Abstract
The recognition of dominantly inherited micro-satellite instable (MSI) cancers caused by pathogenic variants in one of the four mismatch repair (MMR) genes MSH2, MLH1, MSH6 and PMS2 has modified our understanding of carcinogenesis. Inherited loss of function variants in each of these MMR genes cause four dominantly inherited cancer syndromes with different penetrance and expressivities: the four Lynch syndromes. No person has an “average sex “or a pathogenic variant in an “average Lynch syndrome gene” and results that are not stratified by gene and sex will be valid for no one. Carcinogenesis may be a linear process from increased cellular division to localized cancer to metastasis. In addition, in the Lynch syndromes (LS) we now recognize a dynamic balance between two stochastic processes: MSI producing abnormal cells, and the host’s adaptive immune system’s ability to remove them. The latter may explain why colonoscopy surveillance does not reduce the incidence of colorectal cancer in LS, while it may improve the prognosis. Most early onset colon, endometrial and ovarian cancers in LS are now cured and most cancer related deaths are after subsequent cancers in other organs. Aspirin reduces the incidence of colorectal and other cancers in LS. Immunotherapy increases the host immune system’s capability to destroy MSI cancers. Colonoscopy surveillance, aspirin prevention and immunotherapy represent major steps forward in personalized precision medicine to prevent and cure inherited MSI cancer.
Introduction
Ten years ago, revised guidelines were issued for the clinical management of a group of dominantly inherited cancer predisposition syndromes occurring in adults and caused by inherited pathogenic variants of the four mismatch-repair (MMR) genes path_MLH1, path_MSH2, path_MSH6 and path_PMS2 [1, 2]. They were referred to collectively as Lynch syndrome (LS). At the time, penetrance and expressivities of pathogenic variants of the four genes were not well established.
During organogenesis different genes are inactivated in different tissues [3] and, although the functions of the MMR genes are not restricted to tissues or organs derived from embryonic endoderm it appears that these tissues and organs do not have adequate alternative repair systems to compensate for faulty MMR genes.
Except for infrequent brain tumors and osteosarcomas, LS cancers occur in the endoderm-derived lining of the stomach, large and small intestine, the pancreas, bile duct, urinary tract, prostate and endometrium [4, 5]. Ovarian cancer in LS is often of an endometrioid subtype indicating that these cancers may be derived from the cells similar to endometrial cancers [6, 7]. While the EPCAM gene itself is not a cause of LS, the MSH2 promoter is transcriptionally silenced by deletions involving its 3’ region which result in a distinct expression of LS cancers [8]. LS cancers are characterized by loss of the wild type allele following somatic (second hit) mutation, leading to micro-satellite instability (MSI) [9]. MSI cells produce abnormal peptides (neopeptides) which are recognized and targeted by the host immune system [10]. It was originally assumed that adenomas were precursors to all colorectal cancers (CRCs) and removal of adenomas by colonoscopy was advocated in carriers of pathogenic MMR variants (path_MMR) to reduce the incidence of CRC [1]. It soon became apparent, however, that CRC incidence was not reduced as much as expected by colonoscopy in LS. This paper discusses new knowledge reported the last decade.
Epidemiology
The Prospective Lynch Syndrome Database (PLSD)
In 2012 the European Hereditary Tumor Group (www.ehtg.org), at that time denoted the Mallorca Group, [1] decided to compile information on follow-up of path_MMR carriers across multiple specialist centres to answer three questions:
To what degree does colonoscopy surveillance reduce CRC incidence in path_MMR carriers?
What is the penetrance and expressivity of pathogenic variants in each of the four LS-associated genes?
What is the survival of carriers when followed-up as recommended, to facilitate early diagnosis and treatment?
The initial results were published by Møller et al. [11–13]. A more extensive and detailed report confirming the results in the three first reports was recently published [14]. Below we suggest an interpretation of the findings from these and further studies that were triggered by the initial results, and of concomitant tumor biological, prevention and treatment studies and mathematic modelling of the carcinogenetic paradigms that may help to explain what we observe.
The PLSD methods
The PLSD database, it’s structure and the methods for producing the results have been described in detail elsewhere [15–18]. A randomized controlled trial including a control group of path_MMR carriers who would be denied recommended medical interventions was considered impossible. Therefore, we performed an open, prospective observational study. Independently, a complementary retrospective segregation analysis in LS families was performed by the International Mismatch Repair Consortium (IMRC) which enabled estimation of the CRC incidence before surveillance colonoscopy had been widely implemented [19]. The results confirmed that cancer incidence in path_MMR carriers was not increased significantly before 25 years of age.
Our intentions were to examine the accepted paradigms of carcinogenesis and the effects of interventions. In all studies, the results obtained reflect the parameters used for ascertainment and/or the assumptions made when considering the results. To avoid these biases, when designing the PLSD neither the ascertainment model nor the methods used to compile the results included any assumption on carcinogenesis or the effects of interventions. Instead, the methods involved an assumption-free description of the empirical information observed. Compliant with the reporting methods of cancer registries, cancers were scored as discrete events by organ and age, allowing events to be considered as the result of stochastic probabilities in a time dimension.
The results obtained from the PLSD data were therefore empirically observed cancer incidences and subsequent overall survival in path_MMR carriers who were subjected to follow-up including colonoscopy surveillance in expert hereditary cancer centres world-wide. There is no indication that methodological problems could have substantially confounded the main results. Here we provide a brief summary and an interpretation of the results published to date by the PLSD.
The PLSD results
Colonoscopy and incidence of CRC
Compared with published estimates of CRC incidence in former generations before colonoscopy was widely instituted, CRC incidence in LS patients subjected to regular colonoscopy surveillance was increased for path_MLH1 and path_MSH2 carriers, not reduced for path_MSH6 carriers and possibly (but not significantly) reduced in path_PMS2 carriers < 50 years of age [20].
Penetrance and expressivity
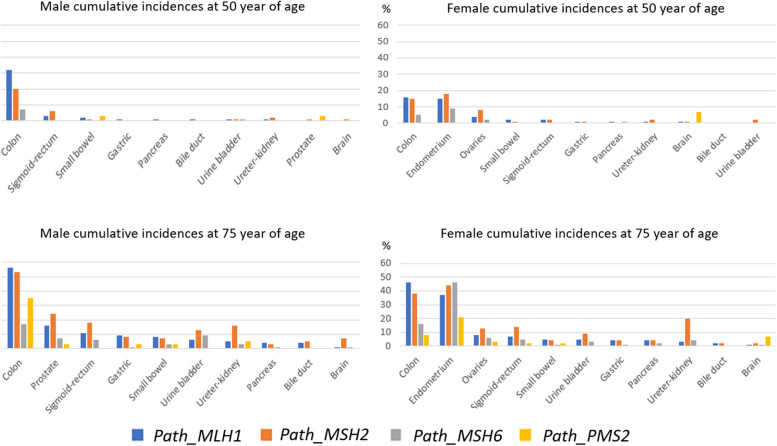
Cumulative cancer incidences stratified by MMR gene, sex, and carriers' age (50 and 75 y.o.a.) when subjected to follow-up, including colonoscopy to achieve early cancer diagnosis, are reported [14] and illustrated in Figs. 1 and 2. Cancers of the endometrium, colon and ovary started to appear in early adult life. Cancers of other organs were diagnosed later and mainly in survivors of earlier cancers [21]. Penetrance and expressivities were specific to each gene.
