Abstract
Cervical cancer remains a leading cause of cancer death for women in low- and middle-income countries. The goal of this study was to evaluate screening and triage strategies, including high-resolution microendoscopy (HRME), to detect cervical abnormalities concerning for precancer at the point-of-care. Women (n=1,824) were enrolled at the Instituto de Cáncer de El Salvador. All underwent screening by both human papillomavirus (HPV) testing using careHPV and visual inspection with acetic acid (VIA). Screen-positives, along with 10% of screen-negatives, were invited to return for a follow-up examination that included triage with VIA, colposcopy, and HRME imaging. Biopsies were taken of any abnormalities identified. If no abnormalities were identified, then the worst scoring site by HRME was biopsied. The sensitivities of HPV testing and VIA to screen for cervical intraepithelial neoplasia grade 2 or more severe diagnoses (CIN2+) were 82.1% and 75% (p=0.77), while the specificities were 90.4% and 80.9% (p<0.001) respectively. The sensitivities of VIA, colposcopy, and HRME as triage tests for CIN2+ were 82.1%, 82.1%, and 71.4% respectively (p≥0.38). HRME had a significantly higher specificity (66.7%) than VIA (51.9%) (p<0.001) and colposcopy (53.3%) (p<0.001). When evaluating different theoretical screening and triage strategies, screening with HPV testing followed by triage with HRME would result in more women receiving appropriate care (97%) compared to screening with VIA (75%) or HPV alone (90%). Our findings demonstrate that screening with HPV is superior to VIA, and that triage with HRME imaging increases the specificity of detecting CIN2+ at the point-of-care in a low-resource setting.
Keywords: cervical cancer, high-resolution microendoscopy, human papillomavirus, visual inspection with acetic acid
Introduction
Cervical cancer continues to be one of the leading causes of cancer and cancer-related deaths among women living in low- and middle-income countries (LMICs).1 In 2013, the World Health Organization (WHO) recommended testing with HPV, VIA, or HPV testing followed by VIA for cervical cancer screening.2 In 2018, the WHO called for the elimination of cervical cancer as a public health problem through prophylactic HPV vaccination in pre-adolescent girls and high-quality cervical screening in mid-adult women.3 There are many challenges with the latter in LMICs, including developing sensitive and specific screening algorithms to maximize cancer prevention and minimize harms due to overtreatment, respectively.
Cervical cancer screening in high-resource settings consists of screening with cytology and/or HPV testing. Women who screen positive generally undergo examination with colposcopy and biopsy at a follow-up visit, followed by treatment at a third visit if CIN2+ is detected. In low-resource settings where cytology and HPV testing are not available, screening is sometimes performed with VIA. The advantages of VIA are that it is low-cost ($0.23) and can be done at the point-of-care with immediate results, allowing for a single visit Screen-and-Treat strategy.4 However, the sensitivity of VIA is reported to be modest and the specificity is poor.5, 6 In comparison, HPV testing has a higher reported sensitivity of 89.9% and specificity of 89.9%.7 The WHO has therefore recommended that LMICs implement screening with primary HPV testing; however, many regions continue to screen with VIA alone due to the high cost and resources needed to implement HPV testing.2, 8 Furthermore, triage tests such as colposcopy and biopsy with associated pathology services are not available in many LMICs. Regions using VIA for screening perform immediate treatment in women with a visible lesion on exam (VIA+).9 In LMICs where HPV testing is available, existing triage strategies (colposcopy, cervical biopsy) are not feasible on a large scale and require additional visits resulting in loss to follow-up. Two different strategies are currently used for HPV+ women: 1) triage with VIA and treat all VIA+ women with ablation (cryotherapy or thermoablation); 2) treat all HPV+ women regardless of whether a lesion is present or not.10 Screen-and-Treat strategies using VIA or HPV followed by the treatment of all women who screen positive result in significant overtreatment due to the low specificity of VIA and because the majority of women who are HPV+ will clear the infection and never develop CIN2+.6, 11 There is therefore a significant need for affordable point-of-care triage/diagnostic tests for women who screen positive by VIA or HPV testing.
The purpose of this study was to evaluate screening and triage strategies, including high-resolution microendoscopy (HRME), to detect cervical abnormalities concerning for precancer at the point-of-care in El Salvador. El Salvador is a LMIC in which cervical cancer is the fourth leading cause of cancer and the second leading cause of cancer in women.12 The estimated annual incidence rate is 18.5 per 100,000 women with an associated mortality rate of 9.4 per 100,000 women. HPV testing is available but follow-up triage strategies (colposcopy, cervical biopsy, and associated pathology services) are not available on a large scale.
