Abstract
Background:
Discrimination can adversely affect health and accelerate aging, but little is known about these relationships in cancer survivors. This study examines associations of discrimination and aging among self-identified African American survivors.
Methods:
A population-based sample of 2232 survivors 20–79 years old at diagnosis were enrolled within 5 years of breast (n = 787), colorectal (n = 227), lung (n = 223), or prostate (n = 995) cancer between 2017 and 2022. Surveys were completed post-active therapy. A deficit accumulation index measured aging-related disease and function (score range, 0–1, where <0.20 is robust, 0.20 to <0.35 is pre-frail, and 0.35+ is frail; 0.06 is a large clinically meaningful difference). The discrimination scale assessed ever experiencing major discrimination and seven types of events (score, 0–7). Linear regression tested the association of discrimination and deficit accumulation, controlling for age, time from diagnosis, cancer type, stage and therapy, and sociodemographic variables.
Results:
Survivors were an average of 62 years old (SD, 9.6), 63.2% reported ever experiencing major discrimination, with an average of 2.4 (SD, 1.7) types of discrimination events. Only 24.4% had deficit accumulation scores considered robust (mean score, 0.30 [SD, 0.13]). Among those who reported ever experiencing major discrimination, survivors with four to seven types of discrimination events (vs. 0–1) had a large, clinically meaningful increase in adjusted deficits (0.062, p< .001) and this pattern was consistent across cancer types.
Conclusion:
African American cancer survivors have high deficit accumulated index scores, and experiences of major discrimination were positively associated with these deficits. Future studies are needed to understand the intersectionality between aging, discrimination, and cancer survivorship among diverse populations.
Keywords: African American persons, aging, Black persons, cancer, deficit accumulation, discrimination, disparities, frailty, survivors
INTRODUCTION
There are complex, bidirectional relationships between aging and cancer.1–4 Aging is a heterogeneous process characterized by accumulated damage to biological systems over the life course, leading to loss of reserve and capacity to respond to challenges, vulnerability to chronic diseases like cancer, and deterioration in function and death.1 Cancer and its treatments can affect the rate of aging of survivors because they destabilize and damage biological systems while attempting to eradicate the disease, in contrast to treatments for other chronic diseases that stabilize systems (e.g., control blood pressure or blood sugar).1,3,4
In noncancer settings, many factors affect aging, health, and health disparities, such as harmful environmental exposures, limits in community resources, socioeconomic opportunity and health care access, and experiencing discrimination.1,5–9 Factors like discrimination are thought to affect aging via chronic stress, upregulation of stress responses, chronic increases in inflammation, shortening of telomeres, and loss of homeostasis in biological systems.1,10–19 Thus, it is possible that experiences of discrimination could explain some of the observed racial disparities in cancer outcomes, including poor age-related function and high cancer-specific and all-cause mortality.18,20
There are virtually no studies of the relationships between discrimination and aging in the setting of cancer survivorship.18 This gap is exacerbated by the continued under-representation of racial and ethnic minorities in cancer research,21 difficulties measuring aging in oncology settings, and observations that levels of frailty, which are thought to reflect aging, are not always clinically apparent.22–24 Frailty is generally measured using one of two types of indices—phenotypic, focused on objective assessments of system failure (e.g., loss of muscle strength)25 and deficit accumulation, focused on comorbidities and self-reported functional problems.23,26,27 Both approaches predict mortality in general populations.23 Deficit accumulation indices are useful because they can readily be constructed from survey and/or clinical data and predict multiple survivorship outcomes, including chemotherapy toxicity, medication adherence and hospitalizations,28,29 cognitive decline,30 quality of life, and all-cause mortality.22,28–31 Deficit accumulation indices are also useful because they are constructed using standardized scaling, facilitating comparisons across studies.23,26
In this cross-sectional study, we tested associations between perceived discrimination and deficit accumulation among self-identified Black or African American breast, lung, prostate, and colorectal cancer survivors who were within 5 years post-diagnosis and had completed active treatment. Survivors were part of the Detroit Research on Cancer Survivors (ROCS) Study, a population-based cohort of self-identified Black or African American adult cancer survivors (hereinafter referred to as African American survivors).32,33 We hypothesized that African American cancer survivors who reported high levels of discrimination would have greater deficit accumulation (i.e., greater frailty) than those reporting lower levels of discrimination. The results are intended to support future studies of multilevel factors affecting aging in cancer survivors from racial minority groups and target testing of interventions to increase racial equity in cancer health outcomes.
MATERIALS AND METHODS
The Detroit ROCS Study has previously been described in detail elsewhere.33 Briefly, African American adults 20–79 years old at diagnosis of a first primary invasive breast, colorectal, lung, prostate, endometrial, or other cancers among individuals ages 20 years and older residing within the metropolitan Detroit area (Wayne, Macomb, and Oakland counties) were identified from the Metropolitan Detroit Cancer Surveillance System cancer registry. Potentially eligible survivors were contacted and 40% consented and completed assessments. The study protocol was reviewed and approved by the institutional review board at Wayne State University (050417M1F).
Study population
For this secondary analysis, we included African American survivors with breast, colorectal, prostate, and lung cancer who were enrolled from September 2017 to April 2022 and were post-active treatment. We focused on these four cancers because they represent the majority of the cancer burden in African American adults, have racial disparities in cancer and all-cause survival and generally affect older age groups where deficit accumulation is most common.
