Abstract
Since the discovery of deep eutectic solvents (DESs) in 2003, significant progress has been made in the field, specifically advancing aspects of their preparation and physicochemical characterization. Their low-cost and unique tailored properties are reasons for their growing importance as a sustainable medium for the resource-efficient processing and synthesis of advanced materials. In this paper, the significance of these designer solvents and their beneficial features, in particular with respect to biomimetic materials chemistry, is discussed. Finally, this article explores the unrealized potential and advantageous aspects of DESs, focusing on the development of biomineralization-inspired hybrid materials. It is anticipated that this article can stimulate new concepts and advances providing a reference for breaking down the multidisciplinary borders in the field of bioinspired materials chemistry, especially at the nexus of computation and experiment, and to develop a rigorous materials-by-design paradigm.
1. Introduction
Biomineralization is a highly dynamic fundamental biological process,1 by which living organisms create mineral-based (amorphous or crystalline) tissues2−4 and is widespread among prokaryotic and eukaryotic organisms. This process is now considered one of the major advances that have critically altered the functional biology, the evolutionary trajectory, and biogeochemical impact of numerous organisms.5,6 During 3.7 billion years of evolution, nature has adapted, and often optimized, biomineralized fine structures, which can serve a wide range of functionalities.7,8 These functionalities include magnetic navigation,9 buoyancy regulation,10 mechanical protection/support,11−14 light scattering15 and transmission,16,17 as well as feeding functions.18−20 Intriguingly, the biologically formed mineral can be designed for specific functions or in numerous cases have multiple functionalities. Living organisms construct biominerals by utilizing a limited range of substances including polysaccharides (i.e., cellulose and chitin), proteins (i.e., keratin, collagen, or silk), and a few minerals (silica, calcium carbonates, hydroxyapatite, iron oxides). These limited substances are arranged into enormous arrays of complex hierarchical structures.21 These examples clearly show that in contrast to many current technologies, natural organisms cannot solely depend on the selection of materials to design a functional system; they instead must generate it from the available existing set of foundational materials, often using a set of principles referred to as the universality-diversity-paradigm (building diverse functions from simple, recurring, and resource-limited building blocks22). Eder21 further emphasizes that the wide range of properties and functions exhibited by biological materials can be attributed less to their varied compositions and more to the diverse structures they can form. In fact, the multifunctionality and high performance of biomineralized tissues arise naturally from their hierarchical structure (structural organizations that span multiple levels from atomic to the macroscale21,23−26), surface morphology, as well as physical and even chemical properties. However, reproduction of the nano- and microstructural features and high degree of hybridization of biomaterials in synthetic materials is not a trivial task. Furthermore, after 50 years of research, current fabrication technologies are still unable to mimic biomaterial fabrication procedures. The mechanistic details underlying biomineralization can serve as inspiration for the development of new material synthesis strategies, such as the use of delicate grain misorientations seen in nature,14 and can be exploited as a design principle in synthetic 2D materials.27 By applying these principles to more applicable foundational materials, it is possible to obtain novel materials and frequently unforeseen combinations of material properties.21,24,28 Therefore, the knowledge that can be extracted from biomineralization is definitively one of the main driving forces for recent progress in biomimetics. As a result, it is on track to becoming a powerful approach in modern materials science, nanotechnologies, and biomimicry.29−32
One of the most effective ways to gain an understanding of the basic principles of biomineralization on a molecular level and their application in materials design is a coherent, synergetic approach using explicit reasoning and well-tested principles of multidisciplinary experience, knowledge, and new technologies.4,33 Broadly speaking, the influence of chemistry, as a scientific discipline, in the realm of biomineralization can be classified into three main distinct domains:34 (i) detailed characterization of the native biominerals encompassing their biochemistry, crystallography, and structural peculiarities; (ii) the design of adequate in vitro model systems to answer fundamental questions and verify the hypotheses regarding the possible roles and interactions of organic matrices in controlling crystallization (nucleation as well as crystal growth stages) of inorganics and biomaterial formation; and (iii) the creation of novel synthetic approaches, inspired by bioinspired systems, to regulate crystal morphology, polymorphism, and material characteristics, resulting in the formation of innovative organic–inorganic hybrids.
We believe that by considering the present state of art regarding computational chemistry, machine learning, and artificial intelligence in chemistry, the fourth area should be defined as (iv) utilization of machine learning and artificial intelligence to corelate structure, chemistry, properties, and function to forecast material properties, construct materials from scratch, and uncover new mechanisms beyond intuition.35
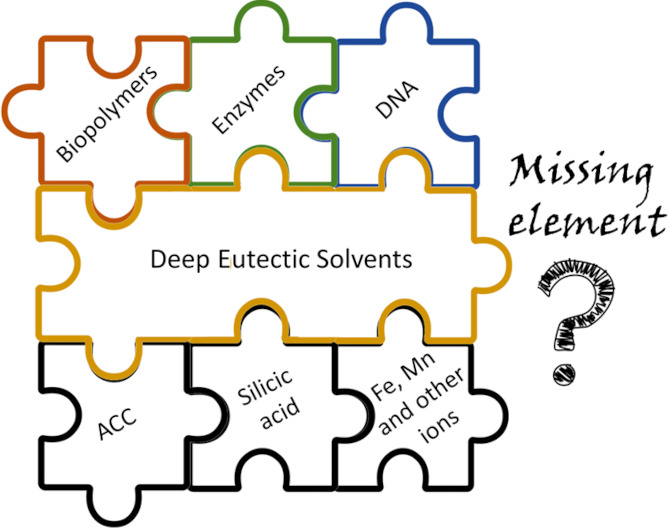
Paraphrasing Cölfen, “A crystal-clear view”,36 we fully agree that “with the biomineralization strategies in the hands of synthetic chemists, the formation of hierarchically structured organic–inorganic composite materials with enhanced material properties will conceivably have the potential to outperform biominerals because of the larger range of materials/solvents and advanced equipment available to synthetic chemists”.36 In fact, the concept of creating bioinspired hybrid materials with advanced properties from fundamental building blocks remains a largely uncharted area in materials science. Currently, much attention is paid to developing bottom-up approaches and soft chemistry methods targeting the synthesis of hybrid materials inspired by biomineralization processes.37 Mimicking of natural biominerals or biomineralization as a phenomenon facets several challenges that include solubilization of biopolymers (chitin, common component of biominerals, is almost insoluble in most solvents) or stabilization of inorganic precursors (i.e., silicic acid) that are crucial to achieve mineral deposition on all levels of structural hierarchy. All these challenges can be effectively solved by deep eutectic solvents (DESs).
DESs are known to be “green”, safe, and inexpensive mixtures and can provide tremendous opportunities as reaction media for modern bioinspired chemistry. These designer mixtures can serve as structure directing agents, protein and inorganic stabilizers, and polymer solvents. All these mechanisms can be applied simultaneously during the development of biomineralization-inspired materials. Therefore, the aim of this article is to highlight the unexplored potential of DESs as a reaction medium for the synthesis of biomineralization-inspired inorganic–organic hybrid materials, including computational design. Surprisingly, despite the wide application of DESs as (i) a medium in the synthesis of inorganic materials and (ii) solvents for biopolymers, this unconventional approach has not been studied in fine detail from the point of view of bioinspired materials chemistry. Due to the preorganized, supramolecular nature of deep eutectic solvents (DESs), they offer a remarkable soft template for directing the creation of biomineralization-inspired hybrids on a spectrum ranging from the atomic to the macroscopic scale. Additionally, the compositional flexibility of DESs lends to limitless compositions and, in turn, an enormous range of attainable properties. Utilization of the properly selected DES combined with the truly multidisciplinary and multiscale understanding of the impact of their structural peculiarities on the properties of biomacromolecules (proteins, enzymes, or polysaccharides) and inorganic building blocks will be game-changing in terms of biomineralization-inspired hybrids. This sustainable approach opens tremendous perspectives and could lead to the next generation of bioinspired hybrids with sophisticated features that can outperform the natural biominerals. We expect that there is a significant potential for fundamental research in exploring the application of DESs in contemporary biomimetics. This article will encourage the scientific society to undertake research that will seek to answer the question: How far can deep eutectic solvents push the boundaries in bio-inspired materials science?
2. Deep Eutectic Solvents: From Chemistry to Bioinspired Synthesis
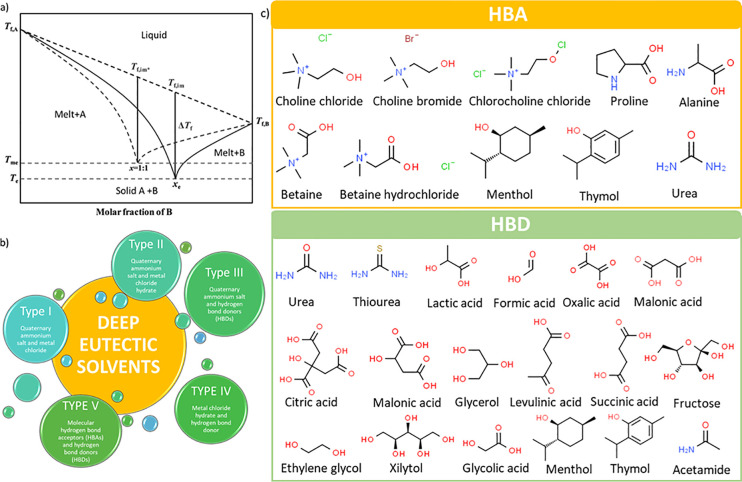
The term “deep eutectic solvent” first appeared in academic literature in 2003, when Abbott et al.38 discovered mixtures of choline chloride and urea with significantly reduced melting points compared to their individual pure components.39 Significant research into the chemistry of DESs has resulted in several changes over time in their definition. To present knowledge and within the context of this article, DESs are defined as mixtures composed of two or more components, which can self-associate to create a new eutectic phase characterized by a significantly reduced melting point (usually below the room temperature) in comparison with the melting points of their individual pure components40−46 (Figure 1a). DESs maintain the identities of their components, which interact via hydrogen bonding, as opposed to covalent bonding. Further, DESs remain in a liquid state: molecular dynamics simulations47 and inelastic neutron scattering studies48,49 revealed that the strength of hydrogen bonds between DES-forming components is weak enough to prevent them settling into a cocrystal.50 So far, depending on the chemical composition, five different categories of DESs are distinguished (Figure 1b). Accordingly, to Hansen et al., these categories are briefly defined as follows: type I, constituted by a quaternary ammonium salt and a metal chloride; type II, constituted by quaternary ammonium salt and a metal chloride hydrate; type III, constituted by a quaternary ammonium salt and a neutral species that acts as a hydrogen bond donor (HBD), typically an organic molecular entity like an amide, carboxylic acid, or polyol; type IV, formed by a metal chloride hydrate and HBD; and type V, represented a recent category exclusively comprised of nonionic, molecular hydrogen bond acceptors (HBAs) and hydrogen bond donors (HBDs), that opens new avenues for the design of hydrophobic DESs.51−53
Figure 1.
(a) Example of DES phase diagram (Tf,A and Tf,B, melting points of individual components A and B; Tf,im* and Tf,im, ideally interpolated temperature at the assumed maximum and the practical eutectic composition; Tme and Te, estimated maximum and the practical eutectic temperature; and xe, the eutectic composition). Reproduced from ref (65). Copyright 2020 American Chemical Society. (b) Diversity of DESs according to Smith et al.39 and Hansen et al.40 (c) Commonly exploited HBAs and HBD in DESs research.
Type III DESs have been the focus of extensive research because of (i) low melting points, which exhibit lower melting temperatures compared to other types of DESs; (ii) composition flexibility, which can be largely designed and customized to achieve DESs with a wide range of physicochemical properties; and (iii) application potential, which has shown promise in a wide range of applications and as a replacement to traditional solvents in numerous industries.
It is worth emphasizing that although type III DESs have received considerable focus, investigations into other DES types, including type I and type II, greatly contribute to the overall advancement and comprehension of deep eutectic solvents. However, recent reports on the eutectic mixtures that fit the definition of DES, but do not fit easily into these five categories, suggest that other types have yet to be discovered.40
DESs have also been described differently throughout literature. Mixtures obtained from natural components are classified as NADES (natural deep eutectic solvents).54−56 Recent reports about new DES subtypes include active pharmaceutical ingredient-DES(API-DES)57/therapeutic-DES (THEDES),58−61 which refer to a combination of two constituents that, at a specific molar ratio, transform into a liquid state at room temperature and in which one of them is an active pharmaceutical ingredient. Additionally, magnetic deep eutectic solvents (MDES) are considered a special category of conventional deep eutectic solvents.62−64 MDES are defined as mixtures comprising two or three components, which include a HBA, HBD, and an additional magnetic component such as a metal chloride.64
We believe that recent identification of DESs in extremophilic organisms66 is an important historical landmark that will lead to discoveries of new DESs classes. Research on DESs has exploded rapidly in recent times, both in the context of fundamental and applied research.
So far, large numbers of excellent critical review articles39,40,43,67,68 and books69−71 describing the chemistry of DESs as well their practical applications in energy storage,72 catalysis,73−75 analytical chemistry,76−78 extraction79−82 or separation,81 environmental monitoring,83 CO2 capture,84−86 biotechnology,87 biocatalysis,88,89 drug discovery and delivery,90,91 materials science,92−94 and biopolymer processing67,95−97 appeared in the past few years. In sum, this work is focused only on the properties that are crucial from the biomineralization-inspired point of view, including biopolymers processing, self-assembly, and inorganic synthesis.
