Abstract
Background
Severe quantitative disorders of consciousness (DoC) due to acute brain injury affect up to 47% of patients upon admission to intensive care and early rehabilitation units. Nevertheless, the rehabilitation of this vulnerable group of patients has not yet been addressed in any German-language guidelines and has only been studied in a small number of randomized clinical trials.
Methods
In an S3 clinical practice guideline project, a systematic literature search was carried out for interventions that could improve consciousness in patients with coma, unresponsive wakefulness syndrome, or minimally conscious state after acute brain injury, and an evidence-based evaluation of these interventions was performed. Recommendations concerning diagnostic methods and medical ethics were issued by consensus.
Results
Misdiagnoses are common in patients with DoC, with minimal consciousness often going unrecognized. Patients with DoC should, therefore, be repeatedly assessed with standardized instruments, particularly the Coma Recovery Scale—Revised. The literature search yielded 54 clinical trials, mostly of low quality; there were two randomized controlled clinical trials providing level 1 evidence. The best available evidence for the improvement of impaired consciousness is for the administration of amantadine (4 studies) and for anodal transcranial direct-current stimulation of the left dorsolateral prefrontal cortex in patients in the minimal conscious state (8 studies, 2 systematic reviews). Further important components of rehabilitation include positioning methods and sensory stimulation techniques such as music therapy.
Conclusion
For the first time, evidence-based German-language clinical practice guidelines have now become available for the neurological rehabilitation of patients with DoC.
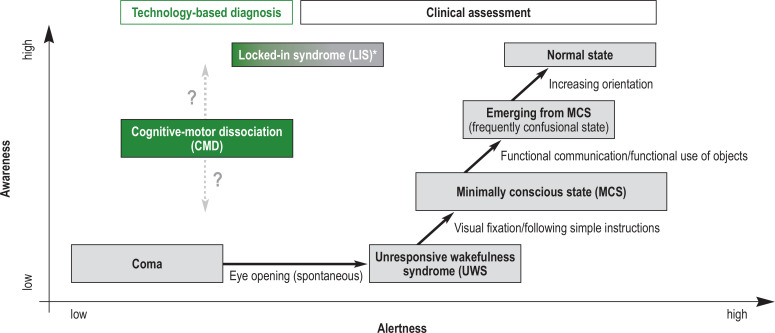
After an acute severe brain injury, patients frequently develop a severe disorder of consciousness (DoC). This manifests as coma, unresponsive wakefulness syndrome (UWS) or minimally conscious state (MCS); (Figure, Box 1) (1, 2).
Figure.
Diagnostic categories for severe disorders of consciousness (DoC), starting from coma through unresponsive wakefulness syndrome (UWS), minimally conscious state (MCS) and cognitive-motor dissociation (CMD) to emerging from MCS. Patients with CMD can only be identified with the help of additional technology-based diagnostic assessment (green); however, the informative value of these investigations is still limited or unclear. Thus, the level of awareness is difficult to assess (symbolized as a question mark). *By contrast, patients with locked-in syndrome (LIS) usually do not have a disorder of consciousness. However, they might be confused with patients with DoC because of their almost complete paralysis and consequent lack of motor response—thus, they have also been included in the Figure.
Adapted from Mokrusch et al.: Currciulum Neurorehabilitation der DGNR e.V.; Hippocampus Verlag 2023.
Box 1. Clinical spectrum of severe disorders of consciousness.
Coma: eyes closed, no alertness
Unresponsive wakefulness syndrome (UWS): Periods of wakefulness but not signs of directed responses (awareness) to oneself or the surroundings (synonyms: apallic syndrome, vegetative state, coma vigile)
Minimally conscious state (MCS): Evidence of conscious responsiveness, for example, visual pursuit or visual fixation (MCS minus) or basic language comprehension (MCS plus), but still without functional communication ability and without correct object use
Cognitive-motor dissociation (CMD): Clinically to be classified as UWS, only in additional technology-supported examinations (e.g., functional magnetic resonance imaging [fMRI], FDG positron emission tomography [FDG-PET], quantitative EEG), signs of an at least partially preserved cognitive processing ability are recognized
In western industrialized countries, the prevalence rates of UWS and MCS vary from 0.2 to 6.1/100 000 population (3). In neurological-neurosurgical early rehabilitation centers with intermediate care and intensive care units, up to 47% of patients suffer from a DoC on admission (4). The most common causes of DoC are (5, 6):
Hypoxic-ischemic encephalopathy (25–45 %)
Stroke (31–38 %)
Traumatic brain injury (TBI) (24–36 %).
DoC is more common in men (62%) than women (38%); the mean age of patients is 49 to 57 years.
In patients with DoC, mortality ranges from 10% to 26% within the first six months and 29% within two years (5– 7). One year after brain injury, 43% of patients have emerged from DoC (that is, they have emerged from MCS, returning to a more normalized consciousness), with younger age, traumatic etiology of brain injury, and MCS already at the start of rehabilitation being the most important predictors of a more favorable outcome (8).
Both nationally and internationally, there is still a lack of comprehensive, evidence-based guidelines for therapeutic interventions that can improve the level of consciousness. Internationally, existing practice recommendations or guidelines mostly contain general recommendations for patients with DoC, but do not focus on evidence-based interventions to improve consciousness (1, 9). In addition, a number of systematic reviews on evidence-based treatment options have been published in recent years (10– 12). In our opinion, the management for patients with DoC in the reality of care practice in Germany is still very heterogeneous and poorly standardized.
