Abstract
2′,3′-Dideoxy-2′,3′-didehydro-β-l(−)-5-fluorocytidine [l(−)Fd4C] was found to be at least 10 times more potent than β-l-2′,3′-dideoxy-3′-thiacytidine [l(−)SddC; also called 3TC, or lamivudine]against hepatitis B virus (HBV) in culture. Its cytotoxicity against HepG2 growth in culture was also greater than that of l(−)SddC (3TC). There was no activity of this compound against mitochondrial DNA synthesis in cells at concentrations up to 10 μM. The dynamics of recovery of virus from the medium of cells pretreated with equal drug concentrations were slower with l(−)Fd4C than with l(−)SddC (3TC). l(−)Fd4C could be metabolized to mono-, di-, and triphosphate forms. The degree of l(−)Fd4C phosphorylation to the 5′-triphosphate metabolite was higher than the degree of l(−)SddC (3TC) phosphorylation when equal extracellular concentrations of the two drugs were used. The apparent Km of l(−)Fd4C phosphorylated metabolites formed intracellularly was higher than that for l(−)SddC (3TC). This may be due in part to a difference in the behavior of l(−)Fd4C and l(−)SddC (3TC) towards cytosolic deoxycytidine kinase. Furthermore, l(−)Fd4C 5′-triphosphate was retained longer within cells than l(−)SddC (3TC) 5′-triphosphate. l(−)Fd4C 5′-triphosphate inhibited HBV DNA polymerase in competition with dCTP with a Ki of 0.069 ± 0.015 μM. Given the antiviral potency and unique pharmacodynamic properties of l(−)Fd4C, this compound should be considered for development as an expanded-spectrum anti-HBV drug.
Hepatitis B virus (HBV) infection is one of the most serious health issues in the world today (1, 3). β-l(−)-2′,3′-Dideoxy-3′-thiacytidine [l(−)SddC; also called 3TC, or lamivudine] (Fig. 1) is the first β-l(−) nucleoside analog identified by us and others to have potent activity against HBV in culture (4, 8, 12, 17). This drug exerts its action by inhibiting HBV DNA synthesis due to the preferential interaction of the l(−)SddC (3TC) 5′-triphosphate metabolite with HBV DNA polymerase (4). Unlike dideoxycytidine (ddC, or zalcitabine), a β-d(+) nucleoside analog with the natural nucleoside conformation in DNA and RNA, l(−)SddC (3TC) does not have potent activity against mitochondrial DNA (mtDNA) synthesis, which is important for maintaining the function of cells (4). Clinical trials of l(−)SddC (3TC) for the treatment of chronic HBV infection are ongoing and look promising (2, 7, 13, 16, 18, 19). Its potential value for HBV-infected patients undergoing liver transplantation is also being evaluated, since l(−)SddC (3TC) can suppress HBV DNA serum levels in these patients. However, apparent l(−)SddC (3TC)-resistant HBV emerged in some patients upon long-term treatment (13, 16, 18). The HBV-resistant genotype appears to be associated with the mutation of methionine to valine or isoleucine in the YMDD motif of HBV DNA polymerase (13, 16, 18). This mutation was previously demonstrated and could render duck HBV resistant to l(−)SddC (3TC) (11). It is not clear if this mutation alone can lead to resistance and, if so, to what degree HBV resistance to l(−)SddC (3TC) develops.
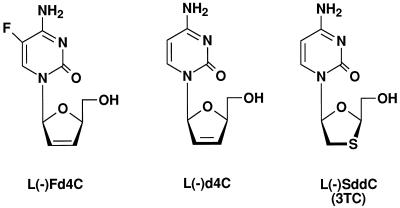
FIG. 1.
Structures of l(−)deoxycytidine analogs.
One approach to overcoming clinical drug resistance is to use higher dosages of l(−)SddC (3TC) given the therapeutic index of the compound in vitro. However, the potency of l(−)SddC (3TC) against HBV in the clinic could be a limiting factor given the dosage application. The antiviral potency is determined not only by its antiviral activity but also by the pharmacodynamic nature of its active metabolite, l(−)SddC (3TC) 5′-triphosphate, in vivo. A more potent compound with more favorable pharmacodynamic behavior of intracellularly active metabolites would be worth exploring.
