Abstract
Background and Purpose
The circle of Willis (COW) is a circulatory anastomosis located at the base of the brain. Little is known about the association between covert vascular brain injury and COW configurations in the general population. We explored this relationship in a community-based Chinese sample.
Methods
A total of 1,055 patients (mean age, 54.8 ± 8.9 years; 36.0% men) without intracranial arterial stenosis were included in the analysis. Magnetic resonance imaging was performed to evaluate the presence of imaging markers of covert vascular brain injury, including white matter hyperintensities (WMHs), lacunes, cerebral microbleeds (CMBs), enlarged perivascular spaces, and brain atrophy. Magnetic resonance angiography was used to classify the COW configurations according to the completeness, symmetry, and presence of the fetal posterior cerebral artery (FTP). The association between vascular lesions and variations in COW was analyzed.
Results
Among the 1,055 patients, 104 (9.9%) had a complete COW. Completeness correlated with age (p = 0.001). Incomplete COW was positively associated with WMH severity (OR = 2.071; 95% CI, 1.004–4.270) and CMB presence (OR = 1.542; 95% CI, 1.012–2.348), independent of age and sex. The presence of FTP was associated with lacunes (OR = 1.878; 95% CI, 1.069–3.298), more severe WMHs (OR = 1.739; 95% CI, 1.064–2.842), and less severe enlarged perivascular spaces (OR = 0.562; 95% CI, 0.346–0.915).
Conclusions
COW configuration was significantly related to various covert vascular brain injuries.
Keywords: Circle of Willis, White matter hyperintensity, Lacunes, Cerebral microbleed, Perivascular space
Introduction
The circle of Willis (COW) is a circulatory anastomosis system connecting both internal carotid and both vertebral arteries. It is located at the base of the brain and forms the primary intracranial collateral circulation. The COW is a highly variable anatomical structure [1, 2, 3]. Although anatomical variants do not directly impair brain perfusion, they may influence collateral capacity and increase the vulnerability to cerebral blood flow changes.
Magnetic resonance imaging (MRI)-detected vascular brain injury, namely, white matter hyperintensities (WMHs), lacunes, cerebral microbleeds (CMBs), enlarged perivascular spaces (EPVSs), and brain atrophy, is much more frequent than clinical stroke and is highly prevalent in community dwellings. Covert vascular brain injury has been proven to increase future risk of stroke, dementia, and death [4]. Although previous research has indicated that incomplete COW variants are associated with ischemic stroke [5], the relationship between COW variation and covert vascular brain injury has not been fully elucidated. Several studies have suggested that an incomplete COW is related to more severe WMHs [6, 7, 8, 9, 10], while others have failed to find a link [11, 12, 13]. Furthermore, few studies have investigated the association between COW variants and CMBs or EPVS [6, 12]. We aimed to determine the prevalence of COW variants in a large community-based Chinese population and assess any association with covert vascular brain injury, including lacunes, WMH, CMBs, EPVS, and cerebral atrophy.
Materials and Methods
Population
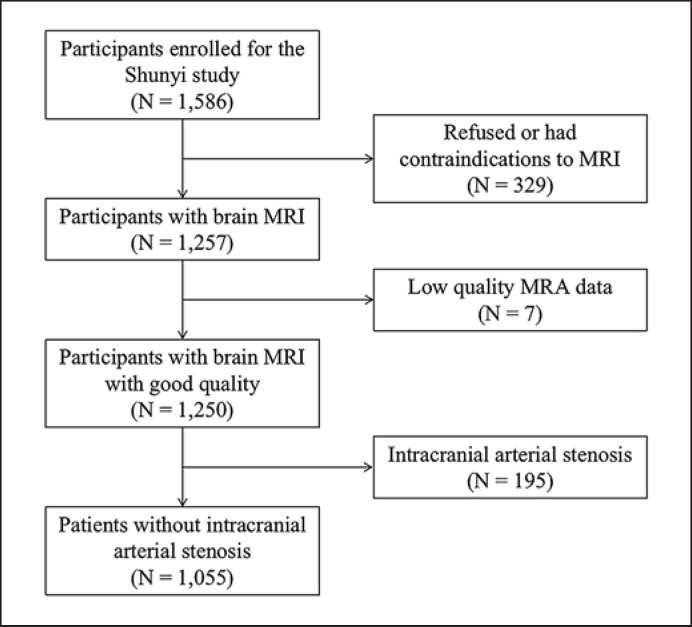
Participants were evaluated from a prospective community-based cohort recruiting inhabitants aged 35 years or older from five villages in Shunyi, a rural district located 20 miles from Beijing, China. From June 2013 to September 2014, the Sunyi Study enrolled 1,586 individuals among the 2,237 eligible residents (response rate, 70.9%). Excluding 329 participants who refused or had contraindications to MRI, 1,257 participants completed the brain MRI. Seven subjects with low-quality MRA data and 195 subjects with intracranial arterial stenosis were excluded, while a subsample of 1,055 subjects was included for further analysis (Fig. 1). This study was approved by the Ethical Committee of the Peking Union Medical College Hospital (No.: B-160).
