Abstract
The world’s population is ageing, and most older adults experience a later life burdened with disease and disability. Frailty is a multidimensional and dynamic condition characterized by declines in reserve and function across multiple physiological systems, such that the ability to cope with every day or acute stressors becomes compromised. It is projected to become one of the most serious public health challenges economically developed societies will face in the coming century. This review provides a comprehensive overview of frailty, exploring its pathophysiology, theoretical and operational definition(s), impact, prevalence, management, and prevention, within the context of its emergence as a major public health challenge, in an increasingly economically developed and ageing world. Further, this review discusses the major limitations, deficiencies, and knowledge gaps presently within the field, and future research directions pertinent to the advancement of frailty research and the promotion of healthy longevity among the increasing global population of older adults.
Keywords: Ageing, Demography, Exercise, Frailty, Rehabilitation
Introduction
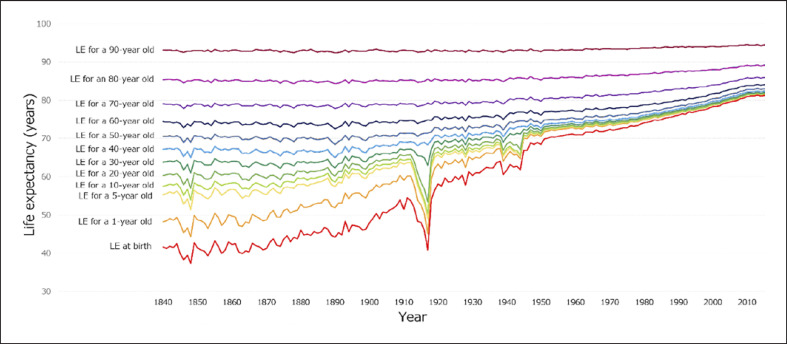
The twentieth and presently twenty-first centuries have been characterized by accelerating medical, pharmacological, and technological advances [1, 2, 3]. In the context of population demographics, one of the most significant outcomes of these advances is the exponential increase in overall population, and the relatively rapid increase in life expectancy [4, 5]. These increases can be partially attributed to improvements in public health that have resulted in a profound reduction in global child mortality rates; with an increasing proportion of the population now living to sexual maturity [3, 6, 7]. However, increases in life expectancy have also occurred in the later part of life, albeit to a relatively lesser extent, in the increased population of older adults [4, 5] (Fig. 1).
Fig. 1.
Estimated life expectancy (LE) by age in the UK and Wales (1841–2016); Human Mortality Database; University of CA, Berkeley (USA), and Max Planck Institute for Demographic Research (Germany). Data available at www.mortality.org; raw data downloaded on February 22, 2020; adapted from [8].
Closely succeeding these increases in life expectancy, another demographic phenomenon has been observed: a substantial reduction in global fertility rates, particularly in developed countries. With most of the developed world now below the population replacement rate of 2.1 births per female for several consecutive decades [9, 10]. The combination of these two demographic phenomena has resulted in a growing, yet increasingly ageing population throughout the developed world; and even in the developing world, the onset of these changes are beginning to be observed [9, 10].
In Europe, current demographic trends indicate that by the year 2030 almost one in six of the European population will be aged 60 years or older, and the number of older people will grow to 247 million by 2050; representing a 35% increase from 2017, with one in four older adults being over 85 by 2040 [11]. The social and economic impacts of this epidemiological transition have yet to be fully experienced, as dependency ratios remain relatively stable, as the increase in the older population is, to an extent, offset by the reduction in youth dependency [5, 12]. However, if present trends persist, over time, dependency ratios in developed countries may shift as the absolute and relative number of those entering older age increases, while the absolute and relative number of those entering from youth dependency to workforce participation decreases [12]. When taken in conjunction with progressive declines in physical activity throughout all stages of the lifespan, this leaves this increasing population of older adults particularly susceptible to the development of disease and comorbidities associated with a lack of physical activity and an increase in sedentary behaviour [13, 14, 15]. This alone, irrespective of future dependency ratios, will have substantial personal and economic impacts as life expectancy increases, while the proportion of the lifespan spent without disease and disability fails to keep pace, or potentially deceases; as has been observed with a number of lifestyle-mediated non-communicable diseases in recent decades [16]. It is in this context that frailty, particularly in older age, has been described as “without question, one of the most serious public health challenges we will face in this coming century” ([17] p. 1376).
Frailty
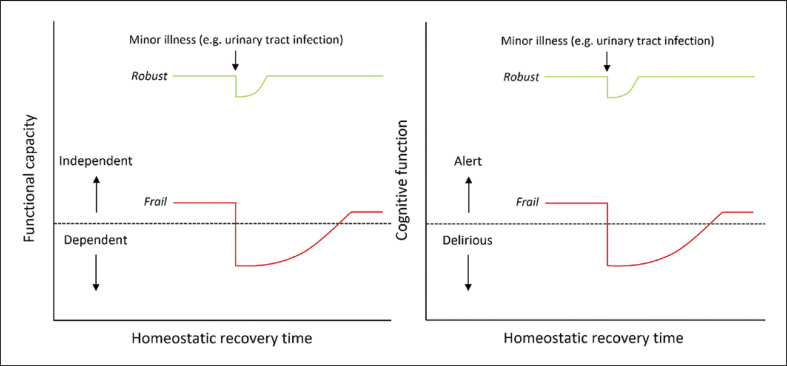
Frailty is a multidimensional and dynamic condition, theoretically defined as “a state of increased vulnerability, resulting from age-associated declines in reserve and function across multiple physiologic systems, such that the ability to cope with every day or acute stressors is compromised” ([18]p. 1, [19]) (Fig. 2).
Fig. 2.
Illustration of the multidimensional nature of frailty as a loss of physiological reserve across multiple systems, such that resilience and homeostatic response to stressors become compromised (adapted from [20]).
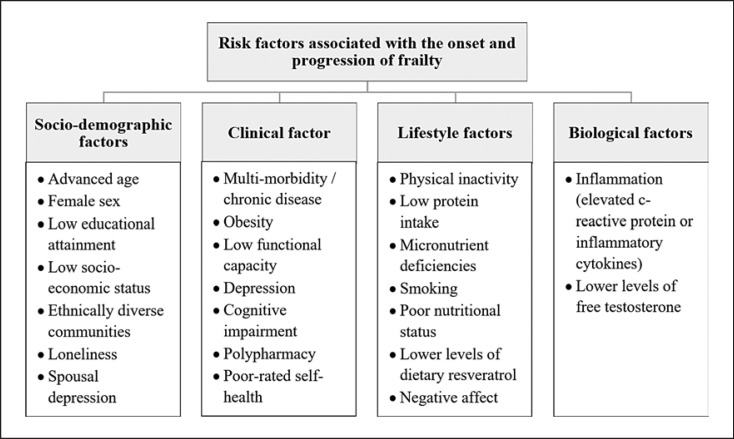
Although declines in physiological reserve are associated with senescence in the normal ageing process, frailty is an extreme consequence of this process, where this decline is accelerated and homeostatic responses begin to fail [21, 22, 23]. Frailty is a common and clinically significant condition among older adults [24]. This is predominantly due to its association with adverse health outcomes, such as hospitalization, falls, disability, and mortality [19, 20, 24, 25, 26, 27, 28]. All older adults are susceptible to the risk of developing frailty, and even their younger counterparts [29, 30]. However, this risk is significantly increased with increases in chronological age, in the presence of comorbidities, low physical activity, poor dietary intake, and low socio-economic status, among a number of other factors (Fig. 3) [19, 31, 32, 33, 34, 35].
Fig. 3.
Risk factors associated with the development and progression of frailty. Derived and adapted from [19, 32, 33, 34, 35, 36], and [37] respectively.
While frailty is a dynamic condition, with the possibility of bidirectional transition between frailty states [28, 37, 38], this transition is more commonly progressive [39]. This is largely due to the association of frailty with a plethora of adverse health outcomes, which can often lead to a spiral of decline. As frailty progresses, interventions to mitigate, manage, or reverse this decline become increasingly difficult to implement, both from practical and physiological perspectives [39, 40]. The relative prevalence of frailty in older adults may be reduced with future improvements in treatment, particularly those identified as effective at mitigating the onset of frailty [17]. However, irrespective of this, the absolute prevalence and overall burden of frailty are projected to increase dramatically in the coming decades as the population ages [36]. Perhaps of most concern in this regard, is that several longitudinal birth cohort studies have reported increases in the relative prevalence of frailty among more contemporary generations of older adults, when compared to their generational predecessors [41, 42, 43].
Operational Definitions of Frailty
Although there is a general consensus regarding the theoretical definition of frailty as a multidimensional and dynamic condition characterized by a loss of reserve across multiple physiological systems which collectively result in a compromised resilience to cope with stressors [17, 18, 19, 20, 36, 44, 45, 46, 47, 48, 49, 50]. Presently, there is no one universally utilized or accepted operational definition for the classification of frailty [27, 51, 52]. However, there are a number of valid operational definitions which exist, i.e., definitions which take into consideration the multidimensional nature of the condition (face and content validity) and have been specifically validated for the assessment of frailty: either through their predictive validity regarding negative health outcomes associated with frailty, or their concurrent validity with existing validated frailty tools [53, 54]. The most commonly utilized and well-regarded of these operational definitions are the Fried frailty phenotype [19], and the Frailty Index (FI) [55, 56, 57]. The Fried frailty phenotype proposes that frailty be defined as a clinical syndrome in which three or more of the five following criteria are present: unintentional weight loss (≥10 lbs in the past year), self-reported exhaustion, weakness (grip strength), slow walking speed, and low physical activity (active kcals expended per week) [19] (Table 1).
Table 1.