In the studies reported herein, we describe the anti-HBV activity, metabolism, and pharmacodynamic properties of 2′,3′-dideoxy-2′,3′-didehydro-β-l(−)-5-fluorocytidine [l(−)Fd4C](Fig. 1) in comparison with those of l(−)SddC (3TC), including its behavior toward deoxycytidine kinase and the interaction of l(−)Fd4C 5′-triphosphate with virion-associated HBV DNA polymerase. Preliminary studies of the anti-HBV and anti-human immunodeficiency virus (HIV) activities of l(−)Fd4C were previously reported by us and others (10, 15).
MATERIALS AND METHODS
l(−)Fd4C and 2′,3′-dideoxy-2′,3′-didehydro-β-l(−)-cytidine [l(−)d4C]were synthesized in the laboratory of the late Tai-Shun Lin at Yale University (15). [3H]l(−)Fd4C, [3H]l(−)SddC ([3H]3TC), and [3H]deoxycytidine were purchased from Moravek Biochemicals (Brea, Calif.), and the β-l(−) nucleoside analogs were further purified by using a chiral column (Cyclobond I 2000, 500 by 10 cm; Advanced Separation Technologies, Inc., Whippany, N.J.) with water as the mobile phase. All other chemicals were of the highest grade available. l(−)Fd4C 5′-triphosphate was synthesized by scientists from Vion Pharmaceuticals (New Haven, Conn.).
The procedures for assessing anti-HBV activity, cell growth, and intracellular mtDNA content were described previously (8). Briefly, 6-day-old cultures of 2.2.15 cells (kindly provided by G. Acs), clonal derivatives of HepG2 that secrete hepatitis B virions, were treated with various drug concentrations for 6 days; fresh medium and drug were added on day 3, and the culture medium was processed for analysis of HBV DNA on day 6. For studies of the reversal of drug anti-HBV activity, 2.2.15 cultures were treated under various drug conditions. After 6 days of drug treatment, fresh medium without drug was added, and the cells were cultured an additional 12 days in the absence of drug. After 6 days without drug and after 12 days without drug, the culture medium was processed for analysis of HBV DNA. Virions secreted into the culture medium were obtained by a polyethylene glycol precipitation method. HBV DNA isolated from these virions was analyzed by Southern blot analysis. Inhibition of viral DNA replication was assessed by comparison of HBV DNA from drug-treated and untreated cultures using hybridization of the blots to an HBV-specific probe followed by autoradiography. Quantitative densitometry and computer generation of images of autoradiographs were performed on a Molecular Dynamics Personal Densitometer SI by using ImageQuaNT image analysis software.
For metabolism studies, HepG2 cells were used 2 days after they reached confluency. Cells were treated under the conditions indicated and were washed twice with ice-cold phosphate-buffered saline at the time of harvesting. Nucleotides were extracted from cells in 60% cold methanol. The samples were centrifuged and supernatant was analyzed as previously described (16) by ion-exchange high-performance liquid chromatography (HPLC) using a Whatman Partisil-SAX column.
Crude extract from aged HepG2 cells was prepared by suspending cells in buffer containing 20 mM Tris-HCl (pH 7.5), 2 mM ATP-MgCl2, and 2 mM dithiothreitol (5). After three freeze-thaw cycles, the extract was centrifuged and the supernatant was removed. Streptomycin sulfate was added to a concentration of 1%, and following centrifugation ammonium sulfate was added to the supernatant to bring the final concentration to 60%. The ammonium sulfate-precipitated pellet was resuspended in the ATP-MgCl2-containing buffer and dialyzed to remove ammonium sulfate. The deoxycytidine kinase assay was performed as previously described (5) by using 100 μM substrate {64 mCi of [3H]deoxycytidine/mmol, 8 mCi of [3H]l(−)Fd4Ci/mmol, or 27 mCi of [3H]l(−)SddC ([3H]3TC)/mmol} in the presence or absence of inhibitor (300 μM bromovinyldeoxyuridine [BVdU] or 200 μM deoxycytidine). Kinetic determinations were performed by Lineweaver-Burk plots using concentration ranges of 0.625 to 10 μM [3H]deoxycytidine, 6.5 to 162 μM [3H]l(−)Fd4C, and 1.25 to 20 μM [3H]l(−)SddC ([3H]3TC).
Hepatitis B viral particles isolated by the polyethylene glycol precipitation method were used to assess the inhibition of HBV DNA polymerase by l(−)Fd4C 5′-triphosphate. The assay mixture consisted of virion-associated HBV DNA polymerase in a solution containing 50 mM Tris-HCl (pH 7.5), 340 mM KCl, 22 mM β-mercaptoethanol, 0.4% Nonidet P-40, 70 μM each dATP, dTTP, and dGTP, and 0.175 μM [α-32P]dCTP (11 Ci/mmol). The details of the assay and gel electrophoresis of the DNA have been described elsewhere (8).