Fig. 1.
Participant selection flowchart.
Image Analysis
MRI was performed using a single 3T Siemens Skyra scanner (Siemens, Erlangen, Germany) according to standard published criteria [14]. Detailed MRI protocols are listed in online supplementary materials (see www.karger.com/doi/10.1159/000527432 for all online suppl. material).
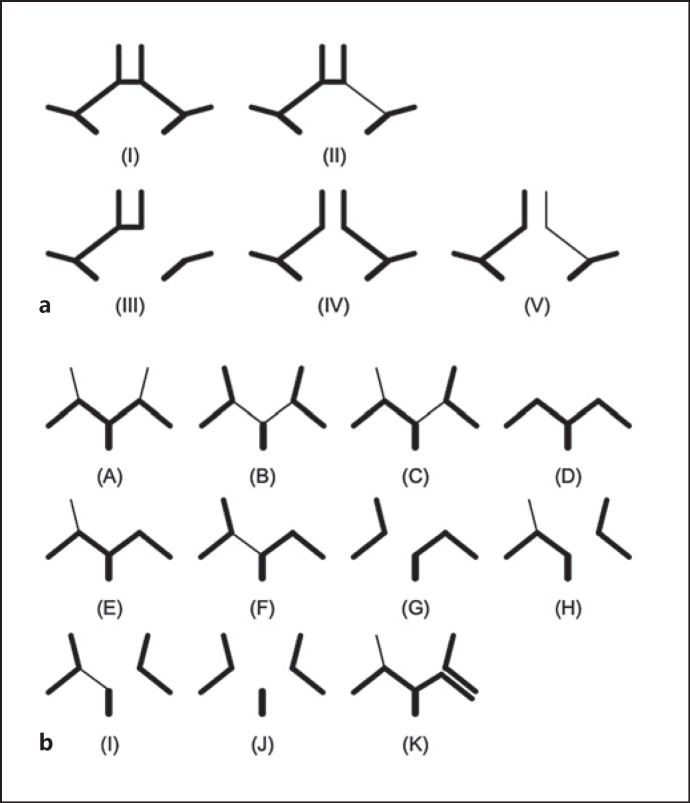
The COW involves the anterior communicating artery (AcomA), right and left pre-communicating anterior cerebral arteries (ACA-A1 segment), posterior communicating artery (PcomA), and right and left pre-communicating posterior cerebral arteries (PCA-P1 segment). The morphology of the COW shown in MRA images was analyzed and evaluated by two experienced neurologists according to the existence and developmental state of the aforementioned blood vessels (Fig. 2). Consensus was reached after discussion when there were any disagreements. We identified 5 variant types of the anterior portion of COW according to the presence of hypoplastic/absent ACA-A1 and AcomA, and 11 variant types of the posterior portion according to the presence of hypoplastic/absent PCA-P1 and PcomA. The diameter of a hypoplastic vessel was less than 50% of the width of the contralateral vessel. A vessel was considered absent if it was invisible or nonconsecutive on MR images.
Fig. 2.
Variants of the COW in anterior and posterior circulation. a Variants in anterior circulation. Type I, two normal A1 + AcomA; type II, normal A1 + hypoplastic A1 + AcomA; type III, normal A1 + missing A1 + AcomA; type IV, two normal A1; type V, normal A1 + hypoplastic A1. b Variants in posterior circulation. Type A, two normal P1 + two PcomA; type B, two hypoplastic P1 + two PcomA; type C, normal P1 + hypoplastic P1 + two PcomA; type D, two normal P1; type E, two normal P1 + one PcomA; type F, normal P1 + hypoplastic P1 + one PcomA; type G, normal P1 + missing P1 + one PcomA; type H, normal P1 + missing P1 + two PcomA; type I, hypoplastic P1 + missing P1 + two PcomA; type J, two missing P1 + two PcomA; type K, two homolateral PCA extended from the internal carotid and basilar arteries.
