Abstract
BACKGROUND
The benefits and risks of augmenting or switching antidepressants in older adults with treatment-resistant depression have not been extensively studied.
METHODS
We conducted a two-step, open-label trial involving adults 60 years of age or older with treatment-resistant depression. In step 1, patients were randomly assigned in a 1:1:1 ratio to augmentation of existing antidepressant medication with aripiprazole, augmentation with bupropion, or a switch from existing antidepressant medication to bupropion. Patients who did not benefit from or were ineligible for step 1 were randomly assigned in step 2 in a 1:1 ratio to augmentation with lithium or a switch to nortriptyline. Each step lasted approximately 10 weeks. The primary outcome was the change from baseline in psychological well-being, assessed with the National Institutes of Health Toolbox Positive Affect and General Life Satisfaction subscales (population mean, 50; higher scores indicate greater well-being). A secondary outcome was remission of depression.
RESULTS
In step 1, a total of 619 patients were enrolled; 211 were assigned to aripiprazole augmentation, 206 to bupropion augmentation, and 202 to a switch to bupropion. Well-being scores improved by 4.83 points, 4.33 points, and 2.04 points, respectively. The difference between the aripiprazole-augmentation group and the switch-to-bupropion group was 2.79 points (95% CI, 0.56 to 5.02; P = 0.014, with a prespecified threshold P value of 0.017); the between-group differences were not significant for aripiprazole augmentation versus bupropion augmentation or for bupropion augmentation versus a switch to bupropion. Remission occurred in 28.9% of patients in the aripiprazole-augmentation group, 28.2% in the bupropion-augmentation group, and 19.3% in the switch-to-bupropion group. The rate of falls was highest with bupropion augmentation. In step 2, a total of 248 patients were enrolled; 127 were assigned to lithium augmentation and 121 to a switch to nortriptyline. Well-being scores improved by 3.17 points and 2.18 points, respectively (difference, 0.99; 95% CI, −1.92 to 3.91). Remission occurred in 18.9% of patients in the lithium-augmentation group and 21.5% in the switch-to-nortriptyline group; rates of falling were similar in the two groups.
CONCLUSIONS
In older adults with treatment-resistant depression, augmentation of existing antidepressants with aripiprazole improved well-being significantly more over 10 weeks than a switch to bupropion and was associated with a numerically higher incidence of remission. Among patients in whom augmentation or a switch to bupropion failed, changes in well-being and the occurrence of remission with lithium augmentation or a switch to nortriptyline were similar. (Funded by the Patient-Centered Outcomes Research Institute; OPTIMUM ClinicalTrials.gov number, NCT02960763.)
Major depression is common in older adults1 and often persists despite appropriate treatment with first-line antidepressants.2 Treatment-resistant depression is typically defined as depression that does not remit despite two adequate trial uses of antidepressant medications3; in older adults, treatment failure is associated with decreased psychological well-being,4 disability,5 and cognitive decline.6–8 Pharmacologic strategies for treatment-resistant depression include augmentation, in which a medication is added to an existing antidepressant, and the replacement of an antidepressant with one from a different class (“switching”). The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial showed that augmenting with, or switching to, bupropion was as effective as or more effective than other strategies.9,10 In a randomized trial involving older adults, augmentation with aripiprazole was more effective than with placebo for reducing depression.11 In the Veterans Affairs Augmentation and Switching Treatments for Improving Depression Outcomes (VAST-D) trial, augmentation with either aripiprazole or bupropion was slightly more effective than a switch to bupropion, 12 but there are limited large comparative-effectiveness studies involving older adults with treatment-resistant depression that would clarify treatment strategies.
There is increasing awareness of the importance of involving patients in the design of clinical trials.13 In a survey involving older adults with treatment-resistant depression, patient stakeholders recommended psychological well-being as an outcome that matters.14 Psychological well-being encompasses satisfaction, happiness, cognitive engagement, meaning, and purpose.15 There is also limited understanding of the comparative safety of antidepressant strategies in older adults,16 including risks of falls,17–21 cardiovascular risks,22 and risk of death23 with different agents used in trials. According to expert opinion, augmentation may lead to more adverse effects and a greater risk of drug interactions.24 There are also safety concerns with respect to using lithium or nortriptyline, approaches to treatment-resistant depression that are used in older adults.25,26 The current trial, Optimizing Outcomes of Treatment-Resistant Depression in Older Adults (OPTIMUM), was designed to investigate the benefits and risks of augmentation as compared with switching strategies for treatment-resistant depression in older adults.27
Methods
Trial Design and Oversight
The OPTIMUM trial was a pragmatic, investigator-initiated trial funded by the Patient-Centered Outcomes Research Institute (PCORI). Its design and procedures have been described previously,27 and the protocol is available with the full text of this article at NEJM.org. The trial had two steps. In step 1, patients were randomly assigned to augmentation of their current antidepressant with aripiprazole or bupropion or a switch to bupropion. Patients who did not have remission or otherwise perceive a benefit from their step 1 treatment or were ineligible for step 1 were randomly assigned in step 2 to augmentation with lithium or a switch to nortriptyline. These treatment options were recommended in surveys of clinicians who treat older adults with treatment-resistant depression.28 We undertook a multistep approach because lithium and nortriptyline are complicated to use, requiring laboratory monitoring and exclusions for cardiac or renal disease. Two years into the trial, at the request of the funder, the protocol was modified to disallow direct entry to step 2 and to increase the threshold for eligibility with respect to the score on the nine-item Patient Health Questionnaire (PHQ-9). Patients received medication from their local pharmacy in an open-label fashion, paid for through insurance or out of pocket. Discussion of the costs that were associated with participation was included in the informed-consent form. Patients and investigators were aware of the trial-group assignments, but outcome assessors were not.
The trial was conducted at five sites — Washington University in St. Louis (coordinating site); Columbia University; the University of California, Los Angeles; the University of Pittsburgh; and the University of Toronto. The institutional review board at each site approved the trial. All the patients provided informed consent before enrollment. An independent data and safety monitoring board governed the trial. The trial was conducted in accordance with the Good Clinical Practice guidelines of the International Council for Harmonisation. The authors vouch for the completeness and accuracy of the data and for the fidelity of the trial to the protocol. There was no commercial involvement in the trial.