Components of the Fried frailty phenotype operational definition for the classification of frailty [19]
| Components | Method of assessment |
|---|---|
| >1. Unintentional weight loss | Self-reported unintentional weight loss of ≥10 lbs in the last year |
|
| |
| >2. Self-reported exhaustion | Centre of Epidemiological Studies Depression Scale: two subjective questions regarding endurance and energy, scored from 0 to 3 (a score >1 on either of these questions signifies confirmation of the exhaustion criteria) |
|
| |
| >3. Weakness | Grip strength measurement. Classification criteria relative to sex and body mass index |
|
| |
| >4. Slow walking speed | 15-foot gait speed assessment. Classification criteria relative to sex and height |
|
| |
| >5. Low physical activity | Short version of the Minnesota leisure time activity questionnaire utilized to estimated active calories expended per week. Classification criteria relative to sex |
The FI proposes that frailty should be operationally defined on a spectrum utilizing a mathematical model which considers frailty in regard to the accumulation of deficits. In this model, deficits represent any symptom, sign, disability, or laboratory measurement regarded as abnormal. The FI score is assessed as the accumulative proportion of these potential deficits that are present. Typically, the list of deficits ranges from approximately 30–70 items related to various aspects of health and well-being [45]. Although these are among the most commonly utilized operational definitions of frailty, there are also a number of other valid operational definitions which are frequently employed (Table 2).
Table 2.
Valid operational definitions for the classification of frailty
| Operational definition | Number of items | Components | Classification criteria |
|---|---|---|---|
| Fried frailty phenotype criteria [19] | 5 | • Unintentional weight loss (≥10 lbs in the last year) • Self-reported exhaustion • Weakness (grip strength) • Slow gait speed • Low levels of physical activity |
0 = Robust (non-frail) 1–2 = Pre-frail ≥3 = Frail |
|
| |||
| Frailty Index (of cumulative deficits) [55] | ~30–70 | Accumulative health deficits (typically 30 or more), with scoring ranging from 0 (the absence of all deficits) to 1 (the presence of all deficits) | Typically reported as a continuous variable; cut-off of >0.25 suggested for frailty |
|
| |||
| Frailty Index (from comprehensive geriatric assessment [CGA]) [56] | 14–52 | Accumulative health deficits, with scoring ranging from 0 to 1, derived from the CGA. 10 domains: • Cognition • Emotion • Communication • Mobility • Balance • Bladder • Bowel • Nutrition • Activities of daily living • Social |
Typically reported as a continuous variable; cut-off of >0.25 suggested for frailty |
|
| |||
| Edmonton Frailty Scale [58] | 11 | • Cognition • Hospital admission • General health • Functional capacity (x2) • Social support • Medication usage (×2)* • Nutrition* • Mood* • Continence* |
0–5 = Non-frail 6–7 = Vulnerable 8–9 = Mild frailty 10–11 = Moderate frailty 12–17 = Severe frailty |
|
| |||
| Reported Edmonton Frailty Scale [59] | 11 | • Cognition • Hospital admission • General health • Functional capacity • Social support • Medication usage (×2)* • Nutrition* • Mood* • Continence* • Self-reported performance |
0–5 = Non-frail 6–7 = Vulnerable 8–9 = Mild frailty 10–11 = Moderate frailty 12–18 = Severe frailty |
|
| |||
| Clinical Frailty Scale [60] | 1 | Visual and written chart scoring frailty on a continuous scale between 1 (very fit) and 9 (terminally ill) | 1–3 = Non-frail 4 = Vulnerable 5–9 = Frail |
|
| |||
| Canadian Study on Health and Ageing (CSHA) Clinical Frailty Scale [60] | 1 | Visual and written chart scoring frailty on a continuous scale between 1 (very fit) and 7 (severely frail) | 1–3 = Non-frall 4 = Vulnerable 5–7 = Frail |
|
| |||
| FRAIL Scale [61] | 5 | • Fatigue • Resistance (ability to climb stairs) • Ambulation (ability to walk one block) • Illnesses • Loss of weight |
0 = Robust (non-frall) 1–2 = Pre-frail ≥3 = Frail |
|
| |||
| Survey of Health, Ageing and Retirement in Europe-Frailty Instrument (SHARE-FI) [62] 5 | • Walking difficulties • Weakness (grip strength) • Exhaustion • Loss of appetite • Low physical activity |
Typically reported as a continuous variable; the following cut-offs are suggested <0.08 = Non-frall ≤0.08 to < 0.25 = Pre-frail ≥0.25 = Frail |
|
|
| |||
| Groningen Frailty Indicator [63] | 15 | 4 domains: • Physical (x9) • Cognitive • Social (x3) • Psychological (x2) |
≥4 = Frail |
|
| |||
| Modified Frailty Index (mFI) [64] | 11 | • Functional status • Diabetes mellitus • Lung problems • Congestive heart failure • Myocardial infarction • Cardiac problems • Hypertension • Impaired sensorium • Prior transient ischaemic attack • History of stroke • Peripheral vascular disease |
0 = Robust (non-frall) >0 to <0.21 = Pre-frail ≥0.21 = Frail |
|
| |||
| Tilburg Frailty Indicator [65] | 15 | 3 domains: • Physical (x8) • Psychological (x5) • Social (x3) |
≥5 = Frail |
|
| |||
| Study of Osteoporotic Fractures (SOF) Index [66] | 3 | • Weight loss • Exhaustion • Chair rise |
0 = Robust 1–2 = Pre-frail ≥2 = Frail |
|
| |||
| Multidimensional Prognostic Index [67] | 8 | • Comorbidity • Nutrition • Polypharmacy • Pressure sore risk • Living status • Activities of daily living • Instrumental activities of daily living |
<0.34 = Robust (non-frail) >0.34–0.66 = Pre-frail >0.66 = Frail |
|
| |||
| Trauma-Specific Frailty Index [68] | 15 | 5 categories: • Comorbidities (×3) • Daily activities (×5) • Health attitude (×5) • Function (×1) • Nutrition (×1) |
≤0.12 = Robust >0.13–0.25 = Pre-frail >0.25 = Frail |
|
| |||
| Emergency General Surgery-Specific Frailty Index [69] | 15 | 5 categories: • Comorbidities (×4) • Daily activities (×5) • Health attitude (×5) • Nutrition (×1) |
≥0.25 = Frail |
|
| |||
| Rockwood frailty assessment [70] | 4 | • Activities of daily living • Bladder function • Bowel function • Cognition |
≥2 = Frail |
|
| |||
| Kihon Checklist [71, 72] | 25 | 7 categories: • Physical strength • Nutrition • Oral function • Socialization • Memory • Mood • Lifestyle |
Dichotomous scoring of all items as per the frailty index. Cut-off of >0.25 suggested for classification of frailty |
|
| |||
| Preoperative Frailty Index (pFI) [73] | 30 | Accumulative health deficits with scoring ranging from 0 (the absence of all deficits) to 1 (the presence of all deficits) | >0.21 = Frail |
|
| |||
| Comprehensive Assessment of Frailty (CAF) [74] | 14 | 4 domains: Laboratory assessment • Serum albumin • Forced expiratory volume • Serum creatine Phenotype assessment • Exhaustion • Physical activity levels • Gait speed • Weakness (grip strength) Modified physical performance assessment** • Balance • Chair rise • Timed ability to put on and remove jacket • Timed ability to pick a pen from the floor • 360-degree turn CSHA CFS assessment** |
1–10 = Non-frail 11–25 = Moderately frail 26–35 = Severely frail |
|
| |||
| Frailty predicts death One yeaR after CArdlac Surgery Test (FORECAST) [44] | 5 | • Chair rise*** • Weakness • Stair climb • CSHA Clinical frailty scale assessment*** • Serum creatine |
0–4 = Non-frail 5–7 = Moderately frail 8–14 = Severely frail |
|
| |||
| Robinson criteria [75] | 7 | • Timed up and go • Katz Index of activities of dally living • Cognition • Charleston Index • Anaemia • Nutrition • Falls |
0–1 = Non-frail 2–3 = Pre-frail 4–7 = Frail |
|
| |||
| National Surgical Quality Improvement Program Frailty Index (NSQIP-FI) [76] | 11 | • History of: - Diabetes - Obstructive pulmonary disease or pneumonia - Cognitive heart failure - Myocardial infarction within 6 months of surgery - Percutaneous coronary intervention cardiac surgery or angina |
>0.25 = Frail |
|
| |||
| • Impaired functional status • Hypertensive medications • Peripheral vascular disease or rest pain • Impaired sensorium • Transient ischaemic attach or cardiovascular accident • History of cardiovascular attack with persistent residual dysfunction |
|||
All criteria on the EFS are scored from 0–2, with the exception of these items which are scored 0–1.
All criteria on the CAF are scored 0–1, with the exception of the modified physical performance assessment, and the CSFHA clinical frailty scale, for which each component is scored 0–4, and 0–7 respectively.
All criteria on the FORECAST are scored 0–1, with the exception of the chair rise, and CSHA clinical frailty scale assessments, which are scored 0–4, and 0–7 respectively.
Further to these validated operational definitions, proxy indicators of frailty are also commonly utilized, such as unidimensional measures of physical function, e.g., the Short Physical Performance Battery (SPPB) [77], Timed Up and Go (TUG) [78], Upper-Extremity Function (UEF) frailty index [79], gait speed [80], and hand grip strength [81]. These measures are associated with frailty and may even possess concurrent validity with existing frailty tools, or predictive validity regarding negative health outcomes associated with frailty. However, they lack the content validity regarding assessment of the multidimensional nature of the condition to be regarded themselves as valid operational definitions. Although strong arguments have been made regarding the pragmatic utility of these tools within various settings and circumstances [82].
Similarly, there are a number of other tools, such as the geriatric 8 questionnaire (G-8) [83], identification of seniors at risk (ISAR) [84], vulnerable elderly survey (VES-13) [85], frailty index for elders (FIFE) [86], frailty risk score [87], hospital frailty risk score [88], and PRISMA 7 [89], which serve as proxy indicators of frailty through identifying “frailty risk,” often with the suggestion of further more comprehensive evaluation. However, they are not valid operational definitions which definitively distinguish between frail, pre-frail, or robust classification states.