Fig. 1.
Cumulative incidences of cancers in male and female carriers subjected to colonoscopy stratified by gene, and sex and age (50 and 75 y.o.a.), ordered by incidence in path_MLH1 carriers. The graphs are based on figures given in [14]
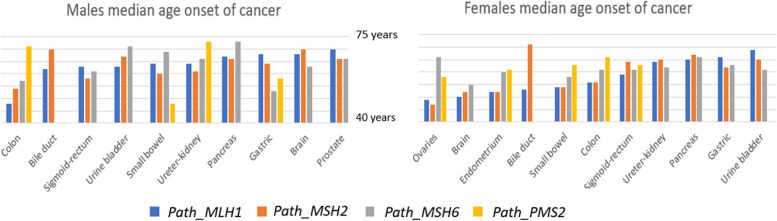
Fig. 2.
Median ages of onset of cancers in male and female carriers subjected to colonoscopy, by gene and sex, ordered by median ages in path_MMR carriers. The graphs are based on figures given in [14]
Survival after cancers detected during follow-up
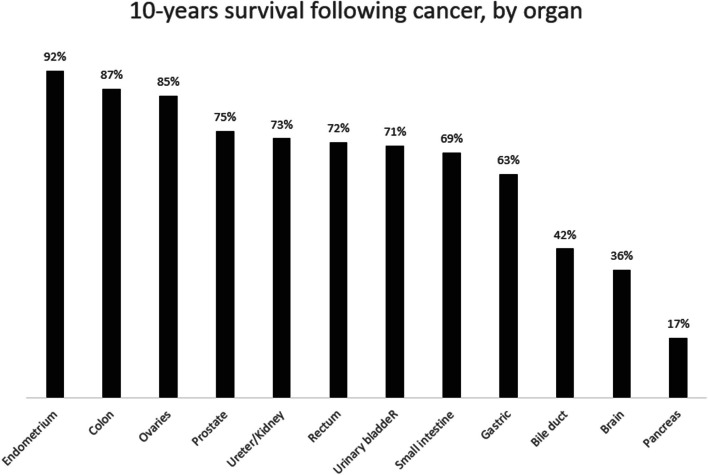
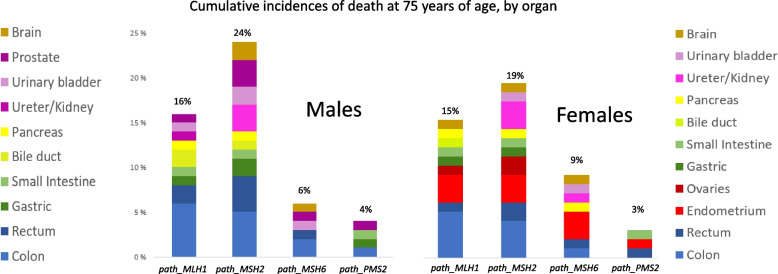
Most early onset cancers in the endometrium, colon and ovaries detected during follow up were cured [14] (Fig. 3). However, later in life, the survivors often developed cancers in other organs most of which were associated with lower overall survival [14] (Fig. 4).
Fig. 3.
10-year survival following cancer in different organs in path_MMR carriers subjected to early diagnosis and treatment including colonoscopy. The graph is based on figures given in [14]. While there was no difference in survival between carriers of path_MMR variants by gene, cancer in path_MSH6 and path_PMS2 carriers were not frequent enough to measure survival apart from after endometrial cancer in path_MSH6 carriers
Fig. 4.
Cumulative incidence of death at 75 years of age following cancer in male and female carriers subjected to colonoscopy, by gene. The graphs are based on figures given in [14]
These results indicated that both penetrance and expressivities of the genes in question have been affected by a substantial time-trend as most cases of CRC are now cured, which was not the case historically. In patients who received colonoscopy surveillance and relatively recent treatments (which in most individuals did not include immunotherapy), the results suggest that there are four different inherited MSI cancer syndromes:
The four Lynch syndromes
The MSH2 Syndrome
The MSH2 Syndrome is autosomal dominantly inherited. Penetrance is high.
Path_MSH2 carriers are at high risk of cancers in all organs that are affected across the Lynch syndromes, with early onset of cancer in endometrium/ovaries and colon. Most carriers survive these first cancers following early detection and treatment. Cancers in other organs are most often diagnosed in survivors of the early onset cancers and include rectal, upper urinary tract, prostate and brain cancers.
Few founder variants – fitness is low
Most cancer deaths are associated with non-CRC cancers, particularly those of the endometrium, rectum, upper urinary tract, prostate and brain
Colonoscopy overdiagnoses colon cancer.
The MLH1 syndrome
The MLH1 Syndrome is autosomal dominantly inherited. Penetrance is high.
Early onset and high incidence of cancer in the colon, endometrium and ovaries. Most carriers survive their first cancer following early detection and treatment. Cancers in other organs are most often diagnosed in survivors of the early onset cancers, and most often include rectal, stomach, small intestine, bile duct and pancreatic cancers.
Few founder variants – fitness is low
Most cancer deaths are associated with non-CRC cancers, particularly those of the endometrium, bile duct and pancreas.
Colonoscopy overdiagnoses colon cancer.
The MSH6 syndrome
The MSH6 Syndrome is autosomal dominantly inherited with sex limitation. Penetrance is high in females but low in males.
There is a high incidence of endometrial and ovarian cancer that occurs at older ages than in path_MSH2/MLH1 carriers. There is an increased incidence of CRC in both sexes that is nonetheless much lower than in path_MSH2/MLH1 carriers. Incidences of cancers in other organs are low.
Few founder variants – fitness is low.
Detection rate by family history is low because of the sex-limited inheritance.
The inconclusive effect of colonoscopy is due to the low number of carriers reported to the PLSD.
The PMS2 syndrome
The PMS2 Syndrome is autosomal dominantly inherited.
Path_PMS2 carriers have a slightly increased incidence of CRC and endometrial cancer in young adults, with higher incidence in older ages. Increased cancer incidence in other organs is not demonstrated.