Patients and Recruitment
Trial patients were 60 years of age or older and had treatment-resistant depression, defined as a lack of remission of major depression after two or more trial uses of antidepressants of adequate dose and duration within the current episode, which was determined by research staff with the use of the PHQ-9 (scores range from 0 to 27, with higher scores indicating greater severity of symptoms). Initially, a score of 6 or more was required for participation, and this was later changed by amendment to 10 or more. Patients had to be receiving one adequately dosed antidepressant at the time of trial enrollment. Full eligibility criteria are provided in the Supplementary Appendix (available at NEJM.org) and the protocol. Patients were recruited by referrals from primary care providers, office advertisements, outreach from the trial team, automated alerts in electronic medical records29 (see the Supplementary Appendix), referrals from psychiatrists, and print, radio, and social media advertising.
Randomization and Trial Groups
In step 1, patients were randomly assigned in a 1:1:1 ratio to augmentation of their existing medication with aripiprazole (starting at 2.5 mg per day and increasing to a maximum of 15 mg per day) (aripiprazole-augmentation group), augmentation of their existing medication with extended-release bupropion (starting at 150 mg per day, with a target of 300 mg per day and a maximum of 450 mg per day) (bupropion-augmentation group), or a taper of their current antidepressant and a switch to extended-release bupropion (same dose as the bupropion-augmentation group) (switch-to-bupropion group). In step 2, patients who did not have remission in step 1 or who were not eligible for step 1 (typically because they had already had a trial of bupropion or aripiprazole) were randomly assigned in a 1:1 ratio to augmentation of their existing antidepressant with lithium (starting at 150 or 300 mg per day, depending on coexisting health conditions and concomitant medications, and increasing to a maximum of 1200 mg per day, with a targeted drug level of 0.6 mmol per liter) (lithium-augmentation group) or a taper of their current antidepressant and a switch to nortriptyline (starting at 25 mg per day, increasing to 1 mg per kilogram of body weight, and targeting a drug level of 80 to 120 ng per milliliter) (switch-to-nortriptyline group). Dose adjustments were made largely on the basis of PHQ-9 scores through recommendations (not obligatory) from the trial research team to treating clinicians.
Both steps used a randomized block design. In step 1, patients were stratified according to the site from which they received their depression care (primary care vs. specialty mental health), age (<70 vs. ≥70 years), and trial institution site; in step 2, patients were stratified according to their step 1 randomization assignment. Patients and investigators were aware of the trial-group assignments, and there was no placebo group.
Patients were followed with calls or in-person visits every other week with a trial clinician, who assessed depression severity using the PHQ-9, as well as adherence to medication and the occurrence of adverse events, in order to provide guidance to the managing provider to adjust the trial medication on the basis of symptoms and side effects (details are provided in the protocol). If preferred by the provider, a trial psychiatrist, instead of the managing provider, could prescribe the trial medication. Each step was 10 weeks in duration, with up to 10 additional weeks allowed to accommodate any delays in initiating treatment changes and assessing outcomes; the median duration was approximately 11 to 12 weeks. The methods of transition between step 1 and step 2 were designed to resemble real-world care; guidance on the speed of tapering of step 1 medications is provided in the Supplementary Appendix.
Outcomes
The effectiveness and safety outcomes were chosen to reflect the stakeholder-driven trial design. The primary effectiveness outcome was psychological well-being, assessed at the beginning and end of each step on the basis of patient report with the use of the National Institutes of Health Toolbox Emotion Battery subscales for Positive Affect and General Life Satisfaction; we calculated a combined T score of the average of these two subscales (normative population mean, 50; with higher scores indicating greater well-being).15
Secondary effectiveness outcomes included remission from depression, changes from the beginning to the end of each step in the score on the Montgomery–Asberg Depression Rating Scale (MADRS; range, 0 to 60, with higher scores indicating greater depression), and changes in social participation and physical function on the basis of the Patient-Reported Outcomes Measurement Information System (PROMIS) scales (mean [±SD] T score, 50±10; with higher scores indicating greater participation or function). Remission was defined as a score of 10 or less on the MADRS at the end of each 10-week step, as assessed by research staff who were trained to use a structured manual30 and who were unaware of the trial-group assignments. When it was not feasible to obtain a MADRS rating because the patient could not be contacted, remission was considered to have occurred if the PHQ-9 score was 5 or less at the week 10 visit. Patients who discontinued the trial before the end of either step were considered to have not had remission.
The primary safety outcomes were falls, including fall-related injuries, and serious adverse events (defined as life-threatening illness, hospitalization, disability or permanent damage, or death). During phone assessments every other week, patients were queried about falls since the last assessment (defined as “a fall, including a slip or trip in which you lost your balance and landed on the floor or ground or lower level,” with choices of 0, 1, 2, or ≥3 falls) and whether falls resulted in an injury (including minor bruising, cuts, or severe injury). Patients were also systematically queried about serious adverse events and adverse effects.
Statistical Analysis
The sample size was adjusted mid-trial because recruitment targets would not be met. Recruitment was stopped on September 21, 2021, short of the original target enrollment of 1500 patients into step 1; therefore, a new power calculation was performed under the assumption of 195 patients in each step 1 group and 124 patients in each step 2 group. This sample would provide the trial with 80% power to detect a between-group difference of 2.6 points in psychological well-being scores for step 1. Details of the revised power calculation are provided in the Supplementary Appendix.
Analyses were conducted according to the intention-to-treat principle. Site and randomization stratification variables were covariates in all the analyses. Psychological well-being was compared with a repeated-measures analysis of variance with time-by-trial-group contrasts comparing changes across pairs of trial groups in step 1. A Benjamini–Hochberg step-down procedure was used to control for the multiple comparisons. If the lowest of the three P values was less than 0.017 (0.05 ÷ 3), it was considered to be significant, and the second lowest P value was considered to be significant if less than 0.025 (0.05 ÷ 2). If both were significant, then the third P value was considered to be significant if less than 0.05. The percentages of patients with remission were compared with generalized linear models with a Poisson link function to estimate risk ratios.31 To handle missing data for MADRS scores at week 10, we considered a PHQ-9 score of 5 or less to indicate remission since the last visit. On the basis of the prespecified definition of remission, when both an MADRS score and a PHQ-9 score at week 10 were unavailable because the step was discontinued prematurely, the patient was considered to have not had remission. Missing values for continuous variables were estimated with the use of multiple imputation with other variables collected at the visit. The widths of the confidence intervals for between-group differences in secondary outcomes were not adjusted for multiple comparisons, and no definite conclusions can be drawn from these results. A sensitivity analysis was conducted by means of multiple imputation for remission that used variables from the baseline visit and the week 10 visit as well as baseline variables.