Under the leadership of the German Society for Neurorehabilitation, with the collaboration of 18 professional societies, associations, and patient organizations (eBox1), evidence-based recommendations for diagnostic investigations and therapeutic interventions aimed at improving the level of consciousness, as well as recommendations for accompanying ethical principles to apply to this extremely vulnerable patient population were developed (13).
eBox 1. Authors (collaborators), medical societies and organizations contributing to the development of the guideline (AWMF reg. no.: 080–006).
Prof. Dr. Dr. Ralf J. Jox
German Academy of Ethics in Medicine (AEM)
Dr. Bernd Eifert, PD Dr. Volker Huge
German Society of Anesthesiology and Intensive Care Medicine (DGAI)
Prof. Dr. Christian Storm, Dr. Jens Nee
German Society of Internal Medicine (DGIM)
Dr. Bernd Hoffmann
German Society for Neurosurgery (DGNC)
Dr. Ulf Bodechtel
German Society for Neurocritical Care and Emergency Medicine (DGNI)
Prof. Dr. Andreas Bender
German Society of Neurology (DGN)
Prof. Dr. Andreas Bender, Dr. Dipl.-Psych Friedemann Müller, Prof. Dr. Stefan Knecht, Prof. Dr. Marcus Pohl, Prof. Dr. Rüdiger Ilg
German Society of Neurorehabilitation (DGNR)
Dr. Thomas van de Weyer
German Society for Physical and Rehabilitation Medicine (DGPRM)
Prof. Dr. Bernhard Elsner
German Society for Physiotherapy Science (DGPTW)
Silja Molle
German Music Therapy Society (DMtG)
Bernd Frittrang
German Federal Association for Academic Speech Therapy and Logopedics (dbs)
Dr. Ilona Rubi-Fessen
German Federal Association for Speech Therapy (dbl)
Birthe Hucke
German Occupational Therapy Association (DVE)
Susanna Freivogel
German Physiotherapy Association (ZVK)
Dr. Pia Wieteck, Dr. Qiumei Jiang-Siebert
Fachgesellschaft Profession Pflege e.V.
Dr. Petra Maurer-Karattup, Dipl.-Psych. Martina Lück
German Society of Neuropsychology (GNP)
Dr. Christoph Stepan
Austrian Society of Neurorehabilitation (OeGNR)
PD Dr. Margret Hund-Georgiadis,
Swiss Society of Neurorehabilitation (SGNR)
Patient representative
Armin Nentwig
Bundesverband Schädel-Hirnpatienten in Not e.V. – German Society of Coma Vigile
Prof. Dr. Eckhard Rickels
CNS – Hannelore Kohl Foundation
Methodological support, coordination and moderation
Dr. Susanne Blödt
AWMF Institute for Medical Knowledge Management
Methods
The comprehensive guideline methodology was published in a guideline report, which is available for download from the Association of the Scientific Medical Societies in Germany (AWMF, Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften; https://register.awmf.org/de/leitlinien/detail/080–006).
Details on the literature search, inclusion criteria for literature selection, and formulation of recommendations can be found in the eMethods section, eBox 2 and in the eFigure. The focus is on interventions that improve the level of consciousness.
eMethods.
Methods of the literature search, literature selection and evidence rating
The full methodology is detailed in a guideline report available via the AWMF website at the following link: https://register.awmf.org/assets/guidelines/080-006m_S3_Neurologische-Rehabilitation-bei-Koma-und-schwerer-Bewusstseinsstoerung-im-Erwachsenenalter_2023-01_1.pdf
The systematic literature search was performed as of 31 January 2021 in two independent scientific literature databases:
MEDLINE/PubMed
Cochrane Library
In order to identify all intervention studies for the target population, regardless of the type of intervention, the following search terms were used:
Search syntax to identify the target population of adult patients with severe persistent disorders of consciousness after acute brain injury: (Coma OR “disorder of consciousness“ OR “disorders of consciousness” OR “vegetative state” OR “unresponsive wakefulness” OR “low awareness” OR “minimal conscious” OR “minimally conscious”)
Search syntax to identify the therapeutic interventions in adult patients with severe persistent disorders of consciousness after acute brain injury: (“clinical trial” OR “observational study” OR “observational trial” OR “meta analysis” OR “meta-analysis” OR “clinical study” OR rehabilitation OR recovery)
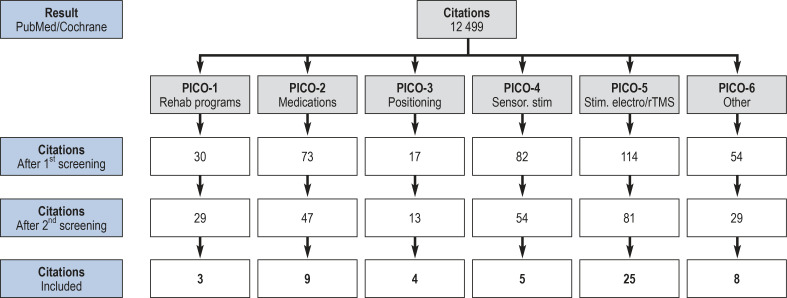
The 12 499 primary search results resulting from the literature search were reviewed and selected in several rounds of literature screening, first at the title and abstract level, then at the full-text level, using the consented inclusion criteria described in eBox 2
After the literature review was completed using the four-eye principle, 54 studies remained; these formed the basis of the evidence rating and formulation of recommendations (eFigure).