Estimates of cancer incidences are difficult because of ascertainment biases.
Founder variants present, fitness seems good.
In contrast to the three other syndromes, the low incidence of CRC in young adults receiving colonoscopy surveillance may suggest that colonoscopy reduces CRC incidence.
Because of the very much lower cancer risks in the PMS2 syndrome and potentially different carcinogenetic mechanisms associated with CRC development, the discussion below may not be pertinent to path_PMS2 carriers.
The PLSD and the InSiGHT variant database
When using the international InSIGHT database that indicates whether variants of the MMR genes are disease-associated or not [2], the PLSD database that indicates the penetrance and expressivity of pathogenic or likely pathogenic variants is displayed by selecting the MMR CANCER RISK tab. The PLSD is also available directly at www.plsd.eu. It interactively displays the remaining life-time risk for cancer in each organ when the user indicates the carrier’s age, sex and genetic variant. This feature of PLSD may be helpful to carriers and health care workers.
Inherited congenital mismatch repair deficiency (CMMRD)
Inherited, biallelic path_PMS2, path_MLH1, path_MSH2 and path_MSH6 variants are recognized to cause the recessively inherited congenital mismatch repair deficiency (CMMRD) syndrome [22]. CMMRD is characterized by a high incidence of MSI non-endodermal malignancies in early life. Path_PMS2 variants are the most frequent cause of CMMRD. The relative rarity of path_MLH1 and path_MSH2 causing CMMRD has caused speculation that most homozygotes or compound heterozygotes may not survive fetal development. A more detailed discussion of CMMRD is outside the scope of this paper.
Relations in time between cancers and consequences of early diagnosis and treatment
Most of the frequent and early onset cancers in the four Lynch syndromes are cured following early diagnosis and treatment, which may be achieved in many cases through colonoscopy and gynaecologic examinations [13] and by promotion of cancer awareness with early consultation in relation to “red flag” symptoms. Most cancer-associated deaths in carriers who are subject to follow-up are now associated with non-CRC cancers [14].
Follow up studies suggest that the occurrence of a non-CRC first cancer was not associated with CRC incidence [12] and neither stage at CRC diagnosis, nor survival following CRC was associated with the interval between colonoscopies [23–26]. These findings were not as expected from the adenoma-carcinoma sequence paradigm of CRC. Instead, they were in keeping with the notion that the observed cancers are the result of stochastic probabilities in time, in conflict with the notion of linear progression from acquisition of an initial somatic pathogenic variant that causes increased mitotic activity, to a dysplastic adenoma and eventually an invasive and metastasizing cancer [27, 28]. If the stochastic model is correct for most LS cancers, the average incidences we have published are valid for groups but may have limited predictive value for an individual. No person has an “average sex “or a pathogenic variant in an “average Lynch syndrome gene” and results that are not stratified by gene and sex will be valid for no one. Modifiers of penetrance may be important in determining which cancers occur and when. More detailed analyses investigating whether there are associations between cancers, or whether they do indeed reflect stochastic chances, would be interesting.
Most cancers diagnosed before 50 years of age in male path_MLH1 carriers are colon cancers, and cancers occurring in other organs reflect the time trend of increased colon cancer survival. Most cancers in young male path_MSH2 carriers are also colon cancers, but rectal, urinary tract, prostate and brain cancers are more frequent later in life and with increased colon cancer survival they have become the major causes of death.
In females, however, endometrial and ovarian cancers together are the first and most frequent cancers in path_MLH1 carriers and even more so in path_MSH2 carriers. Colon cancer has a slightly lower incidence and older onset in path_MSH2 than path_MLH1 carriers. Considering LS as a syndrome of inherited CRC or gastro-intestinal cancers ignores gynaecological cancers as the main manifestation in women, and that gynaecological cancers together with urothelial, prostate and brain cancers are the leading causes of death in path_MMR carriers who receive follow-up with colonoscopy for early diagnosis and treatment.
Ascertainment biases when estimating variant frequencies, penetrance and expressivities
Identification of LS families has been and still is biased. The first clinical criteria to identify affected families were constructed for research purposes to identify the causative gene(s) and reflected the misconception that we were looking for inherited CRC, not a syndrome of inherited cancer in many organs, and they primarily identified path_MLH1 families [29]. The understanding that endometrial cancer was part of the syndrome was reflected in later criteria [30] and led to more path_MSH2 and especially path_MSH6 families being recognized. Because of sex-limited inheritance many path_MSH6 families did not fulfil these clinical criteria which assumed high cancer incidences in both sexes [31]. Path_PMS2 penetrance is so low that discrimination from normal variation by using family history is nearly impossible. A similar pattern of biases occurs when genetic testing is undertaken based on family history and when incident testing is focused on young onset CRC [32]. Only after all incidental cancers are tested for the four genes in question (in all ages and for all the genes in question) and the combined results are assembled, we will be able to fully determine the incidence and expressivities related to the genetic variants of the genes.
Contrary to the assumptions underlying the clinical Amsterdam 1 (AMS1) [29] and Amsterdam 2 (AMS2) [30] criteria, our studies in the PLSD have shown that ovarian cancer can be grouped together with endometrial and colon cancer as the early onset cancers in carriers who are subject to colonoscopy, while rectal cancer can be grouped with the other less frequent cancers that are seen mainly in survivors of the early onset cancers. Therefore, grouping colon and rectal cancer epidemiologically as one organ, as was done by the AMS1/2 criteria might now be considered a mistake. A possible interpretation of our findings is that colonoscopy may prevent rectal but not colon cancer in LS. The PLSD data also show that not including ovarian cancer in the AMS2 criteria was a mistake. And especially so when its treatment often includes hysterectomy which will prevent endometrial cancer. Including cancer in other organs will have little effect on the sensitivity of the criteria in the identification of LS families, because most of these cancers appear in survivors of the early onset cancers [21, 33].
Despite the consensus that colonoscopy reduces CRC incidence in the population as a whole, there is no recognized evidence that this statement is correct in path_MMR carriers, as there is only limited historical evidence based on three publications, all reporting observations made on a cohort of only 22 Finnish families [34–37].