Rates of falls over approximately a 10-week period were compared with a repeated-measures generalized linear model with a Poisson link function; factors were trial group and time (week 2, 4, 6, 8, or 10). The model included all stratification variables and fall history at baseline. Pairwise comparisons between trial groups were computed. Serious adverse events were compared with Cox models of time to event with Anderson and Gill extensions for repeated events. In the safety analyses (falls and serious adverse events), a P value of less than 0.05 was considered to indicate statistical significance. All statistical analyses were conducted with the use of SAS software, version 9.4.
Results
Enrollment and Patient Characteristics
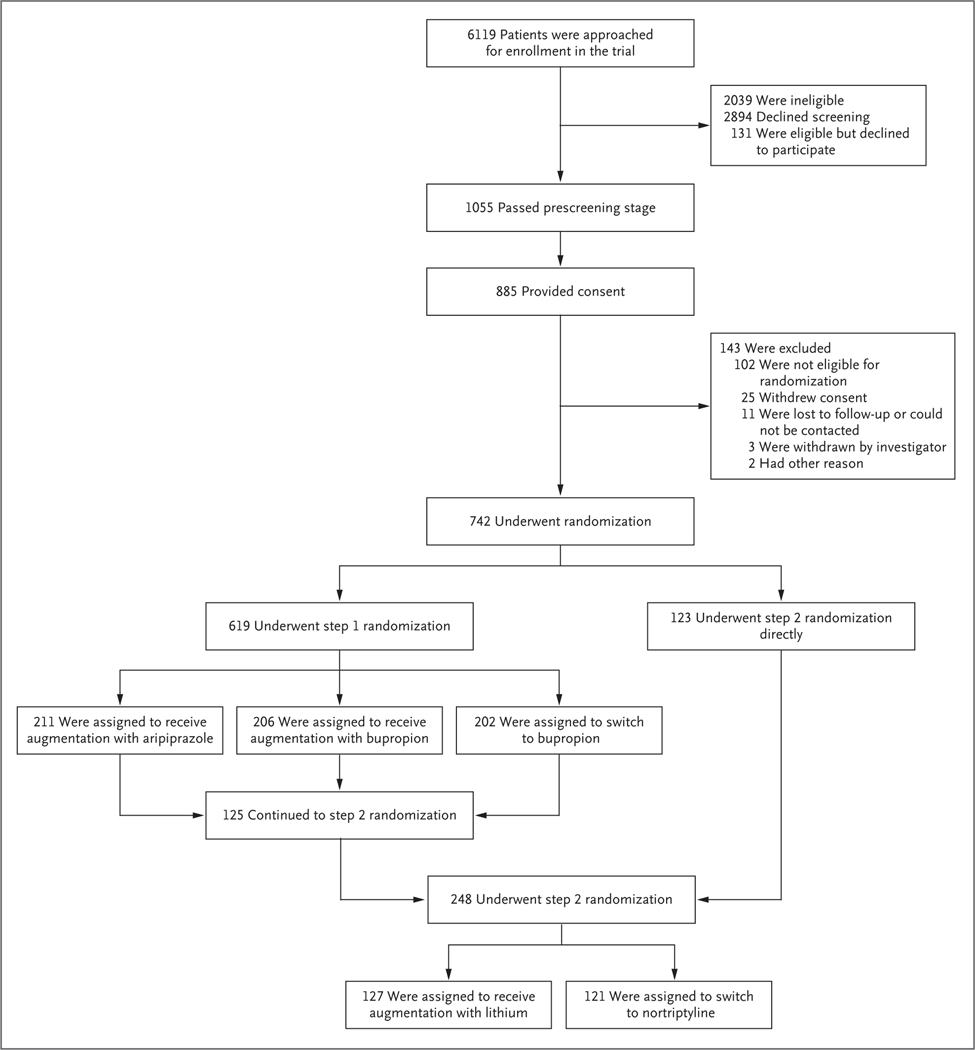
From February 22, 2017, through December 31, 2019, a total of 742 patients were enrolled and assigned to a trial group: 619 in step 1, representing approximately half the originally anticipated enrollment (1500), and 248 in step 2 (125 moved from step 1 to step 2, and 123 were directly enrolled into step 2, the former chiefly because of a previous failed step 1 treatment) (Fig. 1). Full details about the trial flow in steps 1 and 2 are provided in the Supplementary Appendix.
Figure 1. Enrollment and Randomization in Step 1 and Step 2.
Additional details regarding the trial flow of each step are provided in the Supplementary Appendix.
In step 1, the mean age of the patients was 69.3 years; 66.7% were female, 84.3% were White, and 7.4% were Black. The mean number of previous antidepressant trials was 2.3. In step 2, the mean age of the patients was 68.5 years; 69.8% were female, 89.5% were White, and 4.4% were Black. The mean number of previous antidepressant trials was 2.5. Baseline characteristics were similar across the groups (Table 1). The representativeness of the trial population with respect to the population of persons with late-life depression is shown in Table S1 in the Supplementary Appendix. Table S2 shows the existing antidepressant medications (at the time of randomization) in each trial group. In step 1, approximately 70% of the patients were adherent to aripiprazole augmentation and bupropion augmentation, but approximately 40% were adherent to the use of bupropion alone. In step 2, approximately 50% were adherent to medication in each group (Table S7).
Table 1.