The level of evidence of the various studies were determined using the 2011 Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence from 1 (systematic review) to 5 (expert opinion) (e1).
The validity of the included studies was assessed using 14 criteria for individual studies and 13 criteria for systematic reviews based on AMSTAR-2 (e2).
To evaluate the available evidence for an intervention in a synoptic methodological manner, the quality of the evidence was assessed in relation to the endpoint “improvement of the level of consciousness“, using the GRADE approach (e3).
Linking evidence and recommendation
The recommendations formulated were based, on the one hand, on the nature, extent, and quality of the identified evidence for an intervention and, on the other hand, on an additional assessment of other important factors that could result in an up- or downgrading of the grade of recommendation, given the same evidence base (https://www.awmf.org/regelwerk/formulierung-und-graduierung-von-empfehlungen).
For the formulation of recommendations and the assessment of the strength of recommendation, a 3-level graduation system according to AWMF was used, ranging from a strong recommendation (recommendation grade A with the wording “should/should not”) to a recommendation (B, „ought to/ought not to) to an open recommendation (0, “may be considered/no specific recommendation”).
eBox 2. Inclusion criteria for literature selection.
Adult patients (data on children<18 years are not assessed)
Subacute to chronic stage (at least 28 days since acute brain injury)
Clearly defined/operationalized intervention (also several simultaneously)
Language: English/German
Standardized measurement of the level of consciousness using an established clinical scale (e.g. CRS-R, KRS)
A minimum of 3 persons per treatment group and control group
Study types: Randomized clinical trials (RCTs), observational studies, patients as own control group if appropriate intervention and if stable clinical baseline; no case reports
For individual studies with a mixed pediatric-adult study population, the proportion of adult patients should be at least 80% of the total population.
Reviews: Systematic reviews with understandable methodology
eFigure.
Process of literature selection, grouped according to 6 predefined questions of the PICO scheme (PICO, P for patient, I for intervention, C for comparison [control intervention], and O for outcome [target criterion]; senor., sensory; stim, stimulation; rTMS, repetitive transcranial magnetic stimulation.
The available evidence on the topic is generally characterized by moderate quality (at best), with small sample sizes and often sequential study design (pre- versus post-intervention level of consciousness) without true control or sham groups.
Results and recommendations
Diagnosis
In routine clinical practice, incorrect assessments of the level of consciousness of patients with DoC are common, resulting in misdiagnosis rates of approximately 40% (14, 15). In most cases, patients with MCS are wrongly diagnosed as UWS based on non-standard clinical examinations. An evidence-based European guideline on the diagnosis of coma and other disorders of consciousness provides important recommendations, some of which have been adapted to suit the purpose of the guideline presented here (2). Since the misdiagnosis rate is unacceptably high and the correct diagnosis of the level of consciousness is critical, the most important clinical diagnostic recommendations are summarized in Table 1.
Table 1. Recommendations for diagnosis in patients with DOC*.
| Recommendation |
Recommendation grade
(consensus strength) |
| In adult patients with a severe disorder of consciousness due to brain injury … | |
| … the patient‘s eyes should be opened by the examiner during the clinical examination.. | EC (100%) |
| … a mirror should be used to check visual pursuit. | A (100%) |
| … the Coma Recovery Scale – Revised (CRS-R) ought to be used to diagnose the level of consciousness | B (100%) |
| … the standardized clinical examination to diagnose the level of consciousness should be repeated several times over the course of treatment. | A (100%) |
*Adapted from Kondziella et al. 2020 (2)
DOC, disorder of consciousness; EC, expert consensus;
Grades of recommendation: A,“should/should not”; B, “ought/ought not“
Essential for an adequate diagnosis of the level of consciousness is the repeated use of established clinical scales, such as the Coma Recovery Scale-Revised (CRS-R) (Table 2) (16– 18). Furthermore, patients with basal cognitive functions, who may have a more favorable prognosis, can be identified based on structured clinical assessment of spontaneous motor responses, using the Motor Behavior Tools (MBTr) (19). Compared with the gold standard of repeated structured clinical examinations using the CRS-R, the routine clinical neurological examination has a sensitivity of 98% and a specificity of only 57% (positive predictive value 0.61; negative predictive value 0.98) with regard to the correct diagnosis of UWS (15).