The Finnish families were selected on the basis of one case of very early onset CRC with multiple CRC-affected relatives. Some of the additional methodological problems that were not discussed in those reports are as follows: the index clusters were not removed when calculating CRC incidences; it was incorrectly assumed that the family members had 50% carrier probability after the CRC-affected cases were excluded, and lead-time bias in the non-intervention group was not discussed. Therefore, the validity of the conclusions included in those reports is arguable. A later segregation analysis in 70 Finnish families of which 65 had a path_MLH1 variant [38] reported higher CRC incidence than in French families [39] with path_MLH1 variants, and much higher incidence than in a multi-national report on European families with path_MLH1 variants [20]. Additionally, these Finnish papers described findings in carriers of the local Finnish path_MLH1 founder variant which may not be representative of all path_MLH1 variants and may not be representative for carriers of pathogenic variants of the other genes. Findings in a single series should be confirmed in another before they are used to support clinical decision-making. Despite the considerable time since the Finnish reports, there is no other reported evidence that colonoscopy reduces CRC incidence in LS [34].
Ovarian MSI cancer
Ovarian cancer in LS carriers is dramatically different from ovarian cancer in path_BRCA1/2 carriers [40]. The observed risk of dying from gynaecologic cancer diagnosed before 40 years of age for carriers of path_MMR variants was 0%, leading us to conclude that prophylactic hysterectomy and/or oophorectomy before 40 years of age solely for cancer prevention reasons is unwarranted and unethical. Similarly, the observed risk of dying from ovarian cancer in path_MSH6 or path_PMS2 carriers diagnosed before 50 years of age was 0%, and in these carriers, prophylactic oophorectomy before 50 years of age solely for cancer prevention reasons is considered unwarranted and unethical [41]. Etiologic diagnosis of ovarian cancer cases is crucial to select proper treatment for the affected individual, for planning their follow-up for subsequent cancers, and for cascade testing of their relatives when hereditary cancer is demonstrated.
Combined results of epidemiological, biological, interventional and treatment studies
Both the adenoma-carcinoma and stochastic non-linear paradigms are ‘true’
While the PLSD results indicate the assumption that removing colorectal adenomas would prevent most CRC in LS is wrong, they are not in conflict with the adenoma-carcinoma paradigm being true. Rather they indicate that the adenoma-carcinoma pathway is not the only pathway to CRC in LS. Based on currently available information, several possibly interacting carcinogenetic pathways may now be considered [10, 28]. Independent of the PLSD epidemiological studies, tumor biological studies and treatment studies in the last decade have provided new evidence for the mechanisms of colorectal carcinogenesis in path_MMR carriers. In addition to the linear adenoma-carcinoma paradigm, we now know that adult path_MLH1 and path_MSH2 carriers may at any time have a very large number of colonic crypts lacking normal MMR gene products (dMMR) which may develop directly into cancer without going through a macroscopically visible non-invasive tumor stage, such as an adenoma [42, 43]. These dMMR crypts may become MSI and immunogenic as they produce neo-peptides that are identified and attacked by the host immune system. The advances in MSI CRC treatment include immunotherapy which boosts the host immune system to eradicate MSI cells including invasive MSI cancers [44, 45], indicating that the host HLA system is a key barrier to cancer development [46]. Research on making cancer vaccines based on this biological understanding is ongoing [47, 48]. The information from concomitant biological and treatment studies is in keeping with the notion that invasive MSI cancers may be removed by the host immune system which may be in line with the epidemiological findings that frequent colonoscopy over-diagnoses CRC in path_MMR carriers.
Consequences of the stochastic dynamic paradigm for carcinogenesis
The emerging combined picture of carcinogenesis in path_MMR carriers, is of a life-long dynamic situation in which carriers develop a very large number of MSI precursor lesions or tumors which are immunogenic and are controlled by the host immune system. A precursor lesion or tumor may escape by chance and become an invasive cancer. The observed frequency of this occurring may be used as a predictive probability for a group, but has low predictive value for a single individual. The epidemiological information indicates that such cancers might also be removed by the host immune system – a phenomenon which is demonstrated more dramatically when immunotherapy boosts the response and even advanced cancers may be destroyed. In summary, path_MMR carriers may manifest a life-long stochastic dynamic process of tumor initiation by the MSI pathway that is counteracted by the host immune system recognizing and destroying MSI tumors.
Overdiagnosis when screening for cancer as demonstrated by PLSD is not novel [49, 50]. While the mechanisms discussed here are specific for path_MMR carriers, there may be many and different mechanisms leading to over-diagnosis when screening is undertaken for cancers with different etiologies which all may involve a stochastic element, as discussed above.
Modifiers of the stochastic probabilities
Aspirin reduces CRC cancer incidence in path_MMR carriers [51], probably by modifying carcinogenetic pathways [52, 53] or modulating the immune microenvironment. A recent paper reports resistant starch intake to be associated with an overall reduction in upper extracolonic cancers in path_MMR carriers unselected for gene or gender. The major effect was on upper GI cancers [54]. Immunotherapy which increases survival in cancer patients, may be considered an example of an environmental factor modifying the probability that the host immune system will identify and remove a cancer. Aspirin and immunotherapy may be considered environmental factors associated with preventing and curing cancers by modifying the probabilities for stochastic events occurring. Adenomas are not frequent in LS and apparently are not caused by pathogenic variants of the MMR genes. The concept that CRC starts with an adenoma that is later modified to become an MSI cancer, implies that adenomas modified by MSI cause CRC, not the other way around. This is illustrated in the very rare cases of sebaceous skin adenomas which may become cancers in path_MMR carriers (Muir-Torre syndrome) [55]. It is accepted that sporadic CRC is mostly caused by adenomas that by stochastic chance develop into CRC. The assumption that colonoscopy would decrease CRC incidence in LS was based on this concept [1]. In recent years, growing evidence from both epidemiological (the PLSD reports) and biological [10, 27, 28, 56] reports indicate that there is an additional, and perhaps more frequent carcinogenetic pathway to CRC in LS which starts with MSI caused by path_MMR variants and that may not include a non-invasive adenomatous stage before invasive cancer occurs. The existence of the adenoma-carcinoma pathway is no argument against the MSI pathway also being true, and vice-versa. Carcinogenesis is complex, there is no single model to explain it all.
Recognizing that colonoscopy as a modifier probably prevents most CRCs that arise via the adenoma-carcinoma pathway may indicate that in path-MMR carriers undergoing colonoscopy surveillance and polypectomy, the observed CRCs in carriers subjected to colonoscopy arise mostly via the MSI pathway without a macroscopically visible tumor. If invasive MSI cancers are destroyed immunologically by the host, the more frequently colonoscopies are carried out the more frequently CRC will be observed, because some CRCs observed would otherwise have been removed later by the host immune system. Theoretically, this may challenge the normal association between early detection/treatment and improved survival. This may, at least in part, be a consequence of colonoscopy blocking the adenoma-carcinoma pathway, while in LS the immune system might remove invasive cancers. This notion is supported by an early Finnish study which reported the survival in patients receiving three-yearly colonoscopies together with the treatment available at that time [38] and that found comparable survival to that observed by PLSD today [14]. This study, however, described carriers of the local Finnish path_MLH1 founder variant which may not be representative of all path_MLH1 carriers and almost certainly not representative for carriers of pathogenic variants of the other genes. The late progression to metastatic spread and the good prognosis when LS-associated CRCs are removed surgically [57], may reflect that MSI cancer cells are vulnerable to being identified and removed by the host immune system when they are located outside the primary MSI cancer’s local environment.