HRME consists of a low-cost fiber-optic imaging device that can help increase specificity for the detection of CIN2+ by allowing medical providers to visualize the nuclei of cervical epithelial cells in vivo.13–15 The HRME device is equipped with real-time image analysis software to aid in the accurate detection of cervical abnormalities at the point-of-care.16 Recent studies by Hunt et al and Parra et al, demonstrated that using HRME with colposcopy significantly increased the specificity for detecting CIN2+ compared to the use of colposcopy alone.17, 18
In this prospective study, we evaluated the sensitivity, specificity, positive and negative predictive values of different screening and triage strategies to detect CIN2+ at the point-of-care in El Salvador. The modalities evaluated included VIA, HPV DNA testing, and HRME imaging and all were compared to the gold standard of colposcopically directed cervical biopsy. The five different screening and triage strategies evaluated were: 1) Screen with HPV and Treat; 2) Screen with VIA and Treat; 3) Screen with HPV, Triage with VIA, and Treat; 4) Screen with HPV, Triage with HRME, and Treat; and 5) Screen with VIA, Triage with HRME, and Treat.
Materials and Methods
High-Resolution Microendoscopy
HRME has been described in detail previously 16, 18; briefly, it is an in vivo imaging device that employs an optical probe placed on the cervix to allow medical providers to image cervical abnormalities noted during VIA and/or colposcopy revealing information about changes in the morphometry of epithelial nuclei. The HRME device is a low-cost, portable fluorescence microscope; a flexible fiber-optic probe is placed in contact with the cervical tissue to be imaged. HRME is equipped with image analysis software that automatically segments and counts the number of cervical nuclei that are considered abnormal based on pre-determined size and shape criteria. If the number of abnormal nuclei per unit area exceeds a previously established threshold value (120 abnormal nuclei/mm2), the image is classified as HRME positive for high-grade cervical abnormalities, which is defined here as CIN2+; otherwise, the image is classified as HRME negative.
HRME is used following the application of two topically applied contrast agents, proflavine and Lugol’s iodine. Proflavine (0.01%) is a topical antiseptic that fluorescently stains nuclei in cervical epithelial cells; proflavine fluorescence has a maximum excitation near 460 nm and maximum emission near 530 nm. Lugol’s iodine is a strong absorbing dye used in visual inspection (VILI) and colposcopy; regions of columnar tissue and precancerous lesions do not retain Lugol’s iodine. We have previously shown that using proflavine in combination with Lugol’s iodine results in high contrast HRME images because the strong absorption of Lugol’s iodine reduces the penetration depth of light in tissue and reduces out-of-focus signal.
Study Design
We conducted a prospective study to evaluate the performance of two strategies to screen women for CIN2+ (VIA and HPV DNA testing) and three strategies to further triage and diagnose CIN2+ (VIA, colposcopy, and HRME) in El Salvador. The study was conducted at the Instituto del Cáncer de El Salvador (El Salvador Cancer Institute, ICES) in San Salvador, El Salvador in collaboration with Basic Health International. The study was approved by the Comité Nacional de Ética de la Investigación en Salud (National Ethics Committee of Health Research, ID: CNEIS/005/2015) in El Salvador and the institutional review boards at The University of Texas MD Anderson Cancer Center (IRB# 2015–0620), Cleveland Clinic (IRB# 15–1162), and Rice University (IRB# 2017–347). The study was registered with clinicaltrials.gov (NCT04472455).
Women were eligible to participate in the study if they 1) were 30 to 49 years in age; 2) were not pregnant or nursing; 3) had an intact cervix; 4) had no history of invasive cervical cancer; and 5) were willing and able to provide written informed consent. Women were excluded if they had undergone a previous loop electrosurgical excision procedure (LEEP), cone and/or cryotherapy.
Visit 1 – Screening
Women who met the inclusion criteria and provided informed consent underwent cervical cancer screening with provider-collected HPV testing and VIA during an initial visit (Visit 1: screening visit). During Visit 1, a cervical sample was collected for HPV testing (QIAGEN careHPV) and VIA was then performed following the application of 5% acetic acid to the surface of the cervix. Any visible abnormalities detected were noted and the VIA screening result was recorded as positive or negative. The VIA exam was performed by either a local general physician or a local general obstetrician/gynecologist at the ICES with training in VIA. Images of the cervix were taken using a mobile colposcope (MobileODT EVA COLPO).
Each cervical sample for HPV testing was collected using a Qiagen specimen collection brush and placed into careHPV Collection Medium (QIAGEN). Specimens were taken to a local laboratory, Laboritoria Segovia, where they were stored at 8°C for an average of one month prior to being analyzed in batches of 90 samples for high-risk HPV DNA using the careHPV system. If storage ran beyond a month, then specimens were stored at −20°C until the analysis could be run. The careHPV test detects 14 high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) using hybrid-capture technology and chemiluminescent detection. All careHPV testing was performed according to the manufacturers’ instructions.
Visit 2 – Triage and Diagnosis
If a woman had a positive HPV and/or VIA test, she was invited back for a second visit (Visit 2: triage and diagnostic visit). In addition, 10% of women who screened negative (HPV- and VIA-), were invited back for a second visit. The clinical exam at Visit 2 was performed by a local obstetrician/gynecologist at the ICES with expertise in colposcopy. Dilute (5%) acetic acid was applied to the surface of the cervix and any abnormalities observed by VIA were noted. A standard colposcope or mobile colposcope (MobileODT EVA COLPO) was then used to perform colposcopy. A mobile colposcope was used to capture images of the cervix. Any abnormalities detected during colposcopy were noted along with the physician’s clinical impression of the abnormality (benign, low-grade precancer, high-grade precancer, or cancer). 0.01% proflavine was applied before and after the application of Lugol’s iodine to stain cervical nuclei for HRME imaging. HRME images were obtained of each abnormality noted during VIA and/or colposcopy along with one normal area of the cervix. All visual abnormalities were biopsied. The visibly normal area was biopsied if abnormal by HRME. If there were no visible abnormalities during the clinical exam, then an HRME image was taken of each quadrant of the cervix and the worst scoring area by HRME was biopsied, regardless of whether the score was abnormal or normal. An endocervical curettage (ECC) was performed if indicated per standard of care.