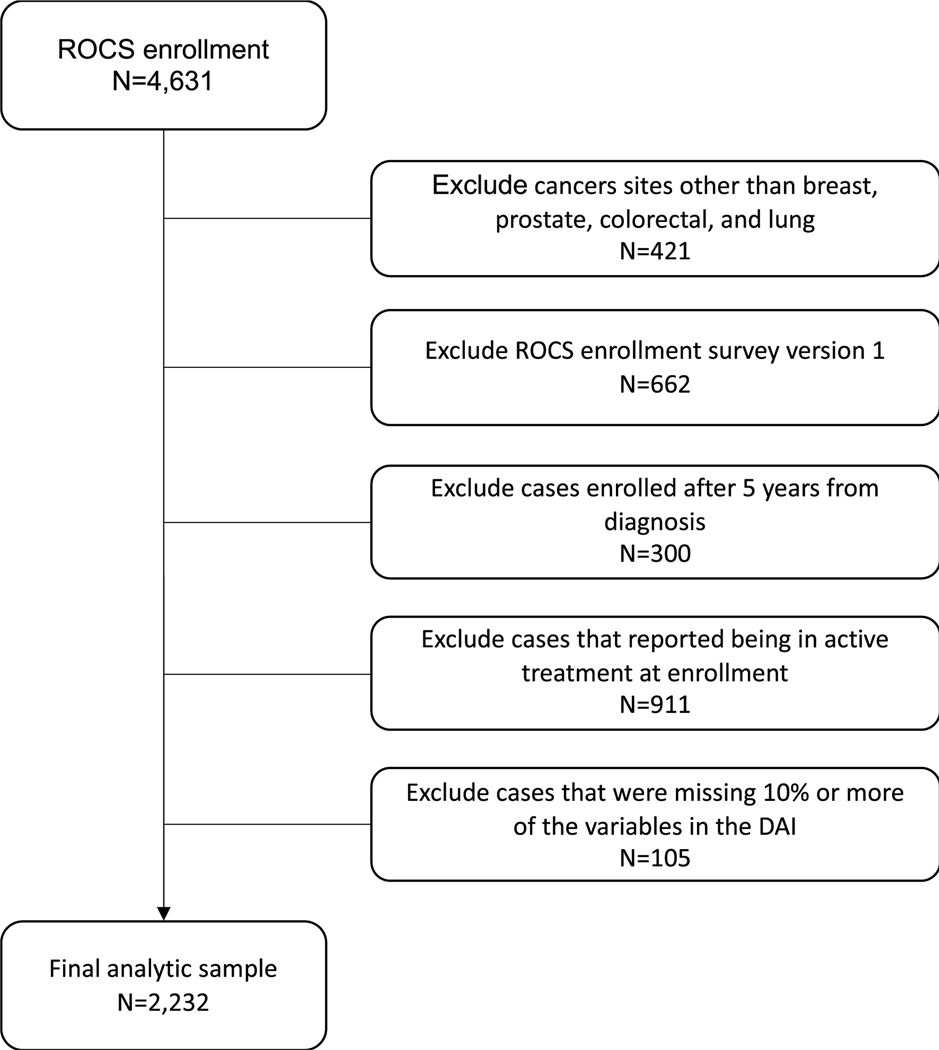
There were 5067 African American survivors enrolled in ROCS by June 2022 and 4631 had data entry and final eligibility confirmation at that time. Among this sample, we excluded participants with other cancers (n = 421), those still in active treatment (n = 911), those enrolled more than 5 years from diagnosis (n = 300), and those enrolled in a hospital recruitment-based pilot before surveys measured discrimination (n = 662). Among the remaining eligible sample of 2337 persons, we excluded 105 (4.5%) participants who were missing sufficient data for calculation of a deficit accumulation index score, for a final analytic sample of 2232 survivors (Figure 1). The 105 survivors with insufficient data to estimate a deficit accumulation index score were similar to the final analytic sample except that they included more persons in lower education categories (≤high school or general equivalency diploma, 49% vs. 35%; p = .013). The survivors enrolled via hospital-based recruitment excluded due to missing discrimination data had higher deficits accumulation scores than the population-based analytic sample (mean, 0.339 [SD, 0.134] vs. 0.305 [SD, 0.133]; p < .001).
FIGURE 1.
CONSORT diagram for analytic sample of self-identified African American breast, colorectal, lung and prostate cancer survivors 20–79 years old at diagnosis.
Data collection
Participants completed an online (16%), written (57%), or phone survey (27%). The survey included data on self-reported sociodemographics, whether active therapy was complete (except for long-term hormonal treatments), self-reported comorbidities, quality of life (FACT G34 and Patient-Reported Outcomes Measurement Information System [PROMIS] depression and anxiety scales),35 perceived discrimination,36,37 and other data.33,38 Participants received a $25 gift card for completion of the questionnaire. Clinical data (i.e., date of diagnosis, cancer site, and stage) were extracted from the registry. Treatment data about surgery, radiotherapy, and systemic therapies were self-reported.
Measures
We were interested in testing the associations of deficit accumulation and discrimination scores. Our deficit accumulation index22,23,26,27,39 included 25 items capturing cardiovascular, metabolic, and other comorbidities, polypharmacy, activity level (e.g., time spent in bed),40 social support (based on marital status and social well-being)34 nutritional status (body mass index and unintentional weight loss), physical, emotional and functional well-being, depression,35 anxiety,35 and fatigue38 (see Table S1). Each deficit item received a score from 0 to 1, where 0 represented absence of the deficit and 1 indicates that the item was present and/or the most severe deficit level. For continuous items, we used a range of scores based on established cutpoint or quartiles, where 0.25–0.75 indicated mild to moderate deficits. Items with interval five-point Likert scales (from never to all the time) were scored as 0, 0.25, 0.5, 0.75, and 1. Item scores are summed and divided by the total number of items available, resulting in a final score ranging from 0 to 1. A higher score indicated greater deficit accumulation. All participants included in our sample had ≥90% of items required for scoring.26 The continuous deficit accumulation index score was our primary outcome. A difference in score of 0.02 is considered a small clinically meaningful difference and 0.06 a large difference.41 We also report categorical scores that have previously been identified with risk of hospitalization and mortality (robust, 0 to <0.2; pre-frail, 0.2 to <0.35; or frail, ≥0.35).23,26
We used a well-validated seven-item42 scale to measure experiences of major discrimination.43–45 Participants were asked if they ever personally experienced discrimination (yes/no). Among those who experienced discrimination, seven experiences of major discrimination were queried, including being unfairly fired or denied a promotion; not being hired for a job; unfairly stopped, searched, questioned, physically threatened or abused by the police; unfairly discouraged by a teacher or advisor from continuing education; unfairly receiving worse medical care than other people; unfairly prevented from moving into a neighborhood because the landlord or a realtor refused to sell or rent a house or apartment; and moved into a neighborhood where neighbors made life difficult. The overall score was a sum of experiences of discrimination ranging from 0 to 7. A score of zero among those who had indicated they had experienced discrimination was included to reflect the fact that the context for their perceived discrimination may not have been captured in the included items.11
We also considered covariates that could be potential confounders of the association between deficit accumulation and discrimination, including age, cancer type, treatment, cancer stage, time from diagnosis, gender, education, employment, income, and insurance at the time of study enrollment.
Analysis
We described the unadjusted distribution of continuous deficit accumulation scores for the overall sample of survivors and survivors by cancer type (breast, colorectal, lung, and prostate). Next, we tested bivariate associations between categories of deficit accumulation scores (robust, 0 to <0.2; pre-frail, 0.2 to <0.35; or frail, ≥0.35) and covariates using χ2 or the Cochran-Armitage trend test as applicable; two-sided p values ≤0.05 were considered statistically significant. In secondary analyses, we also examined unadjusted continuous score distributions separately for each of the four cancer types.
For our primary analysis, we used linear regression models to test the associations of perceived discrimination (number of major discriminatory events among those reported ever having experienced discrimination) with the outcome of continuous deficit accumulation scores, considering age, education, insurance and income level, self-identified sex (for site-specific analyses of lung and colorectal cancer), time from diagnosis, and cancer type and treatment. The number of discrimination events was grouped into 0–1, 2–3, and 4–7 based on sample distributions; results were unchanged using the continuous scores from 0 to 7. In secondary analyses, we repeated the regression models separately for each cancer type to determine if the magnitude of association between major discrimination and deficit accumulation varied across cancer type. Finally, we conducted secondary analyses to test the association of ever versus never reporting discrimination and deficit accumulation scores. Model fit was assessed using R2 values. All analyses were conducted using SAS Version 9.4 (SAS Institute Inc, Cary, North Carolina) and graphs were drawn using R software.