Recently, an alternative approach, separating itself from the phenotypic and accumulation of deficits models, has proposed a focus on intrinsic capacity, i.e., a composite measure of all physical and mental resources which an individual can draw from to overcome environmental, physical, and psychological challenges [90]. The development of this construct was initially supported by the World Health Organization, however, it remains to be empirically validated [90, 91]. While the construct of intrinsic capacity is in its theoretical and operational infancy, it may provide a new paradigm for future exploration, closely aligned with that of frailty research [92].
Presently, one of the major weaknesses in the frailty field is not only a lack of a single standardized operational definition, but also the common utilization of non-validated iterations of the above definitions. This produces a detrimental effect on both the internal and external validity of such studies, resulting in a reduced capacity for accurate evaluation and comparison; even between studies which report to be utilizing the same operational definition [93, 94, 95]. A recently published brief standard checklist for studies reporting frailty data has attempted to address this through outlining, and proposing solution to, some of these persistent issues within the literature, including: (1) studies often report participants as frail without a frailty assessment; (2) studies often claim to utilize validated operational definitions for the classification of frailty, however, adapt these definitions, or classification criteria, which resulted in the definitions becoming not only non-standardized, but also non-validated; (3) the use of the nomenclature for different operational definitions of frailty vary widely, even among studies utilizing the same operational definition; (4) often, useful data regarding prevalence of frailty (such as pre-frailty, a sex breakdown of frailty, or occasionally the overall prevalence of frailty itself) are not reported. To address these issues, the following checklist is proposed: (1) accurate citation of the validation study for the specific operational definition utilized for the classification of frailty; (2) accurate use of the nomenclature of the operational definition of frailty utilized in accordance with the initial validation study to maintain reliability and validity, or prominent subsequent study establishing the nomenclature; (3) reporting of the number of frail, pre-frail (if applicable), and robust participants; (4) a sex breakdown of the number of frail, pre-frail, and robust participants [96].
In this regard, the academic field of frailty is somewhat lacking a desired order and uniformity. This is likely the manifestation of the multidimensional and heterogenous nature of frailty as a combination of a multitude, and often different array, of phenomena which can result from many differential causes and pathways [20]. The breadth of proposed frailty definitions is a manifestation of this complexity. Ultimately, what may be required regarding progress towards the establishment of a universally accepted operational definition, in addition to exploration of emerging constructs [90, 91, 92, 97], is mathematical modelling of large longitudinal datasets which can identify frailty through an abundance of potential multidimensional pathways over time, as it relates to the dynamic ability to cope with acute stressors over these periods. However, to date a universally accepted operational definition for the classification of frailty remains elusive, despite the utility this may provide in the future.
The Prevalence of Frailty
Although the exact prevalence of frailty within geriatric populations is poorly defined due to the lack of a single standardized operational definition, there are a number of systematic reviews and meta-analyses which have attempted to provide well-evidenced estimates of the prevalence of frailty among older adults within a variety of settings [96, 98, 99, 100, 101, 102, 103, 104, 105]. An enhanced understanding regarding the prevalence of a condition within a specific setting has a number of important consequences; including the enhanced ability to contribute towards improvements in the planning and orientation of organizational structures and resources, to meet population needs. This is especially the case regarding the tailoring of service provision within particular settings to the needs of service users. Specifically regarding frailty, this includes for example the provision of exercise rehabilitation services within settings for this population; with physical activity and exercise proposed to offer the best form of treatment for frail older adults [106].
Community-Dwelling Older Adults
Presently, there are several systematic reviews and meta-analyses which have examined the prevalence of frailty in various cohorts of community-dwelling older adults [98, 99, 100, 101, 105] (Table 3). In the single review which examined the overall prevalence of frailty within this population, the pooled prevalence of frailty was 10.7%. However, the reported prevalence of frailty within the studies comprising this review ranged from 4.0 to 59.1%; largely due to inclusion of proxy indicators of frailty, and to a lesser degree the lack of a single standardized operation definition [105]. In the remaining four systematic reviews/meta-analyses of the prevalence frailty in various specific cohorts of community-dwelling older adults, the overall pooled prevalence of frailty ranged from 7.4% among community-dwelling older adults in Japan, to 68% among the overall population of undernourished community-dwelling older adults [100, 101]. Along a similar line of inquiry, a recent systematic review and meta-analysis found the global incidence of frailty, and pre-frailty, among community-dwelling older adults (aged ≥60 years) to be 43.4, and 150.6 per 1,000 person-years respectively [107].
Table 3.
Systematic reviews and meta-analyses examining the prevalence of frailty among community-dwelling older adults
| Author(s) | Study design | Population | Minimum age, years | Included studies | Pooled sample | Pooled prevalence of frailty (95% Cl) (%) | Pooled prevalence of pre-frailty (95% Cl) (%) | Range of reported frailty prevalence (%) | Range of reported pre-frailty prevalence (%) |
|---|---|---|---|---|---|---|---|---|---|
| He et al., 2019 [98] | Systematic review and meta-analysis | Community-dwelling older adults in China | ≥65 | 14 | 81,258 | 10 (8.0–12.0) | 43 (37–50) | >5.9–17.4 | >26.8–52.4 |
| Siriwardhana et al., 2018 [99] | Systematic review and meta-analysis | Community-dwelling older adults in low- and middle-income countries | ≥60 | 47 | 75,133 | >17.4 (14.4–20.7) | >49.3* (46.4–52.2) | >3.9–51.4 | >13.4–71.6* |
| Kojima et al., 2017 [100] | Systematic review and meta-analysis | Community-dwelling older adults in Japan | ≥65 | 5 | 11,414 | >7.4 (6.1–9.0) | >48.1 (41.6–54.8) | >4.6–9.5 | >38.0–65.2 |
| Verlaan et al., 2017 [101] | Systematic review and meta-analysis | Malnourished community-dwelling older adults | ≥50 | 10 | 128 | 68 (59.9–76.1)** | >25.8(18.2–33.4)** | n/a | n/a |
| Collard et al., 2012 [105] | Systematic review | Community-dwelling older adults | ≥65 | 21 | 61,500 | >10.7 (10.5–10.9) | >41.6* | 4–59.1 | >18.7–53.1*** |
Data only available for 42/47 studies (47,302/75,133 participants).
Not reported in the original paper, derived from available data.
Data only available for 15/21 studies (53,727/61,500 participants).
Older Adults in Residential Care (Assisted Living Facility and Nursing Home Residents)
As previously described by Doody et al. [108], presently there are no well-evidenced pooled estimates of the overall prevalence of frailty among older adults in assisted living facilities. Although, it could be postulated that this prevalence would likely be higher than that of community-dwelling older adults, given that older adults within assisted living facilities typically tend to be chronologically older, and often exhibit a greater number of comorbidities and a reduced functional capacity than their community-dwelling counterparts. However, these differences routinely become non-significant once standardized for age [109]. Additionally, the estimated prevalence of frailty, and pre-frailty, in nursing homes (where qualified nursing care is required, in addition to care assistance) is approximately 52.3%, and 40.2%, respectively [104]. As such, the prevalence of frailty in assisted living facilities likely lies somewhere in between that of community-dwelling older adults and nursing home residents, given the inherent nature of these respective settings and the demographics of the individuals who occupy them. However, presently, there appears a lack of individual studies which have examined the prevalence of frailty specifically within assisted living facilities.
Hospitalized Older Adults
A recently published systematic review and meta-analysis produced the first well-evidenced pooled estimates of the prevalence of frailty among geriatric hospital inpatients [98]. This review found that approximately 47.4% (95% CI 43.7–51.1%) of all geriatric hospital inpatients are frail; and another 25.8% (95% CI 22.0–29.6%) pre-frail. Prevalence varied significantly based on age, clinical population, morbidity, ward type, and the operational definition utilized for the classification of frailty [98]. The authors noted that the overall pooled prevalence estimate of frailty of 47.4% reported within this review, placed the prevalence of frailty among geriatric inpatients between that reported for community-dwelling older adults at 10.7% [105], and older adults in nursing homes at 52.3% [104]; outlining an increase in the relative prevalence of frailty with progression through the healthcare system [98]. Further the authors noted the overall pooled prevalence of pre-frailty of 25.8% was lower than that reported for both community-dwelling older adults at 41.6% [105], and nursing home residents at 40.2% [104]; while the combined prevalence estimates of both frailty and pre-frailty increased from 52.3% among community-dwelling older adults, to 73.2% among geriatric inpatients, and to 92.5% among nursing home residents. The authors concluded that this underscores differences in the relative prevalence of frailty status between community, and hospital inpatient settings, are the result of an increase in the relative prevalence of frailty, and similar decreases in the relative prevalence of pre-frailty and robustness. However, differences in the relative prevalence of frailty status between hospital inpatient and nursing home settings, appear primarily the result of a relative increase in the prevalence of pre-frailty, and decrease in the prevalence of robustness [98].
The overall pooled frailty, and pre-frailty, prevalence estimates of 47.4% (95% CI: 43.7–51.1%), and 25.8% (95% CI: 22.0–29.6%) reported within this review were consistent with estimates of a systematic review and meta-analysis examining the prevalence of frailty and pre-frailty among hospitalized older adults in several studies which also assessed undernutrition risk, at 47% (95% CI: 37–57%) and 36% (95% CI: 29–44) respectively [110]. Similarly, the pooled prevalence estimates of frailty on acute wards of 51.1% (95% CI: 35.9–66.2%), and among all acute hospital inpatients of 47.3% (95% CI: 42.8–51.8%) were outlined to be relatively consistent with findings of a recent scoping review, which reported a median frailty prevalence of 49% (range 34–69%) in acute care hospital settings [111]. Further, this review reported no significant differences in the prevalence of frailty stratified by sex. The authors noted that this contrasts systematic reviews and meta-analysis regarding the prevalence of frailty among community-dwelling older adults [98, 99, 105]. However, further noted these findings are consistent with systematic reviews and meta-analysis among other clinical populations of older adults such as nursing home residents [104]; concluding that these findings further contribute to the evidence that sex differences in the prevalence of frailty among community-dwelling older adults may dissipate among clinical geriatric populations [98].