Histopathology and Follow-up
All pathology specimens underwent standard processing with hematoxylin and eosin staining as per standard procedure. All cervical biopsies and ECCs were reviewed by two expert pathologists: the local institutional pathologist at the ICES and a second pathologist from the United States. Each specimen was classified as being normal/benign (diagnoses including normal, negative, and inflammation), CIN1, CIN2, CIN3, adenocarcinoma in situ (AIS), or cancer. Discrepant results were resolved by a third pathologist from the United States, with the final result being based on 2/3 agreement. If all three pathologists arrived at different diagnoses, all three met in person to review the specimen and reach a consensus diagnosis. Patients were treated or scheduled for follow-up based on the final histopathology results per standard of care.
Statistical analysis
The final histopathology diagnosis was used as the study endpoint to assess the performance of all screening and triage tests. The positivity rate for each screening method (HPV testing and VIA) was calculated by dividing the total number who screened positive by the total number who had completed Visit 1. The rate of CIN2+ was calculated by dividing the number who had been diagnosed with CIN2+ by the total number who had completed Visit 1. The positivity rates for each screening method and the rate of CIN2+ were calculated throughout the duration of the study.
The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), with binomial exact 95% confidence intervals (95% CI), of each screening method and each triage method (VIA, colposcopy, and HRME) to detect for CIN2+ were calculated on a per-patient basis. In this analysis, the final HPV result, the worst impression by VIA, colposcopy, and HRME were compared to the worst histopathology result for that patient. A colposcopic impression of low-grade precancer and more severe was used as the threshold for positivity when evaluating the diagnostic performance of colposcopy. McNemar’s exact test was used to compare the sensitivity and specificity values between different screening and triage methods. When the number of discordant pairs exceeded 20, the generalized score statistic proposed by Leisenring et al. (2000) was used to compare PPV and NPV values.19 Differences were considered significant at p<0.05.
Data were used to compare the theoretical performance of five different strategies to screen and immediately identify women requiring treatment, including: 1) Screen with HPV and Treat; 2) Screen with VIA and Treat; 3) Screen with HPV, Triage with VIA, and Treat; 4) Screen with HPV, Triage with HRME, and Treat; and 5) Screen with VIA, Triage with HRME, and Treat. For each strategy, study results were used to track the number of women with biopsy-proven CIN2+ who would have been referred for treatment (number appropriately treated) and who would have not been referred for treatment (number missed), as well as the number of women with biopsy-proven <CIN2 who would have been referred for treatment (number over-treated) and who would have not been referred for treatment (number appropriately not treated). Finally, we calculated the number of women who would have received appropriate care (appropriately treated and appropriately not treated).
Results
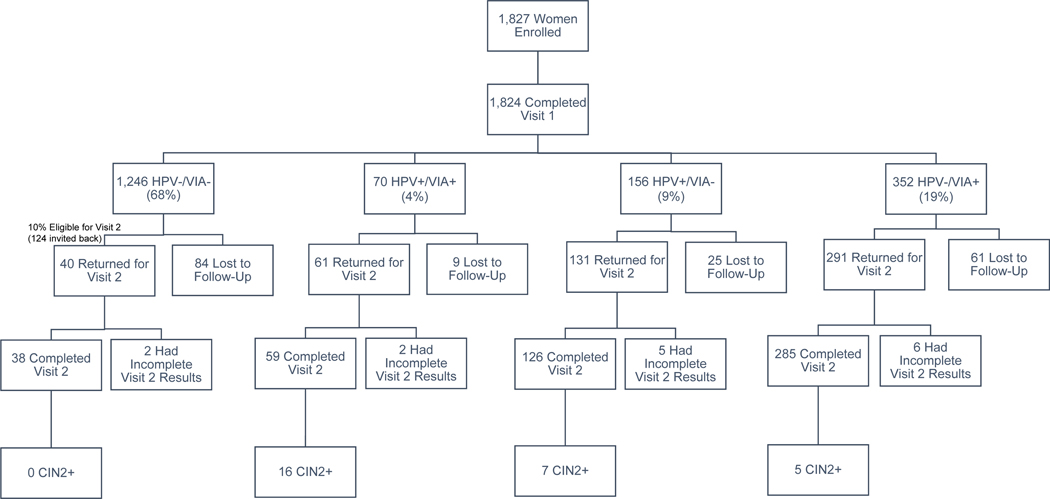
A total of 1,827 women were enrolled in the study. A flowchart mapping their participation and distribution in the study is shown in Figure 1. Of the women enrolled, three women did not complete Visit 1. Of the 1,824 women who completed Visit 1, 708 were invited to return for Visit 2: all the women who screened positive (n=578) and 10% of the women who screened negative (n=124). Of those invited back, 508 of 708 women (72%) returned and completed Visit 2. Of the 508 women who completed Visit 2, 28 were diagnosed with CIN2+ (1.5% of the screened population). The final histopathology diagnoses for all those that completed Visit 2 are summarized in Table 1.