RESULTS
Comparison of the anti-HBV activities of l(−)Fd4C, l(−)d4C, and l(−)SddC (3TC).
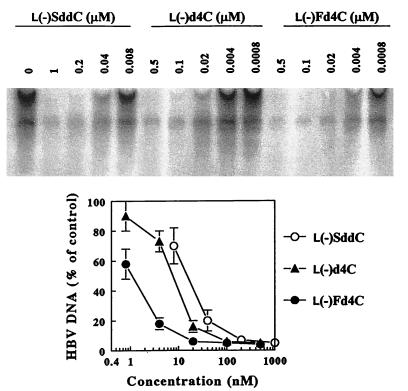
The HBV producer cell line HepG2 2.2.15 was used to evaluate the anti-HBV activities of l(−)Fd4C, l(−)d4C, and l(−)SddC (3TC). Our studies showed that l(−)Fd4C and l(−)d4C had potent anti-HBV activities. Antiviral effects were measured by analysis of extracellular HBV DNA obtained from secreted viral particles. Aged 2.2.15 cells were exposed to l(−)Fd4C, l(−)d4C, or l(−)SddC (3TC) for 6 days as indicated (Fig. 2). The amount of HBV DNA secreted from these cells was analyzed as previously described (8). The results show that each β-l(−) nucleoside analog decreased the amount of extracellular HBV DNA in a dose-dependent manner. Secretion of HBV DNA from 2.2.15 cells was decreased by 50% at a concentration of 1 nM by l(−)Fd4C, which was approximately 7 and 15 times more potent than l(−)d4C and l(−)SddC (3TC), respectively. There was no effect of any of these analogs on cellular mtDNA content at concentrations up to 10 μM. The concentration required to inhibit 50% of HepG2 growth in 3-day cultures was estimated to be 20 μM for l(−)Fd4C, 14 μM for l(−)d4C, and more than 50 μM for l(−)SddC (3TC). None of these compounds had any effect on HBV surface antigen secretion from 2.2.15 cells at concentrations up to 10 μM.
FIG. 2.
HBV inhibition by β-l(−) nucleoside analogs. (Top) Computer-generated autoradiograph of samples isolated from HepG2 2.2.15 culture medium hybridized to a HBV DNA probe. (Bottom) Graph of densitometric data from the autoradiograph: HBV DNA secretion in drug-treated cultures. The DNA was processed and analyzed as described in Materials and Methods.
To address the issue of the reversibility of the anti-HBV activity upon removal of the drug from culture, 2.2.15 cells were treated with drug for 6 days and cultured an additional 12 days in the absence of drug, with medium changes every 3 days. The levels of HBV DNA secreted into the culture medium were assessed on days 6 and 12 post-drug removal. The results indicated that the anti-HBV activities of all three analogs are reversible and that the time to reversal of the anti-HBV activity is dependent upon the initial drug concentration (Table 1). At equal concentrations, the degree of reversal at day 12 was much lower for l(−)Fd4C than for l(−)d4C or l(−)SddC (3TC). This suggests that the continual presence of the nucleoside analog is required to maintain inhibitory activity.
TABLE 1.
Reversibility of β-l(−) nucleoside analog anti-HBV activitya
| Drug and concn (μM) | Level of HBV DNAb at the following time post-drug removal:
|
|
|---|---|---|
| Day 6 | Day 12 | |
| l(−)Fd4C | ||
| 0.02 | 9 | 86 |
| 0.10 | <5 | 47 |
| 0.40 | <5 | 12 |
| l(−)d4C | ||
| 0.10 | 6 | 67 |
| 0.50 | <5 | 35 |
| 2.00 | <5 | 9 |
| l(−)SddC | ||
| 0.20 | 15 | 63 |
| 1.00 | 5 | 50 |
| 4.00 | <5 | 24 |
Aged 2.2.15 cells were treated with drug for 6 days as indicated and cultured an additional 12 days in the absence of drug. Levels of HBV DNA secreted into the culture medium were assessed 6 and 12 days after drug removal.
Values are represented as percentages of HBV DNA levels in untreated control cultures.
Intracellular formation of phosphorylated metabolites in HepG2 cells.