Table 2. Coma Recovery Scale-Revised*.
| Score points | Item/function | Defines |
| Subscale: auditory function | ||
| 4 | Consistent movement to command | MCS+ |
| 3 | Reproducible movement to command | MCS+ |
| 2 | Localization to sound | |
| 1 | Auditory Startle | |
| 0 | None | |
| Subscale: visual function | ||
| 5 | Object recognition | MCS– |
| 4 | Object localization: reaching | MCS– |
| 3 | Visual pursuit | MCS– |
| 2 | Fixation | MCS– |
| 1 | Visual startle | |
| 0 | None | |
| Subscale: motor function | ||
| 6 | Functional object use | eMCS |
| 5 | Automatic motor response | MCS– |
| 4 | Object manipulation | MCS– |
| 3 | Localization to noxious stimulation | MCS– |
| 2 | Flexion withdrawal | |
| 1 | Abnormal posturing | |
| 0 | None/flaccid | |
| Subscale: oromotor/verbal function scale | ||
| 3 | Intelligible verbalization | MCS+ |
| 2 | Vocalization/oral movement | |
| 1 | Oral reflexive movement | |
| 0 | None | |
| Subscale: communication | ||
| 2 | Functional: accurate | eMCS |
| 1 | Non-functional: intentional | MCS+ |
| 0 | None | |
| Subscale: arousal | ||
| 3 | Attention | |
| 2 | Eye opening without stimulation | |
| 1 | Eye opening with stimulation | |
| 0 | Unarousable | |
* Coma Recovery Scale – revised (CRS-R) for the standardized assessment of the level of consciousness (adapted from [16] and [18]). The total score ranges from a minimum of 0 points (no responsiveness, cannot be awakened) to a maximum of 23 points. More important than the total score is the achievement of specific abilities that either define the syndrome of minimal consciousness (MCS) or the emergence from it (eMCS). A further distinction within the diagnostic MCS category is made into “MCS plus” with at least partially preserved speech function and “SMB minus” with speech-independent minimally conscious functions, such as visual pursuit. The CRS-R score should be obtained several times during the course of treatment.
Interventions
All recommendations for the use of interventions to improve the state of consciousness are summarized in Box 2.
Box 2. Overview of all recommendations for interventions in patients with DOC due to acute brain injury.
Provision of multiprofessional neurological rehabilitation (B; 100%)
Sufficiently long primary rehabilitation, also in chronic stages of the disease, if necessary (B; 94%)
Use of sensitive and valid assessment tools (e.g. CRS-R) rather than independence scales (e.g. Barthel index) to evaluate rehabilitation duration (B; 100%)
Discontinuation of sedating medications, if possible (EC; 100%)
Treatment attempt with amantadine with gradual dose escalation to a maximum of 400 mg (e.g. 200 mg bid) enterally (B; 100%)
Single treatment attempt with zolpidem, continuation only in responders (0; 94%)
Treatment attempt with intrathecal baclofen in case of concomitant spasticity/vegetative instability (0; 100%)
Verticalization, e.g. using a tilt table (B; 100%)
Multisensory stimulation employing stimuli with high emotional/autobiographical relevance (B; 100%)
Music therapy and auditory stimulation with personal relevance (B; 100%)
In MCS: Application of anodal tDCS at the left dorsolateral prefrontal cortex for a minimum of 5 days (B; 94%)
Anodal tDCS at the precuneus for a minimum of 5 days may be considered (0; 100%)
Serial rTMS at the dorsolateral prefrontal cortex/angular gyrus may be considered (0; 100%)
Deep brain stimulation only in controlled studies/compassionate use in specialized centers with ethical counseling (0; 100%)
EC, expert consensus; B, “ought to”/0, “may be considered”; grades of recommendation according to guideline methods; percentages in parentheses correspond to consensus strength; the original recommendations are available via the guideline publication at AWMF (https://register.awmf.org/assets/guidelines/080–006l_S3_Neurologische-Rehabilitation-bei-Koma-und-schwerer-Bewusstseinsstoerung-im-Erwachsenenalter_2023–01.pdf) (13) CRS-R, Coma Recovery Scale-Revised; DoC, disorder of consciousness; rTMS, repetitive transcranial magnetic stimulation; MCS, minimally conscious state; tDCS, transcranial direct-current stimulation
Comprehensive rehabilitation programs
Adult patients with severe disorders of consciousness caused by acute brain injury ought to (B) receive multiprofessional neurological rehabilitation. In contrast to individual interventions, comprehensive rehabilitation programs consist of several interventions or a complex, usually all-day therapeutic regime provided by a multiprofessional care team. The effectiveness of such integrated programs is currently supported by weak evidence only; however, some studies and the clinical experience of the contributing guideline authors indicate that such programs are indeed effective.
A retrospective study showed that a treatment protocol, including at least three hours of therapy every working day—consisting of variable combinations of physiotherapy, occupational therapy and speech therapy—, drug therapy and electrical neuromodulation techniques, was associated with an improved level of consciousness at week 12 compared to baseline (20). In this small case series (n = 41), 100% of patients in MCS emerged from minimally conscious state compared to only 38% in comparable historical control groups treated with standard approaches. Over the course of the intensive rehabilitation program, the state of consciousness improved in as many as 81% of patients with UWS, whereas this would have been expected in only 42% of patients based on historical controls.
When determining the duration of rehabilitation treatment for patients with DoC, it should be taken into account that recovering from a severe disorder of consciousness may take some time (months to years after the index event) (21). A prospective observational study with 39 patients with DoC found relevant improvements in neurological examination findings after a median of 22 months. After a median of 485 days, 69% of patients who were initially in UWS, achieved at least MCS, and 14% of patients with initial MCS recovered from DoC. Improvements in the level of consciousness were noted even after more than three years post brain injury. Thus, in addition to a sufficiently long primary inpatient neurological rehabilitation program, rehabilitation should (B) also be offered to patients in chronic phases of the disease.