Typical DES synthesis methods are relatively simple and fast as they are based on heating and stirring the constituents together until a homogeneous liquid is formed (Figure 2). This makes DESs extremely interesting and desirable alternatives to conventional organic solvents and ionic liquids (ILs). There are countless possible combinations and ratios of HBAs and HBDs that can constitute DESs, and these combinations can be finetuned to exhibit desired properties. Further, it is predicted that DESs can be successfully adapted for various applications, including dissolution and processing biopolymers,98 inorganic synthesis,99 or 3D printing.100 Alongside the aforementioned reasons, low costs and low maintenance make DESs excellent candidates for utilization on the industrial scale, which is the main barrier in the case of most ILs.101−104
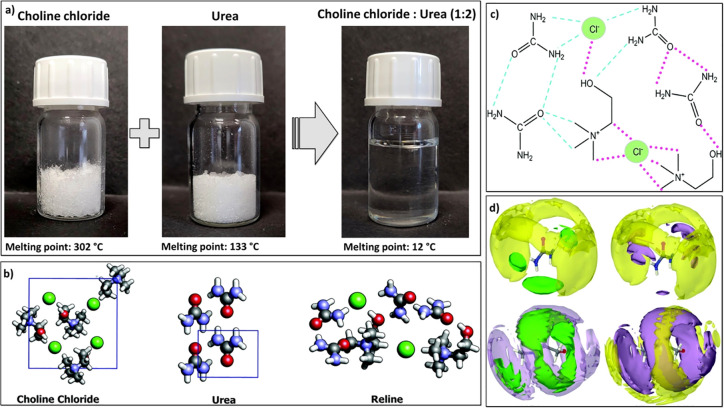
Figure 2.
(a) Schematic representation of the preparation of choline chloride/urea-based DES and differences in the melting points. (b) Molecular representation of the crystal lattices of choline chloride and urea, together with the model of reline (choline chloride:urea, 1:2) after geometry optimization. Adapted with permission from ref (127). Copyright 2017 Royal Society of Chemistry. (c) Schematic representation of the choline chloride:urea cluster with the intermolecular interactions in the formed DES (dashed lines) and those already present in the pure solids (dotted lines). Adapted with permission from ref (127). Copyright 2017 Royal Society of Chemistry. (d) Spatial density functions demonstrate the probability of the 3D structures of the various parts of (choline chloride:urea, 1:2). Reprinted with permission under a Creative Commons Attribution (CC-BY) 3.0 from ref (128). Copyright 2016 Royal Society of Chemistry.
However, such a tremendous level of tunability of DESs is also considered an impediment that requires significant resources, including time to allow for the discovery of mixtures with beneficial or desired performance. In effect, the scientific community is focusing on QSAR (quantitative structure–activity relationship) and QSPR (quantitative structure–property relationship) models that in the future will be able to predict and select the most promising DES, thus eliminating the need for hundreds of hours of expensive laboratory work.105
Various physicochemical properties of DESs have been studied and described lately because such data is considered crucial in terms of developing known and seeking new fields of their application. Taking into consideration that DESs’ properties can alter significantly at different compositions, analyzing not only the eutectic composition but also other combinations is often considered a reasonable solution. However, an analysis of the literature indicates that studies are most often conducted for one HBA to HBD ratio, which is likewise not a eutectic point (which refers to composition characterized by the lowest melting point). This fact significantly hinders the discovery and indication of favorable physicochemical parameters from the application point of view. Within the framework of discovery potential, modeling plays a crucial role, including quantum mechanical106,107 and deep learning approaches.108−117 Specifically, a new generation of generative language models can provide powerful avenues to explore the potentiality of DES structures that have not yet been discovered, specifically focused on de novo design.
The following subsections of this article will provide a general overview of the properties of DESs and the role these properties play in the context of biomineralization-inspired materials.
2.1. Lowered Melting Point
One of the key parameters determining the usefulness of DESs in various applications is its ability to lower the melting point. As was established by Abbott et al.,118 the combination of choline chloride (Tm = 302 °C) with urea (Tm = 133 °C) in a ratio of 1:2 leads to a DES that is a liquid at room temperature (Tm = 12 °C). This crucial discovery showed that DESs can be successfully applied as excellent solvents for processes conducted even at room temperature. Soon after, it was noticed that the melting point of the formed DES varies greatly and depends on the chemical structures of both the HBA and HBD, (e.g., choline chloride:thiourea (1:2) has Tm = 69 °C, choline chloride:1,3-dimethyl urea (1:2) has Tm = 70 °C, choline chloride:ethylene glycol (1:2) has Tm = 66 °C, choline chloride:glycerol (1:2) has Tm = −40 °C, choline fluoride:urea (1:2) has Tm = 1 °C, ZnCl2:ethylene glycol (1:4) has Tm = −30 °C, and H2O:DMSO (1:4) has Tm = −69 °C).40,68,119,120
Conducting processing at ambient temperatures can contribute to strengthening the hydrogen-bonding accepting ability, which theoretically improves the ability to dissolve biomass, such as cellulose, starch, or chitin. Typically, dissolution processes are performed at temperatures ranging from 50 to 100 °C, due to high viscosity of DESs.121,122 This means that both the composition and ratio between HBA and HBD in DESs must be properly selected so that the obtained system is in a liquid state under the assumed conditions. As was reported previously, exposition of DESs consisting of choline chloride and urea on the temperature above 120 °C for a longer period of time leads to decomposition of urea, which can be successfully applied for synthesis of novel inorganic materials, like MnCO3,123 [NH2(CH3)2]2Sn3Se7·0.5NH(CH3)2, [NH4]2Sn4Se9 and [NH4]3AgSn3Se8,124 (VO)2P2O7 and VOPO4,125 or Zn(O3PCH2CO2)]·NH4.126
On the contrary, utilization of DESs at lower temperatures can lead to formation of various inorganic compounds exhibiting unique characteristics, such as nanofibrous networks of RuCo2O4,102 (N-doped) ceria nanoparticles, calcium phosphate nanoparticles,129 or NiCo2O4 nanooctahedrons.130
Interestingly, low melting points of DESs obtained from choline chloride and ethylene glycol131,132 or polymerizable HBD, like acrylic acid,133 maleic acid, and acrylamide,134 were successfully utilized for fabrication of 3D-printed conductive organogels, which have proven to be attractive sensitive strain sensors.
2.2. Intramolecular and Intermolecular Hydrogen Bonding
It is assumed that the sum of interactions and effects, originating from the unique structures of both HBA and HBD are responsible for achieving the depression of melting temperature in DESs. Besides the steric effects related to the spatial arrangement of DES components, hydrogen bonds between the HBA and HBD are believed to play a major role, which contributes to weakening ionic attractions between cation and anion in HBA.118,135−137 Moreover, other forces, particularly van der Waals interactions, were recognized as particularly important in terms of physicochemical properties of DESs, since they can significantly influence the mobility of molecules incorporated into the eutectic system.138,139 This underscores the general relevance of H-bonding for biological and bioinspired materials.140−144
Interestingly, the strength of hydrogen bonding between HBA and HBD in various binary DESs has been correlated to its capability to extract chitin from crustacean waste. A purity level of 93% was attained for chitin extracted by DES consisting of choline chloride and formic acid (1:2).145 These findings are also supported by the fact that DESs of choline chloride and ethylene glycol, or glycerol, were found to be ineffective for dissolving this biopolymer, whereas DES compositions of choline chloride and urea or thiourea achieved satisfactory solubility at a level of 8–9%. Interestingly, these DESs were more effective than compositions comprising various dialkylimidazolium halides or betaine hydrochloride, which suggests that the structure of HBA could significantly influence the process of chitin dissolution.146 Moreover, the presence of imidazolium rings in this case hindered the efficacy, which contrasts with previous reports demonstrating their excellent polysaccharide-dissolving ability.147
It has been found that very strong hydrogen bond interactions in DESs facilitate their homogeneous dispersion and help to obtain a stable morphology of various inorganic nanomaterials.102 For instance, Zhang et al.148 stated that since the chloride ion was incorporated into hydrogen bond networks in DESs, it has a structure-directing ability, which induced the morphology of fabricated advanced nano electrocatalysts consisting of NiCo2O4. The crucial role of hydrogen bonding in acceleration of the polymerization rate was noticed during the fabrication of 3D-printed ultratough transparent conductive elastomers via stereolithography. The preparation strategy enabled the acceleration of the photopolymerization rate of a mixture consisting of maleic acid:choline chloride and acrylamide:choline chloride and the rise of the double bond conversion. Since choline chloride can form hydrogen bonds with both HBDs, the mixed solution before polymerization was transparent, contributed to the formation of uniform polymers.149 As demonstrated by Cai et al., the utilization of acrylic acid as HBD leads also to transparent and uniform materials, which was confirmed by analysis of the collected optical images.133
2.3. Thermal Stability/Volatility
DESs are often compared to ILs because they share many of the same properties, e.g., generally high thermal stability and low volatility because of strong interactions between HBA-HBA, HBD-HBD, and HBA-HBD. These properties are considered especially desirable for possible replacement of volatile organic compounds (VOCs) currently used as solvents in industry.150 It should be noted that the thermal stability of DESs limits the maximum operating temperature, in which they can be effectively applied. In accordance with the literature, while the vapor pressures of DESs are multiple times lower than that of common VOCs (e.g., hexane or acetonitrile), they were simultaneously found to be significantly greater compared to ILs equivalents.40 These dependencies were described in more detail by Ravula et al.,151 who ranked the following fluids with increasing volatility: dicationic ILs < aprotic ILs < polymeric ILs < protic ILs < DESs < long-chain PEGs < triglyme, short-chain PEGs < VOCs.
On one hand, the high thermal stability of DESs allows their use at higher temperatures, which can significantly increase the reaction rate. Furthermore, the solvent-dissolving ability generally notably increases at higher temperatures, due to the reduction of viscosity and surface tension, which eventually contribute to enhanced diffusivity.152,153 On the other hand, high volatility or low stability of some DESs constituents can alter their composition or HBA:HBD ratio, which significantly reduces their application potential. Particularly, the nonionic nature of most common HBDs makes them more susceptible to decomposition or evaporation compared to ionic HBAs. For instance, at around 125 °C, urea degrades rapidly, while ChCl begins to decompose at about 225 °C.154 Other examples confirm that tendency, e.g., thiourea, menthol, and acetamide decompose at 110 °C,155 181 °C,156 and 188 °C,157 respectively.
As DESs represent mixtures rather than pure chemical compounds, thermogravimetric analysis (TGA) could only provide data regarding “apparent” vapor pressures, mainly because of plausible preferential species volatilization leading to change in the composition of the analyzed mixture. This phenomenon is particularly valid for DESs obtained from compounds that are more susceptible to evaporation or sublimation than decomposition, like menthol, thymol, or coumarin.55 Analysis of literature data leads to the conclusion that thermal stability of DESs depends on the stability of both ingredients used for their preparation as well as on the mutual interactions between them (mainly hydrogen bonds network).
2.4. Viscosity
Viscosity, as one of the most essential parameters describing DESs, represents the resistance to deformation under the influence of shear forces. Extensive hydrogen bond networks occurring between constituents of DESs together with others (ionic and van der Waals interactions) significantly affect the mobility of ions/molecules and, as a result, DESs are usually characterized by considerably high viscosities, usually exceeding 50 mPa s. Generally, at ambient temperature this parameter varies from 2.6 mPa s for nonionic DES, menthol:dichloroacetic acid (1:2), to more than 80 000 mPa s for ionic–ionic DES, choline chloride:ZnCl2 (1:2). Detailed values have been summarized previously and can be found elsewhere.40,158−161 It was established that higher viscosities of DESs containing ionic moieties can be attributed to the Coulombic forces between the cation and anion in HBA.162
As was previously
stated, viscosity is directly associated with transport properties
within the liquids phase, such as diffusion of compounds dissolved
in a DES. Therefore, analogously as in the case of ILs, much effort
is being devoted to reducing the viscosity of DESs to increase their
efficacy as working media in the dissolution of biopolymers, synthesis
of inorganic, and organic compounds as well as sophisticated hybrid
nanomaterials.163 To achieve that goal,
various strategies have been elaborated, such as the following: (i)
Selection of appropriate HBA: due to strong van der Waals interactions,
viscosity generally increases as the alkyl chain in HBA increases,
therefore short alkyls contribute to improvement in the mobility of
DES constituents.160,164 (ii) Selection of appropriate
HBD: due to the fact that a strong hydrogen165 network leads to a rise of viscosity, compounds with fewer functional
groups able to form H-bonds are preferable (this fact explains extremely
large viscosities of sugar-based DESs that exceed 10 000 mP s even
at elevated temperatures). (iii) Addition of water: it is beneficial
for significantly reducing hydrogen-bonding interactions, which subsequently
leads to a decrease in viscosity. In the case of some hydrophobic
DESs, viscosity reduction can be achieved by addition of organic solvents
(methanol, acetone, or DMSO). (iv) Increase in temperature: similarly
as in the case of ILs, DESs demonstrate an exponential decrease in
viscosity with a rise of temperature, which can be described by the
Arrhenius model: 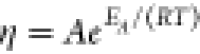 is the activation energy for viscous low, A is a cofactor, and R is the molar gas
constant. The nonlinear characteristic of this relationship indicates
that even a slight increase in temperature can contribute to substantial
reduction of viscosity. It should also be noted that reduction of
viscosity with increasing temperature is more pronounced in the case
of DESs compared to conventional VOCs.166,167 (v) Addition
of third component in the mixture: depending on the type of DES, this
action can decrease the overall viscosity (e.g., for ionic–ionic
DES 1-butyl-3-methyl-imidazolium:ZnCl2 addition of acetamide
reduces viscosity 3–5 fold; for ionic-nonionic DES choline
chloride:xylitol addition of glycerol reduces viscosity 2–3
fold) or can increase it (e.g., for ionic-nonionic DESs choline chloride:urea
and choline chloride:malic acid addition of glycerol provides a rise
of viscosity by at least 50%).165,168,169
is the activation energy for viscous low, A is a cofactor, and R is the molar gas
constant. The nonlinear characteristic of this relationship indicates
that even a slight increase in temperature can contribute to substantial
reduction of viscosity. It should also be noted that reduction of
viscosity with increasing temperature is more pronounced in the case
of DESs compared to conventional VOCs.166,167 (v) Addition
of third component in the mixture: depending on the type of DES, this
action can decrease the overall viscosity (e.g., for ionic–ionic
DES 1-butyl-3-methyl-imidazolium:ZnCl2 addition of acetamide
reduces viscosity 3–5 fold; for ionic-nonionic DES choline
chloride:xylitol addition of glycerol reduces viscosity 2–3
fold) or can increase it (e.g., for ionic-nonionic DESs choline chloride:urea
and choline chloride:malic acid addition of glycerol provides a rise
of viscosity by at least 50%).165,168,169
Excessive viscosity not only impedes the mixing and separation processes carried out in DESs but also influences the dynamic interaction between heat and substances, thereby severely restricting their extensive practical use. Despite utilization of cost-effective substrates and facile method of preparation of DESs, their high viscosity is still often considered as the greatest disadvantage and impediment to their commercialization; therefore, all studies focused on overcoming this important issue are strongly justified.