The Impact of Frailty
Frailty is associated with a myriad of adverse health outcomes, which have both personal and economic consequences. Among these adverse outcomes include the increased occurrence of falls, fractures, worsening mobility, disability, cognitive decline, dementia, depression, hospitalization, institutionalization, and mortality [69, 112, 113, 114, 115, 116, 117]. Moreover, frailty has been consistently shown to be associated with increased healthcare cost and usage [118, 119, 120]. For example, a cross-sectional analysis of approximately 2,600 older adults aged ≥60 years in Germany found that the mean 3-month healthcare expenditure was almost sixfold higher among the frailest participants (five positive Fried frailty phenotype criteria), at € 3,659, compared to the least frail participants (no positive Fried frailty phenotype criteria), at € 642 [121]. A subsequent 3-year longitudinal analysis of over 1,600 older adults within the same cohort found that progression from a non-frail to a frail state was associated with an average of 54%–101% increase in healthcare cost in those with 3, and 4 or 5 positive frailty criteria respectively; including a 200% increase in inpatient costs from those who transitioned from non-frail (no positive Fried frailty phenotype criteria) to low levels of frailty (three positive Fried frailty phenotype criteria) [122]. Similarly, a recent analysis of 5,300 community-dwelling older adults aged ≥60 years in China, found frailty to be an independent predictor of increased health expenditure [118]. However, the impact of frailty on an individual’s life extends further than the clinical manifestation or economic impact of these adverse health outcomes, with frailty additionally being associated with a reduced quality of life, and loneliness [123, 124].
The Associations between Frailty and Socio-Economic Variables
While at the individual level there is evidence of the association between socio-economic status and frailty onset and progression [34], at the societal level the association between economic variables and frailty is less well evidenced. Preliminary research into this area has shown the prevalence of frailty in the community to be correlated with national economic indicators such as gross domestic product (GDP) per capita purchasing power parity (PPP) and healthcare expenditure per capita PPP. However, noted that more research is needed to better understand the relationship between macroeconomic indicators and the prevalence of frailty [125]. A recent systematic review and meta-analysis found no association between the prevalence of frailty among geriatric hospital inpatients and GDP per capita PPP, or healthcare expenditure per capita PPP [98]. The authors postulated it possible that these associations, while present in the community, are not present in inpatient hospital settings, and that given the inherent nature of hospital inpatient settings, i.e., institutions for chronically or acutely unwell patients, such association may be more sensitive among community-dwelling older adults. However the authors noted more large-scale and comprehensive studies are required in a variety of settings as a limitation of these analyses was that included studies were predominantly from economically developed countries due to limited evidence presently from low-income countries [98, 103]. The authors further noted that while it has been postulated that increases in economic prosperity may limit the prevalence and burden of frailty [129], these findings bring this postulation into question, and as such reliance of non-direct intervention such as economic development to improve the prevalence and burden of frailty on health systems alone, appear to be misplaced, and suggests the need for more direct interventions to address the burden of frailty among this population [98].
The Prevention, Treatment, and Management of Frailty
Presently, care plans specifically for frail individuals have yet to be extensively developed or assessed. However, there are several proposed treatments and care pathways involved in the prevention, treatment, and management of frailty. Initial establishment of agreed goals of care may be assisted in clinical settings in particular by a comprehensive geriatric assessment, which can provide a framework from which to develop a management and intervention plan for frail individuals. Further, as frailty progresses patients will develop different care needs, and require different forms of care, often in different settings (Table 4).
Table 4.
Trajectory of care for frail individuals (adapted from [36])
| Primary care | Advanced age older adult Adoption/continuation of unhealthy lifestyle behaviours Accumulation of frailty deficits and risk factors for disease |
Primary prevention |
|---|---|---|
| Diagnosis of chronic disease | Secondary prevention | |
|
|
||
| Acute care | Acute decompensation of disease Cycle of stabilization and destabilization |
|
|
|
||
| Specialist care | Progression of disease to advanced stage Intensive medical or surgical therapy |
|
|
|
||
| Iatrogenic complication from therapy Prolonged hospitalization |
Tertiary prevention | |
|
|
||
| Post-acute care | Functional decline Institutionalization |
|
|
|
||
| Palliative care | Readmission to hospital Death |
|
Regular physical activity and exercise have been shown to provide a degree of protection against multiple components of frailty in both sexes, at all stages of the condition, and all stages of the life cycle [126, 127]. Further, exercise interventions have been proposed as potentially offering the best form of treatment for frail older adults [106], with promising results in a variety of settings and geriatric populations [128, 129], and even shown to mediate the reversal of frailty in some cases [130, 131]. However, more research is needed to determine the feasibility and efficacy of exercise interventions in different settings and clinical populations [25, 131].
Exercise Interventions for Frail Geriatric Populations
Regular physical activity and exercise have been shown to consistently improve cognition, physical function, sarcopenia (low muscle quantity, strength, and performance), and mood in both non-frail and frail older adults [126]. While inactivity is a modifiable risk factor for frailty onset and progression, physical activity and exercise are known to improve function across multiple physiological systems, including the muscle, heart, brain, endocrine system, and inflammation response [132]. In this regard, exercise can improve function in all physiological systems known to be dysregulated with the onset and progression of frailty [133]. However, while there is evidence of the benefits of exercise regarding the prevention, treatment, and potential reversal of frailty, it is universally noted that there needs to be more studies within this area to truly assess the feasibility and efficacy of exercise in frail geriatric populations within different settings, and particularly in clinical settings [25, 134]. Further, to increase external validity of such studies, particularly those among clinical cohorts, it is imperative that prospective studies attempt to recruit as representative a sample as possible, so that feasibility and efficacy assessments are extrapolatable to real-world settings. In this regard for example, a recent systematic review examining exclusion rates in 305 randomized controlled trials involved in the treatment of 31 physical conditions, reported that a quarter of all trials excluded 89% of patients with the specific condition to be treated within that trial, while half excluded 77.1% of patients with the condition. Those excluded were primarily attributed to advanced age, and those with significant comorbidity and co-prescription; characteristics which are ubiquitous among those treated in clinical practice [135]. Though it is often required to exclude certain cohorts to define the clinical population and control for confounding factors, particularly with regard to exercise interventions which pose a low likelihood of contraindication, it is essential that representative samples are examined, which among frail older adults, and particularly in certain settings, invariably includes those with significant comorbidities and polypharmacy.
Interventions among Community-Dwelling Older Adults
Exercise, or exercise and nutrition interventions combined, have been shown to be capable of reversing frailty [130, 131, 136], or limiting its progression [137, 138], among cohorts of community-dwelling older adults.
Interventions among Older Adults in Residential Care (Assisted Living and Nursing Home Residents)
The implementation of exercise interventions in nursing home settings has been shown to be effective in improving strength, gait speed, and balance in older adults residing in these settings [139, 140]. Further, individualized and progressive multicomponent exercise interventions at a moderate intensity have been shown to be effective in the prevention of falls, and the reduction of frailty and mortality among older nursing home residents [129].
Interventions among Hospitalized Older Adults
As previously described by Doody et al. [141], acute hospital admission for older adults is associated with further loss of physical activity and represents a period of increased susceptibility to sarcopenia and frailty [142]. Frailty is associated with longer stay and increased rates of mortality in hospitalized older adults, as well as serving as a predictor of readmission [143, 144]. As such there is a crucial need to examine the feasibility of such interventions within this setting, and whether these interventions can be effectively implemented to improve the health of frail older populations in inpatient hospital ward settings. Preliminary evidence has shown some success in the implementation of exercise interventions to reverse functional decline among general geriatric inpatient populations [145, 146], and walking during hospitalization has been shown to be associated with a shorter length of stay [147]. However, to date, presently, there are no studies which have attempted to assess the feasibility or efficacy of such an intervention in operationally defined frail participants with more significant initial impairments.
Future Directions
There are several research directions which are pertinent to the advancement of the understanding of frailty and the promotion of healthy longevity among the increasing global population of older adults. More generally within the frailty field, further work towards a universally accepted operational definition of the construct, to practically complement the theoretical definition [18], is of paramount interest to the field. Additionally, the association between frailty and other related composite measures such as allostatic load [148, 149, 150] and intrinsic capacity [90, 92], and the potential utilization of these constructs as inexpensive proxy measures for biological ageing (identified through associations with the pattern of DNA methylation at different cytosine-phospho-guanine sites which correlate with mortality and time [151, 152], morbidity and lifespan [153, 154, 155, 156], and the pace of ageing [157]) is of interest for future research. The initial validation of cost-effective assessments as valid proxy measures of biological ageing may allow for a better understanding of ageing, not only in economically developed nations, but also throughout the globe, and especially among less economically developed areas of the world. The lack of data in these regions in particular will become increasingly important from a global perspective, given that these are the regions of the world projected to undergo the largest population growth in the coming century (e.g., the population of sub-Saharan Africa is projected to grow 298% from 2017 to 2100, from 1.03 to 3.07 billion), while conversely, many economically developed regions are projected to experience marked population decline (e.g., Europe’s population is projected to decline 19.2% from 2017 to 2100, from 758 to 613 million, and China’s population is projected to decline 48% over the same period, from 1.41 billion to 732 million) [158].
Further, as frailty is a relatively new concept, particularly as an operationally defined one, with most studies cited within this review published in the past 20 years, the potential change in frailty over time, particularly as it relates to national policy directives, and economic indicators are of interest for future research. Although at the individual level there is evidence of the association between socio-economic status and the frailty onset and progression [34], at the societal level, the association between economic variables and frailty is less well evidenced. More large-scale and comprehensive research is needed in this regard to better understand this relationship between macroeconomic indicators and the prevalence of frailty in a variety of settings. Further, more comprehensive systematic analyses of this association between frailty and national economic indicators among community-dwelling older adults, older adults in residential care settings, and hospitalized older adults, may help to further elucidate this relationship within relevant settings.