RESULTS
African American cancer survivors in this study were an average of 21 months (SD, 14) from diagnosis and most had breast (35.3%) or prostate cancer (44.6%) (Table 1). Two-thirds of the survivors had their cancers diagnosed at local stages. The mean age at enrollment was 62 years (range, 23–84): breast cancer, 60 years (28–84); colorectal cancer, 61 years (23–80); lung cancer, 65 years (39–83); and prostate cancer, 64 years (42–81). Two-thirds of these African American cancer survivors reported ever experiencing major discrimination. Among those reporting discrimination, the mean number of types of events was 2.4 (SD, 1.7), with 24.2% reporting four to seven types of events (Table 2).
TABLE 1.
Characteristics of self-identified African American breast, colorectal, lung, and prostate cancer survivors by deficit accumulation score category
| All cases |
Robust (<0.20) |
Pre-frail (0.20 to <0.35) |
Frail (0.35+) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Col % | No. | Row % | No. | Row % | No. | Row % | p * | |
| Total | 2232 | 100 | 544 | 24.4 | 954 | 42.7 | 734 | 32.9 | |
| Demographics | |||||||||
| Sex | .001 | ||||||||
| Male | 1200 | 53.8 | 309 | 25.8 | 538 | 44.8 | 353 | 29.4 | |
| Female | 1032 | 46.2 | 235 | 22.8 | 416 | 40.3 | 381 | 36.9 | |
| Age at enrollment, years | <.001 | ||||||||
| <50 | 216 | 9.7 | 71 | 32.9 | 87 | 40.3 | 58 | 26.9 | |
| 50–59 | 565 | 25.3 | 168 | 29.7 | 228 | 40.4 | 169 | 29.9 | |
| 60–69 | 918 | 41.1 | 213 | 23.2 | 389 | 42.4 | 316 | 34.4 | |
| 70+ | 533 | 23.9 | 92 | 17.3 | 250 | 46.9 | 191 | 35.8 | |
| Mean (std) | 62 (9.6) | 60 (9.7) | 63 (9.8) | 64 (8.9) | |||||
| Range | 23–84 | 30–83 | 23–84 | 32–82 | |||||
| Education | <.001 | ||||||||
| Less than high school | 190 | 8.6 | 25 | 13.2 | 73 | 38.4 | 92 | 48.4 | |
| High school or GED | 577 | 26.2 | 116 | 20.1 | 244 | 42.3 | 217 | 37.6 | |
| Some college | 871 | 39.6 | 205 | 23.5 | 381 | 43.7 | 285 | 32.7 | |
| 4-year degree | 251 | 11.4 | 80 | 31.9 | 106 | 42.2 | 65 | 25.9 | |
| Graduate/professional degree | 313 | 14.2 | 112 | 35.8 | 137 | 43.8 | 64 | 20.4 | |
| Employment status | <.001 | ||||||||
| Employed (full, part time) | 710 | 31.9 | 306 | 43.1 | 308 | 43.4 | 96 | 13.5 | |
| Unemployed or disability | 574 | 25.8 | 51 | 8.9 | 208 | 36.2 | 315 | 54.9 | |
| Retired | 859 | 38.6 | 163 | 19.0 | 403 | 46.9 | 293 | 34.1 | |
| Other | 85 | 3.8 | 23 | 27.1% | 33 | 38.8 | 29 | 34.1 | |
| Income (household) | <.001 | ||||||||
| <$20,000 | 737 | 35.6 | 100 | 13.6 | 281 | 38.1 | 356 | 48.3 | |
| $20,000–39,999 | 456 | 22.0 | 99 | 21.7 | 195 | 42.8 | 162 | 35.5 | |
| $40,000–59,999 | 339 | 16.4 | 102 | 30.1 | 149 | 44.0 | 88 | 26.0 | |
| $60,000–79,999 | 210 | 10.1 | 68 | 32.4 | 107 | 51.0 | 35 | 16.7 | |
| ≥$80,000 | 327 | 15.8 | 131 | 40.1 | 151 | 46.2 | 45 | 13.8 | |
| Insurance at enrollment | <.001 | ||||||||
| Medicare only | 432 | 19.4 | 81 | 18.8 | 190 | 44.0 | 161 | 37.3 | |
| Medicare plus private | 424 | 19.0 | 79 | 18.6 | 188 | 44.3 | 157 | 37.0 | |
| Medicare plus Medicaid | 291 | 13.1 | 33 | 11.3 | 93 | 32.0 | 165 | 56.7 | |
| Medicaid alone | 352 | 15.8 | 63 | 17.9 | 145 | 41.2 | 144 | 40.9 | |
| Private insurance or VA | 703 | 31.6 | 279 | 39.7 | 323 | 45.9 | 101 | 14.4 | |
| Other | 25 | 1.1 | 8 | 32.0 | 11 | 44.0 | 6 | 24.0 | |
| Cancer characteristics | |||||||||
| Cancer site | <.001 | ||||||||
| Breast | 787 | 35.3 | 195 | 24.8 | 315 | 40.0 | 277 | 35.2 | |
| Colorectal | 227 | 10.2 | 70 | 30.8 | 95 | 41.9 | 62 | 27.3 | |
| Lung | 223 | 10.0 | 26 | 11.7 | 91 | 40.8 | 106 | 47.5 | |
| Prostate | 995 | 44.6 | 253 | 25.4 | 453 | 45.5 | 289 | 29.0 | |
| SEER summary stage | .705 | ||||||||
| Local | 1513 | 68.3 | 368 | 24.3 | 639 | 42.2 | 506 | 33.4 | |
| Regional | 608 | 27.5 | 153 | 25.2 | 263 | 43.3 | 192 | 31.6 | |
| Distant | 93 | 4.2 | 18 | 19.4 | 44 | 47.3 | 31 | 33.3 | |
| Treatments received | |||||||||
| Surgery | .023 | ||||||||
| Yes | 1470 | 66.3 | 384 | 26.1 | 612 | 41.6 | 474 | 32.2 | |
| No | 748 | 33.7 | 156 | 20.9 | 337 | 45.1 | 255 | 34.1 | |
| Chemotherapy | .782 | ||||||||
| Yes | 627 | 28.3 | 147 | 23.4 | 270 | 43.1 | 210 | 33.5 | |
| No | 1591 | 71.7 | 395 | 24.8 | 678 | 42.6 | 518 | 32.6 | |
| Radiation | <.001 | ||||||||
| Yes | 1199 | 54.0 | 261 | 21.8 | 501 | 41.8 | 437 | 36.4 | |
| No | 1023 | 46.0 | 282 | 27.6 | 448 | 43.8 | 293 | 28.6 | |
| Hormone therapy | .365 | ||||||||
| Yes | 438 | 19.9 | 106 | 24.2 | 176 | 40.2 | 156 | 35.6 | |
| No | 1768 | 80.1 | 436 | 24.7 | 763 | 43.2 | 569 | 32.2 | |
| Immunotherapy | .226 | ||||||||
| Yes | 76 | 3.5 | 13 | 17.1 | 33 | 43.4 | 30 | 39.5 | |
| No | 2100 | 96.5 | 524 | 25.0 | 896 | 42.7 | 680 | 32.4 | |
| Time from diagnosis to enrollment | .288 | ||||||||
| 2–12 months | 817 | 36.6 | 182 | 22.3 | 352 | 43.1 | 283 | 34.6 | |
| 13–24 months | 669 | 30.0 | 177 | 26.5 | 289 | 43.2 | 203 | 30.3 | |
| 25–60 months | 746 | 33.4 | 185 | 24.8 | 313 | 42.0 | 248 | 33.2 | |
| Mean (std) | 21 (14) | 21 (14) | 21 (14) | 21 (14) | |||||
| Median | 16 | 17 | 16 | 15 | |||||
| Diagnosis year | .647 | ||||||||
| 2014–2016 | 813 | 36.4 | 201 | 24.7 | 346 | 42.6 | 266 | 32.7 | |
| 2017–2018 | 805 | 36.1 | 207 | 25.7 | 338 | 42.0 | 260 | 32.3 | |
| 2019–2021 | 614 | 27.5 | 136 | 22.1 | 270 | 44.0 | 208 | 33.9 | |
Note: Not reported or unknown values omitted from the table; other insurance includes those who reported no insurance (<1% of the sample). The last date of diagnosis was December 31, 2021, or earlier and the last enrollment and survey completion date was in 2022.