To estimate the necessary duration of rehabilitation, clinical tools, such as the CRS-R, should (B) be used to assess the level of consciousness rather than relying alone on scales assessing independence in activities of daily living (for example, the Barthel index) because of the low sensitivity of the latter (2, 16, 18).
Pharmacotherapy
Prior to initiating a specific drug therapy to increase the level of consciousness, any medication ought to (B) be discontinued that had sedation as its primary indication, for example, in patients with agitation or dysautonomic reactions. This does not apply to medications that are clearly indicated to treat existing diseases or symptoms, such as anticonvulsants or antispasticity drugs. Even though this recommendation is not evidence-based given the absence of related studies, clinical experience shows that in some patients an improvement in the level of consciousness can already be achieved with this step.
The most important drug-treatment recommendation concerns the substance amantadine which is approved as amantadine sulfate for intravenous or oral use to treat post-coma vigilance impairment. To improve the state of consciousness, treatment ought to (B) be attempted with escalating enteral doses of amantadine up to a maximum dose of 400 mg daily. This recommendation is based on a randomized controlled trial (RCT) with 184 patients with traumatic brain injury who were treated with escalating doses (initially 200 mg/day) over a period of four weeks (23). The level of consciousness of patients in the amantadine group improved faster compared to the placebo group, with both groups approaching each other again during the follow-up period after discontinuation of the medication. Statements about the effect of amantadine treatment over a period of more than four weeks cannot be made. Amantadine was well tolerated and not associated with an increased incidence of epileptic seizures. In a small, retrospective case-control study in patients with UWS due to intracerebral hemorrhage, treatment with amantadine was also associated with a faster regain of consciousness; however, after five months there was no longer a difference in the rate of patients with improved consciousness (24). Several systematic reviews and international guidelines/ practice recommendations see an additional benefit in treatment with amantadine (1, 9, 10).
Paradoxically, treatment with the hypnotic agent zolpidem may also be considered off-label to improve the level of consciousness. Several studies found responder rates (defined as improved responsiveness for several hours) between 4% and 10% when zolpidem was administered during the daytime (25). In the rare cases where the level of consciousness improves for some hours after a single oral dose of 10 mg zolpidem, this drug could be used in a targeted manner, for example in the context of activating therapies or interactions with family members.
Positioning methods
Verticalization of patients with severe DoC, for example by means of a tilt table, standing board or standing bed, has been one of the established treatment concepts for decades, used both to improve the level of consciousness and to prevent or treat complications, such as the development of equinovarus foot, pressure ulcers and orthostatic dysregulation.
Ten one-hour verticalization sessions within a period of three weeks, using either a tilt table with integrated robotic leg training (device: Erigo) or a conventional tilt table/standing board, led to an improvement in the level of consciousness in 44 patients with DoC at eight weeks after brain injury (26). After the three-week intervention phase, 64% of patients had already emerged from UWS and 32% even emerged from MCS. No differences in effectiveness were found between the various verticalization devices used. The strength of evidence of the study is significantly limited by the lack of a control group without verticalization.
A systematic review identified ten studies with 233 patients with DoC and concluded that repetitive passive verticalization with tilt tables/standing boards can lead to improvements in consciousness; however, the supporting evidence was not yet classified as convincing (27). This view is also in line with a recent prospective RCT of 47 patients, showing a highly significant correlation of effective verticalization time with CRS-R improvement (R = 0.49; p<0.001) (28). This study found no advantage in the use of a robotic tilt table rather than standard physiotherapy which also aimed at bringing patients from the bed into an upright position.
Despite the poor available evidence, verticalization ought to (B) be performed to improve the level of consciousness, given the clinical experience, low potential for harm and potential positive effects, albeit beyond an increase in the level of consciousness.
Sensory stimulation techniques and music therapy
Multisensory stimulation techniques involve auditory, visual, tactile, olfactory, and gustatory stimuli that lead to improvements in the level of consciousness and more complex clinically observable responses, especially where these stimuli are of high emotional or autobiographical relevance. These include, for example, having relatives read a story aloud, looking at family photos, or oral stimulation with preferred flavors. The available studies on this intervention are small (41 patients with DoC) and of low quality. In a sequential study design, however, the results confirmed positive effects of targeted sensory stimulation on the activation of relevant brain regions and patient responsiveness (29, 30). Due to the low potential for harm and the fact that the method is readily available, multisensory stimulation with high emotional or autobiographical relevance ought to (B) be performed.
For music therapy and auditory stimulation techniques, three studies of moderate quality suggest improvements in level of consciousness related to auditory stimuli of personal relevance; however, it is still unknown how stable these clinical effects will be over time (31– 33). Despite the methodological weaknesses of these studies, music therapy and auditory stimulation with biographical reference ought to (B) be used. They are not associated with relevant side effects and support the active involvement of relatives in the treatment.
Neuromodulation techniques with direct current
Transcranial direct-current stimulation (tDCS) is a non-invasive neuromodulation technique (34). Depending on where the two electrodes are placed, different areas of the brain can be activated (anodal stimulation) or inhibited (cathodal stimulation). There were 12 RCTs and two meta-analyses in total addressing the effect of anodal tDCS in the left dorsolateral prefrontal cortex on consciousness in patients with DoC (35, 36).
