available with the corresponding author upon a reasonable request.
Acknowledgments
All the funding bodies including the Ministry of Science and Technology, Taiwan (NMRPD1H0031-2), and Chang Gung Memorial Hospital, Taiwan (CMRPG3K2421), and National Science Foundation, Sri Lanka (RPHS/2016/CKDu/06), are acknowledged.
Funding Statement
This study was conducted with financial support from the Ministry of Science and Technology, Taiwan (NMRPD1H0031-2), and Chang Gung Memorial Hospital, Taiwan (CMRPG3K2421), and National Science Foundation, Sri Lanka (RPHS/2016/CKDu/06).
References
- 1.Elledge MF, Redmon JH, Levine KE, Wickremasinghe RJ, Wanigasariya KP, Peiris-John RJ. Chronic kidney disease of unknown etiology in Sri Lanka: quest for understanding and global implications. RTI Press Research Brief; 2014. [Google Scholar]
- 2.Premarathne S, Chandrajith R, Nanayakkara N, Gamage CD, Ratnatunga N, Wijetunge S, et al. Could consumption of trace element–contaminated rice Be a risk factor for acute interstitial nephritis with uncertain etiology in the dry zone of Sri Lanka? Biol Trace Elem Res. 2022;200((6)):2597–605. doi: 10.1007/s12011-021-02880-2. [DOI] [PubMed] [Google Scholar]
- 3.Gamage CD, Sarathkumara YD. Chronic kidney disease of uncertain etiology in Sri Lanka: are leptospirosis and Hantaviral infection likely causes? Med Hypotheses. 2016;91:16–19. doi: 10.1016/j.mehy.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Daher EF, Lima RS, Silva Júnior GB, Silva EC, Karbage NN, Kataoka RS, et al. Clinical presentation of leptospirosis: a retrospective study of 201 patients in a metropolitan city of Brazil. Braz J Infect Dis. 2010;14((1)):03–10. [PubMed] [Google Scholar]
- 5.Srisawat N, Sitprija V. Leptospirosis and the kidney: an overview. Transl Res Biomed. 2019;7:1–9. [Google Scholar]
- 6.Soo ZMP, Khan NA, Siddiqui R. Leptospirosis: increasing importance in developing countries. Acta Trop. 2020;201:105183. doi: 10.1016/j.actatropica.2019.105183. [DOI] [PubMed] [Google Scholar]
- 7.Perolat P, Chappel RJ, Adler B, Baranton G, Bulach DM, Billinghurst ML, et al. Leptospira fainei sp. nov., isolated from pigs in Australia. Int J Syst Bacteriol. 1998;48((Pt 3)):851–858. doi: 10.1099/00207713-48-3-851. [DOI] [PubMed] [Google Scholar]
- 8.Brenner DJ, Kaufmann AF, Sulzer KR, Steigerwalt AG, Rogers FC, Weyant RS. Further determination of DNA relatedness between serogroups and serovars in the family Leptospiraceae with a proposal for Leptospira alexanderi sp. nov. and four new Leptospira genomospecies. Int J Syst Bacteriol. 1999;49((Pt 2)):839–858. doi: 10.1099/00207713-49-2-839. [DOI] [PubMed] [Google Scholar]
- 9.Lehmann JS, Matthias MA, Vinetz JM, Fouts DE. Leptospiral pathogenomics. Pathogens. 2014;3((2)):280–308. doi: 10.3390/pathogens3020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nityananda K, Harvey T. Leptospirosis in Ceylon-epidemiological and laboratory studies. Cey J Med Sci. 1971;((1)):5–14. [Google Scholar]
- 11.Naotunna C, Agampodi SB, Agampodi TC. Etiological agents causing leptospirosis in Sri Lanka: a review. Asian Pac J Trop Med. 2016;9((4)):390–394. doi: 10.1016/j.apjtm.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Bandara M, Ananda M, Wickramage K, Berger E, Agampodi S. Globalization of leptospirosis through travel and migration. Global Health. 2014;10((1)):61. doi: 10.1186/s12992-014-0061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Covic A, Goldsmith DJ, Gusbeth-Tatomir P, Seica A, Covic M. A retrospective 5-year study in Moldova of acute renal failure due to leptospirosis: 58 cases and a review of the literature. Nephrol Dial Transplant. 2003;18((6)):1128–1134. doi: 10.1093/ndt/gfg095. [DOI] [PubMed] [Google Scholar]