Abbreviations: GED, general equivalency diploma; SEER, Surveillance, Epidemiology, and End Results; Std, standard deviation; VA, Veteran’s Administration.
p values are χ2 tests, tests for trend, or t-tests.
TABLE 2.
Perceived major discrimination among self-identified African American breast, colorectal, lung, and prostate cancer survivors by unadjusted deficit accumulation score category
| All cases |
Robust (0 to <0.20) |
Pre-frail (0.2 to <0.35) |
Frail (0.35+) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Col % | No. | Row % | No. | Row % | No. | Row % | p * | |
| Perceived discrimination | .570 | ||||||||
| Ever | 1378 | 63.2 | 345 | 25.0 | 584 | 42.4 | 449 | 31.5 | |
| Never | 803 | 36.8 | 186 | 23.2 | 355 | 44.2 | 262 | 32.6 | |
| Perceived discrimination count (among those who reported ever) | <.001 | ||||||||
| 0–1 | 485 | 35.2 | 148 | 30.5 | 215 | 44.3 | 122 | 25.2 | |
| 2–3 | 560 | 40.6 | 144 | 25.7 | 243 | 43.4 | 173 | 30.9 | |
| 4–7 | 333 | 24.2 | 53 | 15.9 | 126 | 37.8 | 154 | 46.2 | |
| Mean (std) | 2.4 (1.7) | 2.0 (1.5) | 2.3 (1.5) | 2.8 (1.8) | |||||
| Median | 2 | 2 | 2 | 3 | |||||
Abbreviation: Std, standard deviation.
p values calculated from Cochran-Armitage trend test, Mantel-Haenszel χ2, or ANOVA (as applicable); 51 people missing data on discrimination.
Deficit accumulation
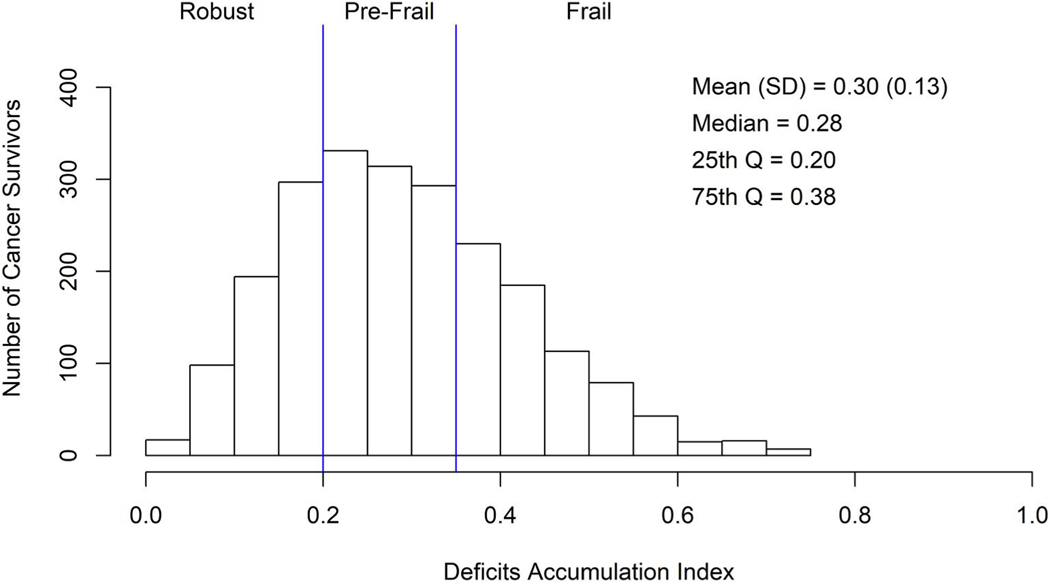
The majority of survivors had unadjusted deficit accumulation scores in the pre-frail (42.7%) or frail category (32.9%); only 24.4% had scores in the robust score range (Table 1 and Figure 2), with a mean deficit accumulation score of 0.30 (SD, 0.13). The proportion of survivors with scores in the frail category did not differ by disease stage (p = .705), although there were small numbers with distant disease. Deficit scores did vary somewhat across cancer types, with the smallest proportion in the frail category among colorectal and prostate cancer survivors (27.3% and 29.0%, respectively) and the highest rates among breast (35.2%) and lung cancer survivors (47.5%, p = .001) (Table 1 and Figure S1).
FIGURE 2.
Distribution of unadjusted deficit accumulation index scores among self-identified African American breast, colorectal, lung, and prostate cancer survivors 20–79 years old at diagnosis. Deficit accumulation scores range from 0 to 1, with scores between 0 to <0.20 considered robust; 0.20 to <0.35 considered pre-frail; and 0.35+ considered frail.
Is discrimination associated with deficit accumulation index score?