For further analysis, we classified these variants according to the completeness and symmetry of the configuration and the presence of a fetal-type posterior cerebral artery (FTP). Complete anterior circulation refers to the presence of AcomA and bilateral ACA-A1s (types I and II); complete posterior circulation refers to the presence of bilateral PcomAs and PCA-P1s (types A, B, and C). Symmetric anterior circulation describes the presence of two balanced ACA-A1s (types I and IV). In cases of FTP, PCA-P1 was absent (type J) or its diameter was smaller than that of the ipsilateral PcomA (type B). Both single- and double-sided FTPs were included in the group.
Lacunes were defined as focal deep infarcts 3–15 mm in size on T1WI. WMH, including periventricular hyperintensity (PVH) and deep white matter hyperintensity (DWMH), was graded according to the Fazekas scale on FLAIR. Severe PVH or DWMH was defined as a Fazekas scale score ≥2. The WMH volume was computed using the lesion segmentation toolbox (http://www.statistical-modeling.de/lst.html) for statistical parametric mapping at κ = 0.15. We defined CMB as a round or ovoid hypointense lesion on SWI. EPVS in the basal ganglia and white matter was evaluated according to a 4-level score [14]. Severe EPVS was defined as degrees 3 and 4. The brain parenchymal fraction was defined as the ratio of the brain parenchymal volume (gray matter + white matter) to the total intracranial volume. The total intracranial volume was computed as the sum of the volumes of gray matter, white matter, and cerebrospinal fluid, which were automatically segmented on T1WI using Statistical Parametric Mapping 12 (http://www.fil.ion.ucl.ac.uk/spm/) and the CAT12 toolbox (http://www.neuro.uni-jena.de/vbm/). Four well-trained neurologists independently rated these imaging markers with the κ value listed earlier [15, 16].
Statistical Analysis
Continuous variables were expressed as means with standard deviations (SD), and categorical variables were expressed as frequencies and proportions. T tests were used to compare continuous variables, and the χ2 and rank-sum tests were used for categorical variables. WMH volume was adjusted for total cranial volume and natural log transformed to normalize the skewness. To assess the relationship between the COW and covert vascular brain injury, a binary logistic regression analysis with adjustment for age and sex was performed with COW variants (symmetry, completeness, and FTP) as determinants and imaging markers of lesions (lacunes, WMH, CMBs, and EPVS) as outcome variables. Statistical significance was set at p < 0.05. All analyses were performed using SPSS version 25.0.0.0 (IBM Co., Armonk, NY, USA).
Results
A total of 1,055 subjects without intracranial arterial stenosis were included in the analysis. The baseline characteristics of the study population are presented in Table 1. The mean ± standard deviation for age was 54.8 ± 8.9 years, and 36.0% were men. A total of 131 (12.4%) participants had lacunes. Severe PVH and DWMH were observed in 16.7% and 9.5% of the subjects, respectively. One hundred and three (9.8%) subjects had at least one CMB. The overall prevalence of severe EPVS in the basal ganglia was 12.3% and in the white matter, 13.7%.
Table 1.
Baseline characteristics of the study population and the prevalence of covert vascular brain injury
| Characteristics | Value |
|---|---|
| Age, mean (SD), years | 54.8 (8.9) |
| Male, n (%) | 380 (36.0) |
| Body mass index, mean (SD), kg/m2 | 26.4 (3.8) |
| Current smoker, n (%) | 231 (21.9) |
| Hypertension, n (%) | 484 (45.9) |
| Diabetes mellitus, n (%) | 144 (13.6) |
| Hyperlipidemia, n (%) | 477 (45.2) |
| Covert vascular brain injury | |
| Lacunes, n (%) | 131 (12.4) |
| WMH volume, mL, median (IQR)* | 0.7 (0.2, 2.3) |
| PVH | |
| Degree 0 | 314 (29.8) |
| Degree 1 | 565 (53.6) |
| Degree 2 | 131 (12.4) |
| Degree 3 | 45 (4.3) |
| DWMH | |
| Degree 0 | 381 (36.1) |
| Degree 1 | 574 (54.4) |
| Degree 2 | 81 (7.7) |
| Degree 3 | 19 (1.8) |
| CMB, n (%) | 103 (9.8) |
| EPVS – BG† | |
| Degree 1 | 289 (27.5) |
| Degree 2 | 633 (60.2) |
| Degree 3 | 127 (12.1) |
| Degree 4 | 2 (0.2) |
| EPVS – WM† | |
| Degree 1 | 404 (38.4) |
| Degree 2 | 503 (47.9) |
| Degree 3 | 122 (11.6) |
| Degree 4 | 22 (2.1) |
| BPF‡ | 0.77 (0.03) |
WMH, white matter hyperintensity; PVH, periventricular hyperintensity; DWMH, deep white matter hyperintensity; CMB, cerebral microbleed; EPVS, enlarged perivascular space; BG, basal ganglia; WM, white matter; SD, standard deviation; BPF, brain parenchymal fraction.