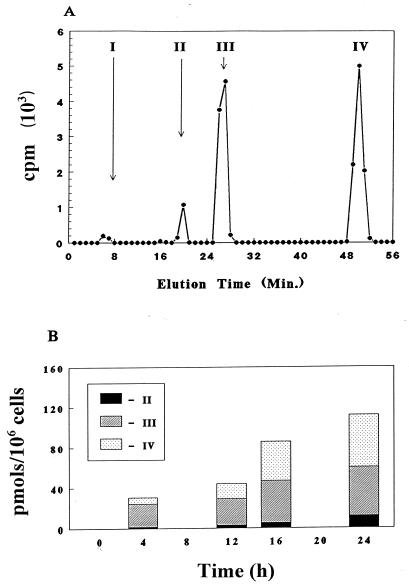
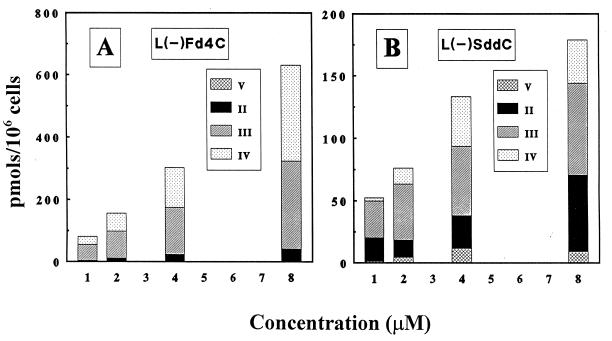
Because the 5′-triphosphate metabolite of l(−)Fd4C is a potential substrate for viral DNA polymerase, it is important to determine its phosphorylation profile in cell culture. The experiment was done by incubating the human hepatocellular carcinoma cell line HepG2 with 2 μM [3H]l(−)Fd4C. At the indicated times, cells were harvested, washed, and methanol extracted, and the soluble fraction was analyzed by ion-exchange HPLC as described in Materials and Methods. There was no detectable [3H]l(−)Fd4C associated with the methanol-insoluble fraction (less than 0.1 pmol/106 cells) with up to 24 h of drug exposure. Radioactivity was associated with four peaks that were subsequently identified as l(−)Fd4C, l(−)Fd4C 5′-monophosphate, l(−)Fd4C 5′-diphosphate, and l(−)Fd4C 5′-triphosphate (Fig. 3A). The time-dependent formation of intracellular l(−)Fd4C phosphorylated metabolites is depicted in Fig. 3B. There was a linear increase in the amount of l(−)Fd4C phosphorylated metabolites up to 24 h. Although the proportions of the phosphorylated metabolites varied with time, l(−)Fd4C 5′-diphosphate was the predominant metabolite at 4 h, whereas the proportion of l(−)Fd4C 5′-triphosphate increased with time. The dose-dependent formation of phosphorylated metabolites of l(−)Fd4C was compared with that of l(−)SddC (3TC) at 24 h post-drug exposure. As shown in Fig. 4, there were concentration-dependent increases in the phosphorylated metabolites of both compounds. The amount of l(−)Fd4C phosphorylated metabolites formed is greater than that of l(−)SddC (3TC) phosphorylated metabolites. When the reciprocal of total intracellular phosphorylated metabolite formation was plotted against the reciprocal of the extracellular drug concentration, a linear relationship was observed, with apparent Kms of 100 μM for l(−)Fd4C and 4.4 μM for l(−)SddC; the relative maximum amount of formation was 33 for l(−)Fd4C and 1 for l(−)SddC (3TC).
FIG. 3.
(A) Resolution by ion-exchange HPLC of intracellular metabolites detected in HepG2 cells exposed to 2 μM [3H]l(−)Fd4C (11 mCi/mmol). The metabolites were identified as l(−)Fd4C (I), l(−)Fd4C 5′-monophosphate (II), l(−)Fd4C 5′-diphosphate (III), and l(−)Fd4C 5′-triphosphate (IV). (B) Time course of intracellular metabolites detected in HepG2 cells after incubation with 2 μM [3H]l(−)Fd4C (11 mCi/mmol).
FIG. 4.
Intracellular concentrations of metabolites detected in HepG2 cells after 24 h of incubation with either [3H]l(−)Fd4C (11 mCi/mmol) (A) or [3H]l(−)SddC ([3H]3TC) (40 mCi/mmol) (B) at the indicated concentrations. The metabolites were identified as the 5′-monophosphate (II), the 5′-diphosphate (III), the 5′-triphosphate (IV), and an unidentified metabolite that eluted between the nucleoside and the monophosphate (V).
Retention of l(−)Fd4C phosphorylated metabolites in comparison with that of l(−)SddC (3TC) phosphorylated metabolites in HepG2 cells.