Figure 1:
Flowchart outlining the number of women that completed each part of the study stratified by their initial screening results at Visit 1.
Table 1.
Number of women who completed Visit 2 stratified by final pathology diagnosis and initial screening test results.
| Initial Screening Results | |||||
|---|---|---|---|---|---|
| HPV−/VIA− (n=38) | HPV+/VIA+ (n=59) | HPV+/VIA− (n=126) | HPV−/VIA+ (n=285) | ||
| Final Pathology Diagnosis | <CIN2 (n=480) | 38 (100%) | 43 (73%) | 119 (94%) | 280 (98%) |
| CIN2 (n=6) | 0 (0%) | 4 (7%) | 2 (2%) | 0 (0%) | |
| CIN3 (n=21) | 0 (0%) | 12 (20%) | 5 (4%) | 4 (1.5%) | |
| AIS (n=1) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.5%) | |
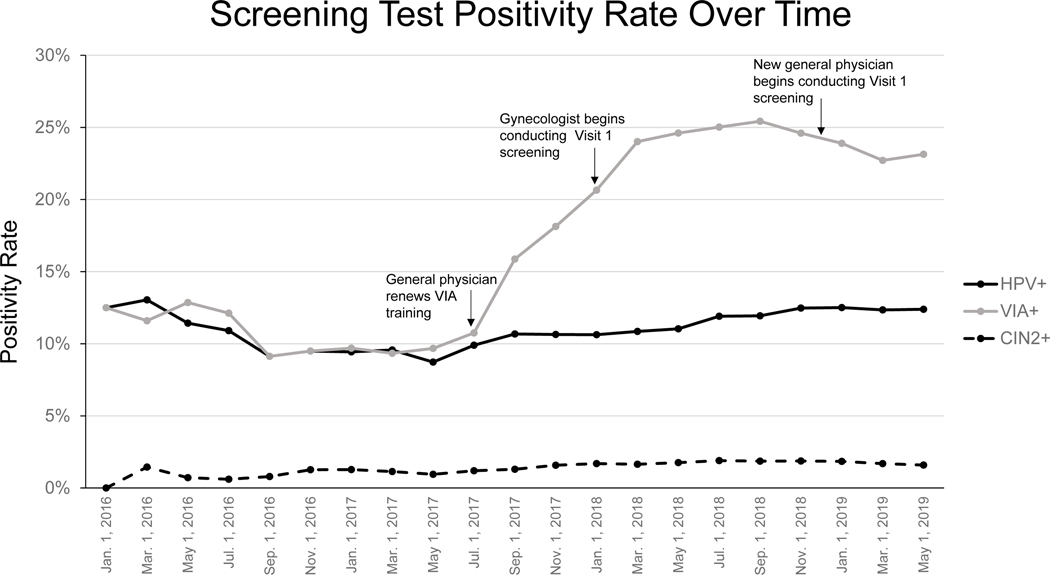
Figure 2 shows the overall positivity rate of HPV and VIA screening at Visit 1 throughout the duration of the study compared to the overall rate of CIN2+ diagnosed by histopathology. The proportion of women diagnosed with CIN2+ was relatively stable between 1–2% throughout the study period. The HPV positivity rate also remained consistent throughout the study period, ranging between 9–13%. However, the VIA positivity rate fluctuated widely. It was between 9–13% for the first 18 months and then steadily increased to 20–25% for the last 17 months of the study period. As shown in the figure, these fluctuations coincided with additional training and/or a new physician joining the study.
Figure 2:
Cumulative positivity rate of HPV and VIA screening compared to the cumulative rate of CIN2+ over the study’s duration. The positivity rate for each was calculated by dividing the total number who screened positive or were diagnosed by CIN2+ by the total number who had completed Visit 1 by the date specified. Significant dates corresponding to changes in clinic personnel or physician training are indicated.