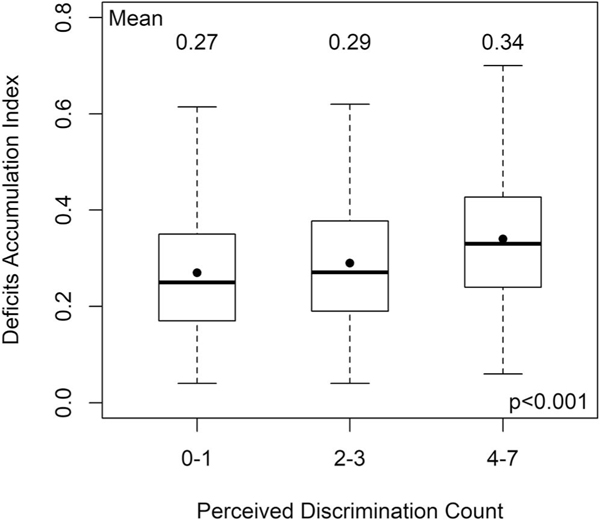
Among those who reported ever experiencing discrimination, as the number of types of major discrimination events increased, the adjusted deficit accumulation scores increased, with those who reported four to seven types of major discrimination events having a large clinically meaningful increase in deficits, controlling for covariates (0.062 higher than those reporting 0–1 types of major discrimination events, p < .001) (Figure 3 and Table 3).
FIGURE 3.
Association between number of perceived major discrimination events among persons reporting ever experiencing discrimination and unadjusted deficit accumulation score category among self-identified African American breast, colorectal, lung, and prostate cancer survivors 20–79 years old at diagnosis (n = 1378; 485 reporting 0–1, 560 reporting 2–3, and 333 reporting 4–7 discrimination events). Zero discrimination events among those reported ever experiencing discrimination reflects that their specific experience was not included in the items included on the scale
TABLE 3.
Adjusted associations of continuous deficit accumulation scores and number of perceived discrimination events and among African American cancer survivors reporting ever experiencing personal discrimination (n = 1257)
| β | SE | Lower confidence limit | Upper confidence limit | p | |
|---|---|---|---|---|---|
| Perceived discrimination (count of major discrimination events) | |||||
| 0–1 | Ref | — | — | — | — |
| 2–3 | 0.025 | 0.007 | 0.010 | 0.040 | .001 |
| 4–7 | 0.062 | 0.009 | 0.045 | 0.079 | <.001 |
| Sex | |||||
| Male | Ref | — | — | — | — |
| Female | 0.046 | 0.015 | 0.016 | 0.076 | .003 |
| Age group at enrollmenta | 0.003 | 0.005 | −0.006 | 0.013 | .471 |
| Education | |||||
| <High school | 0.034 | 0.017 | 0.001 | 0.067 | .044 |
| High school or GED | 0.014 | 0.012 | −0.009 | 0.037 | .228 |
| Some college | 0.019 | 0.010 | −0.001 | 0.038 | .058 |
| 4-year degree | 0.007 | 0.012 | −0.016 | 0.030 | .534 |
| Graduate/professional degree | Ref | — | — | — | — |
| Employment status | |||||
| Employed (full or part time) | Ref | — | — | — | — |
| Unemployed or disability | 0.108 | 0.010 | 0.088 | 0.129 | <.001 |
| Retired | 0.045 | 0.010 | 0.026 | 0.064 | <.001 |
| Other | 0.026 | 0.018 | −0.010 | 0.061 | .152 |
| Income (household) | −0.011 | 0.003 | −0.017 | −0.006 | <.001 |
| Insurance at enrollment | |||||
| Medicare only | 0.016 | 0.011 | −0.005 | 0.038 | .126 |
| Medicare plus private | 0.024 | 0.011 | 0.003 | 0.045 | .024 |
| Medicare plus Medicaid | 0.052 | 0.013 | 0.027 | 0.078 | <.001 |
| Medicaid alone | −0.001 | 0.012 | −0.025 | 0.023 | .942 |
| Private insurance or VA | Ref | — | — | — | — |
| Cancer site | |||||
| Female breast | −0.017 | 0.017 | −0.051 | 0.016 | .314 |
| Colorectal | −0.022 | 0.013 | −0.048 | 0.005 | .107 |
| Lung | 0.005 | 0.014 | −0.024 | 0.033 | .753 |
| Prostate | Ref | — | — | — | — |
| Radiation therapy (vs. none) | 0.023 | 0.007 | 0.009 | 0.037 | .001 |
| Time from diagnosis | 0.000 | 0.000 | −0.001 | 0.000 | .640 |
| Model fit, R2 | 0.286 | ||||
Abbreviations: GED, general equivalency diploma; SE, standard error; VA, Veteran’s Administration.
See age groups in Table 1. Other insurance set to missing (<1%). A total of 121 persons missing one of more model covariates. R2 (from 0 to 1) indicates the percent of variance in deficit accumulation scores explained by the model covariates. Here, 28.6% of the variability in deficit accumulation scores was explained by the model covariates.
Several sociodemographic factors were also independently associated with deficit accumulation, including sex (β 0.46 [SD, 0.015] for females vs. males; p = .003) and education level (β 0.034 for <high school vs. graduate degree; p = .044), but age was not significantly associated with adjusted deficit scores after considering other covariates. Among clinical variables, cancer type or time from diagnosis was not related to adjusted deficit accumulation scores and receipt of radiation therapy (vs. not) was the only treatment modality independently associated with a small clinically meaningful increase in deficit accumulation (β 0.023 [SD0.007], p < .001) (Table 3).
The pattern of adjusted associations between level of perceived discrimination and deficit accumulation scores was consistent across survivors with all four cancer types (Supplement Table 2). The magnitude of effects of discrimination ranged from small (breast and colorectal cancer) to large (lung and prostate cancer) clinically meaningful increases in deficit accumulation scores among those reporting four to seven (vs. 0-1) types of discrimination events, and these relationships were statistically significant for all cancer types except the type with the smallest sample size (colorectal cancer) (Table S2). The independent effects of radiotherapy on deficit accumulation score seen in the all cancer model was mainly driven by effects among breast and prostate cancer survivors (Table S2); other treatment modalities were not related to deficit accumulation. Finally, in models considering ever (vs. never) reporting any discrimination, discrimination was also significantly associated with adjusted deficit accumulation score (p = .041) (Table S3).
DISCUSSION
This is the first study to examine the relationship between deficit accumulation, a measure of aging, and perceived discrimination in African American survivors of breast, colorectal, lung, and prostate cancer. We found that two-thirds of African American survivors had deficit accumulation scores in the pre-frail and frail range. More than 60% of these African American cancer survivors also reported experiencing major discrimination. There was a large clinically meaningful association of reporting more types of major discrimination events and greater deficit accumulation. Finally, socioeconomic indicators and receipt of radiation therapy were also independent predictors of deficit accumulation.