Regarding the provision of well-evidenced estimates of the prevalence of frailty within various settings, presently, there are no currently published well-evidenced pooled estimates of the prevalence of frailty among older adults residing in assisted living facilities. Further research is required to elucidate the prevalence of frailty among older adults in these settings. Further, adapted exercise interventions among frail hospital and intermediate-care patients are also of interest for future research. Particularly, the continuation of these activities and assessments following patient discharge from hospital over a prolonged period, and the impact on these activities on measures of multidimensional health and other health-related outcomes, such as readmissions, and cost-effectiveness. Exercise interventions have been shown to be effective at reducing functional decline among general hospital inpatients during hospitalization [145]; however, to date no research has been conducted among specifically frail inpatients, or providing continuity of the interventions, post-inpatient discharge. Further, adapted exercise interventions may also be ideally suited within more stable clinical environments, such as those of intermediate-care facilities, assisted living facilities, nursing homes, or hospital at home settings.
Conclusion
Frailty and healthy longevity have become increasingly important fields of research, which, if present global demographic trends persist, will continue to grow in importance as the world’s population ages. Further elucidation of frailty, and its exploration in the context of emerging constructs, is pertinent to the advancement of frailty research and the promotion of healthy longevity among the increasing population of older adults.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This review was supported by the European Commission Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement (675003); of which Dr. Paul Doody was a Marie Sklodowska-Curie doctoral research fellow; Professor Anna Whittaker, Professor Janet Lord, and Professor Carolyn Greig doctoral supervisors; and Professor Anna Whittaker the grant’s principal investigator. The funding source had no role in the design, conduct, or reporting of the review, or the decision to publish the manuscript.
Author Contributions
Dr. Paul Doody is the guarantor and lead reviewer. Dr. Paul Doody drafted the initial manuscript with supervision, input, and feedback from Professor Anna Whittaker, Professor Carolyn Greig, and Professor Janet Lord. All the authors have read and approved the final manuscript.
Funding Statement
This review was supported by the European Commission Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement (675003); of which Dr. Paul Doody was a Marie Sklodowska-Curie doctoral research fellow; Professor Anna Whittaker, Professor Janet Lord, and Professor Carolyn Greig doctoral supervisors; and Professor Anna Whittaker the grant’s principal investigator. The funding source had no role in the design, conduct, or reporting of the review, or the decision to publish the manuscript.
References
- 1.Mack CA. Fifty years of moore's law. IEEE Trans Semicond Manufact. 2011;24((2)):202–207. [Google Scholar]
- 2.Heath G, Colburn WA. An evolution of drug development and clinical pharmacology during the 20th century. J Clin Pharmacol. 2000;40((9)):918–929. doi: 10.1177/00912700022009657. [DOI] [PubMed] [Google Scholar]
- 3.Mackenbach JP. The contribution of medical care to mortality decline McKeown revisited. J Clin Epidemiol. 1996;49((11)):1207–1213. doi: 10.1016/s0895-4356(96)00200-4. [DOI] [PubMed] [Google Scholar]
- 4.Human MD. Human mortality database. berkeley (USA) university of california; 2020. and max planck institute for demographic research (Germany) Available From: www.mortality.org (data downloaded on 22/02/2020. [Google Scholar]
- 5.Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations the challenges ahead. Lancet. 2009;374((9696)):1196–208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GBD 2016 Mortality Collaborators, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F. Global and national under-5 mortality adult mortality age-specific mortality and life expectancy, 1970-2016 a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390((10100)):1084–150. doi: 10.1016/S0140-6736(17)31833-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutler D, Miller G. The role of public health improvements in health advances the twentieth-century United States. Demography. 2005;42((1)):1–22. doi: 10.1353/dem.2005.0002. [DOI] [PubMed] [Google Scholar]
- 8.Roser M, Ortiz-Ospina E, Ritchie H. Life expectancy. Our World in Data. 2013 [Google Scholar]
- 9.United Nations, Department of Economic, Affairs S Division P United nations population prospects 2019. ST/ESA/SER.A/423. 2019.
- 10.GBD 2017 Population and Fertility Collaborators Population and fertility by age and sex for 195 countries and territories, 1950–2017 a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392((10159)):1995–2051. doi: 10.1016/S0140-6736(18)32278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.United Nations Department of Economic and Social Affairs Population Division World population ageing 2017. ST/ESA/SER.A/408) 2017. https://Www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2017_Report.pdf.(accessed: 17th april 2019)
- 12.Omran AR. The epidemiologic transition a theory of the epidemiology of population change. Milbank Q. 2005;83((4)):731–757. doi: 10.1111/j.1468-0009.2005.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ford ES, Caspersen CJ. Sedentary behaviour and cardiovascular disease a review of prospective studies. Int J Epidemiol. 2012;41((5)):1338–1353. doi: 10.1093/ije/dys078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide an analysis of burden of disease and life expectancy. Lancet. 2012;380((9838)):219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caspersen CJ, Pereira MA, Curran KM. Changes in physical activity patterns in the United States by sex and cross-sectional age. Med Sci Sports Exerc. 2000;32((9)):1601–1609. doi: 10.1097/00005768-200009000-00013. [DOI] [PubMed] [Google Scholar]
- 16.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A. Global and national incidence and years lived with disability for 310 diseases and injuries, 1990-2015 a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388((10053)):1545–602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty opportunities, challenges, and future directions. Lancet. 2019 Oct 12;394((10206)):1376–1386. doi: 10.1016/S0140-6736(19)31785-4. [DOI] [PubMed] [Google Scholar]
- 18.Xue QL. The frailty syndrome definition and natural history. Clin Geriatr Med. 2011;27((1)):1–15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56((3)):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 20.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381((9868)):752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taffett GE. In Geriatric medicine. New York, NY: Springer; 2003. Physiology of aging; pp. p. 27–35. [Google Scholar]
- 22.Ferrucci L, Cavazzini C, Corsi A, Bartali B, Russo CR, Lauretani F, et al. Biomarkers of frailty in older persons. J Endocrinol Invest. 2002;25((10 Suppl l)):10–15. [PubMed] [Google Scholar]
- 23.Fried LP, Cohen AA, Xue QL, Walston J, Bandeen-Roche K, Varadhan R. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat Aging. 2021;1((1)):36–46. doi: 10.1038/s43587-020-00017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival a systematic literature review. Ageing Res Rev. 2013;12((2)):719–736. doi: 10.1016/j.arr.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Mañas L, Fried LP. Frailty in the clinical scenario. Lancet. 2015;385((9968)):e7–e9. doi: 10.1016/S0140-6736(14)61595-6. [DOI] [PubMed] [Google Scholar]
- 26.Sourial N, Bergman H, Karunananthan S, Wolfson C, Payette H, Gutierrez-Robledo LM, et al. Implementing frailty into clinical practice a cautionary tale. J Gerontol A Biol Sci Med Sci. 2013;68((12)):1505–1511. doi: 10.1093/gerona/glt053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sternberg SA, Wershof Schwartz A, Karunananthan S, Bergman H, Mark Clarfield A. The identification of frailty a systematic literature review. J Am Geriatr Soc. 2011;59((11)):2129–2138. doi: 10.1111/j.1532-5415.2011.03597.x. [DOI] [PubMed] [Google Scholar]
- 28.Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166((4)):418–423. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 29.Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality a prospective analysis of 493 737 UK biobank participants. Lancet Public Health. 2018;3((7)):e323–e332. doi: 10.1016/S2468-2667(18)30091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bagshaw SM, Majumdar SR, Rolfson DB, Ibrahim Q, McDermid RC, Stelfox HT. Erratum to a prospective multicenter cohort study of frailty in younger critically ill patients. Crit Care. 2016;20((1)):223. doi: 10.1186/s13054-016-1393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendonça N, Kingston A, Granic A, Jagger C. Protein intake and transitions between frailty states and to death in very old adults the Newcastle 85+ study. Age Ageing. 2019;49((1)):32–38. doi: 10.1093/ageing/afz142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng Z, Lugtenberg M, Franse C, Fang X, Hu S, Jin C, et al. Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults a systematic review of longitudinal studies. PLoS One. 2017;12((6)):e0178383. doi: 10.1371/journal.pone.0178383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gale CR, Westbury L, Cooper C. Social isolation and loneliness as risk factors for the progression of frailty the English longitudinal study of ageing. Age Ageing. 2018;47((3)):392–397. doi: 10.1093/ageing/afx188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stolz E, Mayerl H, Waxenegger A, Rásky É, Freidl W. Impact of socioeconomic position on frailty trajectories in 10 european countries evidence from the survey of health, ageing and retirement in europe (2004–2013) J Epidemiol Community Health. 2017;71((1)):73–80. doi: 10.1136/jech-2016-207712. [DOI] [PubMed] [Google Scholar]
- 35.Bandeen-Roche K, Seplaki CL, Huang J, Buta B, Kalyani RR, Varadhan R, et al. Frailty in older adults a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70((11)):1427–1434. doi: 10.1093/gerona/glv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty implications for clinical practice and public health. Lancet. 2019 Oct 12;394((10206)):1365–1375. doi: 10.1016/S0140-6736(19)31786-6. [DOI] [PubMed] [Google Scholar]
- 37.Pollack LR, Litwack-Harrison S, Cawthon PM, Ensrud K, Lane NE, Barrett-Connor E, et al. Patterns and predictors of frailty transitions in older men the osteoporotic fractures in men study. J Am Geriatr Soc. 2017;65((11)):2473–2479. doi: 10.1111/jgs.15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trevisan C, Veronese N, Maggi S, Baggio G, Toffanello ED, Zambon S, et al. Factors influencing transitions between frailty states in elderly adults the progetto veneto anziani longitudinal study. J Am Geriatr Soc. 2017;65((1)):179–184. doi: 10.1111/jgs.14515. [DOI] [PubMed] [Google Scholar]
- 39.Fried LP, Xue QL, Cappola AR, Ferrucci L, Chaves P, Varadhan R, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64((10)):1049–1057. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puts MTE, Toubasi S, Andrew MK, Ashe MC, Ploeg J, Atkinson E, et al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults a scoping review of the literature and international policies. Age Ageing. 2017;46((3)):383–392. doi: 10.1093/ageing/afw247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mousa A, Savva GM, Mitnitski A, Rockwood K, Jagger C, Brayne C, et al. Is frailty a stable predictor of mortality across time? Evidence from the cognitive function and ageing studies. Age Ageing. 2018;47((5)):721–727. doi: 10.1093/ageing/afy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu R, Wong M, Chong KC, Chang B, Lum CM, Auyeung TW, et al. Trajectories of frailty among Chinese older people in Hong Kong between 2001 and 2012 an age-period-cohort analysis. Age Ageing. 2018;47((2)):254–261. doi: 10.1093/ageing/afx170. [DOI] [PubMed] [Google Scholar]
- 43.Yang Y, Lee LC. Dynamics and heterogeneity in the process of human frailty and aging evidence from the US older adult population. J Gerontol B Psychol Sci Soc Sci. 2010;65B((2)):246–255. doi: 10.1093/geronb/gbp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173((5)):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62((7)):722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 46.van Kan GA, Rolland Y, Houles M, Gillette-Guyonnet S, Soto M, Vellas B. The assessment of frailty in older adults. Clin Geriatr Med. 2010;26((2)):275–286. doi: 10.1016/j.cger.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Rodríguez-Mañas L, Féart C, Mann G, Viña J, Chatterji S, Chodzko-Zajko W, et al. Searching for an operational definition of frailty a delphi method based consensus statement. the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci. 2013;68((1)):62–67. doi: 10.1093/gerona/gls119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.World Health Organization World report on ageing and health. World Health Organization. 2015.