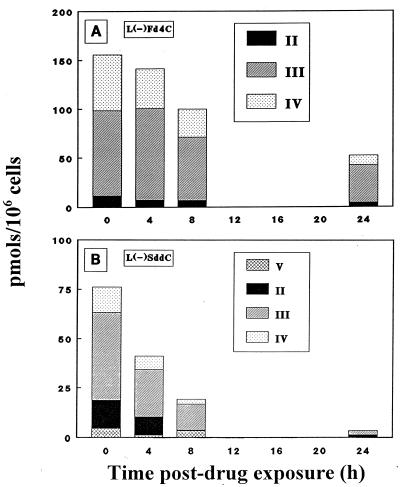
Retention of l(−)Fd4C phosphorylated metabolites in HepG2 cells was compared with that of l(−)SddC (3TC) phosphorylated metabolites by incubating HepG2 cells with 2 μM [3H]l(−)Fd4C or [3H]l(−)SddC ([3H]3TC). After 24 h of exposure to radiolabeled drug, the cells were washed, and fresh culture medium was added. At various times following drug removal, cells were harvested and analyzed for phosphorylated metabolites. Fresh medium without drug was replaced at 8 h to prevent the reuptake of drug secreted from cells. Figure 5 shows the intracellular content of phosphorylated metabolites at various times following drug removal. The apparent intracellular half-life of the β-l(−) nucleoside analog phosphorylated metabolite was approximately 20 h for l(−)Fd4C and 4 h for l(−)SddC (3TC). At 24 h after drug removal, there was still approximately 10 μM l(−)Fd4C 5′-triphosphate in cells.
FIG. 5.
Intracellular concentration of metabolites detected in HepG2 cells after removal of drug from the culture medium. HepG2 cells were exposed to either 2 μM [3H]l(−)Fd4C (11 mCi/mmol) (A) or 2 μM [3H]l(−)SddC ([3H]3TC) (40 mCi/mmol) (B) for 24 h. The metabolites were identified as explained for Fig. 3 and 4.
Phosphorylation of l(−)Fd4C and l(−)SddC (3TC) by HepG2 cellular extracts.
Extracts of HepG2 cells were prepared in order to examine the phosphorylation of l(−)Fd4C and l(−)SddC (3TC) as described in Materials and Methods. BVdU, a potent inhibitor of mitochondrial deoxypyrimidine nucleoside kinase, could not inhibit the phosphorylation of l(−)Fd4C and l(−)SddC (3TC), whereas deoxycytidine inhibited their phosphorylation. This suggests that neither of these β-l(−) nucleoside analogs serves as a substrate for mitochondrial deoxypyrimidine nucleoside kinase in these cell extracts (9). The concentration-dependent phosphorylation of l(−)Fd4C and l(−)SddC (3TC) was examined in the cellular extracts. The apparent Kms of l(−)Fd4C and l(−)SddC (3TC) were determined to be 100 and 11 μM, respectively, in crude HepG2 cellular extracts and were not dissimilar to the values obtained from purified deoxycytidine kinase for l(−)Fd4C and l(−)SddC (3TC) (9). The difference in the relative Vmax values for l(−)Fd4C and l(−)SddC (3TC) was about 36-fold (Table 2).
TABLE 2.
Phosphorylation of β-l(−) nucleoside analogs by HepG2 cellular extracts
| Inhibitor | Phosphorylation (pmol/mg of protein/min) ofa:
|
||
|---|---|---|---|
| dCyd | l(−)Fd4C | l(−)SddC | |
| None | 59.8 ± 0.7 | 42.1 ± 5.5 | 7.5 ± 0.6 |
| BVdU (300 μM) | 33.0 ± 2.4 | 34.9 ± 7.0 | 7.3 ± 0.8 |
| dCyd (200 μM) | 27.6 ± 0.7 | 0.9 ± 0.6 | 0.1 ± 0.06 |
| Km (μM)b | 100 ± 1.0 | 11 ± 2.1 | |
| Relative Vmaxc | 1 | 2.2 ± 0.8 | 0.06 ± 0.01 |
Each substrate was used at 100 μM. dCyd, deoxycytidine.
Values were determined by Lineweaver-Burk plots and are the means ± standard deviations of three determinations.
Values are relative to maximum velocity measurements of deoxycytidine.
Inhibition of virion-associated HBV DNA polymerase by l(−)Fd4C 5′-triphosphate.