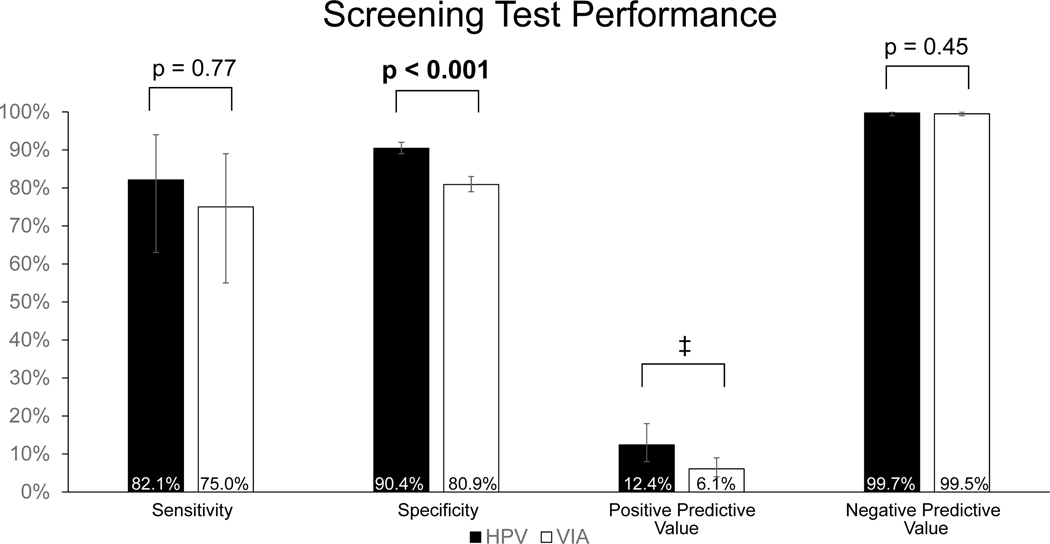
Figure 3 shows the sensitivity, specificity, PPV, and NPV for HPV testing and VIA as screening modalities to detect CIN2+. This analysis was based on the 508 women that completed Visit 2 and the 1,208 women that screened negative by both HPV and VIA and did not complete Visit 2. For the analysis, those that screened negative by both HPV and VIA and did not complete Visit 2 were assumed to be <CIN2. There was no significant difference in sensitivity between HPV testing (82.1%) and VIA (75.0%) to detect women with CIN2+ (p=0.77). However, the specificity of HPV testing (90.4%) was significantly higher than that of VIA (80.9%) (p<0.001). The PPV (12.4%) and NPV (99.7%) of HPV testing were also higher than that of VIA, which had a PPV of 6.1% and NPV of 99.5%. However, there were too few discordant pairs to calculate a significant difference in PPV and there was no significant difference in NPV (p=0.45).
Figure 3:
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of HPV and VIA screening to detect women with biopsy proven CIN2+. Analysis included the 508 women with complete screening and pathology results and the 1208 women that screened negative by HPV and VIA and are assumed to be <CIN2. Error bars represent 95% exact binomial confidence intervals. ‡Number of discordant pairs was less than or equal to 20.
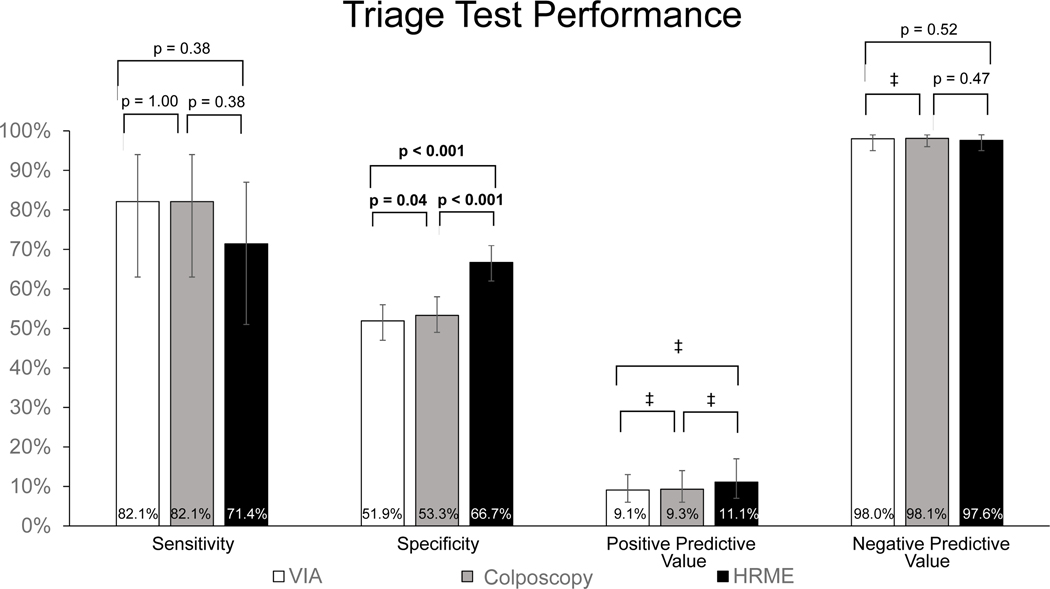
Figure 4 shows the sensitivity, specificity, PPV, and NPV for VIA, colposcopy, and HRME as triage modalities to detect CIN2+ in the 508 women who completed Visit 2. There were no significant differences between the sensitivities of VIA (82.1%), colposcopy (82.1%) and HRME (71.4%) (p≥0.38). However, the specificity of HRME (66.7%) was significantly higher than that of VIA (51.9%, p<0.001) and colposcopy (54.4%, p<0.001). Of note, the specificity of colposcopy (53.3%) was also significantly higher than VIA (51.9%, p=0.04). HRME had the highest PPV (11.1%) in comparison to VIA (9.1%) and colposcopy (9.3%), but there were too few discordant pairs to calculate a significant difference. The NPV (97.6%) for HRME was not significantly lower than VIA (98.0%, p=0.52) and colposcopy (98.1%, p=0.47).