2.5. pH
Depending on the chemical structure of the starting materials, the obtained DESs can be available in a wide range of pH values. DESs obtained from neutral compounds, like choline chloride and sugars exhibit a neutral pH, whereas compositions containing polyols and organic acids can be characterized by acidic behavior.165,170,171 On the other hand, urea and potassium carbonate act dominantly over choline chloride and glycerol which contribute to the basic nature of such DESs.172 Literature surveys171,173 indicate also that Kamlet–Taft solvatochromic parameters are being determined to describe the character of the obtained DESs. Hence, the calculated parameters α, β, and π* are associated with hydrogen-bond acidity (hydrogen bond donating strength), hydrogen-bond basicity (hydrogen bond accepting strength), and dipolarity/polarizability of the solvents, respectively. Interestingly, the value of the Kamlet–Taft β parameter is positively correlated with the rate of dissolution of polysaccharides in DESs. In the case of chitin, a relatively acidic environment was more favorable for the extraction of chitin from crustacean waste. Additionally, it was discovered that purity of chitin is positively correlated with the HBD acidity. According to another study, pH values had a measurable effect on the extraction efficiency of the proteins with the use of binary and ternary DESs, thus showing the importance of this parameter also in separation processes.174 Moreover, the stability of various enzymes, like lipase is also correlated with DESs’ hydrogen bond acidity, which in the future can be utilized in synthesis of novel materials via biocatalysis.175
2.6. Density
Another critical parameter of DESs in various applications is their density. Density is utilized in the development of various thermodynamical models, process simulations, and engineering estimations that are required for studying fluid flow, mass transfer, heat transfer, and reaction kinetics of DES. State-of-the-art indicates that the most of the described DESs are characterized by higher densities compared with water, with values ranging from 1.0 to 1.6 g cm–3 at 25 °C.176,177 Nonetheless, some exceptions can exhibit even greater densities (FeCl3 6H2O:glycine (2:1) = 1.677 g cm–3) or extraordinarily low densities (borneol:thymol (1:2) = 0.800 g cm–3).178 It was also noted that densities lower than water can be obtained for hydrophobic DESs, which generally contain structurally more bulky constituents, and consequently, the packing fraction of atoms is strongly reduced.179,180 Generally, the density of DESs decreases linearly with increasing temperature, in addition to relying on the presence of vacancies in the DES network.176,181 Previous studies have extensively documented the densities of known DESs.40,158,161,177,180,182−191 In recent years, many computational methods have been developed to predict with high accuracy the density of DESs using the classical quantitative structure–property relationships (QSPR) approach with suitable mathematical equations,183,185,192−194 artificial intelligence,186 and machine learning.180,184,195 Interestingly, Alkhatib et. al196 and Kovács et. al54 described state-of-the-art, perspectives and guidelines on modeling the physicochemical properties of DES, including density. With such a substantial number of models, model performance can be compared with, e.g., Tak et al.,183 who compared results calculated from five of the existing models with experimental results to reveal the one that is the most precise. Researchers noted also that models based on the cohesion factor of the cubic equation of state (predictive Soave–Redlich–Kwong (PSRK) equation of state with NSM1 alpha function model (NM-PRNSM1), PSRK equation of state with original derived alpha function model (NMSRK))197,198 are able to compute density with higher precision compared to models based on correlations like the Reid (RR) model, McHaweh (MH) model, and linear generalized model (LGM).199 The high level of scientific interest in computer-based predictive methods clearly demonstrates that further progress in this field is inevitable and will help to increase in understanding of the interactions occurring between the respective components of DESs.
2.7. Conductivity
Electrical conductivity is a fundamental physical property that represents how easily a material can conduct electrical current. Nowadays, it is also considered to be the most important factor for the design, control, and optimization of electrochemical processes.200 For example, in the manufacturing of bioinspired materials made of ultrathin nanofibers by an electrospinning technique, conductivity of solvent is considered to be a crucial parameter affecting the course of the process and the morphology of the resulting fibers.201,202 Additionally, electrical conductivity provides essential information for designing the cathodic protection required to prevent corrosion.200 When measured in a broad temperature range, the conductivity of DESs usually exhibits non-Arrhenius behavior, typical for glass-forming liquids.203 DESs and their solutions are usually characterized by conductivity between 0.01 and 30 mS cm–1. These values are higher than pure solvent conductivity (0.0001–0.0050 mS cm–1 for distilled water) but similar to ILs (0.01 to 100 mS cm–1) or nonaqueous electrolytes (around 10 mS cm–1 for Li-ion electrolytes) and lower in comparison with aqueous electrolytes (50 to 250 mS cm–1).39,200,204−206 In accordance with many reports, the conductivity of DESs rises with an increase of the temperature as well as the amount of water, which is generally a decrease in viscosity of tested compositions.68,170,181,207 Interestingly, the electrical conductivity of DES–solvent systems generally show a maximum value at a relatively low DES concentration (Figure 3).208,209
Figure 3.
Electrical conductivity as a function of (a) temperature and (b) glycine mole fraction. (c) Temperature dependence of the composition (x1max) of maximum conductivity obtained for glyceline and reline. Reproduced from ref (208). Copyright 2021 American Chemical Society.
These observations imply that DESs systems are unique compared with either organic solvent mixtures or aqueous electrolyte solutions, in which concentration and conductivity are often directly proportional to each other.209 Recently, promising attempts have been made to build computer models based on multiple linear regression or using artificial neural networks to predict the conductivity of DES.170,200,210−212 However, it should be noted that these models are based on a relatively small amount of data, which limits their applicability. It can be assumed that in the coming years, with more data and use of DESs, these models will be significantly improved.
2.8. Polarity
The most widely applied method to describe polarity properties of organic compounds, including DESs and ionic liquids, is the Kamlet–Taft empirical polarity scale. To capture the complexity of interactions, this scale, based upon linear solvation energy relationships, is composed of the complementary scales of hydrogen bond acidity (α), hydrogen bond basicity (β), and dipolarity/polarizability effects (π*). Generally, polarity is associated with multiple overlapping elements, such as the hydrogen-bonding, electron pair donor–acceptor interactions, Columbic interactions, and various dipole interactions. This parameter is rather complex as shown in solvent-dependent phenomena, e.g., the rate and selectivity of many chemical reactions in DESs, interpreting the interaction of DESs with various organic and inorganic materials as well as biomass.213−215 Moreover, since the polarity increases with an increase in the intermolecular interactions, this parameter is often described as a unique solubilization property of a solvent, which is crucial in studies focused on utilization of DESs as a “green” alternative to common solvents.161 The solvent polarity, separately or together with hydrophobicity and hydrogen bond characteristics, can also affect the extraction capacity or protein stability and activity. These findings clearly indicate the need for conscious selection of a specific HBA:HBD pair, which should certainly be based on the current state of knowledge in a specific application.189,216
The role, significance, and dependencies in all three parameters α, β, and π* has been thoroughly outlined in other insightful review articles.40,68,161,176,214 On the basis of the above mentioned reports, the following polarity of DESs can be arranged in the following order: monoacid-based hydrophilic DESs < ionic or neutral hydrophobic DESs < diacid–based hydrophilic DESs < alcohol-based hydrophilic DESs < water. The HBD structure and water content have also been shown to affect the polarity of the systems. Simple replacement of the HBD or change in the water content can significantly alter solvent polarity, likewise with just water or methanol.215,217 Interestingly, results summarized by J. Cao and E. Su161 indicate also that the polarity of hydrophobic DESs mainly depends on the specific properties of HBA that originate from its chemical structure.
2.9. Surface Tension
Surface tension is a measure of cohesive forces in liquid on the surface and plays a pivotal role in diverse processes relying on wetting, permeability, lubrication, and bubbling. Therefore, it is considered a key physicochemical property for the application of DESs in the field of interface and colloid processes,218−220 including biomineralization-inspired material syntheses.221,222 It has been proved that the solution surface tension plays a significant role (besides the already identified parameters, such as the biopolymer functional groups and the biopolymer concentration) in the crystallization of biominerals.221
There are few reports discussing the influence of this parameter on application DESs in other important areas, like biopolymer processing or synthesis of various organic/inorganic materials. The majority of DESs are characterized by surface tension (γ) between 30 and 70 mN m–1 in standard conditions,219 and the stronger hydrogen-bonding interactions usually lead to a higher value of this physicochemical property (e.g., for choline chloride:lactic acid (1:2) γ = 47.4 mN/m, for betaine:lactic acid (1:2) γ = 46.8 mN/m, for choline chloride:glycerol (1:2) γ = 57.8 mN/m).219 Interestingly, the temperature and the molar ratios between the components are among the primary factors that strongly affect γ and can be easily adjusted.218 Similarly, to traditional VOCs or ILs, the γ of DES linearly decreases with increasing temperature.176,219 Obviously, γ in DES can be lowered by addition of surface-active compounds, which possess amphiphilic structures.223 Interestingly, the presence of crystal water in HBAs would contribute to a slight decrease in the surface tension. However, the presence of a small amount of DES significantly decreases the surface tension of water.219 Additionally, due to strong hydrogen-bonding interactions, small addition of solvents like alcohols, acetone, or ethyl acetate can lead to decrease in surface tension of DES; nevertheless, a small addition of DES to these solvents does not affect their surface tension.219 There are many studies focused on investigation of the surface tension of DESs based on which of several predictive models were created.218 It should be stressed that the most general surface tension and density models developed by Haghbakhsh et al.218 allow us to predict these properties at various temperatures, even for novel, not yet discovered DESs. This area needs to be further explored and related to the biopolymer processing and formation of biopolymer-mineral hybrid materials.
2.10. Self-Assembly
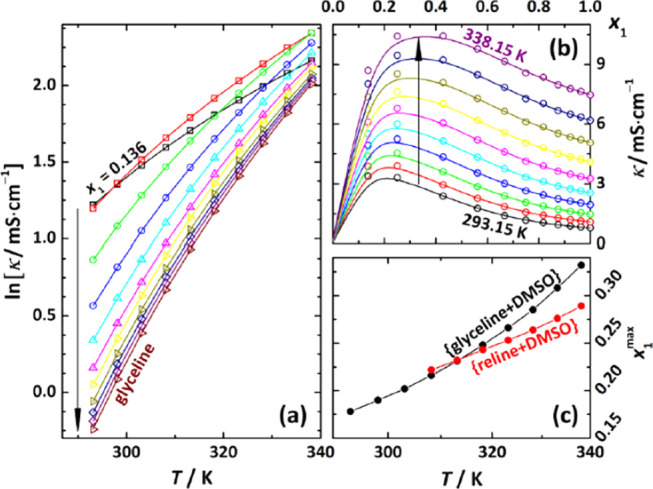
It is hypothesized that the DESs generally do not contain amphiphilic molecules or ions and therefore lack the beneficial self-assembled nanostructures found in numerous ILs. On the other hand, formation of intra- and intermolecular interactions, including Coulomb forces, hydrogen bonding, van der Waals interactions, electrostatics, dispersion forces, and a polar–polar segregation in DESs dictates their distinct bulk liquid and interfacial nanostructure.224 DES nanostructures demonstrate different spatial arrangements of the distinct species and exist over several length scales, from molecular- to nano- and mesoscales.224 The nanostructure of DESs is inspiringly discussed in a review by Bryant et al.224 In their review, the authors excellently described the structure–function relationship and highlight the recent challenges and pointed out the crucial importance in the understanding of nanostructure and self-assembly of DESs. McDonald et al.225 have shown that using a methyl-terminated primary cation “switches on” the solvophobic self-assembly of cation alkyl chains in DESs to produce nanostructured solvents. Buzolic et al.226 discovered that an amphiphilic HBD, specifically C4 and C6 acids, can induce nanostructure formation in DESs. Furthermore, it was observed that the definition of the nanostructure improves with an increase in HBD chain length (Figure 4).
Figure 4.
(a) Representative schematic of the transition from the multilayer regime to the boundary regime for ChCl/C6OOH on mica. Reprinted with permission from ref (226). Copyright 2022 Elsevier. (b) EPSR model snapshots of nanostructured propylammonium bromide:glycerol (1:2). Adapted from ref (225). Copyright 2018 American Chemical Society.