- 14.Yang HY, Chang CH, Yang CW. Leptospirosis and chronic kidney disease. In: Yang CW, Pan MJ, Yang HY, editors. Transl Res Biomed. Karger Publishers; 2019. pp. p. 27–36. [Google Scholar]
- 15.Jeffree MS, Mori D, Yusof NA, Atil AB, Lukman KA, Othman R, et al. High incidence of asymptomatic leptospirosis among urban sanitation workers from Kota Kinabalu, Sabah, Malaysian Borneo. Sci Rep. 2020;10((1)):19442. doi: 10.1038/s41598-020-76595-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrade L, Cleto S, Seguro AC. Door-to-dialysis time and daily hemodialysis in patients with leptospirosis: impact on mortality. Clin J Am Soc Nephrol. 2007;2((4)):739–744. doi: 10.2215/CJN.00680207. [DOI] [PubMed] [Google Scholar]
- 17.Forbes AE, Zochowski WJ, Dubrey SW, Sivaprakasam V. Leptospirosis and Weil's disease in the UK. QJM. 2012;105((12)):1151–1162. doi: 10.1093/qjmed/hcs145. [DOI] [PubMed] [Google Scholar]
- 18.Herath NJ, Kularatne SA, Weerakoon KG, Wazil A, Subasinghe N, Ratnatunga NV. Long term outcome of acute kidney injury due to leptospirosis? A longitudinal study in Sri Lanka. BMC Res Notes. 2014;7:398. doi: 10.1186/1756-0500-7-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu MY, Wu MS. Pathophysiology of leptospirosis kidney disease. Transl Res Biomed. 2019;7:10–19. [Google Scholar]
- 20.Badurdeen Z, Nanayakkara N, Ratnatunga NV, Wazil AW, Abeysekera TD, Rajakrishna PN, et al. Chronic kidney disease of uncertain etiology in Sri Lanka is a possible sequel of interstitial nephritis! Clin Nephrol. 2016;86((2016))((13)):106–109. doi: 10.5414/CNP86S115. [DOI] [PubMed] [Google Scholar]
- 21.Yang CW. Leptospirosis renal disease: emerging culprit of chronic kidney disease unknown etiology. Nephron. 2018;138((2)):129–136. doi: 10.1159/000480691. [DOI] [PubMed] [Google Scholar]
- 22.McDonough LK, Meredith KT, Nikagolla C, Middleton RJ, Tan JK, Ranasinghe AV, et al. The water chemistry and microbiome of household wells in Medawachchiya, Sri Lanka, an area with high prevalence of Chronic Kidney Disease of unknown origin (CKDu) Sci Rep. 2020;10((1)):18295. doi: 10.1038/s41598-020-75336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubasinghe R, Gunatilake SK, Chandrajith R. Geochemical characteristics of groundwater in different climatic zones of Sri Lanka. Environ Earth Sci. 2015;74((4)):3067–3076. [Google Scholar]
- 24.Chandrajith R, Diyabalanage S, Dissanayake CB. Geogenic fluoride and arsenic in groundwater of Sri Lanka and its implications to community health. Groundwater Sustainable Development. 2020;10:100359. [Google Scholar]
- 25.Niwattayakul K, Homvijitkul J, Niwattayakul S, Khow O, Sitprija V. Hypotension, renal failure, and pulmonary complications in leptospirosis. Ren Fail. 2002;24((3)):297–305. doi: 10.1081/jdi-120005363. [DOI] [PubMed] [Google Scholar]
- 26.Jayasundara D, Senavirathna I, Warnasekara J, Gamage C, Siribaddana S, Kularatne SAM, et al. 12 Novel clonal groups of Leptospira infecting humans in multiple contrasting epidemiological contexts in Sri Lanka. PLoS Negl Trop Dis. 2021;15((3)):e0009272. doi: 10.1371/journal.pntd.0009272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chou LF, Chen TW, Yang HY, Tian YC, Chang MY, Hung CC, et al. Transcriptomic signatures of exacerbated progression in leptospirosis subclinical chronic kidney disease with secondary nephrotoxic injury. Am J Physiol Renal Physiol. 2021;320((5)):F1001–18. doi: 10.1152/ajprenal.00640.2020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set used or analyzed during the current study is not publicly available due to the pending examination of the postgraduate student (SP) but is available with the corresponding author upon a reasonable request.