The most comprehensive meta-analysis found a large effect size for the subgroup of patients with MCS for improvement in the level of consciousness by anodal tDCS of the left dorsolateral prefrontal cortex, measured with CRS-R (MCS 0.88; 95% confidence interval: [0.37; 1.39]; p = 0.0008) (36). It is still unknown whether the effects of neuromodulation will persists beyond the end of stimulation.
Given that tDCS is comparatively easy to apply and has already been routinely used for several years in the context of other diseases in a safe manner and largely without side effects, a series of anodal tDCS of the left dorsolateral prefrontal cortex ought to (B) be administered as an intervention for improving level of consciousness in patients in MCS in the context of a parallel activating therapy, such as physiotherapy, occupational therapy, speech therapy, and music therapy.
Other interventions
Given the lack of data from studies, it was not possible to make a recommendation for or against the following interventions:
Transcranial laser therapy and shock wave therapy
Hyperbaric oxygen therapy
Acupuncture
Spinal stimulation therapy
Median-nerve stimulation therapy.
Fetal cell transplantation is fraught with significant medical ethical and legal concerns and limitations. It is not recommended to treat patients with DoC after acute brain injury with fetal cell transplantation.
Ethics
The vulnerability of patients with DoC is so great that it raises relevant questions about ethical implications. The ethical recommendations presented here are based on expert consensus in the literature and in the Guideline Development Group. On principle, it is ethically required to aim for an increase in the level of consciousness, provided that this is done under a continuing risk-benefit assessment focusing on the well-being of the person. While this can increase the intensity of suffering or create suffering in the first place, increased awareness and the improved communication that comes with it also opens up a variety of possibilities for relieving suffering; moreover, a conscious human existence may be regarded as a value in itself (37).
For any treatment intended to improve the level of consciousness, the proxy consent of a representative, which must be based on the patient‘s living will or presumed will, is indispensable. Here, the model of shared decision making is to be followed (38). Finally, the ethical principle of justice obliges to provide equal access to therapies intended to improve the level of consciousness for all persons concerned and to consider the legitimate interests of other patients, relatives and other persons according to their ethical weight (39).
Discussion
Patients with DoC are a vulnerable patient population which, in our opinion, is currently receiving very heterogeneous rehabilitation, if any. Therefore, it is all the more important to consistently use the few treatment methods for which evidence of a positive effect on the level of consciousness is available. With hardly any RCTs of adequate power at hand, there is an urgent need for larger and methodologically sound clinical trials in this area of medicine.
Beyond the above recommendations for the diagnosis and management of DoC, it is indisputable that many medical, nursing, and therapeutic interventions are also required that, while not necessarily improving the level of consciousness, do improve the overall health of patients with DoC. These measures include preventing or treating complications. Needless to say, these clinically established and proven nursing and therapeutic interventions should be further applied in clinical practice in addition to the above-mentioned evidence-based methods and should also be subjected to evidence evaluation in the future so that comprehensive care guidelines can be developed for the severely affected population of patients with DoC. Examples of nursing and therapeutic interventions include:
Positioning for pressure ulcer prevention
Multimodal spasticity management
Preservation of joint mobility
Promotion of perception
Nutrition therapy
Tracheostomy tube management
Dysphagia treatment.
Given the limited number of intervention studies, we believe that in the population of patients with DoC lack of evidence does not necessarily mean that an intervention is ineffective.
Several of the positive intervention studies we have cited here included patients with DoC in a chronic stage, about 6–9 months after they suffered the acute brain injury (20, 21, 29, 32, 35). The vast majority of patients with DoC, however, have already been discharged from inpatient neurological-neurosurgical early rehabilitation into a nursing setting after this time, at least in Germany (40). It is unknown whether therefore the rehabilitation potential of these patients is not fully realized. This should be taken into account when developing clinical treatment pathways in the future. Until then, these recommendations should be implemented to the extent possible even in chronic stages of the disease.
Acknowledgments
Clinical guidelines are not peer-reviewed in Deutsches Ärzteblatt, as well as in many other journals, because clinical (S3) guidelines are texts which have already been repeatedly evaluated, discussed and broadly consented by experts (peers).
Translated from the original German by Ralf Thoene, MD.
Footnotes
Conflict of interest statement
AB received funding from the German Federal Ministry of Education and Research (BMBF) for the PerBrain study on the multimodal diagnosis of DoC patients (pts), external funds for a scientific project focused on rTMS therapy in DoC pts from the CNS – Hannelore Kohl Foundation, and from the EU Horizon 2020 Research Framework Program for the DOCMA project evaluating the effect of tDCS. Payments were made to the respective institution. He received lecture fees from BMS. He received reimbursement of congress fees and travel expenses from the German Society for Neurorehabilitation (DGNR). He is a Member of the Presidium of the German Society for Neurorehabilitation (DGNR). He received VR goggles from the manufacturer CUREosity GmbH for planning and conducting clinical rehabilitation studies using VR technology.
PMK received fees for teaching activities from Neuroraum and SWAN. She received reimbursement of congress fees from the International Brain Injury Association (IBIA).
IRF is the second chair of the Society for Aphasia Research and Treatment (GAB).
FM received consultancy and lecture fees as well as reimbursement of congress fees and travel expenses from Ipsen and Merz.
The remaining authors declare that no conflict of interest exists.