Figure 4:
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of VIA, colposcopy, and HRME to immediately diagnose women with biopsy proven CIN2+. Analysis included the 508 women who completed Visit 2. Error bars represent 95% exact binomial confidence intervals. ‡Number of discordant pairs was less than or equal to 20.
Table 2 compares the number of women that would have been appropriately treated/not treated, missed, and over-treated based on the five theoretical Screen-and-Treat or Screen-Triage-Treat strategies. The values tabulated are based on the actual screening, triage and histopathology results for the 508 women that completed Visit 2 and the 1,208 women that screened negative by both HPV and VIA and are assumed to be <CIN2. A Screen-and-Treat strategy based on HPV DNA testing detects the most cases of CIN2+ (23/28, 82%), but over-treats 10% of disease-free women (162/1688). Screen-and-Treat based on VIA detects a similar number of cases (21/28, 75%) but substantially increases the number of women over-treated (323/1688, 19%). A Screen-Triage-Treat strategy based on sequential combination of HPV DNA testing and VIA detects slightly fewer cases (19/28, 68%) but reduces the number of women who are over-treated (63/1688, 4%). A Screen-Triage-Treat strategy combining HPV DNA testing with HRME detects 16/28 cases (57%) and results in the lowest number of women who are over-treated (43/1688, 3%). In comparison, combining VIA screening with HRME detects the fewest number of cases of CIN2+ (14/28, 50%) and results in a higher number of women receiving unnecessary treatment (124/1688, 7%). Of these different theoretical strategies, a Screen-Triage-Treat strategy combining HPV DNA testing with HRME would have resulted in the most women receiving appropriate care (1661/1716, 97%).
Table 2.
Number of women who would have been appropriately treated, appropriately not treated, missed or over-treated for CIN 2+ vs. point-of-care detection method.
| True Positives Number of Women with CIN2+ Diagnosed and Appropriately Treated (out of 28 women total with CIN2+) | False Negatives Number of Women with CIN2+ Missed (out of 28 women total with CIN2+) | False Positives Number of Women Over-treated (out of 1688 women without CIN2+) | True Negatives Number of Women Appropriately Not Treated (out of 1688 women without CIN2+) | Number of Women who Received Appropriate Care (out of 1716 women) | ||
|---|---|---|---|---|---|---|
| Screening Test | Triage Test | |||||
| HPV | - | 23 (82%) | 5 (18%) | 162 (10%) | 1526 (90%) | 1549 (90%) |
| VIA | - | 21 (75%) | 7 (25%) | 323 (19%) | 1365 (81%) | 1386 (81%) |
| HPV | VIA | 19 (68%) | 9 (32%) | 63 (4%) | 1625 (96%) | 1644 (96%) |
| HPV | HRME | 16 (57%) | 12 (43%) | 43 (3%) | 1645 (97%) | 1661 (97%) |
| VIA | HRME | 14 (50%) | 14 (50%) | 124 (7%) | 1564 (93%) | 1578 (92%) |
Discussion
The accurate detection and treatment of high-grade cervical abnormalities is important to prevent cervical cancer. For LMICs without access to HPV and cytology screening, the WHO recommends using VIA as a screening strategy. However, studies have shown VIA to be highly subjective and have poor specificity, resulting in the overtreatment of significant numbers of women when using Screen-and-Treat programs based on VIA. Our results are consistent with these findings; over the duration of the study, the positivity rate of VIA testing fluctuated widely following changes in the study physician or when the study physician received additional training. In contrast, the rate of HPV positivity and CIN2+ remained consistent. A meta-analysis by Driscoll et al.20 revealed that provider type was a significant predictor of VIA sensitivity and components of provider training were significant predictors of VIA sensitivity and specificity. Again, consistent with other studies21, we found that the specificity of HPV testing was significantly higher than VIA, and the results of this study support the use of HPV testing in LMICs to more accurately screen for cervical cancer.
Our evaluation of triage tests showed that HRME had a significantly higher specificity than colposcopy and VIA alone. Like VIA, colposcopy is a subjective test that depends on the skills and experience of the provider.22, 23 In contrast, HRME offers an objective way to evaluate cervical abnormalities by incorporating automatic image analysis software and obviating the need for biopsy and pathology services.
Screen-and-Treat strategies allow women to be screened and treated for high-grade cervical abnormalities within the same clinic visit.24 This approach to cervical cancer prevention is ideal for low-resource areas where women who screen positive at a first visit can be lost to follow-up when asked to return for a second triage visit and then a third treatment visit. In this study we compared the theoretical results of five different Screen-and-Treat/Screen-Triage-Treat strategies. Of these strategies, HPV screening followed by immediate treatment yielded the highest number of women appropriately treated for CIN2+, however HPV screening alone led to 162 (10%) of disease-free women being inappropriately treated. Adding a triage step with HRME led to seven fewer cases of CIN2+ being detected, but decreased the number of over-treated women to 43 (3%) and resulted in the greatest number of women receiving appropriate care (97%) of the five strategies compared.