Demographic and Clinical Characteristics of the Patients at Baseline.*
| Characteristic | Step 1 | Step 2 | |||
|---|---|---|---|---|---|
| Aripiprazole-Augmentation Group (N = 211) | Bupropion-Augmentation Group (N = 206) | Switch-to-Bupropion Group (N = 202) | Lithium-Augmentation Group (N = 127) | Switch-to-Nortriptyline Group (N = 121) | |
| Age — yr | 69.1±6.5 | 69.1±7.1 | 69.7±7.7 | 69.0±6.0 | 68.0±5.7 |
| Female sex — no. (%) | 144 (68.2) | 142 (68.9) | 127 (62.9) | 90 (70.9) | 83 (68.6) |
| Race — no. (%)† | |||||
| White | 173 (82.0) | 174 (84.5) | 175 (86.6) | 116 (91.3) | 106 (87.6) |
| Black | 16 (7.6) | 17 (8.3) | 13 (6.4) | 7 (5.5) | 4 (3.3) |
| Other | 22 (10.4) | 15 (7.3) | 14 (6.9) | 4 (3.1) | 11 (9.1) |
| Ethnic group — no. (%)† | |||||
| Hispanic or Latino | 22 (10.4) | 17 (8.3) | 13 (6.4) | 3 (2.4) | 10 (8.3) |
| Other | 189 (89.6) | 189 (91.7) | 189 (93.6) | 124 (97.6) | 111 (91.7) |
| Education | |||||
| No. of patients evaluated | 208 | 203 | 198 | 121 | 120 |
| Mean — yr | 14.4±3.0 | 14.4±3.0 | 15.1±2.8 | 15.3±2.6 | 14.6±2.8 |
| PHQ-9 score‡ | 16.2±4.2 | 15.9±4.1 | 15.2±4.4 | 14.4±4.3 | 14.4±4.4 |
| No. of adequate trials of antidepressant treatment§ | 2.3±0.8 | 2.2±0.7 | 2.4±0.9 | 2.5±0.9 | 2.6±1.1 |
| Age at first onset of MDD | |||||
| No. of patients evaluated | 192 | 182 | 186 | 101 | 103 |
| Mean — yr | 30.5±19.1 | 34.5±21.3 | 33.0±20.3 | 30.3±18.8 | 29.0±19.3 |
| CIRS-G total score¶ | |||||
| No. of patients evaluated | 207 | 206 | 201 | 127 | 121 |
| Mean score | 8.8±4.9 | 8.7±4.7 | 8.7±4.7 | 8.3±4.4 | 8.1±4.1 |
| Falls during past 6 mo — no./total no. (%) | |||||
| 0 | 117/208 (56.2) | 130/204 (63.7) | 117/199 (58.8) | 76/127 (59.8) | 79/120 (65.8) |
| ≥1 | 91/208 (43.8) | 74/204 (36.3) | 82/199 (41.2) | 51/127 (40.2) | 41/120 (34.2) |
Plus–minus values are means ±SD. Percentages may not total 100 because of rounding. MDD denotes major depressive disorder.
Race and ethnic group were reported by the patients.
Scores on the nine-item Patient Health Questionnaire (PHQ-9) range from 0 to 27, with higher scores indicating greater severity of symptoms.
Adequate trials met criteria for minimal adequate dose and treatment duration. Psychotherapy treatment was not considered to indicate a failed antidepressant trial. Data were collected at trial entry; for step 2 patients, the trial drugs received in step 1 were not included.
The Cumulative Illness Rating Scale–Geriatric (CIRS-G) instrument captures information about the severity of physical problems divided into 14 categories based on body system (e.g., vascular, renal, and neurologic). Ratings for the severity of each category range from 0 to 4, with higher scores indicating greater severity. Scores for each category are added to calculate the total score (range, 0 to 56).
Effectiveness Outcomes
In step 1, increases (improvements) from baseline in the psychological well-being T score were 4.83 points (95% confidence interval [CI], 3.28 to 6.38) in the aripiprazole-augmentation group, 4.33 (95% CI, 2.76 to 5.91) in the bupropion-augmentation group, and 2.04 (95% CI, 0.43 to 3.66) in the switch-to-bupropion group. The difference in the change from baseline in psychological well-being between the aripiprazole-augmentation group and the switch-to-bupropion group was 2.79 points (95% CI, 0.56 to 5.02); the P value of 0.014 was the lowest P value for the three between-group comparisons in the step-down procedure and was lower than the prespecified P value of 0.017 and therefore was significant (Cohen’s d, 0.37; 95% CI, 0.07 to 0.67). The difference between the bupropion-augmentation group and the switch-to-bupropion group was 2.29 points (95% CI, 0.01 to 4.57); the P value of 0.049 was above the prespecified threshold of 0.025 and therefore was not significant. The difference between the aripiprazole-augmentation group and the bupropion-augmentation group was 0.50 points (95% CI, −1.69 to 2.69) and was not significant because of the failure of the step-down hierarchical procedure. In step 2, improvements in the psychological well-being T score were 3.17 points (95% CI, 1.12 to 5.22) in the lithium-augmentation group and 2.18 (95% CI, 0.10 to 4.26) in the switch-to-nortriptyline group (difference, 0.99; 95% CI, −1.92 to 3.91). Table S3 shows scores on each subscale.
Changes in MADRS scores and remission in both steps are shown in Table 2. In step 1, changes from baseline in MADRS scores were −7.60 (95% CI, −9.20 to −5.99) in the aripiprazole-augmentation group, −7.23 (95% CI, −8.86 to −5.59) in the bupropion-augmentation group, and −4.14 (95% CI, −5.81 to −2.48) in the switch-to-bupropion group. The percentage of patients with remission was 28.9% in the aripiprazole-augmentation group (risk ratio vs. the switch-to-bupropion group, 1.50; 95% CI, 1.06 to 2.13), 28.2% in the bupropion-augmentation group (risk ratio, 1.49; 95% CI, 1.04 to 2.12), and 19.3% in the switch-to-bupropion group (risk ratio, 1.00 [reference]), without correction for multiple comparisons. In step 2, changes in MADRS scores were −4.63 (95% CI, −6.78 to −2.49) in the lithium-augmentation group and −5.33 (95% CI, −7.52 to −3.14) in the switch-to-nortriptyline group. The percentage of patients with remission was 18.9% in the lithium-augmentation group and 21.5% in the switch-to-nortriptyline group (risk ratio, 0.84; 95% CI, 0.53 to 1.36). In both steps, secondary outcomes of changes in PROMIS measures of social participation and physical function were numerically similar in the trial groups (Table 2).
Table 2.