This paves the way for DES design, control of forces between ions, and the dissolution of more hydrophobic solutes. Additionally, such an approach provides a mechanism for the improvement of the functionality of DESs by incorporating properties related to nanostructures from their ionic liquid cousins while retaining their crucial advantages intrinsic to DESs (low cost and easy preparation). Amphiphilic nanostructured DESs will unravel their intrinsic properties with respect to synthesis bioinspired hybrid materials that are based on organic precursors (TEOS, TBOT, TIP, TEOG, etc.). In this respect, DESs amphiphiles can be employed to take advantage of (i) increasing solubility of organic precursors,227 (ii) DESs self-assembly into nanostructures with well-defined size and geometry but also to exploit (iii) their natural organization in ordered arrangements, which play a role of structure driving agents.228 The amphiphilic nature of DESs can also enhance enzymatic activity in enzyme-driven biomineralization studies.229
2.11. Impact of Water on DESs Properties
The majority of DES systems based on organic salts, like choline chloride and urea, were found to be extremely hygroscopic and when exposed directly to the atmosphere can absorb up to 20% (w/w) of water. Hence, it is difficult to generalize DES behavior since different combinations of HBA and HBD can result in DESs with different affinity for water. Therefore, many scientists agree with the fact that the influence of water on the properties of DESs as well as their application should be considered individually.214,230 Multiple studies unambiguously demonstrated that the addition of H2O rapidly changes the heterogeneous 3D structures of pure DESs, leading to alteration of physicochemical characteristics and their behavior. The experimental data gathered for DES–H2O systems, thoroughly summarized by C. Ma et al. in 2018, clearly indicate that the water content can exhibit a significant impact on density, viscosity, and electrical conductivity.231 Therefore, tailoring properties of DESs with water was proposed as a promising strategy to design for applications. Recent studies performed on choline chloride:urea DESs composed of dry components under moisture-free conditions have shown altered values of phase transitions and thermal instability. In this study, it was noticed that the very low water contents result in increased viscosity and decreased molecular mobility in the DES, making it less mobile, which directly influences the observed thermal properties.230 Additionally, neutron total scattering and empirical potential structure refinement studies for a mixture of choline chloride:urea (1:2) with water revealed that the nanostructure of the DES is retained at a remarkably high level of water (ca. 42% of H2O). However, above 51% of the hydration level, the DES–water mixture should be described as an aqueous solution of DES components. Therefore, the plausible phenomenon of transition from ionic mixture to aqueous solution upon the increase in the water content should be taken into consideration in the case of development of DESs in the field of bioinspired materials.232
2.12. Biocompatibility and Environmental Effects of DESs
The significance of solvent biodegradability, nontoxicity, and biocompatibility cannot be overstated in the realm of bioinspired materials. These materials are designed to emulate the extraordinary properties and functions observed in nature, presenting immense potential across diverse fields, including medicine. Therefore, ensuring the safety and compatibility of these materials is crucial to their successful translation into practical applications. Moreover, considering the increasing recognition of solvents’ impact on pollution, energy consumption, air quality, and climate change, the principles of green chemistry and sustainability233 demand a comprehensive understanding of solvent toxicity, biocompatibility, and environmental fate. Scientists must adopt a holistic approach, considering the implications of solvents on both human health and the ecosystem, to develop bioinspired materials that meet stringent environmental and ethical standards.234
DESs are commonly asserted to be environmentally sustainable, with their characteristics often described in terms of “low toxicity, high biodegradability, low cost, and sourcing from renewable feedstock.” Nevertheless, this broad assertion tends to be unquestioningly accepted in scientific papers that discuss novel advancements and applications of DESs. Furthermore, the perceived sustainability of DESs can vary considerably depending on the specific components present in the eutectic mixture. Nejrotti et al.235 recently provided a comparative, broad-spectrum review on the sustainability of widely employed DESs (or DES-like mixtures), sharing ChCl as their common HBA component. The toxicity of a DES could then be different from that of its components. Authors pointed out that the combination of components within a DES, even if they are individually nontoxic, can give rise to synergistic effects that alter the toxicity of the mixture. This can be attributed to the emerging interactions within the supramolecular structure of the eutectic mixture. According to Nejrotti et al.235 who holistically analyzed toxicity and biodegradability of DESs (simultaneously analyzing the impact of DESs production on the environment), the sustainability of the choline chloride (ChCl)-based deep eutectic solvent can be ordered as follow ChCl:glycine > ChCl:urea > ChCl:ethylene glycol > ChCl:malonic acid.
The results presented in several corresponding articles describing DESs toxicity236,237 clearly show that that the initial belief that DESs are universally environmentally friendly, solely based on the nature of their constituents, is somewhat simplistic. The findings highlighting the potential toxicity of DESs emphasize the need for rigorous toxicological assessments specific to bioinspired materials that will be dedicated for biomedical applications. These assessments should encompass comprehensive studies to understand the potential risks, identify safe exposure limits, and develop appropriate guidelines for the use of DESs in biomedical contexts. Consequently, there is an urgent necessity to thoroughly assess the toxicity of DESs and establish comprehensive and standardized protocols that align with their potential applications. The potential residues of DESs in bioinspired materials and its impact must be carefully analyzed. Indeed, the development of DESs-based bioinspired materials, including eutectogels intended for direct contact with biological systems like implantable medical devices or biocompatible coatings, require careful evaluation of DESs’ effects on cellular responses, tissue compatibility, and long-term biocompatibility. This thorough assessment is crucial to ensure the safety of patients and the efficacy of biomedical interventions. Holistic approach to DESs’ toxicity entails examining factors such as cell viability, proliferation, adhesion, and functionality when exposed to DES-based materials. Additionally, evaluating the compatibility of DESs with different types of tissues and their ability to integrate seamlessly within the biological environment is crucial. Conducting comprehensive evaluations of DESs’ biocompatibility ensures that these materials not only mimic natural systems but also exhibit the necessary biocompatibility and safety required for effective integration within the human body.
A distinguishing factor that unequivocally establishes the safety and environmental friendliness of DESs is their extremely low vapor pressure, which effectively eliminates the risk of atmospheric contamination and inhalation-related hazards.235,238 Lastly, DES implementation in industry also requires development of proper methods for solvent recovery and recycling and development of closed-loop systems and circular economy principles. By implementing efficient recovery techniques, the potential of DESs as environmentally friendly solvents can be fully realized.
3. Applications of Deep Eutectic Solvents
3.1. Biopolymer Processing
In various organisms, the formation of ordered arrays of inorganic crystals is facilitated through regulated nucleation occurring at the interfaces of the crystals and substrate biomacromolecules. Therefore, efficient processing of biomacromolecules is of crucial importance for the practical development of biomineralization-inspired hybrid materials. Chitin, collagen, and silk are identified as main biopolymers templating the biomineralization phenomenon. Chitin has been found in calcium carbonate239 and siliceous biominerals240 and collagen is a main organic component of bones241 and siliceous spicules of sponges,242 while chitin together with silklike proteins according to the famous Levi-Kalisman model243 regulate calcium carbonate formation in the bivalve mollusk shell. According to recent scientific reports,241 the importance of designing the biomineral-inspired materials is to understand the structural hierarchy as well chemistry of the biomineral on all length-scales.244−246 As biomineral deposition occurs on the nano, micro, and macroscale, by mimicking this process, researchers need to ensure efficient hybridization of the inorganic with selected biopolymers.247 This is not a trivial task if we consider, for example, that chitin is nonsoluble in the most known solvents.248−250 DESs are recently reported as a green, sustainable, and efficient chitin solvents and do not cause significant impact on the chitin crystallinity after its precipitation from DES.251−254 The hypothesized mechanism of chitin dissolution is explained as the interaction between the HBA and HBD components of a DES with inter- and intramolecular H-bonds of chitin that results in the breaking of the H-bond network of chitin and, as a result, facilitating its dissolution.255 This feature opens the new synthesis possibilities for creation of chitin-based bioinspired inorganic–organic materials. Among polysaccharides, cellulose is also widely used as a template for development of advanced biomineralization-inspired materials.256−260 Numerous reports describe the dissolution, nanofibrilation, and modification of cellulose in DESs,261−267 which consequently open an entirely new way for creating such composites.
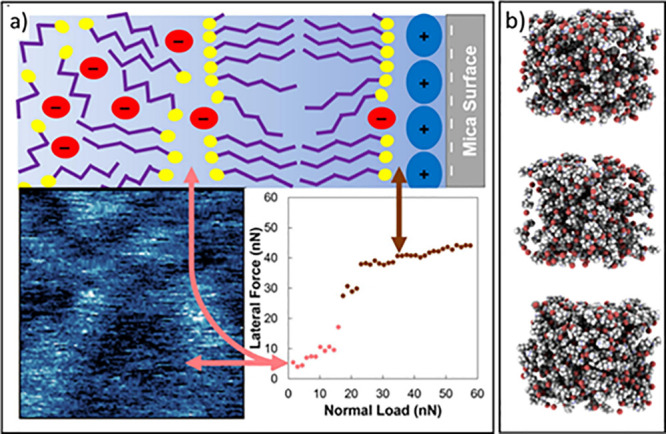
DESs have also been efficiently applied in the processing of chitin (Figure 5a), cellulose, collagen (Figure 5b,c), and silk (Figure 5d–g). Silk fibroins were recently explored in the design biomimetic biomineralization-inspired multilayered materials268 and functional biomaterials.269 With the assistance of computational simulation, the materials that arise from multiscale self-assembly and comprise silk nanofibrils (SNFs), hydroxyapatite (HAP), and chitin nanofibrils (CNFs) exhibit nacrelike structures. These structures possess mechanical strength and toughness that surpass that of the majority of natural nacre and nacrelike nanocomposites. We hypothesize here that biopolymer solubility in DESs can ensure their efficient hybridization with selected inorganic phases, and this inorganic–organic molecular recognition process will be beneficial for practical applications. It should be noted that inorganic precursors dissolved in DESs can be effectively stabilized, and this will ensure effective mineral deposition on various levels of structural organization. Stabilization of inorganic precursors is achieved through several mechanisms. One mechanism is the formation of hydrogen bonds between HBD and the precursor, which can prevent the precursor from undergoing unwanted reactions or decomposition. Another mechanism is the coordination of the precursor with the HBA. This can occur when the HBA contains a coordinating group, such as a carboxylate or amine, that can interact with the metal center of the precursor. This coordination can also stabilize the precursor and prevent unwanted reactions and can have an impact on the mechanisms of reaction with dissolved biomacromolecules.
Figure 5.
(a) Solubility of chitin (wt %) in selected DESs, according to ref (146). (b) Efficiency of collagen extraction from cod skin with respect to applied DES and (c) mechanism of interaction of DES with collagen. Reproduced from ref (272). Copyright 2017 American Chemical Society. Impact of the (d) DES composition and (f) treatment time on the water-soluble fraction and silk yield. The photographs of supernatants collected after DES treatment with various molar ratios of DES components (e) and various processing time (g). Reproduced from ref (273). Copyright 2020 American Chemical Society.
Moreover, according to the green chemistry rules,270,271 scientific efforts should be shifted toward using solvation ability of DESs with respect to silk, collagen, cellulose, and chitin in materials chemistry. This will represent new, rational, low-cost, and efficient strategies for the combining of all these building blocks together with inorganic precursors and design and preparation of robust, hierarchical, and functional nanomaterials that will meet a variety of application requirements in modern technologies. Such an approach will reduce or remove the need for harmful chemical additives and energy-inefficient equipment.
However, the delay in implementing DESs in this case is due to the complex dissolution of biopolymers by DESs, which is affected by numerous factors such as viscosity, pH, and polarity. Thus, there is urgent need to find the DESs structure relationship with biopolymer-dissolving ability.
3.2. Electrospinning and Additive Manufacturing
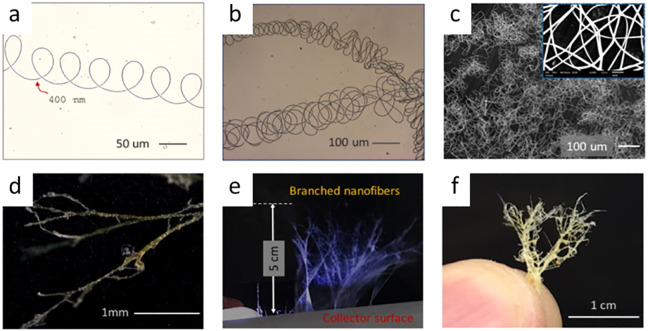
Electrospinning is commonly employed as a straightforward and adaptable approach for producing bioinspired materials made of ultrathin nanofibers274 that allows control over various morphologies of the electrospun fibers. It has been already proven that the electrospinning of biopolymers from DESs lead to development of sophisticated materials with outstanding properties and morphologies. Application DESs in electrospinning eliminates the need for toxic or flammable volatile organic solvents (that are usually used for biopolymer processing). Additionally, most of the precursors and waste are designed to be recycled, therefore it fulfills the demand of sustainability and is a promising step torward zero-waste technology.275 Electrospinning of proteins using DESs enables unique control over the fiber morphology (Figure 6a–e). It has been observed that HBA in DESs preserve the α-helix configuration during electrospinning and leads to formation of unique coil-shaped fibers, and in some extreme examples electrospun fibers form sophisticated 3D architectures resembling the cedar leaf structure (Figure 6f).276
Figure 6.
Tunability of fibers produced by electrospinning DES-Zein presenting (a) helical loops, (b) multiple helical loops, (c) multilayered nanofibers with (d) branches, (e) branched nanofibers, and (f) structures resembling cedar leaf morphology. Reprinted with permission under a Creative Commons Attribution (CC-BY) 4.0 from ref (276). Copyright 2020 Springer Nature.
Surprisingly, there is a lack of reports reporting electrospinning of inorganic–organic biomineralization-inspired materials from ILs or DESs. This area needs urgent exploration as it can be a milestone in the development of biomimetic hybrid materials for water purification and enzyme immobilization. Obtained materials may have excellent mechanical properties that will extend their practical importance.
Another application of DESs is in 3D printing. 3D printing is a ground-breaking additive manufacturing technique already used in various advanced applications and broadening the prospects of a technology from nano to even industrial macroscale277−280 and could be envisioned to help synthesize biomineralization-inspired materials.
Bioinspired 3D printing of biomineralization-related or biomineralization-inspired structures is focused on (i) calcium phosphates281 because of their abundance in natural organisms and their compatibility with bone-implant applications282 or (ii) silica bioinspired structures with nanoscale precision.283 These materials also mimic the hierarchical structures found in native biominerals producing their unique mechanical properties such as high toughness.284−289 Considering that DESs can facilitate biopolymers dissolution, stabilization of inorganic precursors, and even can undergo polymerization, it is a natural consequence that the scientific community should shift their attention to explore the potential of DESs-based pastes that will combine biomacromolecules and inorganic precursors. By understanding the structure–property relations in biominerals, as synthetic chemists and materials engineers, it would be beneficial to use 3D-printing and a wider palette of building blocks (in contrast to natural organisms) that will enable the development of a new generation of biomimetic materials that will even outperform the natural structures. Therefore, much attention should be paid to the 3D-printing-assisted fusion of biopolymers, electroconductive DESs, and inorganic constituents (i.e., MXenes,290,291 MOFs,292 perovskites, POSS,293 MxOy294−296) that will mimic the structural hierarchy found in biominerals. Such a new fabrication technique combined with unique stability of DESs could enable the design and fabrication of the smart structures that are lightweight yet strong for biomimetically soft robotics.297 This unexplored direction will definitively require the comprehensive analysis and understanding of the flow behavior of above-mentioned biopolymers in DESs, which will open a new avenue for research that will have a fundamental impact on the additive manufacturing technologies.