References
- 1.Giacino JT, Katz DI, Schiff ND, et al. Practice guideline update recommendations summary: disorders of consciousness: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology. 2018;91:450–460. doi: 10.1212/WNL.0000000000005926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kondziella D, Bender A, Diserens K, et al. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur J Neurol. 2020;27:741–756. doi: 10.1111/ene.14151. [DOI] [PubMed] [Google Scholar]
- 3.van Erp WS, Lavrijsen JC, van de Laar FA, Vos PE, Laureys S, Koopmans RT. The vegetative state/unresponsive wakefulness syndrome: a systematic review of prevalence studies. Eur J Neurol. 2014;21:1361–1368. doi: 10.1111/ene.12483. [DOI] [PubMed] [Google Scholar]
- 4.Boltzmann M, Schmidt SB, Gutenbrunner C, et al. The influence of the CRS-R score on functional outcome in patients with severe brain injury receiving early rehabilitation. BMC Neurol. 2021;21 doi: 10.1186/s12883-021-02063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grill E, Klein AM, Howell K, et al. Rationale and design of the prospective German registry of outcome in patients with severe disorders of consciousness after acute brain injury. Arch Phys Med Rehabil. 2013;94:1870–1876. doi: 10.1016/j.apmr.2012.10.040. [DOI] [PubMed] [Google Scholar]
- 6.Estraneo A, Fiorenza S, Magliacano A, et al. Multicenter prospective study on predictors of short-term outcome in disorders of consciousness. Neurology. 2020;95:e1488–e1499. doi: 10.1212/WNL.0000000000010254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estraneo A, Magliacano A, Fiorenza S, et al. Risk factors for 2-year mortality in patients with prolonged disorders of consciousness: an international multicentre study. Eur J Neurol. 2022;29:390–399. doi: 10.1111/ene.15143. [DOI] [PubMed] [Google Scholar]
- 8.Magliacano A, Liuzzi P, Formisano R, et al. Predicting long-term recovery of consciousness in prolonged disorders of consciousness based on coma recovery scale-revised subscores: validation of a machine learning-based prognostic index. Brain Sci. 2022;13 doi: 10.3390/brainsci13010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Royal College of Physicians. Prolonged disorders of consciousness following sudden onset brain injury. National Clinical Guidelines. London: Royal College of Physicians. 2020 [Google Scholar]
- 10.Thibaut A, Schiff N, Giacino J, Laureys S, Gosseries O. Therapeutic interventions in patients with prolonged disorders of consciousness. Lancet Neurol. 2019;18:600–614. doi: 10.1016/S1474-4422(19)30031-6. [DOI] [PubMed] [Google Scholar]
- 11.Schnakers C, Monti MM. Disorders of consciousness after severe brain injury: therapeutic options. Curr Opin Neurol. 2017;30:573–579. doi: 10.1097/WCO.0000000000000495. [DOI] [PubMed] [Google Scholar]
- 12.Edlow BL, Claassen J, Schiff ND, Greer DM. Recovery from disorders of consciousness: mechanisms, prognosis and emerging therapies. Nat Rev Neurol. 2021;17:135–156. doi: 10.1038/s41582-020-00428-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bender A, Blödt S, Bodechtel U, et al. S3-LL Neurologische Rehabilitation bei Koma und schwerer Bewusstseinsstörung im Erwachsenenalter. Leitlinien für die Neurorehabilitation. Deutsche Gesellschaft für Neurorehabilitation e.V. (DGNR) 2022 [Google Scholar]
- 14.van Erp WS, Lavrijsen JC, Vos PE, Bor H, Laureys S, Koopmans RT. The vegetative state: prevalence, misdiagnosis, and treatment limitations. J Am Med Dir Assoc. 2015;16 doi: 10.1016/j.jamda.2014.10.014. e9-e14. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Hu X, Hu Z, Sun Z, Laureys S, Di H. The misdiagnosis of prolonged disorders of consciousness by a clinical consensus compared with repeated coma-recovery scale-revised assessment. BMC Neurol. 2020;20 doi: 10.1186/s12883-020-01924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maurer-Karattup P, Giacino J, Luther M, Eifert B. Diagnostik von Bewusstseinsstörungen anhand der deutschsprachigen Coma Recovery Scale-Revised (CRS-R) Neurol Rehabil. 2010;16:232–246. [Google Scholar]
- 17.Wannez S, Heine L, Thonnard M, Gosseries O, Laureys S Coma Science Group collaborators. The repetition of behavioral assessments in diagnosis of disorders of consciousness. Ann Neurol. 2017;81:883–889. doi: 10.1002/ana.24962. [DOI] [PubMed] [Google Scholar]
- 18.Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004;85:2020–2029. doi: 10.1016/j.apmr.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 19.Pincherle A, Johr J, Chatelle C, et al. Motor behavior unmasks residual cognition in disorders of consciousness. Ann Neurol. 2019;85:443–447. doi: 10.1002/ana.25417. [DOI] [PubMed] [Google Scholar]
- 20.DeFina PA, Fellus J, Thompson JWG, et al. Improving outcomes of severe disorders of consciousness. Restor Neurol Neurosci. 2010;28:769–780. doi: 10.3233/RNN-2010-0548. [DOI] [PubMed] [Google Scholar]