Effectiveness Outcomes.*
| Outcome | Step 1 | Step 2 | |||
|---|---|---|---|---|---|
| Aripiprazole-Augmentation Group (N = 211) | Bupropion-Augmentation Group (N = 206) | Switch-to-Bupropion Group (N = 202) | Lithium-Augmentation Group (N = 127) | Switch-to-Nortriptyline Group (N = 121) | |
| Primary outcome | |||||
| Psychological well-being† | |||||
| Baseline | |||||
| No. of patients evaluated | 183 | 180 | 176 | 113 | 108 |
| Least-squares mean T score (95% CI) | 33.32 (32.23 to 34.42) | 33.68 (32.58 to 34.78) | 33.22 (32.11 to 34.32) | 31.62 (30.16 to 33.09) | 32.42 (30.92 to 33.92) |
| Wk 10 | |||||
| No. of patients evaluated | 170 | 159 | 140 | 96 | 95 |
| Least-squares mean T score (95% CI) | 38.16 (37.02 to 39.29) | 38.02 (36.87 to 39.16) | 35.26 (34.04 to 36.48) | 34.79 (33.21 to 36.38) | 34.60 (33.00 to 36.19) |
| Change from baseline (95% CI) ‡ | 4.83 (3.28 to 6.38) | 4.33 (2.76 to 5.91) | 2.04 (0.43 to 3.66) | 3.17 (1.12 to 5.22) | 2.18 (0.10 to 4.26) |
| Secondary outcomes § | |||||
| Remission¶ | |||||
| No. (%) | 61 (28.9) | 58 (28.2) | 39 (19.3) | 24 (18.9) | 26 (21.5) |
| Risk ratio vs. switch group (95% CI) | 1.50 (1.06 to 2.13) | 1.49 (1.04 to 2.12) | 1.00 (reference) | 0.84 (0.53 to 1.36) | 1.00 (reference) |
| MADRS score | |||||
| Baseline | |||||
| No. of patients evaluated | 203 | 199 | 194 | 126 | 120 |
| Least-squares mean score (95% CI) | 23.55 (22.44 to 24.67) | 22.97 (21.85 to 24.10) | 22.67 (21.53 to 23.81) | 23.61 (22.08 to 25.15) | 24.45 (22.87 to 26.03) |
| Wk 10 | |||||
| No. of patients evaluated | 183 | 175 | 163 | 116 | 108 |
| Least-squares mean score (95% CI) | 15.96 (14.79 to 17.12) | 15.74 (14.55 to 16.94) | 18.53 (17.29 to 19.76) | 18.98 (17.32 to 20.64) | 19.12 (17.44 to 20.80) |
| Change from baseline (95% CI)‖ | −7.60 (−9.20 to−5.99) | −7.23 (−8.86 to −5.59) | −4.14 (−5.81 to −2.48) | −4.63 (−6.78 to −2.49) | −5.33 (−7.52 to−3.14) |
| Social participation** | |||||
| Baseline | |||||
| No. of patients evaluated | 179 | 176 | 174 | 113 | 107 |
| Least-squares mean T score (95% CI) | 41.20 (40.09 to 42.31) | 42.09 (40.97 to 43.22) | 41.17 (40.03 to 42.32) | 42.43 (41.02 to 43.85) | 42.10 (40.65 to 43.56) |
| Wk 10 | |||||
| No. of patients evaluated | 168 | 159 | 140 | 96 | 94 |
| Least-squares mean T score (95% CI) | 44.29 (43.14 to 45.44) | 44.55 (43.40 to 45.70) | 43.12 (41.88 to 44.36) | 43.89 (42.36 to 45.43) | 43.67 (42.12 to 45.22) |
| Change from baseline (95% CI)†† | 3.09 (1.51 to 4.68) | 2.46 (0.86 to 4.06) | 1.95 (0.29 to 3.60) | 1.46 (−0.53 to 3.44) | 1.57 (−0.45 to 3.58) |
| Physical function** | |||||
| Baseline | |||||
| No. of patients evaluated | 183 | 180 | 177 | 113 | 108 |
| Least-squares mean T score (95% CI) | 42.14 (41.01 to 43.27) | 40.93 (39.82 to 42.05) | 41.44 (40.29 to 42.59) | 41.30 (39.83 to 42.76) | 41.46 (39.94 to 42.97) |
| Wk 10 | |||||
| No. of patients evaluated | 170 | 159 | 140 | 97 | 95 |
| Least-squares mean T score (95% CI) | 42.10 (40.95 to 43.25) | 41.46 (40.25 to 42.67) | 41.11 (39.83 to 42.39) | 42.79 (41.21 to 44.37) | 42.45 (40.84 to 44.06) |
| Change from baseline (95% CI)‡‡ | −0.03 (−1.64 to 1.57) | 0.53 (−1.10 to 2.15) | −0.33 (−2.06 to 1.40) | 1.50 (−0.56 to 3.56) | 1.00 (−1.09 to 3.09) |
Shown are best estimates of change (least-squares mean and 95% confidence interval) for each trial group. Observed mean change scores are provided in Table S4.
Scores were calculated as the average T scores of two fixed-length surveys from the National Institutes of Health Toolbox Emotion Battery subscales for Positive Affect and General Life Satisfaction. The normative population mean is 50, with higher scores indicating higher levels of psychological well-being.
In step 1, the difference between the aripiprazole-augmentation group and the switch-to-bupropion group was 2.79 (95% CI, 0.56 to 5.02; P = 0.014, with a threshold P value of 0.017; Cohen’s d, 0.37; 95% CI, 0.07 to 0.67). The difference between the bupropion-augmentation group and the switch-to-bupropion group was 2.29 (95% CI, 0.01 to 4.57; P = 0.05, with a threshold P value of 0.025). The difference between the aripiprazole-augmentation group and the bupropion-augmentation group was 0.50 (95% CI, −1.69 to 2.69; P = 0.66). In step 2, the difference between the lithium-augmentation group and the switch-to-nortriptyline group was 0.99 (95% CI, −1.92 to 3.91).
For secondary outcomes, the widths of the confidence intervals for between-group differences were not adjusted for multiple comparisons, and no definite conclusions can be drawn from these data.
Remission was defined as a reduction in depressive symptoms over a period of 10 weeks with a final Montgomery–Asberg Depression Rating Scale (MADRS) score of 10 or less (range, 0 to 60, with higher scores indicating greater severity of depressive symptoms). If no MADRS score was available but a PHQ-9 score was available, a PHQ-9 score of 5 or less was considered to indicate remission.