- 49.Cesari M, Prince M, Thiyagarajan JA, De Carvalho IA, Bernabei R, Chan P, et al. Frailty an emerging public health priority. J Am Med Dir Assoc. 2016;17((3)):188–192. doi: 10.1016/j.jamda.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 50.Bracchitta LM, Angioni D, Celotto S, Cesari M. In The role of family physicians in older people care. Springer; 2022. Frailty and sarcopenia in primary care current issues; pp. p. 141–154. [Google Scholar]
- 51.Buta BJ, Walston JD, Godino JG, Park M, Kalyani RR, Xue QL, et al. Frailty assessment instruments systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. 2016;26:53–61. doi: 10.1016/j.arr.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice a review. Eur J Intern Med. 2016;31:3–10. doi: 10.1016/j.ejim.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 53.Rockwood K. What would make a definition of frailty successful? Age Ageing. 2005;34((5)):432–434. doi: 10.1093/ageing/afi146. [DOI] [PubMed] [Google Scholar]
- 54.Bandeen-Roche K, Gross AL, Varadhan R, Buta B, Carlson MC, Huisingh-Scheetz M, et al. Principles and issues for physical frailty measurement and its clinical application. J Gerontol A Biol Sci Med Sci. 2020;75((6)):1107–1112. doi: 10.1093/gerona/glz158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Scientific World Journal. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc. 2004;52((11)):1929–1933. doi: 10.1111/j.1532-5415.2004.52521.x. [DOI] [PubMed] [Google Scholar]
- 57.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8((1)):24–10. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Velanovich V, Antoine H, Swartz A, Peters D, Rubinfeld I. Accumulating deficits model of frailty and postoperative mortality and morbidity its application to a national database. J Surg Res. 2013;183((1)):104–110. doi: 10.1016/j.jss.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 59.Robinson TN, Wu DS, Pointer L, Dunn CL, Cleveland JC, Jr, Moss M. Simple frailty score predicts postoperative complications across surgical specialties. Am J Surg. 2013;206((4)):544–550. doi: 10.1016/j.amjsurg.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sündermann S, Dademasch A, Rastan A, Praetorius J, Rodriguez H, Walther T, et al. One-year follow-up of patients undergoing elective cardiac surgery assessed with the comprehensive assessment of frailty test and its simplified form; 21378017. Interact Cardiovasc Thorac Surg. 2011;13((2)):119–123. doi: 10.1510/icvts.2010.251884. discussion 123. [DOI] [PubMed] [Google Scholar]
- 61.Sündermann S, Dademasch A, Praetorius J, Kempfert J, Dewey T, Falk V, et al. Comprehensive assessment of frailty for elderly high-risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2011;39((1)):33–37. doi: 10.1016/j.ejcts.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 62.McIsaac DI, Wong CA, Huang A, Moloo H, van Walraven C. Derivation and validation of a generalizable preoperative frailty index using population-based health administrative data. Ann Surg. 2019 Jul;270((1)):102–108. doi: 10.1097/SLA.0000000000002769. [DOI] [PubMed] [Google Scholar]
- 63.Satake S, Senda K, Hong YJ, Miura H, Endo H, Sakurai T, et al. Validity of the K ihon checklist for assessing frailty status. Geriatr Gerontol Int. 2016;16((6)):709–715. doi: 10.1111/ggi.12543. [DOI] [PubMed] [Google Scholar]
- 64.Tomata Y, Hozawa A, Ohmori-Matsuda K, Nagai M, Sugawara Y, Nitta A, et al. Validation of the kihon checklist for predicting the risk of 1-year incident long-term care insurance certification the ohsaki cohort 2006 study. Nihon Koshu Eisei Zasshi. 2011;58((1)):3–13. [PubMed] [Google Scholar]
- 65.Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hébert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet. 1999;353((9148)):205–206. doi: 10.1016/S0140-6736(98)04402-X. [DOI] [PubMed] [Google Scholar]
- 66.Orouji Jokar T, Ibraheem K, Rhee P, Kulavatunyou N, Haider A, Phelan HA, et al. Emergency general surgery specific frailty index a validation study. J Trauma Acute Care Surg. 2016;81((2)):254–260. doi: 10.1097/TA.0000000000001120. [DOI] [PubMed] [Google Scholar]
- 67.Joseph B, Pandit V, Zangbar B, Kulvatunyou N, Tang A, O'Keeffe T, et al. Validating trauma-specific frailty index for geriatric trauma patients a prospective analysis. J Am Coll Surg. 2014;219((110)):>10.e1–>17.e1. doi: 10.1016/j.jamcollsurg.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 68.Pilotto A, Ferrucci L, Franceschi M, D'Ambrosio LP, Scarcelli C, Cascavilla L, et al. Development and validation of a multidimensional prognostic index for one-year mortality from comprehensive geriatric assessment in hospitalized older patients. Rejuvenation Res. 2008;11((1)):151–161. doi: 10.1089/rej.2007.0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ensrud KE, Ewing SK, Taylor BC, Fink HA, Stone KL, Cauley JA, et al. Frailty and risk of falls and mortality in older women the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2007;62((7)):744–751. doi: 10.1093/gerona/62.7.744. [DOI] [PubMed] [Google Scholar]
- 70.Gobbens RJ, van Assen MA, Luijkx KG, Wijnen-Sponselee MT, Schols JM. The tilburg frailty indicator psychometric properties. J Am Med Dir Assoc. 2010;11((5)):344–355. doi: 10.1016/j.jamda.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 71.Tsiouris A, Hammoud ZT, Velanovich V, Hodari A, Borgi J, Rubinfeld I. A modified frailty index to assess morbidity and mortality after lobectomy. J Surg Res. 2013;183((1)):40–46. doi: 10.1016/j.jss.2012.11.059. [DOI] [PubMed] [Google Scholar]
- 72.Stevernik N, Slaets J, Schuurmans H, Van Lis M, Steverink N, Slaets J, et al. Measuring frailty development and testing the GFI (Groningen Frailty Indicator) 2001 [Google Scholar]
- 73.Romero-Ortuno R, Walsh CD, Lawlor BA, Kenny RA. A frailty instrument for primary care findings from the Survey of Health, Ageing and Retirement in Europe (SHARE) BMC Geriatr. 2010;10((1)):57. doi: 10.1186/1471-2318-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle agedAfrican Americans. J Nutr Health Aging. 2012;16((7)):601–608. doi: 10.1007/s12603-012-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hilmer SN, Perera V, Mitchell S, Murnion BP, Dent J, Bajorek B, et al. The assessment of frailty in older people in acute care. Australas J Ageing. 2009;28((4)):182–188. doi: 10.1111/j.1741-6612.2009.00367.x. [DOI] [PubMed] [Google Scholar]
- 76.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the edmonton frail scale. Age Ageing. 2006;35((5)):526–529. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49((2)):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 78.Podsiadlo D, Richardson S. The timed “Up and go” a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39((2)):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 79.Joseph B, Toosizadeh N, Orouji Jokar T, Heusser MR, Mohler J, Najafi B. Upper-extremity function predicts adverse health outcomes among older adults hospitalized for ground-level falls. Gerontology. 2017;63((4)):299–307. doi: 10.1159/000453593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matsuzawa Y, Konishi M, Akiyama E, Suzuki H, Nakayama N, Kiyokuni M, et al. Association between gait speed as a measure of frailty and risk of cardiovascular events after myocardial infarction. J Am Coll Cardiol. 2013;61((19)):1964–1972. doi: 10.1016/j.jacc.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 81.Syddall H, Cooper C, Martin F, Briggs R, Aihie Sayer A. Is grip strength a useful single marker of frailty? Age Ageing. 2003;32((6)):650–656. doi: 10.1093/ageing/afg111. [DOI] [PubMed] [Google Scholar]
- 82.Cesari M, Landi F, Calvani R, Cherubini A, Di Bari M, Kortebein P, et al. Rationale for a preliminary operational definition of physical frailty and sarcopenia in the SPRINTT trial. Aging Clin Exp Res. 2017;29((1)):81–88. doi: 10.1007/s40520-016-0716-1. [DOI] [PubMed] [Google Scholar]
- 83.Bellera CA, Rainfray M, Mathoulin-Pelissier S, Mertens C, Delva F, Fonck M, et al. Screening older cancer patients first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23((8)):2166–2172. doi: 10.1093/annonc/mdr587. [DOI] [PubMed] [Google Scholar]
- 84.McCusker J, Bellavance F, Cardin S, Trepanier S, Verdon J, Ardman O. Detection of older people at increased risk of adverse health outcomes after an emergency visit the ISAR screening tool. J Am Geriatr Soc. 1999;47((10)):1229–1237. doi: 10.1111/j.1532-5415.1999.tb05204.x. [DOI] [PubMed] [Google Scholar]
- 85.Saliba D, Elliott M, Rubenstein LZ, Solomon DH, Young RT, Kamberg CJ, et al. The vulnerable elders survey a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001;49((12)):1691–1699. doi: 10.1046/j.1532-5415.2001.49281.x. [DOI] [PubMed] [Google Scholar]
- 86.Tocchi C, Dixon J, Naylor M, Jeon S, McCorkle R. Development of a frailty measure for older adults the frailty index for elders. J Nurs Meas. 2014;22((2)):223–240. doi: 10.1891/1061-3749.22.2.223. [DOI] [PubMed] [Google Scholar]
- 87.Pijpers E, Ferreira I, van de Laar RJ, Stehouwer CD, Nieuwenhuijzen Kruseman AC. Predicting mortality of psychogeriatric patients a simple prognostic frailty risk score. Postgrad Med J. 2009;85((1007)):464–469. doi: 10.1136/pgmj.2008.073353. [DOI] [PubMed] [Google Scholar]