Although recent epidemiological studies indicate that surveillance colonoscopy in path_MMR carriers has not decreased CRC incidence as hoped for, prevention of CRC-associated death is its true objective and this is largely being achieved. The PLSD results indicate that colonoscopy surveillance should be continued, but question the benefit of colonoscopy being performed more frequently than every three years. Immunotherapy will hopefully increase survival following MSI cancers, including the later onset cancers with currently poor survival. Aspirin reduces MSI cancer incidence, and in future anti-cancer vaccination may so do as well.
In general, cancer incidences increase exponentially with increasing age. Why, in path_MMR carriers, there is close to zero increase in cancer incidence before 25 years of age and why cancer incidence does not increase substantially after 50 years is not understood. It may possibly be associated with maturation and ageing of the host immune system and its interactions with MSI cells [58].
Are there any additional genes that cause inherited MSI cancers?
Variants of other genes may cause inherited MSI cancers through interactions with the four MMR genes described here. As mentioned in the introduction above, inherited deletion of the EPCAM tail causes silencing of the MSH2 promoter [8]. The POLE genes variants may cause somatic mutations in the MMR genes which in effect may cause inherited MSI cancers [59].
Different pathobiology of the two most common inherited cancer syndromes
The knowledge-based development of immunotherapy tailored to treat both inherited and sporadic MSI cancers, comes in addition to the knowledge-based development of PARP inhibitors to treat both inherited and somatic BRCA1/2 deficient DNA double-stranded break-associated cancer [60].
Because about 15% of all CRCs are MSI and may benefit from immunotherapy, there is a growing consensus that all CRC cases should be tested for MSI [61]. Germline testing for LS may be restricted to patients with MSI cancers and time-consuming family history documentation may become less important for other cases, enabling genetic counsellor time to be focused on those with proven germline predisposition and their relatives.
We now have knowledge-based personalized precision treatment for the two most frequent inherited cancer groups, and for biologically similar but sporadic cancers. All individuals with these types of cancer will benefit from testing to determine which carcinogenetic mechanisms have caused their cancer, enabling the selection of the most appropriate treatment, follow-up for subsequent cancers when needed, and for cascade testing in their families. Ovarian cancer, which is common to both path_MMR and path_BRCA1/2 carriers, and where both non-inherited MSI and path_BRCA1/2 associated cancers occur, is an example.
Conclusions
This paper identifies, delineates and denotes the group of dominantly inherited Lynch syndromes by their shared inherited trait which is MSI cancers (https://www.genome.gov/genetics-glossary/Mendelian-Inheritance). Corresponding with OMIM (https://www.ncbi.nlm.nih.gov/omim/?term=LYNCH+SYNDROME), we recognize four different Lynch syndromes caused by the four MMR genes, as described in this paper, and consider 3’ EPCAM deletions only as an alternative mechanism by which MSH2 may be silenced. The numbering of the Lynch syndromes in OMIM is confusing and in conflict with how Henry Lynch grouped and numbered them. Instead, we name the distinct syndromes by the four genes that cause them which is precise and self-explanatory, and gives room for more groups to be named in future if more genes are found to cause MSI cancers.
No person has an “average sex “or a pathogenic variant in an “average Lynch syndrome gene” and results that are not stratified by gene and sex will be valid for no one. The penetrance and expressivities of the genetic variants causing the Lynch syndromes are age-dependent, and discussions of penetrance or expressivity without considering age gives limited information. Average ages at which cancers are diagnosed reflect the ages of cases ascertained, not necessarily the ages at which cancers usually occur. Reports that are not stratified on genetic variants, sex and age may have limited utility. Compiling series with enough carriers to consider age, gene and sex needs wide international collaboration, as has been achieved by the PLSD.
Improved survival following early diagnosis and treatment of MSI cancers of the colon, endometrium and ovary has led to carriers living on and contracting subsequent cancers in other organs. These have worse prognoses. Tests for MSI have generally been optimised to demonstrate MSI colon cancers, but the different Lynch syndrome genes have different organ specific penetrance and expressivities. The prevalence of MSI in cancers in these other organs is not well studied, with respect to either how to test for MSI cancers in these organs or to estimate the frequency of MSI cancers. Identifying such MSI cancers would be of interest to select cases for immunotherapy tailored against MSI cancers. The obvious next steps for clinical research on the MSH2 and MLH1 syndromes include determining the effects of immunotherapy for cancers with currently poor prognoses.
When the MMR genes were identified as the causes of the Lynch syndromes, it was assumed that colonoscopy and removal of pre-invasive adenomas would prevent colon cancer. However, despite wide implementation of these measures, reduction in colorectal cancer incidence by colonoscopy surveillance has not been documented. Colonoscopy should be advocated to improve the prognosis when colon cancer is diagnosed, not to reduce colon cancer incidence in the Lynch syndromes.
Colon cancer may be asymptomatic and MSI colon cancer typically spreads late. Reports on colon cancer incidence that have not controlled for lead time bias should be interpreted with caution.
The host immune system may remove invasive MSI cancers, explaining overdiagnosis of colon cancer when colonoscopy surveillance is undertaken. A randomized control trial of colonoscopy versus no colonoscopy would not be possible for ethical reasons. What we could and should do, is to conduct a trial of one year versus three years intervals between colonoscopies and measure colon cancer incidences and survival. The quality of colonoscopy is subjected to time-trends. Whether improved techniques will reduce MSI colon cancer incidence remains to be seen.
Tumours are mostly tested genetically for structural gene changes and less commonly for epigenetic silencing, despite knowledge that the somatic “second hit” needed to abrogate mismatch repair is often epigenetic. Most MSI colon cancers are sporadic and result from bi-allelic somatic epigenetic silencing of an MMR gene. It may be of interest to discover more about the mechanisms causing second hits that lead to cancer in path_MMR carriers, and MSI sporadic cancers in non-carriers. These factors may be stochastic events, but they may also have inherited genetic components.
Acknowledgements
EHTG is a non-profit charity company registration number SC048407 in Scotland. EHTG is the legal host for PLSD. Anyone may contribute to and become part of the PLSD – for details see www.ehtg.org.
Authors’ contributions
Pål Møller initiated and designed the PLSD and wrote the paper. All authors who includes all board members of the European Hereditary Tumour Group and most contributors to the PLSD substantially contributed to the conception and design of the review article and interpreting the relevant literature, and were involved in writing the review article or revised it for intellectual content, and approved it.