In step 1, the between-group differences were as follows: aripiprazole-augmentation group and switch-to-bupropion group, −3.45 (95% CI, −5.76 to −1.14); bupropion-augmentation group and switch-to-bupropion group, −3.08 (95% CI, −5.42 to −0.75); and aripiprazole-augmentation group and bupropion-augmentation group, −0.37 (95% CI, −2.66 to 1.93). In step 2, the between-group difference was 0.70 (95% CI, −2.37 to 3.76).
Scores are based on the Patient-Reported Outcomes Measurement Information System (PROMIS) scales for social participation and physical function. Mean (±SD) T scores are 50±10, with higher scores indicating higher levels of social participation or physical function.
In step 1, the between-group differences were as follows: aripiprazole-augmentation group and switch-to-bupropion group, 1.15 (95% CI, −1.14 to 3.44); bupropion-augmentation group and switch-to-bupropion group, 0.51 (95% CI, −1.74 to 2.77); and aripiprazole-augmentation group and bupropion-augmentation group, 0.63 (95% CI, −1.61 to 2.88). In step 2, the between-group difference was −0.11 (95% CI, −2.94 to 2.72).
In step 1, the between-group differences were as follows: aripiprazole-augmentation group and switch-to-bupropion group, 0.30 (95% CI, −2.03 to 2.62); bupropion-augmentation group and switch-to-bupropion group, 0.86 (95% CI, −1.49 to 3.21); and aripiprazole-augmentation group and bupropion-augmentation group, −0.56 (95% CI, −2.83 to 1.70). In step 2, the between-group difference was 0.50 (95% CI, −2.42 to 3.42).
A post hoc sensitivity analysis was conducted for remission with the use of multiple imputation to account for patients who did not have an MADRS score at week 10. Findings were similar to those for the original analysis but with slightly higher incidences of remission and generally lower relative risks (Table S5). Some patients reported exposure before the trial to one of the step 1 medications; a post hoc sensitivity analysis that excluded those patients did not substantially change the primary findings (Table S6). The results of a post hoc sensitivity analysis that categorized patients according to whether or not they were “adherent” (i.e., started the medication, reached the target dose [e.g., ≥300 mg per day for bupropion], and kept taking it until the end of the step) were similar to those of the intention-to-treat analysis (Table S7). The percentage of patients who both were adherent and had remission was less than 10% in the switch-to-bupropion group in step 1 and the lithium-augmentation group in step 2.
Safety Outcomes
In step 1, fall rates during the acute phase over a period of approximately 10 weeks were 0.33 per patient in the aripiprazole-augmentation group, 0.55 in the bupropion-augmentation group, and 0.38 in the switch-to-bupropion group (Table 3). The risk ratio for falls in the aripiprazole-augmentation group as compared with the bupropion-augmentation group was 0.59 (95% CI, 0.38 to 0.92; P = 0.02), in the aripiprazole-augmentation group as compared with the switch-to-bupropion group was 0.77 (95% CI, 0.49 to 1.22; P = 0.27), and in the bupropion-augmentation group as compared with the switch-to-bupropion group was 1.32 (95% CI, 0.88 to 1.96; P = 0.17). Further details about falls are provided in Table S8. Rates of overall serious adverse events were 0.07 in the aripiprazole-augmentation group (hazard ratio vs. the switch-to-bupropion group, 0.59; 95% CI, 0.31 to 1.11), 0.08 in the bupropion-augmentation group (hazard ratio, 0.61; 95% CI, 0.32 to 1.15), and 0.12 in the switch-to-bupropion group (hazard ratio, 1.00 [reference]), with similar rates of serious adverse events in the three groups.
Table 3.
Safety Outcomes.
| Outcome | Step 1 | Step 2 | |||
|---|---|---|---|---|---|
| Aripiprazole-Augmentation Group (N = 211) | Bupropion-Augmentation Group (N = 206) | Switch-to-Bupropion Group (N = 202) | Lithium-Augmentation Group (N = 127) | Switch-to-Nortriptyline Group (N = 121) | |
| Falls * | |||||
| Rate per patient | 0.33 | 0.55 | 0.38 | 0.47 | 0.38 |
| Total no. of falls† | 70 | 114 | 77 | 60 | 46 |
| No. of injurious falls | 36 | 52 | 38 | 27 | 16 |
| Serious adverse events | |||||
| Rate per patient | 0.07 | 0.08 | 0.12 | 0.10 | 0.09 |
| Total no. of eventsद | 15 | 16 | 24 | 13 | 11 |
| Psychiatric event | 0 | 3 | 0 | 0 | 2 |
| Nonpsychiatric event | 15 | 13 | 24 | 13 | 9 |
| Death | 1‖ | 1** | 1†† | 0 | 0 |
| Relation of events to intervention — no.‡‡ | |||||
| Probably or possibly related | 1 | 7 | 3 | 5 | 4 |
| Not likely to be related | 14 | 9 | 21 | 8 | 7 |
| Adverse events | |||||
| Rate per patient | 2.82 | 2.20 | 2.55 | 2.73 | 3.12 |
| Total no. of events | 596 | 453 | 515 | 347 | 377 |
| Most common adverse events — no. | |||||
| Dizziness or impaired balance | 36 | 41 | 40 | 28 | 21 |
| Gastrointestinal distress | 27 | 35 | 37 | 20 | 20 |
| Reduced salivation | 15 | 30 | 23 | 13 | 51 |
| Tension, inner unrest, or anxiety | 30 | 20 | 29 | 8 | 9 |
| Reduced or disturbed sleep | 39 | 18 | 33 | 6 | 6 |
Falls were assessed during each trial call or visit every other week.
In step 1, the risk ratio for the aripiprazole-augmentation group as compared with the bupropion-augmentation group was 0.59 (95% CI, 0.38 to 0.92; P = 0.02), for the aripiprazole-augmentation group as compared with the switch-to-bupropion group was 0.77 (95% CI, 0.49 to 1.22; P = 0.27), and for the bupropion-augmentation group as compared with the switch-to-bupropion group was 1.32 (95% CI, 0.88 to 1.96; P = 0.17). In step 2, the risk ratio for the lithium-augmentation group as compared with the switch-to-nortriptyline group was 1.22 (95% CI, 0.62 to 2.39; P = 0.57).