The strengths of this study include that it provides a direct comparison of different screening and triage methods, including a novel triage strategy based on HRME to identify women with CIN2+ in a LMIC. Due to the large number of patients being enrolled in the study without disease, we are able to compare the specificity of HRME to those of VIA and colposcopy with a power of 100% (α=0.05).
A limitation of this study was the number of CIN2+ cases (n=28), which resulted in a study power of 9.6% (α=0.05) when comparing the sensitivities of different triage strategies. Future work evaluating HRME will focus on prescreened populations to ensure an adequate number of CIN2+ cases are included to draw more statistically significant conclusions on sensitivity and on developing better image analysis algorithms that can more accurately detect CIN2+.
Novelty and Impact:
The high-resolution microendoscope (HRME) is an in vivo imaging device that allows medical providers to further examine and triage cervical abnormalities to detect cervical precancer at the point-of-care. This study evaluated different screening and triage strategies to detect cervical precancer in El Salvador. The results demonstrate the superiority of HPV screening in comparison to VIA, and that triage with HRME increases the specificity of detecting cervical precancer in a low-resource setting.
Acknowledgements:
The authors would like to thank all the physicians and medical personnel at the Instituto del Cáncer de El Salvador that made this study possible, including Dr. Silvia Bennett, Dr. Maria Calderon, Dr. Claudia Polanco, and Dr. Brenda Ramirez. Special thanks to Alexa Juarez from Rice University, Dr. Melvin Rodriguez from Basic Health International El Salvador, and Jessica Gallegos, Ana Lopez, Cindy Melendez, Juana Rayo and Mark Munsell from The University of Texas MD Anderson Cancer Center for their assistance in data collection and coordinating study efforts. Funding for this study was provided by NIH R01 CA186132-01. Dr. Parra would also like to acknowledge the support of the Baylor College of Medicine Medical Scientist Training Program.
Dr. Castle has received HPV tests and assays at a reduced or no cost for research from Roche, Becton Dickinson, Cepheid, and Arbor Vita Corporation. Dr. Cremer is the President/Founder of BHI and sits on the Merck Advisory Board and Speakers Bureau. Dr. Richards-Kortum is an inventor on patents related to optical diagnostic technologies that have been licensed to Remicalm LLC.
Abbreviation List
- AIS
adenocarcinoma in situ
- CI
confidence intervals
- CIN
cervical intraepithelial neoplasia
- ECC
endocervical curettage
- HPV
human papillomavirus
- HRME
high-resolution microendoscopy
- ICES
Instituto del Cáncer de El Salvador
- LEEP
loop electrosurgical excision procedure
- LMICs
low- and middle-income countries
- NPV
negative predictive value
- PPV
positive predictive value
- VIA
visual inspection with acetic acid
- VILI
visual inspection with Lugol’s iodine
- WHO
World Health Organization
Footnotes
Conflict of Interest: All other authors have no conflicts of interest to disclose.
Ethics Statement: This study was approved by the Comité Nacional de Ética de la Investigación en Salud (National Ethics Committee of Health Research, ID: CNEIS/005/2015) in El Salvador and the institutional review boards at The University of Texas MD Anderson Cancer Center (IRB# 2015-0620), Cleveland Clinic (IRB# 15-1162), and Rice University (IRB# 2017-347). Written informed consent was obtained from all eligible participants prior to enrollment. The study was registered with clinicaltrials.gov (NCT04472455).
Data Availability Statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.American Cancer Society. Global cancer facts & figures. 3rd ed. Atlanta: American Cancer Society; 2015. [Google Scholar]
- 2.World Health Organization. WHO guidelines for screening and treatment of precancerous lesions for cervical cancer prevention. South Africa: World Health Organization; 2013. [PubMed] [Google Scholar]
- 3.Cervical cancer: An NCD we can overcome. World health organization; 2018. [Google Scholar]
- 4.Perkins RB, Langrish SM, Stern LJ, Burgess JF, Simon CJ. Impact of patient adherence and test performance on the cost-effectiveness of cervical cancer screening in developing countries: The case of honduras. Womens Health Issues 2010. Jan-Feb;20(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ardahan M, Temel AB. Visual inspection with acetic acid in cervical cancer screening. Cancer Nurs 2011. Mar-Apr;34(2):158–63. [DOI] [PubMed] [Google Scholar]
- 6.Mustafa RA, Santesso N, Khatib R, Mustafa AA, Wiercioch W, Kehar R, Gandhi S, Chen Y, Cheung A, Hopkins J, et al. Systematic reviews and meta-analyses of the accuracy of HPV tests, visual inspection with acetic acid, cytology, and colposcopy. Int J Gynaecol Obstet 2016. Mar;132(3):259–65. [DOI] [PubMed] [Google Scholar]
- 7.Koliopoulos G, Nyaga VN, Santesso N, Bryant A, Martin-Hirsch PP, Mustafa RA, Schunemann H, Paraskevaidis E, Arbyn M. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst Rev 2017. Aug 10;8:CD008587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta R, Gupta S, Mehrotra R, Sodhani P. Cervical cancer screening in resource-constrained countries: Current status and future directions. Asian Pac J Cancer Prev 2017. Jun 25;18(6):1461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paul P, Winkler JL, Bartolini RM, Penny ME, Huong TT, Nga le T, Kumakech E, Mugisha E, Jeronimo J. Screen-and-treat approach to cervical cancer prevention using visual inspection with acetic acid and cryotherapy: Experiences, perceptions, and beliefs from demonstration projects in peru, uganda, and vietnam. Oncologist 2013;18(12):1278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Salvador Ministry of Health. Lineamientos técnicos para la prevención y control del cáncer cérvico uterino y de mama. San Salvador, El Salvador; 2015. [Google Scholar]
- 11.Rositch AF, Koshiol J, Hudgens MG, Razzaghi H, Backes DM, Pimenta JM, Franco EL, Poole C, Smith JS. Patterns of persistent genital human papillomavirus infection among women worldwide: A literature review and meta-analysis. Int J Cancer 2013. Sep 15;133(6):1271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.GLOBOCAN 2018: El Salvador [Internet]; c2018 [cited 2020 May 25]. Available from: https://gco.iarc.fr/today/data/factsheets/populations/222-el-salvador-fact-sheets.pdf.