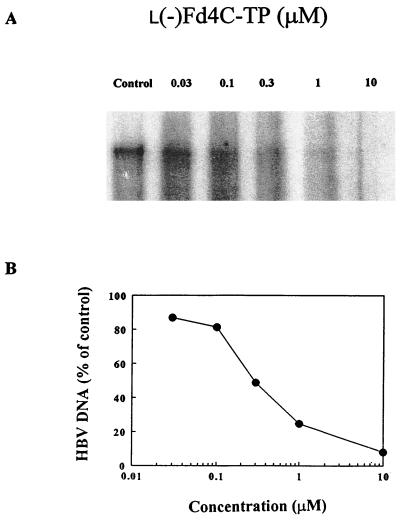
The effect of l(−)Fd4C 5′-triphosphate on the activity of virion-associated HBV DNA polymerase in vitro was examined. There is a dose-dependent inhibition of [α-32P]dCTP incorporation into viral DNA, as shown in Fig. 6. The Ki was calculated to be 69 ± 15 nM by using the competitive inhibition equation as previously described (6).
FIG. 6.
Inhibition of endogenous HBV DNA polymerase by l(−)Fd4C 5′-triphosphate. (A) Computer-generated autoradiograph of HBV DNA analyzed by agarose gel electrophoresis. (B) Quantitation of HBV DNA by densitometry. The assay was performed as described in Materials and Methods.
DISCUSSION
l(−)Fd4C is among the most potent anti-HBV compounds discovered. The introduction of a fluorine atom at the 5 position of cytosine enhances the antiviral activity against both HBV and HIV with minimal effects on cytotoxicity. The cytotoxicity of l(−)Fd4C is dependent on the cell line used. The concentration required to inhibit 50% of the growth of HepG2 cells is about 2 to 3 times greater than that for CEM cells. Based on the concentration required to inhibit HBV or HIV replication and that required to inhibit cell growth in culture, l(−)Fd4C is at least as impressive as l(−)SddC (3TC) as an antiviral compound. The anti-HBV activity of l(−)Fd4C is reversible, like that of l(−)SddC (3TC). However, at equal concentrations, it takes longer for drug-treated 2.2.15 cells to resynthesize viral DNA after removal of l(−)Fd4C than after removal of l(−)SddC (3TC). This could be due to differences in the rates of removal of these compounds from the termini of HBV DNA, a possibility which is currently under investigation, and/or to differences in the retention of intracellular active metabolites.
Like l(−)SddC (3TC), l(−)Fd4C could be phosphorylated to mono-, di-, and triphosphate metabolites. The level of formation of phosphorylated metabolites in cells is higher for l(−)Fd4C than for l(−)SddC (3TC). Also, the apparent Km and the relative amount of phosphorylated metabolites in whole cells are similar to those with HepG2 extracts. Deoxycytidine inhibits the formation of l(−)Fd4C and l(−)SddC (3TC) metabolites in HepG2 cell extracts, suggesting that l(−)Fd4C, l(−)SddC (3TC), and deoxycytidine share a common kinase for their phosphorylation to monophosphate metabolites. The inhibition of deoxycytidine phosphorylation by BVdU suggests the presence of mitochondrial deoxypryrimidine nucleoside kinase in the HepG2 cell extracts. However, the absence of an effect of BVdU on l(−)Fd4C and l(−)SddC (3TC) phosphorylation implies that mitochondrial deoxypyrimidine nucleoside kinase does not phosphorylate these two β-l(−) nucleoside analogs. Indeed, the enzyme responsible for the phosphorylation of l(−)Fd4C has been shown to be cytosolic deoxycytidine kinase (9). That study also shows that mitochondrial deoxypyrimidine nucleoside kinase does not phosphorylate l(−)Fd4C. The major metabolite of l(−)Fd4C and l(−)SddC (3TC) is the diphosphate nucleotide as early as 4 h after drug exposure. This suggests that, for both β-l(−) nucleoside analogs, the step of conversion of the diphosphate metabolite to the triphosphate metabolite is not efficient. However, this step appears to be more efficient for l(−)Fd4C than for l(−)SddC (3TC) when equal extracellular drug concentrations are compared. This is supported by the observation that longer drug exposure times decrease the diphosphate-to-triphosphate ratio of l(−)Fd4C faster than that of l(−)SddC (3TC). Whether this is due to a difference between l(−)SddC (3TC) 5′-diphosphate and l(−)Fd4C 5′-diphosphate in behavior towards the enzyme for phosphorylation or a difference in the intracellular levels of these two diphosphate metabolites is not clear. l(−)Fd4C metabolites have a much longer retention time in HepG2 cells than l(−)SddC (3TC) metabolites. This could partly explain why the time to reappearance of HBV DNA after drug removal is greater for l(−)Fd4C-treated cells than for l(−)SddC (3TC)-treated cells. l(−)Fd4C 5′-triphosphate is inhibitory to the incorporation of dCTP into viral DNA by HBV DNA polymerase. The Ki is estimated to be much lower than the intracellular content of l(−)Fd4C 5′-triphosphate at a 2 μM extracellular concentration of l(−)Fd4C. It is likely that l(−)Fd4C 5′-triphosphate is an alternative substrate for the viral enzyme and can be incorporated into viral DNA as a DNA chain terminator. This is currently under investigation, including the interaction of l(−)Fd4C 5′-triphosphate with human DNA polymerase α, β, γ, and δ (14).