3.3. Enzyme-Mediated Biomineralization in DES?
Recently various research groups298,299 confirm that DESs can be viable enzyme activators and stabilizers that lead toward more sustainable and energy efficient biotechnological processes. Huang et al. studied Penicillium expansum lipase performance in water solutions of 24 DESs. It has been found that by selecting a proper DES as an additive with an optimal salt/HBD ratio, an enzyme can be effectively activated and/or stabilized. The case of the choline acetate:glycerol (1:2) lipase showed a 1.4-fold increase in activity and a 17.4-fold enhancement in stability. However, the impact of DESs can be protein/enzyme-specific. The same DES induces different behaviors with different proteins/enzymes. Molecular dynamics simulations indicate that the intermolecular hydrogen-bonding network within DES components could effectively prevent the diffusion of DES components into the enzyme molecule, thereby decreasing the denaturation.300 However, findings reported by Cao et al.301 for nearly anhydrous DESs indicate that ChCl-acid DESs are not biocompatible solvents for enzymatic catalysis. They also found that impact of DESs on the enzyme activity and thermal stability is directly linked with the polarity, α and β parameters, and hydroxyl group content in DESs.301
This article is more focused on highlighting potential avenues for enzymatic-mediated synthesis of biomineralization-inspired materials. Therefore, for better understanding and insight toward enzyme stabilization and activation by DESs, readers are kindly referred to following review articles (refs (301−304)).
One advantage of using DESs as solvents for enzymes is their ability to stabilize enzymes in nonaqueous environments. Some of the reports, confirm that enzymes have higher activity in some DESs than in aquatic environments.305 This is crucial regarding studying biomineralization phenomenon in extremophiles (see section 3.5). Such an approach will enable one to take the full advantages and new opportunities offered by DESs. Especially when we consider that (i) choline chloride (component of most studied DESs) is an effective stabilizing agent of inorganic precursors,306,307 (ii) DESs effectively coordinate metal ions,82 and (iii) DESs have the potential to facilitate novel biocatalytic synthesis pathways that are not achievable in traditional reaction media such as aqueous buffers.308
Considering the utility of enzymes in biomineralization-inspired syntheses, it is essential to investigate how the enzymatic control over the mineral nucleation and crystallization is affected by different DESs.
3.4. DNA-Origami
DNA as a biomacromolecule has also been successfully used as template or mold in the bottom-up fabrication of well-defined mineralized nanostructures, ranging from nanometers to submicrometers, with sophisticated geometrical geometries.309−313 Recently, “scaffolded DNA origami” has become widely recognized as one of the most promising methods for assembling techniques and paving the way for unconventional synthesis approaches of nano-objects with tailored shape and functionality.314 In the laboratory, scientists can fold a long single DNA strand by DNA short strands (staple strand) that are complementary to the desired template strand. With the careful design, supported by computational molecular dynamics, the possibilities of DNA-origami are practically unlimited. The fundamental background knowledge regarding DNA-origami nanotechnology including origami design, synthesis, functionalization, and characterization is described in the comprehensive reviews:314−317 readers are strongly encouraged to follow these fundamental papers for a better overview of this extremely versatile technology.
This section is highlighting the potential of DNA-origami technology that provides a programmable platform for the development of hybrid, inorganic–organic hierarchical structures, inspired by the mostly distributed biominerals (calcium phosphates and silica). Last findings demonstrate that extracellular DNA functions as an initiator of collagen intrafibrillar mineralization.318 Recently, Liu et al.311 reported successful utilization of the DNA chiral scaffold for precise calcium phosphate crystallization that guides the mineralization of calcium phosphate with prescribed two-dimensional and three-dimensional shapes on the nanolevel (10–100 nm), Figure 7a,b.
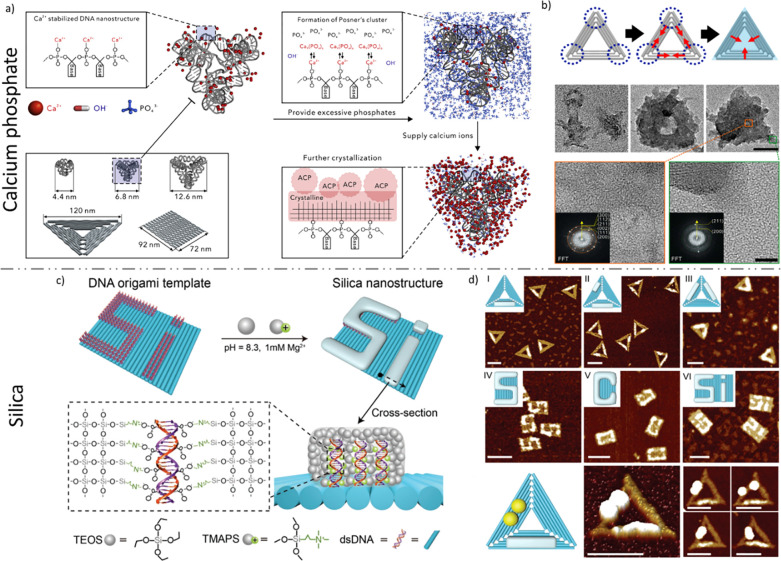
Figure 7.
(a) Schematic representation of DNA-framework-templated crystallization of hydroxyapatite that involves DNA stabilization by calcium ions, formation of amorphous calcium phosphate due to local supersaturation, and further aging and crystallization of nanohydroxyapatite. (b) Transmission electron microscopy images showing the triangular DNA origami-hydroxyapatite nanocomposites with triangular morphology. Interestingly, the triangle tips center the crystallization trend. Reprinted with permission from ref (311). Copyright 2020 Elsevier. (c) Schematic representation on the proposed mechanism of DNA-origami silicification. (d) AFM imaging of various structures obtained experimentally. Reproduced with permission from ref (312). Copyright 2020 John Wiley and Sons.
It has been established that morphological features (size and shape) of DNA-calcium phosphate hybrid nanostructures are programmed through the utilization of structural information that is encoded within DNA sequences, in conjunction with electrostatic interactions between the DNA phosphate backbone and mineral counterparts (Figure 7a).311 Additionally, it has been found that DNA is able for atomic-scale control of calcium phosphate formation, due to the exceptional match between crystal plane distance calcium phosphate (hydroxyapatite, synthetic,319 as well biogenic320) along the c axis (002) and distance between adjacent base pair of DNA that is equal 0.34 nm.311 The biggest challenge in this method results from high density of phosphate groups in DNA that cause local calcium ions supersaturation and lead to relatively fast nucleation, crystallization, and uncontrolled overgrowth of hydroxyapatite nanocrystals, losing the shape of the DNA-template. Wu et al.321 in their recent study, overcome this issue by using the particle attachment method and using magnesium ions that stabilize DNA templates and slow down the spontaneous crystallization of hydroxyapatite.322 This allowed for the precisely controlled formation of the calcium phosphate layer with fine-tuned morphological features and functions through transcription of DNA-origami. Corresponding to the biominerals, obtained hybrids possess improved thermal stability and mechanical properties.
Customized 2D and 3D hierarchical silica-DNA hybrid nanostructures (with size 10–1000 nm) that replicate the wide range of geometric information on DNA-origami chiral scaffolds were successfully reported309,312,323 and are definitively a step forward in the mimicking of the complex geometrical architectures of natural silica-based biominerals like diatom frustules or skeletons of radiolarians (silicoflagellata). Liu et al.323 proved that the thickness of the amorphous silica (opal-like) layer deposited on the DNA surface can be simply tuned by adjusting the silicification time. Interestingly, the nanomechanical studies of formed DNA-silica hybrid structures possess ten-fold higher Young’s modulus than the DNA template while maintaining its original structural flexibility323 that are correspondingly found in naturally occurring biominerals.324 Shang et al.312 with use of a molecular dynamics simulation found that double-stranded DNA scaffolds possess electrostatic affinity to the positively charged silicic acid precursors. This was used as an advantage in development of a novel strategy to site-specific synthesis fabrication of nanostructured DNA-silica hybrids with nanoscale precision (Figure 7 c,d).312
Also, the DNA-templated metal (gold, silver, copper) nanoclusters mimicking the extreme biomineralization (for review, see ref (3)) are now emerging as a new type of functional nanomaterial with a wide spectrum of unique applications in modern technologies.325 All these examples clearly demonstrate that the DNA-origami technique is a groundbreaking nanofabrication strategy that opens a new avenue for bioinspired materials science and precise, programmable bottom-up inorganic material synthesis. However, a potential limitation of DNA-origami nanotechnology is the fact that it is mostly limited to aqueous or substantially hydrated media; application of organic solvents usually causes alterations of the DNA helical structure or the loss of base pairing.
Recent advances in this field done by a research group headed by Professor Nicholas V. Hud proved that anhydrous DESs composed of choline chloride:urea (1:2 molar ratio) and chloride chloride:glycerol (1:4) are suitable environments in which DNA can form stable forms of duplex, triplex, and G-quadruplex structures.326 It was observed that DNA maintains B-form helical structures in DES, and the regenerated DNA shows a high thermal and pH stability. According to Gállego et al.,326 a plausible mechanism for DNA solvation involves the interaction of choline cations with the DNA phosphate backbone (Figure 8).
Figure 8.
AFM images showing the DNA origami folding in DES (choline chloride-glycerol, 1:4) at 20 °C at selected time points. Reprinted with permission from ref (326). Copyright 2015 John Wiley and Sons.
Additionally, given previous reports that DESs support both enzymatic catalysis and controlled crystallization of inorganic nanostructures, the possibility that catalytic nucleic acids and enzyme–DNA complexes could be used as a tool for development of biomineralization inspired materials and better understanding of biomineralization. Bringing the exquisite control of DNA through DESs paving the way for development of new approaches in the synthesis nanostructured bioinspired materials and their efficient application in the new generation of nanoelectronics, nanophotonics, catalysis and many other areas improving the quality of human life.
3.5. Extreme Biomimetics
The impressive capacity of living beings to adapt and thrive in harsh or extreme environments has captivated and inspired numerous scientists from various research disciplines. Exploration of the mechanisms underlying biomineralization strategies in extremophiles and transferring this knowledge into laboratory practice in the development of new hybrid materials has become a fundamental driving force for the development of recent extreme biomimetics.327−329 This direction, pioneered by Prof. Hermann Ehrlich in 2010, is now a vibrant area of research that led to the synthesis of a new generation of biomaterials and biocomposites that are characterized by unusual and rather unexpected functional properties.330,331 Extreme biomimetics encompasses a range of subjects, including the study of biosilicification in Antarctic diatoms that thrive in temperatures below −20 °C, the examination of diverse biomineralizing organisms that inhabit the freezing point of seawater (−1.9 to 4 °C), and the investigation of fauna found in hydrothermal vents and hot springs with temperatures ranging from 60 to 121 °C.
Gaining a comprehensive understanding of the fundamental principles of biomineralization that allow living organisms to thrive in extreme environments has the potential to pave the way for the development of innovative principles of extreme biomimetics. By applying these principles in various technological applications, researchers can harness the remarkable adaptive mechanisms of living organisms and improve the performance of materials and devices in harsh conditions.
Recent findings published by Gertrudes et al.66 revealed that metabolites of some extremophiles are forming natural deep eutectic solvents (NADES) that play the role as cryoprotectants in the cells. NADES are also responsible for dissolution, transport, and biotransformation of poorly water-soluble molecules, improving the activity and stabilization of enzymes. This might be a missing key to understanding the puzzling biomineralization (calcification and silicification) phenomenon in psychrophilic organisms. Detailed studies of biosilicification, biocalcification, and iron- or manganese biomineralization in psychrophilic organisms are of high importance to close the gap in knowledge about this mysterious phenomenon. We strongly believe that a deep insight into the role of DES/NADES in stabilization of biomacromolecules (chitin,332 collagen,242 ferritin,334,335 actin336); enzymes (silicatein,337−339 glassin340,341); and inorganic precursors (silicic acid or ACC, iron, or manganese ions) will shine new light on the psychrophilic biomineralization pathways. As a result, this will trigger new research oriented on the development of a new class of biomaterials, with unusual properties, according to extreme biomimetics philosophy.342
Open questions: (i) Can deep eutectic solvents act as stabilizers for inorganic precursors (silicic acid, ACC, phosphate, and metal ions)? (ii) How do deep eutectic solvents affect the stability of enzymes (e.g., silicatein, urease, phosphatase) and proteins (e.g., collagen, silk, actin) that are identified as responsible for biomineralization phenomenon? (iii) What is the DESs structure–solvation relationship with respect to the chitin, silk, collagen? Which mechanism underlies the solubility of biomacromolecules in deep eutectic solvents? (iv) How do DESs impact the structural peculiarities and dynamics of proteins in terms of molecular and fibrillar levels? (v) How do DESs nanostructures guide the synthesis of biomineralization-inspired materials? (vi) How can predictive multiscale modeling be utilized effectively, especially generative deep learning, to discover new compounds including an assessment of synthesizability?
4. Computational and Artificial Intelligence Perspectives
The development of next-generation biomaterials, using DESs, biopolymers (chitin, collagen, silk, DNA), enzymes, and inorganic precursors, must incorporate the principles of green chemistry and engineering into a broader definition of performance, which includes sustainability considerations. Achieving this ambitious aim will require modern tools such as computational multiscale modeling and artificial intelligence. This synergy will inform predictions on how even subtle modifications on the molecular scale chemistries or nanostructural organization of DESs will impact the final product in terms of its properties on the macroscale such as viscosity or self-assembly.