In step 1, a total of 55 serious adverse events occurred in 49 patients. In the switch-to-bupropion group, 3 patients had 2 serious adverse events each and 1 patient had 3 serious adverse events. In step 2, a total of 24 serious adverse events occurred in 22 patients. In the lithium-augmentation group, 1 patient had 2 serious adverse events; in the switch-to-nortriptyline group, 1 patient had 2 serious adverse events.
I n step 1, the hazard ratios were 0.59 (95% CI, 0.31 to 1.11) in the aripiprazole-augmentation group, 0.61 (95% CI, 0.32 to 1.15) in the bupropion-augmentation group, and 1.00 (reference) in the switch-to-bupropion group. In step 2, the hazard ratios were 1.30 (95% CI, 0.58 to 2.92) in the lithium-augmentation group and 1.00 (reference) in the switch-to-nortriptyline group.
In step 1, P = 0.93 for the aripiprazole-augmentation group as compared with the bupropion-augmentation group, P = 0.10 for the aripiprazole-augmentation group as compared with the switch-to-bupropion group, and P = 0.13 for the bupropion-augmentation group as compared with the switch-to-bupropion group. In step 2, P = 0.52 for the lithium-augmentation group as compared with the switch-to-nortriptyline group.
One patient died of an unknown cause; this patient had not started randomized treatment.
One serious adverse event was a fall and resulted in death, which was deemed to be related to benzodiazepine and alcohol use.
One operation was followed by a fatal postsurgical pneumonia. This was counted as two serious adverse events. This patient was no longer taking the trial medication (bupropion) at the time of death.
The relationship of the serious adverse event to the intervention was determined by the site principal investigator.
In step 2, fall rates were 0.47 per patient in the lithium-augmentation group and 0.38 in the switch-to-nortriptyline group (risk ratio, 1.22; 95% CI, 0.62 to 2.39; P = 0.57), and rates of serious adverse events were 0.10 and 0.09, respectively (hazard ratio, 1.30; 95% CI, 0.58 to 2.92). The most common nonserious adverse events and their frequency with each treatment strategy are shown in Table 3. Table S9 provides details of all serious adverse events in both steps, with most considered by the site principal investigators to be unrelated to the trial medications. Table S10 lists all adverse events, as well as severity levels; adverse events occurred at a rate of 2.64 per patient across all groups, with similar rates in the augmentation groups and switch groups.
Discussion
This trial compared the risks and benefits of common antidepressant strategies for older adults with treatment-resistant depression over two 10-week periods. The trial examined psychological well-being as the primary effectiveness outcome on the basis of feedback from older adults with depression, who indicated that this was an important issue to them in a survey we conducted to inform the design of this trial.14 There were three key findings. First, augmentation of existing antidepressant with aripiprazole was significantly better with respect to psychological well-being than a switch to bupropion, and the percentage of patients with remission, not adjusted for multiple comparisons, was numerically higher with either aripiprazole augmentation or bupropion augmentation than with a switch to bupropion. Second, bupropion augmentation was numerically similar in effectiveness to aripiprazole augmentation and was associated with a higher rate of falls than aripiprazole augmentation. Third, lithium augmentation and a switch to nortriptyline were similar in effectiveness and safety in a population of patients who did not have a response to their assigned treatment in the first step of the trial or who were not eligible to enter the first step. These results suggest that in the trial population studied, aripiprazole augmentation may have been a better overall antidepressant strategy than bupropion augmentation or a switch to bupropion. The finding that aripiprazole augmentation was more effective than a switch to bupropion is consistent with the findings of previous studies and trials of aripiprazole augmentation for treatment-resistant depression in older adults.11
The low incidences of remission in both steps of the trial highlight the challenge of treating depression when previous medications have failed. For context, the STAR*D trial showed incidences of remission of 13 to 14% after multiple failed trial uses of medication,32 and the VAST-D trial12 involving patients with treatment-resistant depression showed incidences of remission of less than 30% with all treatments tested.12 In our trial, less than 10% of the patients who switched to bupropion or had augmentation with lithium both reached and maintained the target dose and had remission.
The higher rate of falls with bupropion augmentation than with aripiprazole augmentation may be clinically important, because it included many injurious falls. A higher risk of falls with bupropion augmentation than with other strategies has been previously reported in a treatment trial involving patients with late-life depression.21 Even in the lowest fall-risk group (augmentation with aripiprazole), we observed a rate of 0.33, which means one fall for every three patients during approximately 10 weeks of treatment. These findings warrant further examination to inform prevention strategies. With respect to adverse events and serious adverse events, there was no suggestion in the trial results that patients in the augmentation groups were more likely to have an adverse event than those in the switch groups.
There are several limitations to this trial. First, the trial had no placebo group and patients were aware of their trial-group assignments, so we cannot rule out the possibility that patients may have been reacting positively to receiving two drugs rather than one and cannot determine whether any of the treatment strategies was better than no change in pharmacologic treatment. Second, the trial enrolled approximately half its targeted sample; therefore, tests of effectiveness or safety may have been underpowered. Third, each step of the trial lasted 10 weeks, and we cannot assess whether longer exposure to a trial drug would have had different effectiveness or risks. Fourth, adherence to the treatment strategies was in the range of 50 to 70%, which highlights the challenge of managing treatment-resistant depression in real-world settings. Fifth, the number of patients who belonged to traditionally underrepresented racial or ethnic groups was smaller than planned, possibly related to disparities of access.33 Sixth, our findings do not apply to other augmentation and switching options.
This pragmatic trial involving older persons with treatment-resistant depression showed greater improvement in psychological well-being and a numerically higher incidence of remission with aripiprazole augmentation than with a switch to bupropion. Improvement in psychological well-being and incidences of remission were low but similar with lithium augmentation or a switch to nortriptyline after the failure of initial trial treatment.
Supplementary Material
Acknowledgments
Supported by a Patient-Centered Outcomes Research Institute (PCORI) Award (TRD-1511-33321). No in-kind support was received. Dr. Lenze received additional support from the Taylor Family Institute for Innovative Psychiatric Research at Washington University School of Medicine, as well as the Washington University Institute of Clinical and Translational Sciences grant (UL1TR002345) from the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH). Dr. Mulsant received additional support from the Labatt Family Chair in Biology of Depression in Late-Life Adults at the University of Toronto. Dr. Lavretsky received support from grants (K24 AT009198, R01 AT008383, and R01 MH114981) from the NIH. Dr. Brown received additional support from the National Institute of Mental Health OPTIMUM NEURO grant (5R01MH114980).