In summary, l(−)Fd4C is much more potent than l(−)SddC (3TC) against HBV in cell culture. It is likely that the dosage required to suppress HBV replication in patients will be lower for l(−)Fd4C than for l(−)SddC (3TC). This could be important in view of the current dosage of l(−)SddC (3TC), which is 100 mg per day for the treatment of patients with chronic HBV infection. Escalation of the dosage, if required to overcome viral resistance to l(−)SddC (3TC), could be achieved with much less l(−)Fd4C than l(−)SddC (3TC), assuming that there is the same degree of viral resistance to l(−)Fd4C. The higher potency against HBV of l(−)Fd4C compared to l(−)SddC (3TC) is partly due to the higher levels of phosphorylated metabolites of l(−)Fd4C compared to those of l(−)SddC (3TC), which may reflect differences in the behavior of these two analogs towards cytosolic deoxycytidine kinase. l(−)Fd4C 5′-triphosphate is most likely the active metabolite that can inhibit HBV DNA replication. The intracellular retention time of phosphorylated l(−)Fd4C metabolites is about 5 times longer than that of phosphorylated l(−)SddC (3TC) metabolites. This suggests that less-frequent doses of l(−)Fd4C than of l(−)SddC (3TC) are required, assuming that there is no major difference in pharmacokinetic behavior between these two analogs. This is particularly important in view of the potent activity of l(−)Fd4C against HIV. Currently, two doses per day are required for l(−)SddC (3TC), while one could be required for l(−)Fd4C, making patient compliance easier. If the longer retention time of phosphorylated l(−)Fd4C metabolites in cells compared with that of phosphorylated l(−)SddC (3TC) metabolites also holds for HIV-susceptible cells in patients as for CEM cells (9), then l(−)Fd4C could be more advantageous than l(−)SddC (3TC) for the treatment of patients with HIV infection. Based on the antiviral potency and unique pharmacodynamic properties of l(−)Fd4C, this compound should be explored further for its clinical potential.
ACKNOWLEDGMENTS
This work was supported by grants AI-38204 and CA-63477 from the National Institutes of Health.
REFERENCES
- 1.Beasley R P, Hwang L Y, Lin C C, Chien S. Hepatocellular carcinoma and hepatitis B virus. Lancet. 1981;ii:1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 2.Benhamou Y, Dohin E, Lunel-Fabiani F, Poynard T, Huraus J M, Katlama C, Opolon P, Gentilini M. Efficacy of lamivudine on replication of hepatitis B virus in HIV-infected patients. Lancet. 1994;345:396–397. doi: 10.1016/s0140-6736(95)90388-7. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Cases of selected notifiable diseases. Morbid Mortal Weekly Rep. 1995;1995:43–963. [Google Scholar]
- 4.Chang C-N, Doong S-L, Zhou J H, Beach J W, Jeong L S, Chu C K, Tsai C H, Liotta D C, Schinazi R F, Cheng Y-C. Deoxycytidine deaminase-resistant stereoisomer is the active form of (±)-2′,3′-dideoxy-3′-thiacytidine in the inhibition of hepatitis B virus replication. J Biol Chem. 1992;267:13938–13942. [PubMed] [Google Scholar]
- 5.Cheng Y-C, Domin B, Lee L-S. Human deoxycytidine kinase. Purification and characterization of the cytoplasmic and mitochondrial isozymes derived from blast cells of acute myelocytic leukemia patients. Biochim Biophys Acta. 1977;481:481–492. doi: 10.1016/0005-2744(77)90281-9. [DOI] [PubMed] [Google Scholar]
- 6.Cheng Y-C, Prusoff W H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (ID50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 7.Dienstag J L, Perrillo R P, Schiff E R, Bartholomew M, Vicary C, Rubin M. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med. 1995;333:1657–1661. doi: 10.1056/NEJM199512213332501. [DOI] [PubMed] [Google Scholar]