One approach to such predictions is the use of molecular dynamics (MD) to build large-scale models that simulate dissolving biopolymers in DESs.343,344 MD and related quantum scale techniques such as density functional theory (DFT) have been used to study biomass, and biomaterials will complement this work and can provide direction for further research.343−346 Quantum-level (e.g., DFT) and atomistic or coarse-grained MD simulations could produce data that describes the behavior of numerous solvents and biopolymers without performing time-consuming manual experiments. By using computational methods to simulate the dissolution of biopolymers in DESs, we can rapidly gather data on structure–property relationships that can better inform our procedures in preparing pure biopolymers for bioinspired materials synthesis.
Artificial intelligence approaches, especially machine learning (ML) and deep learning (DL) algorithms, can also be used to discover new combinations of DESs for a variety of applications. ML is emerging as a powerful tool in the fields of chemistry,347 materials and biomaterials science,348−352 and mechanical engineering,353−357 attributed to its power to predict materials properties, design de novo materials,358 and discover new mechanisms beyond intuitions.348 For DESs ML has primarily been used to estimate density and viscosity.184,195 In the cases of predicting density, researchers have applied supervised machine learning techniques and have quantified the accuracy of seven different machine learning methods before determining their highest accuracy tool. For viscosity, another group has applied multiple machine learning algorithms to train a model that can achieve strong predictive performance.195,359,360 The success of these past models further begs the need to advance in this direction of computational approach. Another avenue of computational work would be to utilize generative modeling. Generative neural network models can also be used to generate de novo protein designs (Figure 9) with specific mechanical behaviors by coupling it with coarse grained analysis and has been demonstrated to solve the inverse problem of correlating and tuning polycrystalline materials to mechanical properties in which this framework can be used to guide de novo biomaterial design.361
Figure 9.
(a) Overview of generative deep learning models that use secondary structure design objectives to predict amino acid sequences and 3D protein structures. These models can operate on either the overall content or per-residue structure of the protein. (b) Depiction of the 1D U-net architecture that translates an input Ii into an output Oi under a condition set Ci. This model takes a conditioning description of the desired secondary structures as input and produces various sequences of AAs from random noise vector sources and illustration of the Markov chain of noising (top) and denoising (bottom). Reprinted with permission from ref (97). Copyright 2021 Elsevier.
Similar approaches to what has been accomplished previously can be taken by studying DESs. A model could be developed that is capable of both understanding DESs and even discovering new DESs. Recently, we reported a series of deep learning models to solve forward and inverse design problems in molecular modeling and design.362 Using both diffusion models inspired by nonequilibrium thermodynamics and attention-based transformer architectures implemented via a generative language model, we demonstrate a versatile framework capable of capturing complex chemical structures from commonly used simplified molecular-input line-entry system (SMILES) input. Trained jointly in tasks related to the quantum machines (QM9) data set and DESs, the model can predict various quantum mechanical properties and critical properties to achieve deep eutectic solvent behavior. Several potential combinations of DESs are proposed based on this framework, i.e., monoethylcholine chloride and 4-methylcatechol.362
Further examples of inverse problems that can be addressed are which DESs would best dissolve biopolymers or aid the creation of a specific desired biomineral hybrid material. In order to develop generative models for this purpose, more experimental data on DESs polymer solubility, inorganic crystallization (growth rate) in DESs would significantly support progress. The creation of future versions of generative models centered on DESs would then greatly aid in bioinspired materials synthesis and biopolymer organic–inorganic hybrid synthesis (Figure 10).
Figure 10.
Schematic illustration of the expected impact of using machine learning techniques in the DESs-assisted development of bioinspired hybrid materials synthesis. The potential data set includes results from laboratory experiments, developing synthetic data using molecular dynamics simulations, and a data set on structure–property relationships. The data set may span DESs, biominerals, biopolymers, and minerals. The use of generative models may provide the field with insights into novel material discovery for specific applications and material properties.
It is important to note that high-throughput data set collection is crucial with machine learning. Parameters of interest might be SMILES, melting point, density, viscosity, deep eutectic melting temperature, and the Kamlet–Taft parameters. Experimental data sourced from the literature on DESs can be gathered and cleaned for data set creation. Additionally, experimental data can be produced in which the procedures of experimentation will be openly documented and public.
With readily available data sets, models can be trained and tested with this experimental data serving as ground truth, combined with inputs from simulation data from molecular dynamics or DFT calculations.
Applying computational methods toward studying DESs is a high potential area of research that would accelerate innovation in the fields of biomimetic chemistry and materials science.
Acknowledgments
Dedicated to Professor Hermann Ehrlich on the occasion of his retirement from Technische Unversität Bergakademie Freiberg (Germany). The majority of this work was financially supported by National Science Centre (Poland) in the framework of SONATA Program 2021/43/D/ST5/00853. M.W. is grateful to The Kosciuszko Foundation for the financial support in the Research Exchange Program to the United States. This material is also based upon work supported by the National Science Foundation Graduate Research Fellowship under Grant no. 2141064. We acknowledge support by NIH (5R01AR077793-03), the Office of Naval Research (N000141612333 and N000141912375), AFOSR-MURI (FA9550-15-1-0514), and the Army Research Office (W911NF1920098). The authors would like to thank Eryk Jȩdrzejczak for his technical assistance.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- Gilbert P. U. P. A.; Porter S. M.; Sun C. Y.; Xiao S.; Gibson B. M.; Shenkar N.; Knoll A. H. Biomineralization by Particle Attachment in Early Animals. Proc. Natl. Acad. Sci. U. S. A. 2019, 116 (36), 17659–17665. 10.1073/pnas.1902273116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V.; Srinivasan A.; Nikolajeff F.; Kumar S. Biomineralization Process in Hard Tissues: The Interaction Complexity within Protein and Inorganic Counterparts. Acta Biomater 2021, 120, 20–37. 10.1016/j.actbio.2020.04.049. [DOI] [PubMed] [Google Scholar]
- Ehrlich H.; Bailey E.; Wysokowski M.; Jesionowski T. Forced Biomineralization: A Review. Biomimetics 2021, 6 (3), 46. 10.3390/biomimetics6030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysokowski M.; Zaslansky P.; Ehrlich H. Macrobiomineralogy: Insights and Enigmas in Giant Whale Bones and Perspectives for Bioinspired Materials Science. ACS Biomater Sci. Eng. 2020, 6 (10), 5357–5367. 10.1021/acsbiomaterials.0c00364. [DOI] [PubMed] [Google Scholar]
- Gilbert P. U. P. A.; Bergmann K. D.; Boekelheide N.; Tambutté S.; Mass T.; Marin F.; Adkins J. F.; Erez J.; Gilbert B.; Knutson V.; Cantine M.; Hernández J. O.; Knoll A. H. Biomineralization: Integrating Mechanism and Evolutionary History. Sci. Adv. 2022, 8 (10), eabl9653 10.1126/sciadv.abl9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y.; Faivre D. Diversity of Microbial Metal Sulfide Biomineralization. ChemPlusChem. 2022, 87 (1), e202100457 10.1002/cplu.202100457. [DOI] [PubMed] [Google Scholar]
- Bhushan B. Biomimetics: Lessons from Nature an Overview. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 2009, 367, 1445–1486. 10.1098/rsta.2009.0011. [DOI] [PubMed] [Google Scholar]
- Weiner S.; Nudelman F.; Sone E.; Zaslansky P.; Addadi L. Mineralized Biological Materials: A Perspective on Interfaces and Interphases Designed over Millions of Years. Biointerphases 2006, 1 (2), P12. 10.1116/1.2207607. [DOI] [PubMed] [Google Scholar]
- Faivre D.; Schüler D. Magnetotactic Bacteria and Magnetosomes. Chem. Rev. 2008, 108 (11), 4875–4898. 10.1021/cr078258w. [DOI] [PubMed] [Google Scholar]
- Checa A. G.; Cartwright J. H. E.; Sánchez-Almazo I.; Andrade J. P.; Ruiz-Raya F. The Cuttlefish Sepia Officinalis (Sepiidae, Cephalopoda) Constructs Cuttlebone from a Liquid-Crystal Precursor. Sci. Rep. 2015, 5 (1), 11513. 10.1038/srep11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Shen J.; Wei Q.; Li X. Dynamic Self-Strengthening of a Bio-Nanostructured Armor — Conch Shell. Materials Science and Engineering: C 2019, 103, 109820. 10.1016/j.msec.2019.109820. [DOI] [PubMed] [Google Scholar]
- Naleway S. E.; Taylor J. R. A.; Porter M. M.; Meyers M. A.; McKittrick J. Structure and Mechanical Properties of Selected Protective Systems in Marine Organisms. Mat. Sci. Eng. C 2016, 59, 1143–1167. 10.1016/j.msec.2015.10.033. [DOI] [PubMed] [Google Scholar]
- Li H.; Sun C. Y.; Fang Y.; Carlson C. M.; Xu H.; Ješovnik A.; Sosa-Calvo J.; Zarnowski R.; Bechtel H. A.; Fournelle J. H.; Andes D. R.; Schultz T. R.; Gilbert P. U. P. A.; Currie C. R. Biomineral Armor in Leaf-Cutter Ants. Nat. Commun. 2020, 11, 5792. 10.1038/s41467-020-19566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew A. J.; Stifler C. A.; Tits A.; Schmidt C. A.; Scholl A.; Cantamessa A.; Müller L.; Delaunois Y.; Compère P.; Ruffoni D.; Buehler M. J.; Gilbert P. U. P. A. A Molecular Scale Understanding of Misorientation Toughening in Corals and Seashells. Adv. Mater. 2023, 35, 2300373. 10.1002/adma.202300373. [DOI] [PubMed] [Google Scholar]
- Gal A.; Brumfeld V.; Weiner S.; Addadi L.; Oron D. Certain Biominerals in Leaves Function as Light Scatterers. Adv. Mater. 2012, 24 (10), OP77–OP83. 10.1002/adma.201104548. [DOI] [PubMed] [Google Scholar]
- Monn M. A.; Weaver J. C.; Zhang T.; Aizenberg J.; Kesari H. New Functional Insights into the Internal Architecture of the Laminated Anchor Spicules of Euplectella Aspergillum. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 (16), 4976–4981. 10.1073/pnas.1415502112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich H.; Maldonado M.; Parker A. R.; Kulchin Y. N.; Schilling J.; Köhler B.; Skrzypczak U.; Simon P.; Reiswig H. M.; Tsurkan M. v.; Brunner E.; Voznesenskiy S. S.; Bezverbny A. v.; Golik S. S.; Nagorny I. G.; Vyalikh D. v.; Makarova A. A.; Molodtsov S. L.; Kummer K.; Mertig M.; Erler C.; Kurek D. v.; Bazhenov V. v.; Natalio F.; Kovalev A. E.; Gorb S. N.; Stelling A. L.; Heitmann J.; Born R.; Meyer D. C.; Tabachnick K. R. Supercontinuum Generation in Naturally Occurring Glass Sponges Spicules. Adv. Opt Mater. 2016, 4 (10), 1608–1613. 10.1002/adom.201600454. [DOI] [Google Scholar]
- Lichtenegger H. C.; Schöberl T.; Bartl M. H.; Waite H.; Stucky G. D. High Abrasion Resistance with Sparse Mineralization: Copper Biomineral in Worm Jaws. Science 2002, 298 (5592), 389–392. 10.1126/science.1075433. [DOI] [PubMed] [Google Scholar]