We thank the patients who participated in this trial, referring providers, the staff of the PCORI, members of the independent data and safety management board (Madhukar Trivedi, M.D. [chair], Mim Schwartz, Warren Taylor, M.D., J. Craig Nelson, M.D., and Jeff Williamson, M.D.), members of the stakeholder advisory board, and the entire OPTIMUM trial team for the coordination of the trial.
Footnotes
References
- 1.Mojtabai R Diagnosing depression in older adults in primary care. N Engl J Med 2014; 370: 1180–2. [DOI] [PubMed] [Google Scholar]
- 2.Hamm ME, Karp JF, Lenard E, et al. “What else can we do?” — provider perspectives on treatment-resistant depression in late life. J Am Geriatr Soc 2022; 70: 1190–7. [DOI] [PubMed] [Google Scholar]
- 3.Berlim MT, Turecki G. What is the meaning of treatment resistant/refractory major depression (TRD)? A systematic review of current randomized trials. Eur Neuropsychopharmacol 2007; 17:696–707. [DOI] [PubMed] [Google Scholar]
- 4.Beekman AT, Penninx BW, Deeg DJ, de Beurs E, Geerling SW, van Tilburg W. The impact of depression on the well-being, disability and use of services in older adults: a longitudinal perspective. Acta Psychiatr Scand 2002; 105: 20–7. [DOI] [PubMed] [Google Scholar]
- 5.Lenze EJ, Rogers JC, Martire LM, et al. The association of late-life depression and anxiety with physical disability: a review of the literature and prospectus for future research. Am J Geriatr Psychiatry 2001; 9: 113–35. [PubMed] [Google Scholar]
- 6.Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol 2011; 7:323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF III. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry 2013;202: 329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry 2006;63: 530–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rush AJ, Trivedi MH, Wisniewski SR, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med 2006; 354:1231–42. [DOI] [PubMed] [Google Scholar]
- 10.Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med 2006; 354: 1243–52. [DOI] [PubMed] [Google Scholar]
- 11.Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised, double-blind, placebo-controlled trial. Lancet 2015; 386: 2404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA 2017; 318: 132–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selby JV, Forsythe L, Sox HC. Stake-holder-driven comparative effectiveness research: an update from PCORI. JAMA 2015; 314:2235–6. [DOI] [PubMed] [Google Scholar]
- 14.Lenze EJ, Ramsey A, Brown PJ, et al. Older adults’ perspectives on clinical research: a focus group and survey study. Am J Geriatr Psychiatry 2016; 24: 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salsman JM, Lai JS, Hendrie HC, et al. Assessing psychological well-being: self-report instruments for the NIH Toolbox. Qual Life Res 2014;23: 205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Correll CU, Detraux J, De Lepeleire J, De Hert M. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry 2015; 14: 119–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60: 616–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flint AJ, Iaboni A, Mulsant BH, et al. Effect of sertraline on risk of falling in older adults with psychotic depression on olanzapine: results of a randomized placebo-controlled trial. Am J Geriatr Psychiatry 2014; 22:332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gebara MA, Lipsey KL, Karp JF, Nash MC, Iaboni A, Lenze EJ. Cause or effect? Selective serotonin reuptake inhibitors and falls in older adults: a systematic review. Am J Geriatr Psychiatry 2015; 23: 1016–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gebara MA, Shea MLO, Lipsey KL, et al. Depression, antidepressants, and bone health in older adults: a systematic review. J Am Geriatr Soc 2014; 62: 1434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joo JH, Lenze EJ, Mulsant BH, et al. Risk factors for falls during treatment of late-life depression. J Clin Psychiatry 2002; 63:936–41. [DOI] [PubMed] [Google Scholar]
- 22.Drye LT, Spragg D, Devanand DP, et al. Changes in QTc interval in the citalopram for agitation in Alzheimer’s disease (CitAD) randomized trial. PLoS One 2014;9 (6): e98426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 2009; 360: 225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brender R, Mulsant BH, Blumberger DM. An update on antidepressant pharmacotherapy in late-life depression. Expert Opin Pharmacother 2021; 22: 1909–17. [DOI] [PubMed] [Google Scholar]
- 25.Crossley NA, Bauer M. Acceleration and augmentation of antidepressants with lithium for depressive disorders: two meta-analyses of randomized, placebo-controlled trials. J Clin Psychiatry 2007; 68:935–40. [DOI] [PubMed] [Google Scholar]
- 26.Köhler-Forsberg O, Larsen ER, Butten-schøn HN, et al. Effect of antidepressant switching between nortriptyline and escitalopram after a failed first antidepressant treatment among patients with major depressive disorder. Br J Psychiatry 2019; 215: 494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cristancho P, Lenard E, Lenze EJ, et al. Optimizing outcomes of treatment-resistant depression in older adults (OPTIMUM): study design and treatment characteristics of the first 396 participants randomized. Am J Geriatr Psychiatry 2019; 27: 1138–52. [DOI] [PubMed] [Google Scholar]
- 28.Arandjelovic K, Eyre HA, Lavretsky H. Clinicians’ views on treatment-resistant depression: 2016 survey reports. Am J Geriatr Psychiatry 2016; 24: 913–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rollman BL, Fischer GS, Zhu F, Bel-nap BH. Comparison of electronic physician prompts versus waitroom case-finding on clinical trial enrollment. J Gen Intern Med 2008; 23: 447–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams JB, Kobak KA. Development and reliability of a structured interview guide for the Montgomery Asberg Depression Rating Scale (SIGMA). Br J Psychiatry 2008; 192: 52–8. [DOI] [PubMed] [Google Scholar]
- 31.Zou G A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159: 702–6. [DOI] [PubMed] [Google Scholar]
- 32.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006;163:1905–17. [DOI] [PubMed] [Google Scholar]
- 33.Vyas CM, Donneyong M, Mischoulon D, et al. Association of race and ethnicity with late-life depression severity, symptom burden, and care. JAMA Netw Open 2020;3 (3): e201606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.