Ninety-eight participants had missing WMH volume due to inadequate image quality for automatic WMH segmentation.
Four participants had missing EPVS scores due to inadequate imaging quality for visual assessment.
Seventy-three participants had missing BPF data due to inadequate image quality for automatic structural segmentation.
Variations of the COW and Risk Factors
Among the participants, 104 (9.9%) had a complete COW. The most common COW variations were type IV (343 cases, 32.5%) of anterior circulation, in which AcomA was absent, and type D (421 cases, 39.9%) of posterior circulation, in which both PcomAs were absent (online suppl. Table 1). When considering the common risk factors of vascular disease, patients who had an incomplete COW tended to be older (t = 3.228, p = 0.001). There were no significant associations between COW completeness and sex or vascular risk factors.
After classification of all variants, we found that the completeness ratio was 60.4% in the anterior circulation and 17.8% in the posterior circulation. The symmetry ratio of the anterior circulation was 83.3%. In total, 252 (23.9%) participants were found with FTPs. Among 218 participants with unilateral FTPs, FTPs on the right side were more common (136 vs. 82, χ2 = 13.38, p = 0.001). A significant imbalance was found in the distribution of hypoplastic/absent ACA-A1 and PCA-P1, with more present on the right side (ACA: Z = −4.503, p = 0.001; PCA: Z = −3.448, p = 0.001) (online suppl. Table 2).
Relationship between COW Variations and Covert Vascular Brain Injury
WMH and Lacunes
Univariate analysis showed that asymmetric anterior circulation and incomplete posterior circulation were both associated with severe PVH and severe DWMH (online suppl. Table 3). The volume of the WMH was also higher in patients with incomplete posterior circulation (t = 4.995, p = 0.03). The presence of FTP was related to severe DWMH and lacunes in white matter. After adjusting for age and sex, severity of DWMH increased in patients with FTP (OR = 1.739; 95% CI, 1.064–2.842; p = 0.027) and incomplete posterior circulation (OR = 2.071; 95% CI, 1.004–4.270; p = 0.049). Lacunes in white matter were positively associated with the presence of FTP (OR = 1.878; 95% CI, 1.069–3.298; p = 0.028) (Table 2).
Table 2.
Multivariate analysis of association of variations of the COW with covert vascular brain injury
| Outcomes | Anterior circulation |
Posterior circulation |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| symmetric | asymmetric |
complete | incomplete |
no FTP | FTP |
complete | incomplete |
|||||
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |||||
| Lacune | ||||||||||||
| Any lacune | 1 (Ref) | 1.205 (0.727–1.996) | 0.469 | 1 (Ref) | 1.246 (0.837–1.855) | 0.278 | 1 (Ref) | 1.221 (0.767–1.943) | 0.400 | 1 (Ref) | 0.774 (0.453–1.324) | 0.350 |
| Lacune – BG | 1 (Ref) | 1.322 (0.752–2.327) | 0.332 | 1 (Ref) | 1.243 (0.788–1.962) | 0.349 | 1 (Ref) | 1.015 (0.589–1.750) | 0.956 | 1 (Ref) | 0.778 (0.424–1.428) | 0.418 |
| Lacune-WM | 1 (Ref) | 0.891 (0.462–1.718) | 0.729 | 1 (Ref) | 1.282 (0.775–2.121) | 0.332 | 1 (Ref) | 1.878(1.069–3.298) | 0.028 | 1 (Ref) | 0.998 (0.503–1.978) | 0.995 |
| WMH | ||||||||||||
| Severe PVH | 1 (Ref) | 1.394 (0.886–2.193) | 0.151 | 1 (Ref) | 1.110(0.771–1.599) | 0.574 | 1 (Ref) | 1.348 (0.881–2.061) | 0.168 | 1 (Ref) | 1.744 (0.990–3.073) | 0.054 |
| Severe DWMH | 1 (Ref) | 1.291 (0.757–2.202) | 0.348 | 1 (Ref) | 0.862 (0.551–1.347) | 0.514 | 1 (Ref) | 1.739(1.064–2.842) | 0.027 | 1 (Ref) | 2.071 (1.004–4.270) | 0.049 |
| CMB | ||||||||||||
| Any CMB | 1 (Ref) | 0.718 (0.399–1.295) | 0.271 | 1 (Ref) | 1.542(1.012–2.348) | 0.044 | 1 (Ref) | 1.285 (0.781–2.116) | 0.324 | 1 (Ref) | 1.748 (0.904–3.381) | 0.097 |