- Stegbauer L.; Smeets P. J. M.; Free R.; Wallace S. G.; Hersam M. C.; Alp E. E.; Joester D. Persistent Polyamorphism in the Chiton Tooth: From a New Biomineral to Inks for Additive Manufacturing. Proc. Natl. Acad. Sci. U. S. A. 2021, 118, e2020160118 10.1073/pnas.2020160118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstam H. A. Minerals Formed by Organisms. Science 1981, 211 (4487), 1126–1131. 10.1126/science.7008198. [DOI] [PubMed] [Google Scholar]
- Eder M.; Amini S.; Fratzl P. Biological Composites—Complex Structures for Functional Diversity. Science 2018, 362, 543–547. 10.1126/science.aat8297. [DOI] [PubMed] [Google Scholar]
- Cranford S. W.; Buehler M. J.. Biomateriomics; Springer Netherlands, 2012. [Google Scholar]
- Beniash E.; Stifler C. A.; Sun C. Y.; Jung G. S.; Qin Z.; Buehler M. J.; Gilbert P. U. P. A. The Hidden Structure of Human Enamel. Nat. Commun. 2019, 10 (1), 4383. 10.1038/s41467-019-12185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J.; Wang Y.; Yao Y.; Wang Y.; Fei X.; Qi P.; Lin S.; Kaplan D. L.; Buehler M. J.; Ling S. Biological Material Interfaces as Inspiration for Mechanical and Optical Material Designs. Chem. Rev. 2019, 119 (24), 12279–12336. 10.1021/acs.chemrev.9b00416. [DOI] [PubMed] [Google Scholar]
- Guo K.; Buehler M. J. Nature’s Way: Hierarchical Strengthening through Weakness. Matter 2019, 1 (2), 302–303. 10.1016/j.matt.2019.07.011. [DOI] [Google Scholar]
- Currey J. D. Hierarchies in Biomineral Structures. Science 2005, 309 (5732), 253–254. 10.1126/science.1113954. [DOI] [PubMed] [Google Scholar]
- Lew A. J.; Buehler M. J. A Deep Learning Augmented Genetic Algorithm Approach to Polycrystalline 2D Material Fracture Discovery and Design. Appl. Phys. Rev. 2021, 8 (4), 041414. 10.1063/5.0057162. [DOI] [Google Scholar]
- Elsharkawy S.; Mata A. Hierarchical Biomineralization: From Nature’s Designs to Synthetic Materials for Regenerative Medicine and Dentistry. Adv. Healthc Mater. 2018, 7 (18), 1800178. 10.1002/adhm.201800178. [DOI] [PubMed] [Google Scholar]
- Yao S.; Jin B.; Liu Z.; Shao C.; Zhao R.; Wang X.; Tang R. Biomineralization: From Material Tactics to Biological Strategy. Adv. Mater. 2017, 29 (14), 1605903. 10.1002/adma.201605903. [DOI] [PubMed] [Google Scholar]
- Natalio F.; Corrales T. P.; Panthöfer M.; Schollmeyer D.; Lieberwirth I.; Müller W. E. G.; Kappl M.; Butt H. J.; Tremel W. Flexible Minerals: Self-Assembled Calcite Spicules with Extreme Bending Strength. Science 2013, 339 (6125), 1298–1302. 10.1126/science.1216260. [DOI] [PubMed] [Google Scholar]
- Nudelman F.; Sommerdijk N. A. J. M. Biomineralization as an Inspiration for Materials Chemistry. Angew. Chem., Int. Ed. 2012, 51 (27), 6582–6596. 10.1002/anie.201106715. [DOI] [PubMed] [Google Scholar]
- Zhang B.; Han Q.; Zhang J.; Han Z.; Niu S.; Ren L. Advanced Bio-Inspired Structural Materials: Local Properties Determine Overall Performance. Mater. Today 2020, 41, 177–199. 10.1016/j.mattod.2020.04.009. [DOI] [Google Scholar]
- Wysokowski M.; Jesionowski T.; Ehrlich H. Biosilica as Source for Inspiration in Biological Materials Science. Am. Mineral. 2018, 103, 665–691. 10.2138/am-2018-6429. [DOI] [Google Scholar]
- Estroff L. A. Introduction: Biomineralization. Chem. Rev. 2008, 108, 4329–4331. 10.1021/cr8004789. [DOI] [PubMed] [Google Scholar]
- Guo K.; Yang Z.; Yu C. H.; Buehler M. J. Artificial Intelligence and Machine Learning in Design of Mechanical Materials. Mater. Horiz 2021, 8 (4), 1153–1172. 10.1039/D0MH01451F. [DOI] [PubMed] [Google Scholar]
- Cölfen H. A Crystal-Clear View. Nat. Mater. 2010, 9 (12), 960–961. 10.1038/nmat2911. [DOI] [PubMed] [Google Scholar]
- Pereira R. F. P.; Zehbe K.; Günter C.; Dos Santos T.; Nunes S. C.; Paz F. A. A.; Silva M. M.; Granja P. L.; Taubert A.; De Zea Bermudez V. Ionic Liquid-Assisted Synthesis of Mesoporous Silk Fibroin/Silica Hybrids for Biomedical Applications. ACS Omega 2018, 3 (9), 10811–10822. 10.1021/acsomega.8b02051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott A. P.; Capper G.; Davies D. L.; Rasheed R. K.; Tambyrajah V. Novel Solvent Properties of Choline Chloride/Urea Mixtures. Chem. Commun. 2003, (1), 70–71. 10.1039/b210714g. [DOI] [PubMed] [Google Scholar]
- Smith E. L.; Abbott A. P.; Ryder K. S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114 (21), 11060–11082. 10.1021/cr300162p. [DOI] [PubMed] [Google Scholar]
- Hansen B. B.; Spittle S.; Chen B.; Poe D.; Zhang Y.; Klein J. M.; Horton A.; Adhikari L.; Zelovich T.; Doherty B. W.; Gurkan B.; Maginn E. J.; Ragauskas A.; Dadmun M.; Zawodzinski T. A.; Baker G. A.; Tuckerman M. E.; Savinell R. F.; Sangoro J. R. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121 (3), 1232–1285. 10.1021/acs.chemrev.0c00385. [DOI] [PubMed] [Google Scholar]
- Tomé L. I. N.; Baião V.; da Silva W.; Brett C. M. A. Deep Eutectic Solvents for the Production and Application of New Materials. Appl. Mater. Today 2018, 10, 30–50. 10.1016/j.apmt.2017.11.005. [DOI] [Google Scholar]
- Florindo C.; Lima F.; Ribeiro B. D.; Marrucho I. M. Deep Eutectic Solvents: Overcoming 21st Century Challenges. Curr. Opin Green Sustain Chem. 2019, 18, 31–36. 10.1016/j.cogsc.2018.12.003. [DOI] [Google Scholar]
- Wagle D. V.; Zhao H.; Baker G. A. Deep Eutectic Solvents: Sustainable Media for Nanoscale and Functional Materials. Acc. Chem. Res. 2014, 47 (8), 2299–2308. 10.1021/ar5000488. [DOI] [PubMed] [Google Scholar]
- Marcus Y.The Variety of Deep Eutectic Solvents. In Deep Eutectic Solvents; Springer, Cham, 2019; pp 13–44. [Google Scholar]
- Abbott A. P.; Edler K. J.; Page A. J. Deep Eutectic Solvents—The Vital Link between Ionic Liquids and Ionic Solutions. J. Chem. Phys. 2021, 155 (15), 150401. 10.1063/5.0072268. [DOI] [PubMed] [Google Scholar]
- Martins M. A. R.; Pinho S. P.; Coutinho J. A. P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solution Chem. 2019, 48, 962–982. 10.1007/s10953-018-0793-1. [DOI] [Google Scholar]
- Naseem Z.; Shehzad R. A.; Ihsan A.; Iqbal J.; Zahid M.; Pervaiz A.; Sarwari G. Theoretical Investigation of Supramolecular Hydrogen-Bonded Choline Chloride-Based Deep Eutectic Solvents Using Density Functional Theory. Chem. Phys. Lett. 2021, 769, 138427. 10.1016/j.cplett.2021.138427. [DOI] [Google Scholar]
- Araujo C. F.; Coutinho J. A. P.; Nolasco M. M.; Parker S. F.; Ribeiro-Claro P. J. A.; Rudić S.; Soares B. I. G.; Vaz P. D. Inelastic Neutron Scattering Study of Reline: Shedding Light on the Hydrogen Bonding Network of Deep Eutectic Solvents. Phys. Chem. Chem. Phys. 2017, 19 (27), 17998–18009. 10.1039/C7CP01286A. [DOI] [PubMed] [Google Scholar]
- Nolasco M. M.; Pedro S. N.; Vilela C.; Vaz P. D.; Ribeiro-Claro P.; Rudić S.; Parker S. F.; Freire C. S. R.; Freire M. G.; Silvestre A. J. D. Water in Deep Eutectic Solvents: New Insights From Inelastic Neutron Scattering Spectroscopy. Front Phys. 2022, 10, 40. 10.3389/fphy.2022.834571. [DOI] [Google Scholar]
- Carrasco-Huertas G.; Jiménez-Riobóo R. J.; Gutiérrez M. C.; Ferrer M. L.; del Monte F. Carbon and Carbon Composites Obtained Using Deep Eutectic Solvents and Aqueous Dilutions Thereof. Chem. Commun. 2020, 56 (25), 3592–3604. 10.1039/D0CC00681E. [DOI] [PubMed] [Google Scholar]
- Abranches D. O.; Coutinho J. A. P. Type V Deep Eutectic Solvents: Design and Applications. Curr. Opin Green Sustain Chem. 2022, 35, 100612. 10.1016/j.cogsc.2022.100612. [DOI] [Google Scholar]
- Abranches D. O.; Martins M. A. R.; Silva L. P.; Schaeffer N.; Pinho S. P.; Coutinho J. A. P. Phenolic Hydrogen Bond Donors in the Formation of Non-Ionic Deep Eutectic Solvents: The Quest for Type V DES. Chem. Commun. 2019, 55 (69), 10253–10256. 10.1039/C9CC04846D. [DOI] [PubMed] [Google Scholar]
- Zamora L.; Benito C.; Gutiérrez A.; Alcalde R.; Alomari N.; Bodour A. Al; Atilhan M.; Aparicio S. Nanostructuring and Macroscopic Behavior of Type V Deep Eutectic Solvents Based on Monoterpenoids. Phys. Chem. Chem. Phys. 2021, 24 (1), 512–531. 10.1039/D1CP04509A. [DOI] [PubMed] [Google Scholar]
- Kovács A.; Neyts E. C.; Cornet I.; Wijnants M.; Billen P. Modeling the Physicochemical Properties of Natural Deep Eutectic Solvents. ChemSusChem 2020, 13 (15), 3789–3804. 10.1002/cssc.202000286. [DOI] [PubMed] [Google Scholar]
- Van Osch D. J. G. P.; Dietz C. H. J. T.; Van Spronsen J.; Kroon M. C.; Gallucci F.; Van Sint Annaland M.; Tuinier R. A Search for Natural Hydrophobic Deep Eutectic Solvents Based on Natural Components. ACS Sustain Chem. Eng. 2019, 7 (3), 2933–2942. 10.1021/acssuschemeng.8b03520. [DOI] [Google Scholar]
- Paiva A.; Craveiro R.; Aroso I.; Martins M.; Reis R. L.; Duarte A. R. C. Natural Deep Eutectic Solvents - Solvents for the 21st Century. ACS Sustain Chem. Eng. 2014, 2 (5), 1063–1071. 10.1021/sc500096j. [DOI] [Google Scholar]
- Pedro S. N.; Freire M. G.; Freire C. S. R.; Silvestre A. J. D.. Deep Eutectic Solvents Comprising Active Pharmaceutical Ingredients in the Development of Drug Delivery Systems. Expert Opinion on Drug Delivery; Taylor and Francis Ltd, 2019; pp 497–506. [DOI] [PubMed] [Google Scholar]
- Duarte A. R. C.; Ferreira A. S. D.; Barreiros S.; Cabrita E.; Reis R. L.; Paiva A. A Comparison between Pure Active Pharmaceutical Ingredients and Therapeutic Deep Eutectic Solvents: Solubility and Permeability Studies. Eur. J. Pharm. Biopharm. 2017, 114, 296–304. 10.1016/j.ejpb.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Silva J. M.; Reis R. L.; Paiva A.; Duarte A. R. C. Design of Functional Therapeutic Deep Eutectic Solvents Based on Choline Chloride and Ascorbic Acid. ACS Sustain Chem. Eng. 2018, 6 (8), 10355–10363. 10.1021/acssuschemeng.8b01687. [DOI] [Google Scholar]
- Gutiérrez A.; Aparicio S.; Atilhan M. Design of Arginine-Based Therapeutic Deep Eutectic Solvents as Drug Solubilization Vehicles for Active Pharmaceutical Ingredients. Phys. Chem. Chem. Phys. 2019, 21 (20), 10621–10634. 10.1039/C9CP01408J. [DOI] [PubMed] [Google Scholar]
- Silva J. M.; Pereira C. V.; Mano F.; Silva E.; Castro V. I. B.; Sá-Nogueira I.; Reis R. L.; Paiva A.; Matias A. A.; Duarte A. R. C. Therapeutic Role of Deep Eutectic Solvents Based on Menthol and Saturated Fatty Acids on Wound Healing. ACS Appl. Bio Mater. 2019, 2 (10), 4346–4355. 10.1021/acsabm.9b00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R.; Zhou F.; Chen Y.; Liu Z.; Liu S.; Mu T. Magnetic Deep Eutectic Solvents: Formation and Properties. Phys. Chem. Chem. Phys. 2022, 24 (34), 20073–20081. 10.1039/D2CP01592G. [DOI] [PubMed] [Google Scholar]
- Zainal-Abidin M. H.; Hayyan M.; Matmin J.; Al-Fakih A. M.; Jamaluddin N.; Wan Mahmood W. M. A.; Abdul Wahab R.; Abdullah F. Greening Industrial Applications with Magnetic-Based Deep Eutectic Solvents: A Promising Future. J. Ind. Eng. Chem. 2023, 124, 1–16. 10.1016/j.jiec.2023.04.011. [DOI] [Google Scholar]
- Makoś-Chełstowska P.; Kaykhaii M.; Płotka-Wasylka J.; de la Guardia M. Magnetic Deep Eutectic Solvents - Fundamentals and Applications. J. Mol. Liq. 2022, 365, 120158. 10.1016/j.molliq.2022.120158. [DOI] [Google Scholar]
- Song Z.; Hu X.; Wu H.; Mei M.; Linke S.; Zhou T.; Qi Z.; Sundmacher K. Systematic Screening of Deep Eutectic Solvents as Sustainable Separation Media Exemplified by the CO2 Capture Process. ACS Sustain Chem. Eng. 2020, 8 (23), 8741–8751. 10.1021/acssuschemeng.0c02490. [DOI] [Google Scholar]
- Gertrudes A.; Craveiro R.; Eltayari Z.; Reis R. L.; Paiva A.; Duarte A. R. C. How Do Animals Survive Extreme Temperature Amplitudes? The Role of Natural Deep Eutectic Solvents. ACS Sustain Chem. Eng. 2017, 5 (11), 9542–9553. 10.1021/acssuschemeng.7b01707. [DOI] [Google Scholar]
- Zdanowicz M.; Wilpiszewska K.; Spychaj T. Deep Eutectic Solvents for Polysaccharides Processing. A Review. Carbohydr. Polym. 2018, 200, 361–380. 10.1016/j.carbpol.2018.07.078. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; De Oliveira Vigier K.; Royer S.; Jérôme F. Deep Eutectic Solvents: Syntheses, Properties and Applications. Chem. Soc. Rev. 2012, 41, 7108–7146. 10.1039/c2cs35178a. [DOI] [PubMed] [Google Scholar]
- Cysewski P.; Jeliński T.. Eutectic Solvents; MDPI, 2020. [Google Scholar]