Funding
PM, EH and MD-V received a grant from The Norwegian Cancer Society, Contract 194751–2017. TTS received grants from Cancer Society Finland, Sigrid Juselius Foundation, Jane and Aatos Erkko Foundation, Relander Foundation and the Academy of Finland. EJC and DGE are funded by the Manchester National Institute for Health Research (NIHR) Biomedical Research Centre (IS-BRC-1215–20007), IS-BRC-1215–20007. JRS received grant support previously from Health and Care Research Wales for the Wales Gene Park.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
Toni T. Seppälä: Healthfund Finland (CEO and stock holding), LS Cancer Diag (advisory board and stock holding) and Amgen Finland (speaker honorarium).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vasen HF, Blanco I, Aktan-Collan K, et al. Revised guidelines for the clinical management of Lynch syndrome (HNPCC): recommendations by a group of European experts. Gut. 2013;62(6):812–23, 10.1136/gutjnl-2012-304356. https://pubmed.ncbi.nlm.nih.gov/23408351/. [DOI] [PMC free article] [PubMed]
- 2.InSiGHT variant database, https://www.insight-group.org/variants/databases/ Accessed dates.
- 3.Boland MJ, Nazor KL, Loring F. Epigenetic regulation of pluripotency and differentiation. Circ Res. 2014; 115: 311–324. https://www.ahajournals.org/doi/full/10.1161/CIRCRESAHA.115.301517. [DOI] [PMC free article] [PubMed]
- 4.https://www.britannica.com/science/endoderm Accessed dates.
- 5.https://embryology.oit.duke.edu/GI/GI.html Accessed dates.
- 6.Tanaka T, Takehara K, Yamashita N, et al. Frequency and clinical features of deficient mismatch repair in ovarian clear cell and endometrioid carcinoma. J Gynecol Oncol. 2022;33(5):e67. 10.3802/jgo.2022.33.e67, https://pubmed.ncbi.nlm.nih.gov/36032025/. [DOI] [PMC free article] [PubMed]
- 7.Wiegand KC, Shah SP, Al-Agha OM, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363(16):1532–43. 10.1056/NEJMoa1008433. https://pubmed.ncbi.nlm.nih.gov/20942669/. [DOI] [PMC free article] [PubMed]
- 8.Ligtenberg MJL, Kuiper RP, Geurts van Kessel A. et al. EPCAM deletion carriers constitute a unique subgroup of Lynch syndrome patients. Familial Cancer. 12: 169–174 (2013). https://link.springer.com/article/10.1007/s10689-012-9591-x. [DOI] [PubMed]
- 9.Vasen HF, Möslein G, Alonso A, et al. Recommendations to improve identification of hereditary and familial colorectal cancer in Europe. Fam Cancer. 2010;9(2):109–15. 10.1007/s10689-009-9291-3. https://pubmed.ncbi.nlm.nih.gov/19763885/. [DOI] [PubMed]
- 10.Ahadova A, Seppälä TT, Engel C, et al. The "unnatural" history of colorectal cancer in Lynch syndrome: Lessons from colonoscopy surveillance. Int J Cancer. 2021 Feb 15;148(4):800-811. doi: 10.1002/ijc.33224. https://pubmed.ncbi.nlm.nih.gov/32683684/. [DOI] [PubMed]
- 11.Møller P, Seppälä T, Bernstein I, et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut. 2017;66(3):464–472. 10.1136/gutjnl-2015-309675. https://pubmed.ncbi.nlm.nih.gov/26657901/. [DOI] [PMC free article] [PubMed]
- 12.Møller P, Seppälä T, Bernstein I, et al. Incidence of and survival after subsequent cancers in carriers of pathogenic MMR variants with previous cancer: a report from the prospective Lynch syndrome database. Gut. 2017;66(9):1657–1664. 10.1136/gutjnl-2016-311403. https://pubmed.ncbi.nlm.nih.gov/27261338/. [DOI] [PMC free article] [PubMed]
- 13.Møller P, Seppälä TT, Bernstein I, et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database. Gut. 2018;67(7):1306–1316. 10.1136/gutjnl-2017-314057. https://pubmed.ncbi.nlm.nih.gov/28754778/. [DOI] [PMC free article] [PubMed]
- 14.Dominguez-Valentin M, Haupt S, Seppälä TT, et al. Mortality by age, gene and gender in carriers of pathogenic mismatch repair gene variants receiving surveillance for early cancer diagnosis and treatment: a report from the Prospective Lynch Syndrome Database. eClinicalMedicine. 2023; 101909, 10.1016/j.eclinm.2023.101909, https://www.sciencedirect.com/science/article/pii/S258953702300086X. [DOI] [PMC free article] [PubMed]
- 15.Møller, P., Nakken, S., Hovig, E. Databases: intentions, capabilities, and limitations. In: Valle, L., Gruber, S., Capellá, G. (eds) Hereditary Colorectal Cancer. Cham: Springer;2018. 10.1007/978-3-319-74259-5_26, 10.1007/978-3-319-74259-5_26.
- 16.Møller P, Nakken S, Hovig E. The prospective lynch syndrome database. In: Valle L, Gruber S, Capellá G. (eds) Hereditary colorectal cancer. Cham: Springer;2018. 10.1007/978-3-319-74259-5_28.
- 17.Møller P. The Prospective lynch syndrome database reports enable evidence-based personal precision health care. Hered Cancer Clin Pract. 2020;18:6. 10.1186/s13053-020-0138-0. https://pubmed.ncbi.nlm.nih.gov/32190163/. [DOI] [PMC free article] [PubMed]
- 18.Møller, P. The prospective lynch syndrome database: background, design, main results and complete MySQL code. Hered Cancer Clin Pract. 2022. 20: 37 10.1186/s13053-022-00243-z. [DOI] [PMC free article] [PubMed]
- 19.International Mismatch Repair Consortium. Variation in the risk of colorectal cancer in families with Lynch syndrome: a retrospective cohort study. Lancet Oncol. 2021;22(7):1014–1022. 10.1016/S1470-2045(21)00189-3. https://pubmed.ncbi.nlm.nih.gov/34111421/. [DOI] [PMC free article] [PubMed]
- 20.Møller P, Seppälä T, Dowty JG, et al. Colorectal cancer incidences in Lynch syndrome: a comparison of results from the prospective lynch syndrome database and the international mismatch repair consortium. Hered Cancer Clin Pract. 2022;20(1):36. 10.1186/s13053-022-00241-1. https://pubmed.ncbi.nlm.nih.gov/36182917/. [DOI] [PMC free article] [PubMed]
- 21.Møller P, Dominguez-Valentin M, Sampson JR, et al. Lynch Syndrome, which cancer comes first? https://www.ehtg.org/pdf/EHTG-Meeting-Abstracts-2022.pdf.