- 13.Pierce M, Yu D, Richards-Kortum R. High-resolution fiber-optic microendoscopy for in situ cellular imaging. J Vis Exp 2011. Jan 11;(47). pii: 2306. doi(47): 10.3791/2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pierce MC, Guan Y, Quinn MK, Zhang X, Zhang WH, Qiao YL, Castle P, Richards-Kortum R. A pilot study of low-cost, high-resolution microendoscopy as a tool for identifying women with cervical precancer. Cancer Prev Res (Phila) 2012. Nov;5(11):1273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinn MK, Bubi TC, Pierce MC, Kayembe MK, Ramogola-Masire D, Richards-Kortum R. High-resolution microendoscopy for the detection of cervical neoplasia in low-resource settings. PLoS One 2012;7(9):e44924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quang T, Schwarz RA, Dawsey SM, Tan MC, Patel K, Yu X, Wang G, Zhang F, Xu H, Anandasabapathy S, et al. A tablet-interfaced high-resolution microendoscope with automated image interpretation for real-time evaluation of esophageal squamous cell neoplasia. Gastrointest Endosc 2016. Nov;84(5):834–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunt B, Fregnani JHTG, Schwarz RA, Pantano N, Tesoni S, Possati-Resende JC, Antoniazzi M, de Oliveira Fonseca B, de Macedo Matsushita G, Scapulatempo-Neto C, et al. Diagnosing cervical neoplasia in rural brazil using a mobile van equipped with in vivo microscopy: A cluster-randomized community trial. Cancer Prev Res (Phila) 2018. Jun;11(6):359–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parra SG, Rodriguez AM, Cherry KD, Schwarz RA, Gowen RM, Guerra LB, Milbourne AM, Toscano PA, Fisher-Hoch SP, Schmeler KM, et al. Low-cost, high-resolution imaging for detecting cervical precancer in medically-underserved areas of texas. Gynecol Oncol 2019. Jul 6. [DOI] [PMC free article] [PubMed]
- 19.Leisenring W, Alonzo T, Pepe MS. Comparisons of predictive values of binary medical diagnostic tests for paired designs. Biometrics 2000. Jun;56(2):345–51. [DOI] [PubMed] [Google Scholar]
- 20.Driscoll SD, Tappen RM, Newman D, Voege-Harvey K. Accuracy of visual inspection performed by community health workers in cervical cancer screening. Int J Gynaecol Obstet 2018. Sep;142(3):260–9. [DOI] [PubMed] [Google Scholar]
- 21.Toliman PJ, Kaldor JM, Badman SG, Gabuzzi J, Silim S, Kumbia A, Kombuk B, Kombati Z, Munnull G, Guy R, et al. Performance of clinical screening algorithms comprising point-of-care HPV-DNA testing using self-collected vaginal specimens, and visual inspection of the cervix with acetic acid, for the detection of underlying high-grade squamous intraepithelial lesions in papua new guinea. Papillomavirus Res 2018. Dec;6:70–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mustafa MS, Jindal AK, Singh P. Visual inspection using acetic acid for cervical cancer in low resource settings. Med J Armed Forces India 2010. Oct;66(4):382–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massad LS, Jeronimo J, Schiffman M, National Institutes of Health/American Society for Colposcopy and Cervical Pathology (NIH/ASCCP) Research Group. Interobserver agreement in the assessment of components of colposcopic grading. Obstet Gynecol 2008. Jun;111(6):1279–84. [DOI] [PubMed] [Google Scholar]
- 24.Santesso N, Mustafa RA, Schunemann HJ, Arbyn M, Blumenthal PD, Cain J, Chirenje M, Denny L, De Vuyst H, Eckert LO, et al. World health organization guidelines for treatment of cervical intraepithelial neoplasia 2–3 and screen-and-treat strategies to prevent cervical cancer. Int J Gynaecol Obstet 2016. Mar;132(3):252–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.