There is limited data on deficit accumulation in adult cancer survivors,46 and even less information among African American survivors.20 The average deficit accumulation index scores among the African American cancer survivors in our study were close to a frail range, with two-thirds having scores in the pre-frail and frail categories. These rates are higher than those reported in other analyses of largely White cancer survivors and general populations of adults. For example, studies of predominantly White breast cancer survivors with similar age distributions as our sample reported that only 5% of women have deficit accumulation scores in the frail range,46–48 compared to 35.2% in our population. In one study of gastrointestinal cancer survivors, African American survivors were significantly more likely to have scores in the frail range than White survivors, independent of covariates.20 In the general US population, phenotypic frailty rates in African American adults ages 65 and older were 22.9%,49 lower than the 35.2% rate seen in our cancer survivors with an average age of 62 using a deficits accumulation index, suggesting possible interactions of aging and cancer.
There are many studies linking discrimination and health,6,8,15,16,50 but fewer that have examined how discrimination affects aging or biomarkers of aging processes,51–53 and only one small, inconclusive study of discrimination and an aging marker in breast cancer survivors.18 In addition to a relative paucity of data, the relationships between experiences of discrimination, aging and health outcomes are not straightforward. There are reports that low income, passive coping styles, internalized racism, and medical mistrust can exacerbate the negative effects of discrimination on health among African American individuals.18,54 These relationships have not been studied in the context of aging and cancer survivorship and will be important to consider in future studies.
Beyond discrimination, socioeconomic indicators were independent predictors of deficit accumulation. This result is not unexpected because African American adults in the general population consistently have higher levels of frailty than White adults, and these differences are not explained by socioeconomic factors.49,55 It will be important to study other social determinants of health to illuminate relationships between different factors in their effects on deficit accumulation. Cancer and its therapies can also increase accumulation of aging-related deficits.3,56–58 We found that radiotherapy was independently associated with deficit accumulation, largely due to effects among breast and prostate cancer survivors. We did not find an effect for chemotherapy, but this modality was only used by 28.3% of survivors compared to 54.0% receiving radiotherapy. Further examination of the effects of specific modalities and agents on aging of African American cancer survivors is warranted.
This study has many strengths, including use of data from the Detroit ROCS Study, the largest investigation of multilevel determinants of cancer outcomes exclusively conducted in an African American population. Inclusion of survivors who were post-active treatment and within 5 years from diagnosis allowed recovery and reduced the amount of informative missing data. Longer-term cohort studies that include data from before and after cancer diagnosis, prospective studies that enroll survivors at cancer diagnosis with concurrent noncancer controls and preclinical models will be useful to better understand the interactions of chronic life stressors, stress biology, and cancer on aging-related outcomes.
There are also several caveats that should be considered in evaluating our results. First, because aging was not an initial study focus, the number of items available for the deficit accumulation index were adequate, but fewer than used in some other studies.46 The smaller number of items means that certain aspects of aging may not have been fully captured (e.g., instrumental activities of daily living or vision and hearing impairments), making the index potentially less sensitive than indices having more items and likely biasing results toward the null. Furthermore, we did not have data on specific biomarkers of aging processes that might illuminate the pathways between experiences of discrimination and frailty and other cancer survivorship outcomes. This will be an important next step.18 Second, although the items in our measure of major discrimination has been used over decades and predicts mental and physical health outcomes,7,45,50,52,59 it may not reflect all types of discrimination in current society. A simple count of the types of major discriminatory events may also not capture more subtle aspects of discrimination or the effects of item framing or unwillingness to report unfair events.11 Additionally, our discrimination measure only ascertained if an event occurred, but not the frequency of the experience or when it occurred relative to cancer diagnosis and treatment. This is likely to have under-estimated our observed effect of discrimination on deficits. It will be important to obtain more nuanced data on the dose and timing of discriminatory events in future studies. It is also possible that the emotional, social, and biological impacts of discrimination affect aging differently in cancer versus noncancer groups. To the extent that we may have under-ascertained discrimination or its impact, this would have led to measurement error that should have biased the observed association of discrimination and deficit accumulation toward the null. We also did not specifically assess different dimensions of discrimination, including internalized discrimination, interpersonal versus institutional discrimination and discrimination due to race versus other causes (e.g., weight, disability, and national origin). There is only very limited data on these dimensions in cancer survivors. Moving forward, it will be important to evaluate the impact of multiple discrimination dimensions to better understand mechanisms and identify intervention strategies. Earlier studies of the impact of racism on health suggest that structural discrimination will affect socioeconomic opportunity and access to and quality of cancer care, whereas experiences of discrimination and coping strategies like internalization have been observed in general populations to affect allostatic load, stress, gene expression and inflammatory responses.14–16,18,59 Finally, this was a cross-sectional analysis, and we did not have baseline deficit accumulation data from the time of diagnosis, limiting conclusions about causal relationships.
Overall, the results of this study illustrate that the experience of major discrimination is related to aging as measured by deficit accumulation, and this association is not explained by socioeconomic status. With the largest projected increases in the number of cancer survivors occurring among racial and/or ethnic minority groups,1,4,60 we urgently need transdisciplinary collaborations to study multilevel factors to identify pathways to achieving greater cancer health equity.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the National Cancer Institute at the National Institutes of Health (U01 CA199240 to Ann G. Schwartz). The study was also supported in part by the National Cancer Institute at the National Institutes of Health (R01CA129769 and R35CA197289 to Jeanne S. Mandelblatt and K01CA212056 to Traci N. Bethea) and the National Institute on Aging at the National Institutes of Health (R21AG07500 to Jeanne S. Mandelblatt, Lucile Adams-Campbell, and Ann G. Schwartz). This work was supported by a grant from the Epidemiology Research Core and the National Cancer Institute Center (P30CA022453) awarded to the Karmanos Cancer Institute at Wayne State University. The study sponsor did not have any role in the design of the study, the collection, analysis, and interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication.
Funding information
National Cancer Institute, Grant/Award Numbers: K01CA212056, P30CA022453, R01CA129769, R35CA197289, U01 CA199240; National Institute on Aging, Grant/Award Numbers: R21 AG075008, R21AG075008; National Institutes of Health
Footnotes
CONFLICT OF INTEREST STATEMENT
Lucile Adams-Campbell reports consulting fees from Healios and Ryne Bio. Traci N. Bethea reports grant funding from the National Cancer Institute. Jeanne S. Mandelblatt reports grant funding from National Institutes of Health. Kristen Purrington reports grant funding from the Foundation for the National Institutes of Health. The other authors declare no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The Detroit Research on Cancer Survivors Study (ROCS) data are available for sharing following the National Institutes of Health requirements and Findability, Accessibility, Interoperability, Reproducibility (FAIR) principles for data access. Data access is via requests described at https://detroitrocs.org/dnn/For-Researchers/Data-Requests. The deficit accumulation index used in this study is included in Table S1. The SAS code and data for the analyses included in the article are available on request within the constraints of the Detroit ROCS institutional review board requirements.