| Deep or infratentorial CMB | 1 (Ref) | 0.393 (0.150–1.027) | 0.057 | 1 (Ref) | 1.567(0.900–2.730) | 0.113 | 1 (Ref) | 1.279 (0.658–2.485) | 0.468 | 1 (Ref) | 1.406 (0.624–3.166) | 0.411 |
| Strictly lobar CMB | 1 (Ref) | 1.180 (0.571–2.440) | 0.655 | 1 (Ref) | 1.499 (0.826–2.721) | 0.183 | 1 (Ref) | 1.271 (0.635–2.546) | 0.498 | 1 (Ref) | 2.284 (0.784–6.652) | 0.130 |
| EPVS | ||||||||||||
| Severe EPVS – BG | 1 (Ref) | 0.958(0.577–1.593) | 0.870 | 1 (Ref) | 0.952 (0.648–1.399) | 0.802 | 1 (Ref) | 0.734 (0.449–1.200) | 0.218 | 1 (Ref) | 1.750(0.946–3.240) | 0.075 |
| Severe EPVS – WM | 1 (Ref) | 1.040 (0.639–1.694) | 0.873 | 1 (Ref) | 0.682 (0.468–0.996) | 0.048 | 1 (Ref) | 0.562 (0.346–0.915) | 0.020 | 1 (Ref) | 0.716 (0.445–1.153) | 0.169 |
Models are adjusted for age and sex. OR, odds ratio; Cl, confidence interval; Ref, reference; FTP, fetal-type posterior cerebral artery; WMH, white matter hyperintensity; PVH, periventricular hyperintensity; DWMH, deep white matter hyperintensity; CMB, cerebral microbleed; EPVS, enlarged perivascular space; BG, basal ganglia; WM, white matter.
CMBs and EPVS
Univariate analysis showed that incomplete anterior circulation increased the risk of CMBs. In addition, the absence of FTPs was associated with severe EPVS in the white matter; incomplete posterior circulation was associated with severe EPVS in the basal ganglia (online suppl. Table 3). After adjusting for age and sex, the presence of CMBs was significantly associated with completeness of the anterior circulation (OR = 1.542; 95% CI, 1.012–2.348; p = 0.044). Severe EPVS in the white matter was negatively associated with FTPs (OR = 0.562; 95% CI, 0.346–0.915; p = 0.020) (Table 2).
Cerebral Atrophy
A positive correlation was found between asymmetric ACA-A1 (t = 2.835, p = 0.005), incomplete anterior circulation (t = 2.345, p = 0.02), and lower brain parenchymal fraction. However, after adjustment for age and sex, these associations were no longer significant (β ± SE = −0.288 ± 0.188, p = 0.127 for symmetry; β ± SE = −0.127 ± 0.143, p = 0.375 for completeness).
Discussion
COW Variations
We observed a complete COW on MRA in 9.9% of participants, whereas the remaining 90.1% had at least one missing segment. The prevalence of COW anomalies varies greatly [1] due to limited sample sizes of a few hundred individuals. Two studies involving more than 1,000 participants were conducted in Norwegian and male Chinese populations, with complete COW prevalence rates of 11.9% [2] and 12.24% [3], respectively. The most common COW variant was type IV of anterior circulation, in which AcomA was absent, and type D of posterior circulation, in which two PcomAs were absent. These findings are in agreement with the two large-scale studies mentioned above [2, 3]. Although the COW configuration varies greatly in the general population, the prevalence of a complete COW and the most common variant were similar across populations.
Our study indicated that a complete COW was negatively associated with increasing age, consistent with previous studies [2, 17]. The underlying cause may be the reduction of cerebral flow with increasing age. In addition, the increased tortuosity of cerebral vessels with age may influence the flow pattern in the COW, causing the missing segments on flow-sensitive MRI [2].
Furthermore, we found that the distribution of hypoplastic/absent ACA-A1 and PCA-P1 was unbalanced, with more cases being observed on the right side. Several researchers have determined the same left-dominant asymmetry in the distributions of ACA-A1 [2, 3] and PCA-P1 [3]. One possible reason for the asymmetry is that the left hemisphere is better perfused in people who are right-handed (i.e., the majority of the population).