- 22.Gallon R, Phelps R, Hayes C, et al. Constitutional mismatch repair deficiency. Gastroenterology. 2022:S0016–5085(22)01444–5. doi: 10.1053/j.gastro.2022.12.017. https://pubmed.ncbi.nlm.nih.gov/36586540/.
- 23.Seppälä T, Pylvänäinen K, Evans DG, et al. Colorectal cancer incidence in path_MLH1 carriers subjected to different follow-up protocols: a prospective lynch syndrome database report. Hered Cancer Clin Pract. 2017;15:18. 10.1186/s13053-017-0078-5. https://pubmed.ncbi.nlm.nih.gov/29046738/. [DOI] [PMC free article] [PubMed]
- 24.Engel C, Vasen HF, Seppälä T, et al. No Difference in Colorectal Cancer Incidence or Stage at Detection by Colonoscopy Among 3 Countries With Different Lynch Syndrome Surveillance Policies. Gastroenterology. 2018;155(5):1400–1409.e2. 10.1053/j.gastro.2018.07.030. https://pubmed.ncbi.nlm.nih.gov/30063918/. [DOI] [PubMed]
- 25.Seppälä TT, Ahadova A, Dominguez-Valentin M, et al. Lack of association between screening interval and cancer stage in Lynch syndrome may be accounted for by over-diagnosis; a prospective Lynch syndrome database report. Hered Cancer Clin Pract. 2019;17:8. 10.1186/s13053-019-0106-8. https://pubmed.ncbi.nlm.nih.gov/30858900/. [DOI] [PMC free article] [PubMed]
- 26.Dominguez-Valentin M, Seppälä TT, Sampson JR, et al. Survival by colon cancer stage and screening interval in Lynch syndrome: a prospective Lynch syndrome database report. Hered Cancer Clin Pract. Hered Cancer Clin Pract. 2019;17:28. 10.1186/s13053-019-0127-3. https://pubmed.ncbi.nlm.nih.gov/31636762/. [DOI] [PMC free article] [PubMed]
- 27. Jass JR. Limitations of the Adenoma–Carcinoma Sequence in Colorectum. Clin Cancer Res. 2004; 10 (17): 5969–5970. https://aacrjournals.org/clincancerres/article/10/17/5969/185343/Limitations-of-the-Adenoma-Carcinoma-Sequence-in. [DOI] [PubMed]
- 28.Haupt S, Zeilmann A, Ahadova A, et al. Mathematical modeling of multiple pathways in colorectal carcinogenesis using dynamical systems with Kronecker structure. PLoS Comput Biol. 2021;17(5):e1008970. 10.1371/journal.pcbi.1008970. https://pubmed.ncbi.nlm.nih.gov/34003820/. [DOI] [PMC free article] [PubMed]
- 29.Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis Colon Rectum. 1991;34(5):424–5. 10.1007/BF02053699. https://pubmed.ncbi.nlm.nih.gov/2022152/. [DOI] [PubMed]
- 30.Vasen FA, Watson P, Mecklin J-P, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative Group on HNPCC. Gastroenterol. 1999;116:1453-1456 10.1016/S0016-5085(99)70510-X). [DOI] [PubMed]
- 31.Sjursen W, Haukanes BI, Grindedal EM, et al. Current clinical criteria for Lynch syndrome are not sensitive enough to identify MSH6 mutation carriers. J Med Genet. 2010;47(9):579–85. 10.1136/jmg.2010.077677. https://pubmed.ncbi.nlm.nih.gov/20587412/. [DOI] [PMC free article] [PubMed]
- 32.Cavestro GM, Mannucci A, Balaguer F et al. Delphi Initiative for Early-Onset Colorectal Cancer (DIRECt) international management guidelines. Clin Gastroenterol Hepatol. 2022:S1542–3565(22)01171–5. 10.1016/j.cgh.2022.12.006. https://pubmed.ncbi.nlm.nih.gov/36549470/. [DOI] [PMC free article] [PubMed]
- 33.Peltomäki P, Nyström M, Mecklin JP, Seppälä TT. Lynch Syndrome Genetics and Clinical Implications. Gastroenterology. 2023:S0016–5085(23)00050–1. 10.1053/j.gastro.2022.08.058. https://pubmed.ncbi.nlm.nih.gov/36706841/. [DOI] [PubMed]
- 34.https://hereditarycancer.dfci.harvard.edu/mylynch/_w_aa735df3/assumptions-and-references.pdf Accessed dates.
- 35.Mecklin JP, Järvinen HJ, Peltokallio P. Cancer family syndrome. Genetic analysis of 22 Finnish kindreds. Gastroenterology. 1986;90(2):328–33. https://pubmed.ncbi.nlm.nih.gov/3940911/. [PubMed]
- 36.Järvinen HJ, Mecklin JP, Sistonen P. Screening reduces colorectal cancer rate in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 1995;108(5):1405–11. 10.1016/0016-5085(95)90688-6. https://pubmed.ncbi.nlm.nih.gov/7729632/. [DOI] [PubMed]
- 37.Järvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118(5):829–34. 10.1016/s0016-5085(00)70168-5. https://pubmed.ncbi.nlm.nih.gov/10784581/. [DOI] [PubMed]
- 38.Hampel H, Stephens JA, Pukkala E, et al. Cancer risk in hereditary nonpolyposis colorectal cancer syndrome: later age of onset. Gastroenterology. 2005;129(2):415–21. 10.1016/j.gastro.2005.05.011. https://pubmed.ncbi.nlm.nih.gov/16083698/. [DOI] [PubMed]
- 39.Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305(22):2304–10. 10.1001/jama.2011.743. https://pubmed.ncbi.nlm.nih.gov/21642682/. [DOI] [PubMed]
- 40.Finch AP, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32(15):1547–53. 10.1200/JCO.2013.53.2820. https://pubmed.ncbi.nlm.nih.gov/24567435/. [DOI] [PMC free article] [PubMed]
- 41.Dominguez-Valentin M, Crosbie EJ, Engel C, et al. Risk-reducing hysterectomy and bilateral salpingo-oophorectomy in female heterozygotes of pathogenic mismatch repair variants: a Prospective Lynch Syndrome Database report. Genet Med. 2021;23(4):705–712. 10.1038/s41436-020-01029-1. https://pubmed.ncbi.nlm.nih.gov/33257847/. [DOI] [PMC free article] [PubMed]