REFERENCES
- 1.Mandelblatt JS, Ahles TA, Lippman ME, et al. Applying a life course biological age framework to improving the care of individuals with adult cancers: review and research recommendations. JAMA Oncol. 2021;7(11):1692. doi: 10.1001/jamaoncol.2021.1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Campisi J., aging and cellular senescence. Vivo. 2000;14(1): 183–188. [PubMed] [Google Scholar]
- 3.Hodes RJ, Sierra F, Austad SN, et al. Disease drivers of aging. Ann N. Y Acad Sci. 2016;1386(1):45–68. doi: 10.1111/nyas.13299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guida JL, Ahles TA, Belsky D, et al. Measuring aging and identifying aging phenotypes in cancer survivors. J Natl Cancer Inst. 2019; 111(12):1245–1254. doi: 10.1093/jnci/djz136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward-Caviness CK, Nwanaji-Enwerem JC, Wolf K, et al. Long-term exposure to air pollution is associated with biological aging. Oncotarget. 2016;7(46):74510–74525. doi: 10.18632/oncotarget.12903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams DR, Neighbors HW, Jackson JS. Racial/ethnic discrimination and health: findings from community studies. Am J Public Health. 2008;98(9 Suppl):S29–S37. doi: 10.2105/ajph.98.supplement_1.s29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erving CL, Cobb RJ, Sheehan C. Attributions for everyday discrimination and all-cause mortality risk among older Black women: a latent class analysis approach. Gerontologist. 2022. doi: 10.1093/geront/gnac080 [DOI] [PMC free article] [PubMed]
- 8.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453–1463. doi: 10.1016/s0140-6736(17)30569-x [DOI] [PubMed] [Google Scholar]
- 9.Dennis KK, Auerbach SS, Balshaw DM, et al. The importance of the biological impact of exposure to the concept of the exposome. Environ Health Perspect. 2016;124(10):1504–1510. doi: 10.1289/ehp140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geronimus AT, Hicken MT, Pearson JA, Seashols SJ, Brown KL, Cruz TD. Do US Black women experience stress-related accelerated biological aging? Hum Nat. 2010/03/01 2010;21(1):19–38. doi: 10.1007/s12110-010-9078-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams DR, Lawrence JA, Davis BA. Racism and health: evidence and needed research. Annu Rev Publ Health. 2019;40(1):105–125. doi: 10.1146/annurev-publhealth-040218-043750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boots EA, Feinstein DL, Leurgans S, et al. Acute versus chronic inflammatory markers and cognition in older Black adults: results from the Minority Aging Research Study. Brain Behav Immun. 2022;103: 163–170. doi: 10.1016/j.bbi.2022.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rentscher KE, Carroll JE, Mitchell C. Psychosocial stressors and telomere length: a current review of the science. Annu Rev Publ Health. 2020;41(1):223–245. doi: 10.1146/annurev-publhealth-040119-094239 [DOI] [PubMed] [Google Scholar]
- 14.Rentscher KE, Carroll JE, Repetti RL, Cole SW, Reynolds BM, Robles TF. Chronic stress exposure and daily stress appraisals relate to biological aging marker ply(INK4a). Psychoneuroendocrinology. 2019; 102:139–148. doi: 10.1016/j.psyneuen.2018.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thames AD, Irwin MR, Breen EC, Cole SW. Experienced discrimination and racial differences in leukocyte gene expression. Psychoneuroendocrinology. 2019/08/01/2019;106:277–283. doi: 10.1016/j.psyneuen.2019.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Dyke ME, Baumhofer NK, Slopen N, et al. Pervasive discrimination and allostatic load in African American and White adults. Psychosom Med. 2020;82(3):316–323. doi: 10.1097/psy.0000000000000788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Linden B, Cheval B, Sieber S, Kliegel M, Cullati S. Adverse childhood experiences are associated with frailty in old age. Innov Aging. 2018;2(Suppl_1):892. doi: 10.1093/geroni/igy031.3326 [DOI] [Google Scholar]
- 18.Aghaee S, Allen A, Ramirez J, et al. Everyday discrimination and telomere length in a multiethnic cohort of breast cancer survivors. Ethn Health. 2022;27(3):542–553. doi: 10.1080/13557858.2020.1739231 [DOI] [PubMed] [Google Scholar]
- 19.Chae DH, Wang Y, Martz CD, et al. Racial discrimination and telomere shortening among African Americans: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Health Psychol. 2020;39(3):209–219. doi: 10.1037/hea0000832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams GR, Al-Obaidi M, Harmon C, et al. Racial disparities in frailty and geriatric assessment impairments in older adults with cancer in the Deep South: results from the CARE Registry. Cancer. 2022;128(12):2313–2319. doi: 10.1002/cncr.34178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertagnolli MM, Singh H. Treatment of older adults with cancer - addressing gaps in evidence. N Engl J Med. 2021;385(12):1062–1065. doi: 10.1056/NEJMp2106089 [DOI] [PubMed] [Google Scholar]
- 22.Cohen HJ, Smith D, Sun CL, et al. Frailty as determined by a comprehensive geriatric assessment-derived deficit-accumulation index in older patients with cancer who receive chemotherapy. Cancer. 2016;122(24):3865–3872. doi: 10.1002/cncr.30269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rockwood K, Howlett SE. Fifteen years of progress in understanding frailty and health in aging. BMC Med. 2018;16(1):220. doi: 10.1186/s12916-018-1223-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirkhus L, Šaltytė Benth J, Rostoft S, et al. Geriatric assessment is superior to oncologists’ clinical judgement in identifying frailty. Br J Cancer. 2017;117(4):470–477. doi: 10.1038/bjc.2017.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3): M146–M156. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 26.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008; 8(1):24. doi: 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rockwood K, Howlett SE. Age-related deficit accumulation and the diseases of aging. Mech Ageing Dev. 2019;180:107–116. doi: 10.1016/j.mad.2019.04.005 [DOI] [PubMed] [Google Scholar]
- 28.Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol. 2016; 34(20):2366–2371. doi: 10.1200/jco.2015.65.4327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheppard VB, Faul LA, Luta G, et al. Frailty and adherence to adjuvant hormonal therapy in older women with breast cancer: CALGB protocol 369901. J Clin Oncol. 2014;32(22):2318–2327. doi: 10.1200/jco.2013.51.7367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandelblatt JS, Small BJ, Luta G, et al. Cancer-related cognitive outcomes among older breast cancer survivors in the Thinking and Living with Cancer Study. J Clin Oncol. 2018;36(32):Jco1800140--3222. doi: 10.1200/jco.18.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guerard EJ, Deal AM, Chang Y, et al. Frailty index developed from a cancer-specific geriatric assessment and the association with mortality among older adults with cancer. J Natl Compr Canc Netw. 2017;15(7):894–902. doi: 10.6004/jnccn.2017.0122 [DOI] [PubMed] [Google Scholar]