Relationship between COW Variations and Covert Vascular Brain Injury
For symptomatic ischemic brain injury, a meta-analysis involving 2,718 participants showed that any variation in the COW made patients 1.38 times more likely to develop ischemic stroke [5]. However, when looking at WMH and lacunes, which are generally considered to be asymptomatic ischemic lesions [18], prior reports on the general population showed conflicting results (online suppl. Table 4). Miyazawa et al. [19] found significant correlations between lacunes and COW completeness, while Del Brutto et al. [12] failed to find this relationship. Unlike our findings on WMH, both Hindenes et al. [20] and Del Brutto et al. [12] found that WMH may not be related to the completeness or specific variants of the COW. This might be caused by the classification method of the COW, wherein we distinguished the completeness of the anterior and posterior parts of the COW and found that the latter was related to WMH.
Our findings provide new evidence for susceptibility to silent ischemic brain lesions in people with an incomplete COW. The COW could possibly influence the development of vascular brain injury via collateral circulation. Most COW variants are generally thought not to directly reduce cerebral perfusion. However, the hypoplastic/absent vessels of COW variants can impair the hemodynamic balance [21], making it difficult to establish rapid collateral compensation after changes in cerebral blood flow [22]. This may contribute to ischemic brain injury over time.
Most previous studies on COW configuration have focused on the completeness rather than symmetry. However, mathematical models showed that asymmetry of paired vessels in the COW correlated with higher resistance to flow [21] and may therefore affect brain perfusion and wall shear stress [23]. Our findings on asymmetric anterior circulation and brain atrophy imply the significance of symmetry considerations in further studies.
In this study, the presence of CMBs was higher in patients with incomplete anterior circulation. In a population-based study led by Del Brutto et al. [12], the severity of deep CMB was not related to completeness of the entire COW. This inconsistency might be caused by their exclusion of lobar CMBs, their COW classification method, or limited sample size. The majority of CMBs reflect microhemorrhages of small cerebral blood vessels after their disorganization [18]. The incomplete configuration in some COW variants can predispose blood vessels to higher wall shear stress [21, 24], further damaging the downstream vessel wall and causing microbleeds.
One interesting result of our study was the negative correlation between severe EPVS in the white matter and the presence of FTP. Evidence indicates that the PVS plays an important role in the movement and drainage of fluid in the brain, and the EPVS is a marker of blood-brain barrier breakdown and microvascular functional impairment [25]. Our result showed that the presence of FTP, with the blood of the PCA mainly supplied by the internal carotid system rather than the vertebrobasilar system, can protect individuals from severe EPVS. However, underlying pathogeneses require further investigation.
In this large population-based study, we comprehensively explored the relationship between various imaging markers of covert vascular brain injury and COW variants. Our study had some limitations. First, we chose four imaging markers as outcome variables, and a stringent threshold for statistical significance should be set at a p value < 0.0125. Under this stringent threshold, the association of COW variation and CSVD could not withstand the Bonferroni correction. Although the phenotypes of CSVD being not completely parallel might be one reason, further validation is needed to judge the reliability of the reported results. Second, we used MRA as a noninvasive method that can be applied to the general population. Although it is sensitive to blood flow, the figure resolution is limited, making it difficult to reliably identify vessels <1 mm on MRI. Furthermore, in this community-based sample, we excluded subjects with intracranial arterial stenosis because of possible misleading hypoplasia of the vessel. However, the excluded patients were more likely to depend on collateral perfusion by the COW as their cerebral blood flow was reduced. Last, we did not distinguish lesions of covert vascular brain injury ipsilateral and contralateral to COW variants because of the limited total number of lesions. Therefore, potential side-specific findings might be missed.
Conclusion
This study showed a positive association between specific variations in the COW and susceptibility to silent vascular brain lesions. Therefore, close monitoring may be necessary for people who are found to carry COW variants identified through imaging examinations.
Statement of Ethics
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethical Committee of the Peking Union Medical College Hospital (No: B-160). Informed consent in written format was obtained from all participants.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The study was funded by the National Natural Science Foundation of China (Grant No. 62072045), the Fundamental Research Funds for the Central Universities (Grant No. 33320I20006), and the Research Foundation for Young Scholars of Peking Union Medical College Hospital (Grant No. PUMCH201911275).