- 8.Doong S I, Tsai C H, Schinazi R F, Liotta D C, Cheng Y C. Inhibition of the replication of hepatitis B virus in vitro by 2′,3′-dideoxy-3′-thiapyrimidine and related analogues. Proc Natl Acad Sci USA. 1991;88:8495–8499. doi: 10.1073/pnas.88.19.8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutschman G E, Bridges E G, Liu S-H, Gullen E, Guo X, Kukhanova M, Cheng Y-C. Metabolism of 2′,3′-dideoxy-2′,3′-dehydro-β-l(−)-5-fluorocytidine and its activity in combination with clinically approved anti-human immunodeficiency virus β-d(+) nucleoside analogs in vitro. Antimicrob Agents Chemother. 1998;42:1799–1804. doi: 10.1128/aac.42.7.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faraj A, Schinazi R F, Joudawlkis A, Lesnikowski Z, McMillan A, Morrow C D, Sommadossi J-P. Effects of β-d-2′,3′-didehydro-2′,3′-dideoxy-5-fluorocytidine 5′-triphosphate (β-d-D4FC-TP) and its β-l-enantiomer 5′-triphosphate (β-l-D4FC-TP) on viral DNA polymerases. Antivir Res. 1997;34:A66. [Google Scholar]
- 11.Fischer K P, Tyrrell D L J. Generation of duck hepatitis B virus polymerase mutants through site-directed mutagenesis which demonstrate resistance to lamivudine [(−)-β-l-2′,3′-dideoxy-3′-thiacytidine] in vitro. Antimicrob Agents Chemother. 1996;40:1957–1960. doi: 10.1128/aac.40.8.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furman P A, Davis M, Liotta D C, Paff M, Frick L W, Nelson D J, Dornsife R E, Wurster J A, Wilson L J, Fyfe J A, Tuttle J V, Miller W H, Condreay L, Averett D R, Schinazi R F, Painter G R. The anti-hepatitis B virus activities, cytotoxicities, and anabolic profiles of the (−) and (+) enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob Agents Chemother. 1992;36:2686–2692. doi: 10.1128/aac.36.12.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honkoop P, Niesters H G M, Deman R A M, Osterhaus A D M E, Schalm S W. Lamivudine resistance in immunocompetent chronic hepatitis B. Incidence and patterns. J Hepatol. 1997;26:1393–1395. doi: 10.1016/s0168-8278(97)80476-x. [DOI] [PubMed] [Google Scholar]
- 14.Kukhanova, M., and Y.-C. Cheng. 1997. Unpublished data.
- 15.Lin T-S, Luo M-Z, Liu M-C, Zhu Y-L, Gullen E, Dutschman G E, Cheng Y-C. Design and synthesis of 2′,3′-dideoxy-2′,3′-didehydro-β-l-cytidine (β-l-d4C) and 2′,3′-dideoxy-2′,3′-didehydro-β-l-5-fluorocytidine (β-l-Fd4C), two exceptionally potent inhibitors of human hepatitis B virus (HBV) and potent inhibitors of human immunodeficiency virus (HIV) in vitro. J Med Chem. 1996;39:1757–1759. doi: 10.1021/jm950836q. [DOI] [PubMed] [Google Scholar]
- 16.Ling R, Mutimer D, Ahmed M, Boxall E H, Elias E, Dusheiko G M, Harrison T J. Selection of mutations in the hepatitis B virus polymerase during therapy of transplant recipients with lamivudine. Hepatology. 1996;24:711–713. doi: 10.1002/hep.510240339. [DOI] [PubMed] [Google Scholar]
- 17.Severini A, Liu X-Y, Wilson J S, Tyrrell D L J. Mechanism of inhibition of duck hepatitis B virus polymerase by (−)-β-l-2′,3′-dideoxy-3′-thiacytidine. Antimicrob Agents Chemother. 1995;39:1430–1435. doi: 10.1128/aac.39.7.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tipples G A, Ma M M, Fischer K P, Bain V G, Kneteman N M, Tyrrell D L. Mutation in HBV RNA-dependent polymerase confers resistance to lamivudine in vivo. Hepatology. 1996;24:714–717. doi: 10.1002/hep.510240340. [DOI] [PubMed] [Google Scholar]
- 19.Tyrrell D L J, Mitchell M C, DeMon R, Schalm S W, Main J, Thomas H C, Fevery J, Nevens F, Beranck P, Vicary C. Phase II trial of lamivudine for chronic hepatitis B. Hepatology. 1993;18:112A. [Google Scholar]