- 88.Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, et al. Development and validation of a hospital frailty risk score focusing on older people in acute care settings using electronic hospital records an observational study. Lancet. 2018;391((10132)):1775–1782. doi: 10.1016/S0140-6736(18)30668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raîche M, Hébert R, Dubois MF. PRISMA-7 a case-finding tool to identify older adults with moderate to severe disabilities. Arch Gerontol Geriatr. 2008;47((1)):9–18. doi: 10.1016/j.archger.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 90.Beard, Jotheeswaran AT, Cesari M, Araujo de. Carvalho I The structure and predictive value of intrinsic capacity in a longitudinal study of ageing. BMJ open. 2019;9((11)):e026119. doi: 10.1136/bmjopen-2018-026119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cesari M, Araujo de Carvalho I, Amuthavalli Thiyagarajan J, Cooper C, Martin FC, Reginster JY, et al. Evidence for the domains supporting the construct of intrinsic capacity. J Gerontol A Biol Sci Med Sci. 2018;73((12)):1653–1660. doi: 10.1093/gerona/gly011. [DOI] [PubMed] [Google Scholar]
- 92.Belloni G, Cesari M. Frailty and intrinsic capacity two distinct but related constructs. Front Med. 2019;6:133. doi: 10.3389/fmed.2019.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Theou O, Cann L, Blodgett J, Wallace LMK, Brothers TD, Rockwood K. Modifications to the frailty phenotype criteria systematic review of the current literature and investigation of 262 frailty phenotypes in the survey of health, ageing, and retirement in europe. Ageing Res Rev. 2015;21:78–94. doi: 10.1016/j.arr.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 94.Karunananthan S, Bergman H. Managing frailty in primary care evidence gaps cannot be ignored. CMAJ. 2018;190((38)):E1122–E1123. doi: 10.1503/cmaj.181151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Walston J, Bandeen-Roche K, Buta B, Bergman H, Gill TM, Morley JE, et al. Moving frailty toward clinical practice NIA intramural frailty science symposium summary. J Am Geriatr Soc. 2019;67((8)):1559–1564. doi: 10.1111/jgs.15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Doody P, Asamane EA, Aunger JA, Swales B, Lord JM, Greig CA, et al. The prevalence of frailty and pre-frailty among geriatric hospital inpatients and its association with economic prosperity and healthcare expenditure a systematic review and meta-analysis of 467, 779 geriatric hospital inpatients. Ageing Res Rev. 2022;80:101666. doi: 10.1016/j.arr.2022.101666. [DOI] [PubMed] [Google Scholar]
- 97.Bernabei R, Landi F, Calvani R, Cesari M, Del Signore S, Anker SD, et al. Multicomponent intervention to prevent mobility disability in frail older adults randomised controlled trial (SPRINTT project) BMJ. 2022;377:e068788. doi: 10.1136/bmj-2021-068788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.He B, Ma Y, Wang C, Jiang M, Geng C, Chang X, et al. Prevalence and risk factors for frailty among community-dwelling older people in China a systematic review and meta-analysis. J Nutr Health Aging. 2019 May;23((5)):442–450. doi: 10.1007/s12603-019-1179-9. [DOI] [PubMed] [Google Scholar]
- 99.Siriwardhana DD, Hardoon S, Rait G, Weerasinghe MC, Walters KR. Prevalence of frailty and prefrailty among community-dwelling older adults in low-income and middle-income countries a systematic review and meta-analysis. BMJ open. 2018;8((3)):e018195. doi: 10.1136/bmjopen-2017-018195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kojima G, Iliffe S, Taniguchi Y, Shimada H, Rakugi H, Walters K. Prevalence of frailty in Japan a systematic review and meta-analysis. J Epidemiol. 2017 Aug;27((8)):347–353. doi: 10.1016/j.je.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Verlaan S, Ligthart-Melis GC, Wijers SLJ, Cederholm T, Maier AB, de van. der Schueren MAE High prevalence of physical frailty among community-dwelling malnourished older Adults–A systematic review and meta-analysis. J Am Med Dir Assoc. 2017 May 1;18((5)):374–382. doi: 10.1016/j.jamda.2016.12.074. [DOI] [PubMed] [Google Scholar]
- 102.Handforth C, Clegg A, Young C, Simpkins S, Seymour MT, Selby PJ, et al. The prevalence and outcomes of frailty in older cancer patients a systematic review. Ann Oncol. 2015;26((6)):1091–101. doi: 10.1093/annonc/mdu540. [DOI] [PubMed] [Google Scholar]
- 103.Hewitt J, Moug SJ, Middleton M, Chakrabarti M, Stechman MJ, McCarthy K, et al. Prevalence of frailty and its association with mortality in general surgery. Am J Surg. 2015;209((2)):254–259. doi: 10.1016/j.amjsurg.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 104.Kojima G. Prevalence of frailty in nursing homes a systematic review and meta-analysis. J Am Med Dir Assoc. 2015;16((11)):940–945. doi: 10.1016/j.jamda.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 105.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons a systematic review. J Am Geriatr Soc. 2012;60((8)):1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 106.Theou O, Stathokostas L, Roland KP, Jakobi JM, Patterson C, Vandervoort AA, et al. The effectiveness of exercise interventions for the management of frailty a systematic review. J Aging Res. 2011;2011:569194. doi: 10.4061/2011/569194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ofori-Asenso R, Chin KL, Mazidi M, Zomer E, Ilomaki J, Zullo AR, et al. Global incidence of frailty and prefrailty among community-dwelling older adults a systematic review and meta-analysis. JAMA Netw Open. 2019;2((8)):e198398. doi: 10.1001/jamanetworkopen.2019.8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Doody P, Lord JM, Whittaker AC. Assessing the feasibility and impact of an adapted resistance training intervention aimed at improving the multi-dimensional health and functional capacity of frail older adults in residential care settings protocol for a feasibility study. Pilot Feasibility Stud. 2019;5:86. doi: 10.1186/s40814-019-0470-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shah SM, Carey IM, Harris T, DeWilde S, Cook DG. Quality of chronic disease care for older people in care homes and the community in a primary care pay for performance system retrospective study. BMJ. 2011;342:d912. doi: 10.1136/bmj.d912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ligthart-Melis GC, Luiking YC, Kakourou A, Cederholm T, Maier AB, de van der Schueren MAE. Frailty and malnutrition frequently (co-)occur in hospitalized older adults a systematic review and meta-analysis. J Am Med Dir Assoc. 2020 Sep;21((9)):1216–1228. doi: 10.1016/j.jamda.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 111.Theou O, Squires E, Mallery K, Lee JS, Fay S, Goldstein J, et al. What do we know about frailty in the acute care setting? A scoping review. BMC Geriatr. 2018 Jun 11;18((1)):139. doi: 10.1186/s12877-018-0823-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kojima G, Iliffe S, Walters K. Frailty index as a predictor of mortality a systematic review and meta-analysis. Age Ageing. 2018;47((2)):193–200. doi: 10.1093/ageing/afx162. [DOI] [PubMed] [Google Scholar]
- 113.Kojima G. Frailty as a predictor of future falls among community-dwelling older people a systematic review and meta-analysis. J Am Med Dir Assoc. 2015;16((12)):1027–1033. doi: 10.1016/j.jamda.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 114.Soysal P, Veronese N, Thompson T, Kahl KG, Fernandes BS, Prina AM, et al. Relationship between depression and frailty in older adults a systematic review and meta-analysis. Ageing Res Rev. 2017;36:78–87. doi: 10.1016/j.arr.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 115.Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment a review of the evidence and causal mechanisms. Ageing Res Rev. 2013;12((4)):840–851. doi: 10.1016/j.arr.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 116.Kojima G, Taniguchi Y, Iliffe S, Walters K. Frailty as a predictor of alzheimer disease vascular dementia and all dementia among community-dwelling older people a systematic review and meta-analysis. J Am Med Dir Assoc. 2016;17((10)):881–888. doi: 10.1016/j.jamda.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 117.Kojima G. Frailty as a predictor of fractures among community-dwelling older people a systematic review and meta-analysis. Bone. 2016;90:116–22. doi: 10.1016/j.bone.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 118.Jin HY, Liu X, Xue QL, Chen S, Wu C. The association between frailty and healthcare expenditure among Chinese older adults. J Am Med Dir Assoc. 2020;21((6)):780–785. doi: 10.1016/j.jamda.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 119.Kim DH, Glynn RJ, Avorn J, Lipsitz LA, Rockwood K, Pawar A, et al. Validation of a claims-based frailty index against physical performance and adverse health outcomes in the health and retirement study. J Gerontol A Biol Sci Med Sci. 2019;74((8)):1271–1276. doi: 10.1093/gerona/gly197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ensrud KE, Kats AM, Schousboe JT, Taylor BC, Cawthon PM, Hillier TA, et al. Frailty phenotype and healthcare costs and utilization in older women. J Am Geriatr Soc. 2018;66((7)):1276–1283. doi: 10.1111/jgs.15381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bock JO, König HH, Brenner H, Haefeli WE, Quinzler R, Matschinger H, et al. Associations of frailty with health care costs–results of the ESTHER cohort study. BMC Health Serv Res. 2016;16((1)):128. doi: 10.1186/s12913-016-1360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hajek A, Bock JO, Saum KU, Matschinger H, Brenner H, Holleczek B, et al. Frailty and healthcare costs longitudinal results of a prospective cohort study. Age Ageing. 2018;47((2)):233–241. doi: 10.1093/ageing/afx157. [DOI] [PubMed] [Google Scholar]
- 123.Kojima G, Iliffe S, Jivraj S, Walters K. Association between frailty and quality of life among community-dwelling older people a systematic review and meta-analysis. J Epidemiol Community Health. 2016;70((7)):716–721. doi: 10.1136/jech-2015-206717. [DOI] [PubMed] [Google Scholar]