- 32.Beebe-Dimmer JL, Albrecht TL, Ruterbusch JJ, et al. The Detroit Research on Cancer Survivors (ROCS) study: a focus on outcomes after cancer in a racially diverse patient population. J Clin Oncol. 2018/03/01 2018;36(7_Suppl):177. doi: 10.1200/JCO.2018.36.7_suppl.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beebe-Dimmer JL, Ruterbusch JJ, Harper FWK, et al. Physical activity and quality of life in African American cancer survivors: The Detroit Research on Cancer Survivors study. Cancer. 2020;126(9): 1987–1994. doi: 10.1002/cncr.32725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ware J Jr., Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 35.Patient-Reported Outcomes Measurement Information System (PROMIS). Accessed December 2, 2020. doi:http://www.healthmeasures.net/explore-measurement-systems/promis/intro-to-promis
- 36.Brown TN. Measuring self-perceived racial and ethnic discrimination in social surveys. Socio Spectr. 2001/07/01 2001;21(3):377–392. doi: 10.1080/027321701300202046 [DOI] [Google Scholar]
- 37.Penner LA, Harper FWK, Dovidio JF, et al. The impact of Black cancer patients’ race-related beliefs and attitudes on racially-discordant oncology interactions: a field study. Soc Sci Med. 2017; 191:99–108. doi: 10.1016/j.socscimed.2017.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manag. 1997;13(2):63–74. doi: 10.1016/s0885-3924(96)00274-6 [DOI] [PubMed] [Google Scholar]
- 39.Howlett SE, Rutenberg AD, Rockwood K. The degree of frailty as a translational measure of health in aging. Nature Aging. 2021/08/01 2021;1(8):651–665. doi: 10.1038/s43587-021-00099-3 [DOI] [PubMed] [Google Scholar]
- 40.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185(12):914–919. doi: 10.1001/jama.1963.03060120024016 [DOI] [PubMed] [Google Scholar]
- 41.Jang I, Jung H, Lee H, Park H, Lee E, Kim D. Evaluation of clinically meaningful changes in measures of frailty. J Gerontol. 2020;75(6): 1143–1147. doi: 10.1093/gerona/glaa003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams DR, Gonzalez HM, Williams S, Mohammed SA, Moomal H, Stein DJ. Perceived discrimination, race and health in South Africa. Soc Sci Med. 2008;67(3):441–452. doi: 10.1016/j.socscimed.2008.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown TN. Measuring self-perceived racial and ethnic discrimation in social surveys. Socio Spectr. 2001/07/01 2001;21(3):377–392. doi: 10.1080/027321701300202046 [DOI] [Google Scholar]
- 44.Williams DR, Yan Y, Jackson JS, Anderson NB. Racial differences in physical and mental health: socioeconomic status, stress and discrimination. J Health Psychol. 1997;2(3):335–351. doi: 10.1177/135910539700200305 [DOI] [PubMed] [Google Scholar]
- 45.Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM. Experiences of discrimination: validity and reliability of a self-report measure for population health research on racism and health. Soc Sci Med. 2005;61(7):1576–1596. doi: 10.1016/j.socscimed.2005.03.006 [DOI] [PubMed] [Google Scholar]
- 46.Mandelblatt JS, Zhou X, Small BJ, et al. Deficit accumulation frailty trajectories of older breast cancer survivors and noncancer controls: the Thinking and Living with Cancer Study. J Natl Cancer Inst. 2021;113(8):1053–1064. doi: 10.1093/jnci/djab003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams GR, Deal AM, Sanoff HK, et al. Frailty and health-related quality of life in older women with breast cancer. Support Care Cancer. 2019;27(7):2693–2698. doi: 10.1007/s00520-018-4558-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mandelblatt JS, Cai L, Luta G, et al. Frailty and long-term mortality of older breast cancer patients: CALGB 369901 (Alliance). Breast Cancer Res Treat. 2017;164(1):107–117. doi: 10.1007/s10549-017-4222-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70(11):1427–1434. doi: 10.1093/gerona/glv133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams DR. Race, socioeconomic status, and health. The added effects of racism and discrimination. Ann N Y Acad Sci. 1999;896(1): 173–188. doi: 10.1111/j.1749-6632.1999.tb08114.x [DOI] [PubMed] [Google Scholar]
- 51.Lim S, Nzegwu D, Wright ML. The impact of psychosocial stress from life trauma and racial discrimination on epigenetic aging-a systematic review. Biol Res Nurs. 2022;24(2):202–215. doi: 10.1177/10998004211060561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chae DH, Epel ES, Nuru-Jeter AM, et al. Discrimination, mental health, and leukocyte telomere length among African American men. Psychoneuroendocrinology. 2016/01/01/2016;63:10–16. doi: 10.1016/j.psyneuen.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barcelona de Mendoza V, Huang Y, Crusto CA, Sun YV, Taylor JY. Perceived racial discrimination and DNA methylation among African American women in the InterGEN Study. Biol Res Nurs. 2018;20(2): 145–152. doi: 10.1177/1099800417748759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.LaVeist TA, Sellers R, Neighbors HW. Perceived racism and self and system blame attribution: consequences for longevity. Ethn Dis. 2001;11(4):711–721. [PubMed] [Google Scholar]
- 55.Usher T, Buta B, Thorpe RJ, et al. Dissecting the racial/ethnic disparity in frailty in a nationally representative cohort study with respect to health, income, and measurement. J Gerontol A Biol Sci Med Sci. 2021;76(1):69–76. doi: 10.1093/gerona/glaa061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scuric Z, Carroll JE, Bower JE, et al. Biomarkers of aging associated with past treatments in breast cancer survivors. NPJ Breast Cancer. 2017;3(1):50. doi: 10.1038/s41523-017-0050-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sehl ME, Carroll JE, Horvath S, Bower JE. The acute effects of adjuvant radiation and chemotherapy on peripheral blood epigenetic age in early stage breast cancer patients. NPJ Breast Cancer. 2020/ 06/12 2020;6(1):23. doi: 10.1038/s41523-020-0161-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanoff HK, Deal AM, Krishnamurthy J, et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst. 2014;106(4):dju057. doi: 10.1093/jnci/dju057. Epub 2014 Mar 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chae DH, Lincoln KD, Adler NE, Syme SL. Do experiences of racial discrimination predict cardiovascular disease among African American men? The moderating role of internalized negative racial group attitudes. Soc Sci Med. 2010;71(6):1182–1188. doi: 10.1016/j.socscimed.2010.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Howlader N, Noone AM, Krapcho M, et al. eds. SEER Cancer Statistics Review, 1975-2017. National Cancer Institute. https://seer.cancer.gov/csr/1975_2017/, based on November 2019 SEER data submission, posted to the SEER web site, April 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Detroit Research on Cancer Survivors Study (ROCS) data are available for sharing following the National Institutes of Health requirements and Findability, Accessibility, Interoperability, Reproducibility (FAIR) principles for data access. Data access is via requests described at https://detroitrocs.org/dnn/For-Researchers/Data-Requests. The deficit accumulation index used in this study is included in Table S1. The SAS code and data for the analyses included in the article are available on request within the constraints of the Detroit ROCS institutional review board requirements.