Author Contributions
(1) Conception, design, and administrative support: Yi-Cheng Zhu and Shu-Yang Zhang; (2) provision of study materials or patients: Yi-Cheng Zhu and Li-Xin Zhou; (3) collection and assembly of data: Lu Feng, Fei Han, Li-Xin Zhou, Jun Ni, Ming Yao, Fei-Fei Zhai, and Ming-Li Li; (4) data analysis and interpretation: Lu Feng, Fei Han, Li-Xin Zhou, Li-Ying Cui, Zheng-Yu Jin; (5) manuscript writing: Lu Feng, Fei Han, and Yi-Cheng Zhu; and (6) final approval of manuscript: all authors.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author, Fei Han (lourahan@163.com) or Yi-Cheng Zhu (zhuych910@163.com). A preprint version of this article is available on Research Square [26].
Supplementary Material
Supplementary data
Acknowledgments
The authors are grateful to the study participants and the staff of the Shunyi study.
Funding Statement
The study was funded by the National Natural Science Foundation of China (Grant No. 62072045), the Fundamental Research Funds for the Central Universities (Grant No. 33320I20006), and the Research Foundation for Young Scholars of Peking Union Medical College Hospital (Grant No. PUMCH201911275).
References
- 1.Jones JD, Castanho P, Bazira P, Sanders K. Anatomical variations of the circle of Willis and their prevalence, with a focus on the posterior communicating artery: a literature review and meta-analysis. Clin Anat. 2021;34((7)):978–990. doi: 10.1002/ca.23662. [DOI] [PubMed] [Google Scholar]
- 2.Hindenes LB, Håberg AK, Johnsen LH, Mathiesen EB, Robben D, Vangberg TR. Variations in the Circle of Willis in a large population sample using 3D TOF angiography: the Tromsø Study. PLoS One. 2020;15((11)):e0241373. doi: 10.1371/journal.pone.0241373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qiu C, Zhang Y, Xue C, Jiang S, Zhang W. MRA study on variation of the circle of willis in healthy Chinese male adults. Biomed Res Int. 2015;2015:1–8. doi: 10.1155/2015/976340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rensma SP, van Sloten TT, Launer LJ, Stehouwer CDA. Cerebral small vessel disease and risk of incident stroke, dementia and depression, and all-cause mortality: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;90:164–173. doi: 10.1016/j.neubiorev.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oumer M, Alemayehu M, Muche A. Association between circle of Willis and ischemic stroke: a systematic review and meta-analysis. BMC Neurosci. 2021;22((1)):3. doi: 10.1186/s12868-021-00609-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye H, Wu X, Yan J, Wang J, Qiu J, Wang Y. Completeness of circle of Willis and white matter hyperintensities in patients with severe internal carotid artery stenosis. Neurol Sci. 2019;40((3)):509–514. doi: 10.1007/s10072-018-3683-9. [DOI] [PubMed] [Google Scholar]
- 7.Saba L, Sanfilippo R, Porcu M, Lucatelli P, Montisci R, Zaccagna F, et al. Relationship between white matter hyperintensities volume and the circle of Willis configurations in patients with carotid artery pathology. Eur J Radiol. 2017;89:111–116. doi: 10.1016/j.ejrad.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 8.Saba L, Raz E, Fatterpekar G, Montisci R, di Martino M, Bassareo PP, et al. Correlation between leukoaraiosis volume and circle of Willis variants. J Neuroimaging. 2015;25((2)):226–231. doi: 10.1111/jon.12103. [DOI] [PubMed] [Google Scholar]
- 9.Ryan DJ, Byrne S, Dunne R, Harmon M, Harbison J. White matter disease and an incomplete circle of Willis. Int J Stroke. 2015;10((4)):547–552. doi: 10.1111/ijs.12042. [DOI] [PubMed] [Google Scholar]
- 10.Chuang YM, Huang KL, Chang YJ, Chang CH, Chang TY, Wu TC, et al. Associations between Circle of Willis morphology and white matter lesion load in subjects with carotid artery stenosis. Eur Neurol. 2011;66((3)):136–144. doi: 10.1159/000329274. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Xiong Y, Xu G, Zhang R, Zhu W, Yin Q, et al. The circle of willis and white matter lesions in patients with carotid atherosclerosis. J Stroke Cerebrovasc Dis. 2015;24((8)):1749–1754. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.048. [DOI] [PubMed] [Google Scholar]