- 124.Hoogendijk EO, Suanet B, Dent E, Deeg DJH, Aartsen MJ. Adverse effects of frailty on social functioning in older adults results from the longitudinal aging study amsterdam. Maturitas. 2016;83:45–50. doi: 10.1016/j.maturitas.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 125.Theou O, Brothers TD, Rockwood MR, Haardt D, Mitnitski A, Rockwood K. Exploring the relationship between national economic indicators and relative fitness and frailty in middle-aged and older europeans. Age Ageing. 2013 Sep;42((5)):614–619. doi: 10.1093/ageing/aft010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Landi F, Abbatecola AM, Provinciali M, Corsonello A, Bustacchini S, Manigrasso L, et al. Moving against frailty does physical activity matter? Biogerontology. 2010;11((5)):537–545. doi: 10.1007/s10522-010-9296-1. [DOI] [PubMed] [Google Scholar]
- 127.Peterson MJ, Giuliani C, Morey MC, Pieper CF, Evenson KR, Mercer V, et al. Physical activity as a preventative factor for frailty the health, aging, and body composition study. J Gerontol A Biol Sci Med Sci. 2009;64((1)):61–68. doi: 10.1093/gerona/gln001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fiatarone MA, O'Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330((25)):1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 129.Arrieta H, Rezola-Pardo C, Gil SM, Virgala J, Iturburu M, Antón I, et al. Effects of multicomponent exercise on frailty in Long-term nursing homes a randomized controlled trial. J Am Geriatr Soc. 2019;67((6)):1145–1151. doi: 10.1111/jgs.15824. [DOI] [PubMed] [Google Scholar]
- 130.Ng TP, Feng L, Nyunt MSZ, Feng L, Niti M, Tan BY, et al. Nutritional and combination interventions and frailty reversal among older adults a randomized controlled trial. Am J Med. 2015;128((111225)):>1225.e1–>1236.e1. doi: 10.1016/j.amjmed.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 131.Tarazona-Santabalbina FJ, Gómez-Cabrera MC, Pérez-Ros P, Martínez-Arnau FM, Cabo H, Tsaparas K, et al. A multicomponent exercise intervention that reverses frailty and improves cognition and social networking in the community-dwelling frail elderly a randomized clinical trial. J Am Med Dir Assoc. 2016;17((5)):426–433. doi: 10.1016/j.jamda.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 132.McPhee JS, French DP, Jackson D, Nazroo J, Pendleton N, Degens H. Physical activity in older age perspectives for healthy ageing and frailty. Biogerontology. 2016;17((3)):567–580. doi: 10.1007/s10522-016-9641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fried LP. Interventions for human frailty physical activity as a model. Cold Spring Harb Perspect Med. 2016;6((6)):a025916. doi: 10.1101/cshperspect.a025916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Scheerman K, Raaijmakers K, Otten RHJ, Meskers CGM, Maier AB. Effect of physical interventions on physical performance and physical activity in older patients during hospitalization a systematic review. BMC Geriatr. 2018;18((1)):288–213. doi: 10.1186/s12877-018-0965-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.He J, Morales DR, Guthrie B. Exclusion rates in randomized controlled trials of treatments for physical conditions a systematic review. Trials. 2020;21((1)):228–211. doi: 10.1186/s13063-020-4139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nagai K, Miyamato T, Okamae A, Tamaki A, Fujioka H, Wada Y, et al. Physical activity combined with resistance training reduces symptoms of frailty in older adults a randomized controlled trial. Arch Gerontol Geriatr. 2018;76:41–7. doi: 10.1016/j.archger.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 137.Romera-Liebana L, Orfila F, Segura JM, Real J, Fabra ML, Möller M, et al. Effects of a primary care-based multifactorial intervention on physical and cognitive function in frail elderly individuals a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2018;73((12)):1688–1674. doi: 10.1093/gerona/glx259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Serra-Prat M, Sist X, Domenich R, Jurado L, Saiz A, Roces A, et al. Effectiveness of an intervention to prevent frailty in pre-frail community-dwelling older people consulting in primary care a randomised controlled trial. Age Ageing. 2017;46((3)):401–407. doi: 10.1093/ageing/afw242. [DOI] [PubMed] [Google Scholar]
- 139.Arrieta H, Rezola-Pardo C, Zarrazquin I, Echeverria I, Yanguas JJ, Iturburu M, et al. A multicomponent exercise program improves physical function in long-term nursing home residents a randomized controlled trial. Exp Gerontol. 2018;103:94–100. doi: 10.1016/j.exger.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 140.Ferreira CB, Teixeira PS, Alves dos Santos G, Dantas Maya AT, Americano do Brasil P, Souza VC, et al. Effects of a 12-week exercise training program on physical function in institutionalized frail elderly. J Aging Res. 2018;2018:7218102. doi: 10.1155/2018/7218102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Doody P, Lord JM, Greig CA, Whittaker AC. Assessing the feasibility and impact of specially adapted exercise interventions aimed at improving the multi-dimensional health and functional capacity of frail geriatric hospital inpatients protocol for a feasibility study. BMJ Open. 2019;9((11)):e031159. doi: 10.1136/bmjopen-2019-031159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu J, Reijnierse EM, Ancum J, Verlaan S, Meskers C, Maier AB. Vol. 111. NJ, USA: WILEY; 2018. Acute inflammation is associated with lower muscle strength muscle mass and dependency in activities of daily living in male hospitalised older patients; p. p. 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Khandelwal D, Goel A, Kumar U, Gulati V, Narang R, Dey AB. Frailty is associated with longer hospital stay and increased mortality in hospitalized older patients. J Nutr Health Aging. 2012;16((8)):732–735. doi: 10.1007/s12603-012-0369-5. [DOI] [PubMed] [Google Scholar]
- 144.Hao Q, Zhou L, Dong B, Yang M, Dong B, Weil Y. The role of frailty in predicting mortality and readmission in older adults in acute care wards a prospective study. Sci Rep. 2019;9((1)):1207. doi: 10.1038/s41598-018-38072-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Martínez-Velilla N, Casas-Herrero A, Zambom-Ferraresi F, Sáez de Asteasu ML, Lucia A, Galbete A, et al. Effect of exercise intervention on functional decline in very elderly patients during acute hospitalization a randomized clinical trial. JAMA Intern Med. 2019;179((1)):28–36. doi: 10.1001/jamainternmed.2018.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.McCullagh R, O'Connell E, O'Meara S, Dahly D, O'Reilly E, O'Connor K, et al. Augmented exercise in hospital improves physical performance and reduces negative post hospitalization events a randomized controlled trial. BMC Geriatr. 2020;20((1)):46–11. doi: 10.1186/s12877-020-1436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McCullagh R, Dillon C, Dahly D, Horgan NF, Timmons S. Walking in hospital is associated with a shorter length of stay in older medical inpatients. Physiol Meas. 2016;37((10)):1872–1884. doi: 10.1088/0967-3334/37/10/1872. [DOI] [PubMed] [Google Scholar]
- 148.Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation allostatic load and its health consequences: MacArthur studies of successful aging. Arch Intern Med. 1997;157((19)):2259–2268. [PubMed] [Google Scholar]
- 149.Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk MacArthur studies of successful aging. Proc Natl Acad Sci U S A. 2001;98((8)):4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Castagné R, Garès V, Karimi M, Chadeau-Hyam M, Vineis P, Delpierre C, et al. Allostatic load and subsequent all-cause mortality which biological markers drive the relationship? findings from a UK birth cohort. Eur J Epidemiol. 2018;33((5)):441–458. doi: 10.1007/s10654-018-0364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14((10)):R115–R220. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cel. 2013;49((2)):359–367. doi: 10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McCrory C, Fiorito G, Hernandez B, Polidoro S, O'Halloran AM, Hever A, et al. GrimAge outperforms other epigenetic clocks in the prediction of age-related clinical phenotypes and all-cause mortality. The Journals Gerontol Ser A. 2020;76((5)):741–749. doi: 10.1093/gerona/glaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lu AT, Quach A, Wilson JG, Reiner AP, Aviv A, Raj K, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging. 2019;11((2)):303–327. doi: 10.18632/aging.101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging. 2018;10((4)):573–591. doi: 10.18632/aging.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li X, Ploner A, Wang Y, Magnusson PKE, Reynolds C, Finkel D, et al. Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up. Elife. 2020;9:e51507. doi: 10.7554/eLife.51507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Belsky DW, Caspi A, Arseneault L, Baccarelli A, Corcoran DL, Gao X, et al. Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. Elife. 2020;9:e54870. doi: 10.7554/eLife.54870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vollset SE, Goren E, Yuan CW, Cao J, Smith AE, Hsiao T, et al. Fertility and population scenarios for 195 countries and territories from 2017 to 2100 a forecasting analysis for the global burden of disease study. Lancet. 2020 Oct 17;396((10258)):1285–306. doi: 10.1016/S0140-6736(20)30677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]