- 21.Bareham CA, Allanson J, Roberts N, et al. Longitudinal assessments highlight long-term behavioural recovery in disorders of consciousness. Brain Commun. 2019;1 doi: 10.1093/braincomms/fcz017. fcz017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martens G, Bodien Y, Thomas A, Giacino J. Temporal profile of recovery of communication in patients with disorders of consciousness after severe brain injury. Arch Phys Med Rehabil. 2020;101:1260–1264. doi: 10.1016/j.apmr.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Giacino JT, Whyte J, Bagiella E, et al. Placebo-controlled trial of amantadine for severe traumatic brain injury. N Engl J Med. 2012;366:819–826. doi: 10.1056/NEJMoa1102609. [DOI] [PubMed] [Google Scholar]
- 24.Gao Y, Zhang Y, Li Z, Ma L, Yang J. Persistent vegetative state after severe cerebral hemorrhage treated with amantadine: a retrospective controlled study. Medicine (Baltimore) 2020;99 doi: 10.1097/MD.0000000000021822. e21822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whyte J, Rajan R, Rosenbaum A, et al. Zolpidem and restoration of consciousness. Am J Phys Med Rehabil. 2014;93:101–113. doi: 10.1097/PHM.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 26.Krewer C, Luther M, Koenig E, Müller F. Tilt table therapies for patients with severe disorders of consciousness: a randomized, controlled Trial. PLoS One. 2015;10 doi: 10.1371/journal.pone.0143180. e0143180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng H, King A. A systematic review of head-up tilt to improve consciousness in people with a prolonged disorder of consciousness. Clin Rehabil. 2021;35:13–25. doi: 10.1177/0269215520946696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenfelder MJ, Helmschrott VC, Willacker L, Einhäupl B, Raiser TM, Bender A. Effect of robotic tilt table verticalization on recovery in patients with disorders of consciousness: a randomized controlled trial. J Neurol. 2023;270:1721–1734. doi: 10.1007/s00415-022-11508-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Stefano C, Cortesi A, Masotti S, Simoncini L, Piperno R. Increased behavioural responsiveness with complex stimulation in VS and MCS: preliminary results. Brain Inj. 2012;26:1250–1256. doi: 10.3109/02699052.2012.667588. [DOI] [PubMed] [Google Scholar]
- 30.Cheng L, Cortese D, Monti MM, et al. Do sensory stimulation programs have an impact on consciousness recovery? Front Neurol. 2018;9 doi: 10.3389/fneur.2018.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castro M, Tillmann B, Luauté J, et al. Boosting cognition with music in patients with disorders of consciousness. Neurorehabil Neural Repair. 2015;29:734–742. doi: 10.1177/1545968314565464. [DOI] [PubMed] [Google Scholar]
- 32.O‘Kelly J, James L, Palaniappan R, Taborin J, Fachner J, Magee WL. Neurophysiological and behavioral responses to music therapy in vegetative and minimally conscious states. Front Hum Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pape TL, Rosenow JM, Steiner M, et al. Placebo-controlled trial of familiar auditory sensory training for acute severe traumatic brain injury: a preliminary report. Neurorehabil Neural Repair. 2015;29:537–547. doi: 10.1177/1545968314554626. [DOI] [PubMed] [Google Scholar]
- 34.Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Feng Y, Zhang J, Zhou Y, Bai Z, Yin Y. Noninvasive brain stimulation for patients with a disorder of consciousness: a systematic review and meta-analysis. Rev Neurosci. 2020 doi: 10.1515/revneuro-2020-0033. Doi: 10.1515/revneuro-2020-0033. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 36.Liu S, Gao Q, Guan M, et al. Effectiveness of transcranial direct current stimulation over dorsolateral prefrontal cortex in patients with prolonged disorders of consciousness: a systematic review and meta-analysis. Front Neurol. 2022;13 doi: 10.3389/fneur.2022.998953. 998953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weijer C, Peterson A, Webster F, et al. Ethics of neuroimaging after serious brain injury. BMC Med Ethics. 2014;15 doi: 10.1186/1472-6939-15-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armstrong MJ. Developing the disorders of consciousness guideline and challenges of integrating shared decision-making into clinical practice. J Head Trauma Rehabil. 2019;34:199–204. doi: 10.1097/HTR.0000000000000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wade DT. Using best interests meetings for people in a prolonged disorder of consciousness to improve clinical and ethical management. J Med Ethics. 2018;44:336–342. doi: 10.1136/medethics-2017-104244. [DOI] [PubMed] [Google Scholar]
- 40.Pohl M, Bertram M, Bucka C, et al. [Course of rehabilitation in early neurological/neurosurgical rehabilitation. Results of a 2014 multi-center evaluation in Germany] Nervenarzt. 2016;87:634–644. doi: 10.1007/s00115-016-0093-1. [DOI] [PubMed] [Google Scholar]
- E1.Group OLoEW. The Oxford 2011 Levels of Evidence. Oxford: Oxford Centre for Evidence-Based Medicine. 2011 [Google Scholar]
- E2.Platz T. [Evidence-based Practice Guidelines for the German Society for Neurology (DGN) and the German Society for Neurorehabilitation (DGNR): methods for systematic evidence-to-decision process] Fortschr Neurol Psychiatr. 2021;89:415–423. doi: 10.1055/a-1309-1856. [DOI] [PubMed] [Google Scholar]
- E3.Grading of Recommendations A, Development and Evaluation (GRADE) Working Group. GRADE Handbook—Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Hamilton. 2013 [Google Scholar]