- 12.Del Brutto OH, Mera RM, Zambrano M, Lama J. Incompleteness of the Circle of Willis correlates poorly with imaging evidence of small vessel disease. A population-based study in rural Ecuador (the Atahualpa project) J Stroke Cerebrovasc Dis. 2015;24((1)):73–77. doi: 10.1016/j.jstrokecerebrovasdis.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 13.van der Grond J, van Raamt AF, van der Graaf Y, Mali WP, Bisschops RH. A fetal circle of Willis is associated with a decreased deep white matter lesion load. Neurology. 2004;63((8)):1452–1456. doi: 10.1212/01.wnl.0000142041.42491.f4. [DOI] [PubMed] [Google Scholar]
- 14.Han F, Zhou LX, Ni J, Yao M, Zhai FF, Liu YT, et al. Design of the Shunyi study on cardiovascular disease and age-related brain changes: a community-based, prospective, cohort study. Ann Transl Med. 2020;8((23)):1579. doi: 10.21037/atm-20-4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han F, Zhai FF, Wang Q, Zhou LX, Ni J, Yao M, et al. Prevalence and risk factors of cerebral small vessel disease in a Chinese population-based sample. J Stroke. 2018;20((2)):239–246. doi: 10.5853/jos.2017.02110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhai FF, Yan S, Li ML, Han F, Wang Q, Zhou LX, et al. Intracranial arterial dolichoectasia and stenosis: risk factors and relation to cerebral small vessel disease. Stroke. 2018;49((5)):1135–1140. doi: 10.1161/STROKEAHA.117.020130. [DOI] [PubMed] [Google Scholar]
- 17.Zaninovich OA, Ramey WL, Walter CM, Dumont TM. Completion of the circle of willis varies by gender, age, and indication for computed tomography angiography. World Neurosurg X. 2017;106:953–963. doi: 10.1016/j.wneu.2017.07.084. [DOI] [PubMed] [Google Scholar]
- 18.Ter Telgte A, van Leijsen EMC, Wiegertjes K, Klijn CJM, Tuladhar AM, de Leeuw FE. Cerebral small vessel disease: from a focal to a global perspective. Nat Rev Neurol. 2018;14((7)):387–398. doi: 10.1038/s41582-018-0014-y. [DOI] [PubMed] [Google Scholar]
- 19.Miyazawa N, Shinohara T, Yamagata Z. Association of incompleteness of the anterior part of the circle of Willis with the occurrence of lacunes in the basal ganglia. Eur J Neurol. 2011;18((11)):1358–1360. doi: 10.1111/j.1468-1331.2011.03400.x. [DOI] [PubMed] [Google Scholar]
- 20.Hindenes LB, Håberg AK, Mathiesen EB, Vangberg TR. An incomplete Circle of Willis is not a risk factor for white matter hyperintensities: the Tromsø Study. J Neurol Sci. 2021;420:117268. doi: 10.1016/j.jns.2020.117268. [DOI] [PubMed] [Google Scholar]
- 21.Pascalau R, Padurean VA, Bartos D, Bartos A, Szabo BA. The Geometry of the Circle of Willis anatomical variants as a potential cerebrovascular risk factor. Turk Neurosurg. 2019;29((2)):151–158. doi: 10.5137/1019-5149.JTN.21835-17.3. [DOI] [PubMed] [Google Scholar]
- 22.Alastruey J, Parker KH, Peiró J, Byrd SM, Sherwin SJ. Modelling the circle of Willis to assess the effects of anatomical variations and occlusions on cerebral flows. J Biomech. 2007;40((8)):1794–805. doi: 10.1016/j.jbiomech.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Guilliams KP, Gupta N, Srinivasan S, Binkley MM, Ying C, Couture L, et al. MR imaging differences in the circle of willis between healthy children and adults. AJNR Am J Neuroradiol. 2021;42((11)):2062–2069. doi: 10.3174/ajnr.A7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nam SW, Choi S, Cheong Y, Kim YH, Park HK. Evaluation of aneurysm-associated wall shear stress related to morphological variations of circle of Willis using a microfluidic device. J Biomech. 2015;48((2)):348–353. doi: 10.1016/j.jbiomech.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 25.Wardlaw JM, Benveniste H, Nedergaard M, Zlokovic BV, Mestre H, Lee H, et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol. 2020;16((3)):137–153. doi: 10.1038/s41582-020-0312-z. [DOI] [PubMed] [Google Scholar]
- 26.Feng L, Zhai FF, Li ML, Zhou LX, Ni J, Yao M, et al. Association between anatomical variations of the Circle of Willis and covert vascular brain injury in the general population. 2022 doi: 10.1159/000527432. Research Square rs.3.rs-1685139/v1 (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author, Fei Han (lourahan@163.com) or Yi-Cheng Zhu (zhuych910@163.com). A preprint version of this article is available on Research Square [26].