- 42.Kloor M, Huth C, Voigt AY, et al. Prevalence of mismatch repair-deficient crypt foci in Lynch syndrome: a pathological study. Lancet Oncol. 2012;13(6):598–606. 10.1016/S1470-2045(12)70109-2. https://pubmed.ncbi.nlm.nih.gov/22552011/. [DOI] [PubMed]
- 43.Ahadova A, von Knebel Doeberitz M, Bläker H, Kloor M. CTNNB1-mutant colorectal carcinomas with immediate invasive growth: a model of interval cancers in Lynch syndrome. Fam Cancer. 2016;15(4):579–86. 10.1007/s10689-016-9899-z. https://pubmed.ncbi.nlm.nih.gov/26960970/. [DOI] [PubMed]
- 44.André T, Cohen R, Salem ME. Immune checkpoint blockade therapy in patients with colorectal cancer harboring microsatellite instability/mismatch repair deficiency in 2022. Am Soc Clin Oncol Educ Book. 2022;42:1–9. 10.1200/EDBK_349557. https://pubmed.ncbi.nlm.nih.gov/35471834/. [DOI] [PubMed]
- 45.Cabezón-Gutiérrez L, Custodio-Cabello S, Palka-Kotlowska M, Díaz-Pérez D, Mateos-Dominguez M, Galindo-Jara P. Neoadjuvant immunotherapy for dMMR/MSI-H locally advanced rectal cancer: the future new standard approach? Eur J Surg Oncol. 2022:S0748–7983(22)00710–7. 10.1016/j.ejso.2022.10.018. https://pubmed.ncbi.nlm.nih.gov/36400657/. [DOI] [PubMed]
- 46.Ahadova A, Witt J, Haupt S, et al. Is HLA type a possible cancer risk modifier in Lynch syndrome? Int J Cancer. 2022. 10.1002/ijc.34312. https://pubmed.ncbi.nlm.nih.gov/36214792/. [DOI] [PubMed]
- 47.Gebert J, Gelincik O, Oezcan-Wahlbrink M, et al. Recurrent frameshift neoantigen vaccine elicits protective immunity with reduced tumor burden and improved overall survival in a lynch syndrome mouse model. Gastroenterology. 2021;161(4):1288–1302.e13. 10.1053/j.gastro.2021.06.073. https://pubmed.ncbi.nlm.nih.gov/34224739/. [DOI] [PMC free article] [PubMed]
- 48.Kloor M, Reuschenbach M, Pauligk C, et al. Frameshift peptide neoantigen-based vaccine for mismatch repair-deficient cancers: a phase I/IIa clinical trial. Clin Cancer Res. 2020;26(17):4503–4510. https://pubmed.ncbi.nlm.nih.gov/32540851/. [DOI] [PubMed]
- 49.Ryser MD, Lange J, Inoue LYT, et al. Estimation of breast cancer overdiagnosis in a U.S. breast screening cohort. Ann Intern Med. 2022;175(4):471–478. 10.7326/M21-3577. https://pubmed.ncbi.nlm.nih.gov/35226520/. [DOI] [PMC free article] [PubMed]
- 50.Dunn BK, Woloshin S, Xie H, Kramer BS. Cancer overdiagnosis: a challenge in the era of screening. J Natl Cancer Cent. 2022;2(4):235–242. 10.1016/j.jncc.2022.08.005. https://pubmed.ncbi.nlm.nih.gov/36568283/. [DOI] [PMC free article] [PubMed]
- 51.Talseth-Palmer BA, Wijnen JT, Brenne IS, et al . Combined analysis of three Lynch syndrome cohorts confirms the modifying effects of 8q23.3 and 11q23.1 in MLH1 mutation carriers. Int J Cancer. 2013;132(7):1556–64. 10.1002/ijc.27843. https://pubmed.ncbi.nlm.nih.gov/22987364/. [DOI] [PubMed]
- 52.Burn J, Sheth H, Elliott F, et al. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: a double-blind, randomised, placebo-controlled trial. Lancet. 2020;395(10240):1855–1863. 10.1016/S0140-6736(20)30366-4. https://pubmed.ncbi.nlm.nih.gov/32534647/. [DOI] [PMC free article] [PubMed]
- 53.Zumwalt TJ, Wodarz D, Komarova NL, et al. Aspirin-induced chemoprevention and response kinetics are enhanced by PIK3CA mutations in colorectal cancer cells. Cancer Prev Res (Phila). 2017;10(3):208–218. 10.1158/1940-6207.CAPR-16-0175. https://pubmed.ncbi.nlm.nih.gov/28154202/. [DOI] [PMC free article] [PubMed]
- 54.Mathers JC, Elliott F, Macrae F, et al. Cancer prevention with resistant starch in Lynch Syndrome Patients in the CAPP2-Randomized placebo controlled trial: planned 10-Year follow-up. Cancer Prev Res (Phila). 2022;15(9):623–634. 10.1158/1940-6207.CAPR-22-0044. https://pubmed.ncbi.nlm.nih.gov/35878732/. [DOI] [PMC free article] [PubMed]
- 55.https://rarediseases.info.nih.gov/diseases/6821/muir-torre-syndrome Accessed dates.
- 56.Ahadova A, Gallon R, Gebert J, et al. Three molecular pathways model colorectal carcinogenesis in Lynch syndrome. Int J Cancer. 2018;143(1):139–150. 10.1002/ijc.31300, https://pubmed.ncbi.nlm.nih.gov/29424427/. [DOI] [PubMed]
- 57.Malesci A, Laghi L, Bianchi P, et al. Reduced likelihood of metastases in patients with microsatellite-unstable colorectal cancer. Clin Cancer Res. 2007;13(13):3831–9. 10.1158/1078-0432.CCR-07-0366. https://pubmed.ncbi.nlm.nih.gov/17606714/. [DOI] [PubMed]
- 58.Møller P, Haupt S, Ahadova A, et al An additional carcinogenetic mechanism for colon cancer in Lynch syndrome https://www.ehtg.org/pdf/EHTG-Meeting-Abstracts-2022.pdf.
- 59.Jansen AM, van Wezel T, van den Akker BE, et al. Combined mismatch repair and POLE/POLD1 defects explain unresolved suspected Lynch syndrome cancers. Eur J Hum Genet. 2016;24(7):1089–92. doi: 10.1038/ejhg.2015.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ashworth A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol. 2008;26(22):3785–90. 10.1200/JCO.2008.16.0812. https://pubmed.ncbi.nlm.nih.gov/18591545/. [DOI] [PubMed]
- 61.Gupta R, Sinha S, Paul RN. The impact of microsatellite stability status in colorectal cancer. Curr Prob Cancer. 2018; 42(6):548–559, https://www.sciencedirect.com/science/article/abs/pii/S0147027217301691?via%3Dihub. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.