- Ramón D. J.; Guillena G.. Deep Eutectic Solvents: Synthesis, Properties, and Applications; Wiley, 2019. [Google Scholar]
- Marcus Y.Deep Eutectic Solvents; Springer International Publishing: Cham, 2019. [Google Scholar]
- Wu J.; Liang Q.; Yu X.; Lu Q.-F.; Ma L.; Qin X.; Chen G.; Li B. Deep Eutectic Solvents for Boosting Electrochemical Energy Storage and Conversion: A Review and Perspective. Adv. Funct Mater. 2021, 31 (22), 2011102. 10.1002/adfm.202011102. [DOI] [Google Scholar]
- Ni Y.; Bi Z.; Su H.; Yan L. Deep Eutectic Solvent (DES) as Both Solvent and Catalyst for Oxidation of Furfural to Maleic Acid and Fumaric Acid. Green Chem. 2019, 21 (5), 1075–1079. 10.1039/C8GC04022B. [DOI] [Google Scholar]
- Khandelwal S.; Tailor Y. K.; Kumar M. Deep Eutectic Solvents (DESs) as Eco-Friendly and Sustainable Solvent/Catalyst Systems in Organic Transformations. J. Mol. Liq. 2016, 215, 345–386. 10.1016/j.molliq.2015.12.015. [DOI] [Google Scholar]
- Hooshmand S. E.; Afshari R.; Ramón D. J.; Varma R. S. Deep Eutectic Solvents: Cutting-Edge Applications in Cross-Coupling Reactions. Green Chem. 2020, 22 (12), 3668–3692. 10.1039/D0GC01494J. [DOI] [Google Scholar]
- Shishov A.; Bulatov A.; Locatelli M.; Carradori S.; Andruch V. Application of Deep Eutectic Solvents in Analytical Chemistry. A Review. Microchem. J. 2017, 135, 33–38. 10.1016/j.microc.2017.07.015. [DOI] [Google Scholar]
- Santana-Mayor A.; Rodríguez-Ramos R.; Herrera-Herrera A. v.; Socas-Rodríguez B.; Rodríguez-Delgado M. Á. Deep Eutectic Solvents. The New Generation of Green Solvents in Analytical Chemistry. TrAC Trends in Analytical Chemistry 2021, 134, 116108. 10.1016/j.trac.2020.116108. [DOI] [Google Scholar]
- Silva F. A. e; Freire M. G. Achievements and Perspectives of Using Deep Eutectic Solvents in the Analytical Chemistry Field. Ionic Liquids in Analytical Chemistry 2022, 33–72. 10.1016/B978-0-12-823334-4.00002-3. [DOI] [Google Scholar]
- Benfica J.; Miranda J. S.; Morais E. S.; Freire M. G.; Coutinho J. A. P.; De Cássia Superbi De Sousa R. Enhanced Extraction of Levodopa from Mucuna Pruriens Seeds Using Aqueous Solutions of Eutectic Solvents. ACS Sustain Chem. Eng. 2020, 8 (17), 6682–6689. 10.1021/acssuschemeng.0c00196. [DOI] [Google Scholar]
- Musarurwa H.; Tavengwa N. T. Deep Eutectic Solvent-Based Dispersive Liquid-Liquid Micro-Extraction of Pesticides in Food Samples. Food Chem. 2021, 342, 127943. 10.1016/j.foodchem.2020.127943. [DOI] [PubMed] [Google Scholar]
- Tang B.; Zhang H.; Row K. H. Application of Deep Eutectic Solvents in the Extraction and Separation of Target Compounds from Various Samples. J. Sep Sci. 2015, 38 (6), 1053–1064. 10.1002/jssc.201401347. [DOI] [PubMed] [Google Scholar]
- Cen P.; Spahiu K.; Tyumentsev M. S.; Foreman M. R. S. J. Metal Extraction from a Deep Eutectic Solvent, an Insight into Activities. Phys. Chem. Chem. Phys. 2020, 22 (19), 11012–11024. 10.1039/C9CP05982B. [DOI] [PubMed] [Google Scholar]
- Cardoso I. S.; Pedro A. Q.; Silvestre A. J. D.; Freire M. G. Ionic Liquids and Deep Eutectic Solvents in the Field of Environmental Monitoring. Green Chemistry and Sustainable Technology 2019, 203–240. 10.1007/978-981-13-9105-7_8. [DOI] [Google Scholar]
- Ren H.; Lian S.; Wang X.; Zhang Y.; Duan E. Exploiting the Hydrophilic Role of Natural Deep Eutectic Solvents for Greening CO2 Capture. J. Clean Prod 2018, 193, 802–810. 10.1016/j.jclepro.2018.05.051. [DOI] [Google Scholar]
- Liu Y.; Dai Z.; Zhang Z.; Zeng S.; Li F.; Zhang X.; Nie Y.; Zhang L.; Zhang S.; Ji X. Ionic Liquids/Deep Eutectic Solvents for CO2 Capture: Reviewing and Evaluating. Green Energy & Environment 2021, 6 (3), 314–328. 10.1016/j.gee.2020.11.024. [DOI] [Google Scholar]
- Sarmad S.; Mikkola J. P.; Ji X. Carbon Dioxide Capture with Ionic Liquids and Deep Eutectic Solvents: A New Generation of Sorbents. ChemSusChem 2017, 10 (2), 324–352. 10.1002/cssc.201600987. [DOI] [PubMed] [Google Scholar]
- Mbous Y. P.; Hayyan M.; Hayyan A.; Wong W. F.; Hashim M. A.; Looi C. Y. Applications of Deep Eutectic Solvents in Biotechnology and Bioengineering—Promises and Challenges. Biotechnol Adv. 2017, 35 (2), 105–134. 10.1016/j.biotechadv.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Pätzold M.; Siebenhaller S.; Kara S.; Liese A.; Syldatk C.; Holtmann D. Deep Eutectic Solvents as Efficient Solvents in Biocatalysis. Trends Biotechnol 2019, 37 (9), 943–959. 10.1016/j.tibtech.2019.03.007. [DOI] [PubMed] [Google Scholar]
- Xu P.; Zheng G. W.; Zong M. H.; Li N.; Lou W. Y. Recent Progress on Deep Eutectic Solvents in Biocatalysis. Bioresour Bioprocess 2017, 4, 34. 10.1186/s40643-017-0165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainal-Abidin M. H.; Hayyan M.; Ngoh G. C.; Wong W. F.; Looi C. Y. Emerging Frontiers of Deep Eutectic Solvents in Drug Discovery and Drug Delivery Systems. J. Controlled Release 2019, 316, 168–195. 10.1016/j.jconrel.2019.09.019. [DOI] [PubMed] [Google Scholar]
- Pedro S. N.; Freire M. G.; Freire C. S. R.; Silvestre A. J. D. Deep Eutectic Solvents Comprising Active Pharmaceutical Ingredients in the Development of Drug Delivery Systems. Expert Opin Drug Deliv 2019, 16, 497–506. 10.1080/17425247.2019.1604680. [DOI] [PubMed] [Google Scholar]
- Nahar Y.; Thickett S. C. Greener, Faster, Stronger: The Benefits of Deep Eutectic Solvents in Polymer and Materials Science. Polymers 2021, 13, 447. 10.3390/polym13030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X.; Gu C.; Wang X.; Tu J. Deep Eutectic Solvents (DESs)-Derived Advanced Functional Materials for Energy and Environmental Applications: Challenges, Opportunities, and Future Vision. J. Mater. Chem. A 2017, 5 (18), 8209–8229. 10.1039/C7TA01659J. [DOI] [Google Scholar]
- del Monte F.; Carriazo D.; Serrano M. C.; Gutiérrez M. C.; Ferrer M. L. Deep Eutectic Solvents in Polymerizations: A Greener Alternative to Conventional Syntheses. ChemSusChem 2014, 7 (4), 999–1009. 10.1002/cssc.201300864. [DOI] [PubMed] [Google Scholar]
- Prasad K.; Mondal D.; Sharma M.; Freire M. G.; Mukesh C.; Bhatt J. Stimuli Responsive Ion Gels Based on Polysaccharides and Other Polymers Prepared Using Ionic Liquids and Deep Eutectic Solvents. Carbohydr. Polym. 2018, 180, 328–336. 10.1016/j.carbpol.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente F. A.; Bradić B.; Novak U.; Likozar B. α-Chitin Dissolution, N-Deacetylation and Valorization in Deep Eutectic Solvents. Biopolymers 2020, 111, e23351 10.1002/bip.23351. [DOI] [PubMed] [Google Scholar]
- Özel N.; Elibol M. A Review on the Potential Uses of Deep Eutectic Solvents in Chitin and Chitosan Related Processes. Carbohydr. Polym. 2021, 262, 117942. 10.1016/j.carbpol.2021.117942. [DOI] [PubMed] [Google Scholar]
- Van Osch D. J. G. P.; Kollau L. J. B. M.; Van Den Bruinhorst A.; Asikainen S.; Rocha M. A. A.; Kroon M. C. Ionic Liquids and Deep Eutectic Solvents for Lignocellulosic Biomass Fractionation. Phys. Chem. Chem. Phys. 2017, 19 (4), 2636–2665. 10.1039/C6CP07499E. [DOI] [PubMed] [Google Scholar]
- Zhang T.; Doert T.; Wang H.; Zhang S.; Ruck M. Inorganic Synthesis Based on Reactions of Ionic Liquids and Deep Eutectic Solvents. Angew. Chem., Int. Ed. 2021, 60 (41), 22148–22165. 10.1002/anie.202104035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira V. R. A.; Azenha M. A.; Pereira C. M.; Silva A. F. Cation-Bioimprinted Mesoporous Polysaccharide/Sol-Gel Composites Prepared in Media Containing Choline Chloride-Based Deep Eutectic Solvents. J. Appl. Polym. Sci. 2020, 137 (26), 48842. 10.1002/app.48842. [DOI] [Google Scholar]
- Rashid T.; Sher F.; Rasheed T.; Zafar F.; Zhang S.; Murugesan T. Evaluation of Current and Future Solvents for Selective Lignin Dissolution-A Review. J. Mol. Liq. 2021, 321, 114577. 10.1016/j.molliq.2020.114577. [DOI] [Google Scholar]
- Karade S. S.; Lalwani S.; Eum J. H.; Kim H. Deep Eutectic Solvent-Assisted Synthesis of RuCo2O4: An Efficient Positive Electrode for Hybrid Supercapacitors. Sustain Energy Fuels 2020, 4 (6), 3066–3076. 10.1039/D0SE00340A. [DOI] [Google Scholar]
- Brzȩczek-szafran A.; Gaida B.; Blacha-grzechnik A.; Matuszek K.; Chrobok A. Bioderived Ionic Liquids and Salts with Various Cyano Anions as Precursors for Doped Carbon Materials. Int. J. Mol. Sci. 2021, 22 (19), 10426. 10.3390/ijms221910426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzȩczek-Szafran A.; Erfurt K.; Swadźba-Kwaśny M.; Piotrowski T.; Chrobok A. Beckmann Rearrangement with Improved Atom Economy, Catalyzed by Inexpensive, Reusable, Broηnsted Acidic Ionic Liquid. ACS Sustain Chem. Eng. 2022, 10 (41), 13568–13575. 10.1021/acssuschemeng.2c04409. [DOI] [Google Scholar]
- Ahmadi R.; Hemmateenejad B.; Safavi A.; Shojaeifard Z.; Mohabbati M.; Firuzi O. Assessment of Cytotoxicity of Choline Chloride-Based Natural Deep Eutectic Solvents against Human HEK-293 Cells: A QSAR Analysis. Chemosphere 2018, 209, 831–838. 10.1016/j.chemosphere.2018.06.103. [DOI] [PubMed] [Google Scholar]
- Jha D.; Choudhary K.; Tavazza F.; Liao W.-k.; Choudhary A.; Campbell C.; Agrawal A. Enhancing Materials Property Prediction by Leveraging Computational and Experimental Data Using Deep Transfer Learning. Nat. Comm. 2019, 10, 5316. 10.1038/s41467-019-13297-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogojeski M.; Vogt-Maranto L.; Tuckerman M. E.; Müller K. R.; Burke K. Quantum Chemical Accuracy from Density Functional Approximations via Machine Learning. Nat. Commun. 2020, 11 (1), 5223. 10.1038/s41467-020-19093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo K.; Yang Z.; Yu C.; Buehler M. J. Artificial Intelligence and Machine Learning in Design of Mechanical Materials. Mater. Horiz 2021, 8, 1153–1172. 10.1039/D0MH01451F. [DOI] [PubMed] [Google Scholar]
- Yu C. H.; Chen W.; Chiang Y. H.; Guo K.; Martin Moldes Z.; Kaplan D. L.; Buehler M. J. End-to-End Deep Learning Model to Predict and Design Secondary Structure Content of Structural Proteins. ACS Biomater Sci. Eng. 2022, 8 (3), 1156–1165. 10.1021/acsbiomaterials.1c01343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni B.; Kaplan D. L.; Buehler M. J. Generative Design of de Novo Proteins Based on Secondary Structure Constraints Using an Attention-Based Diffusion Model. Chem. 2023, 9, 1828–1849. 10.1016/j.chempr.2023.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y.; Buehler M. J. Deep Language Models for Interpretative and Predictive Materials Science. APL Machine Learning 2023, 1 (1), 010901. 10.1063/5.0134317. [DOI] [Google Scholar]
- Buehler M. J. Multiscale Modeling at the Interface of Molecular Mechanics and Natural Language through Attention Neural Networks. Acc. Chem. Res. 2022, 55, 3387–3403. 10.1021/acs.accounts.2c00330. [DOI] [PubMed] [Google Scholar]
- Gu G. X.; Chen C. T.; Buehler M. J. De Novo Composite Design Based on Machine Learning Algorithm. Extreme Mech Lett. 2018, 18, 19–28. 10.1016/j.eml.2017.10.001. [DOI] [Google Scholar]
- Yang Z.; Buehler M. J. Words to Matter: De Novo Architected Materials Design Using Transformer Neural Networks. Front Mater. 2021, 8, 740754. 10.3389/fmats.2021.740754. [DOI] [Google Scholar]
- Korendovych I. V.; DeGrado W. F. De Novo Protein Design, a Retrospective. Q. Rev. Biophys. 2020, 53, E3 10.1017/S0033583519000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova M.; Isayev O.; Tropsha A. Deep Reinforcement Learning for de Novo Drug Design. Sci. Adv. 2018, 4, eaap7885 10.1126/sciadv.aap7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehler M. J. Liquified Protein Vibrations, Classification and Cross-Paradigm de Novo Image Generation Using Deep Neural Networks. Nano Futures 2020, 4 (3), 035004. 10.1088/2399-1984/ab9a27. [DOI] [Google Scholar]
- Abbott A. P.; Boothby D.; Capper G.; Davies D. L.; Rasheed R. K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126 (29), 9142–9147. 10.1021/ja048266j. [DOI] [PubMed] [Google Scholar]
- Abbott A. P.; Capper G.; Davies D. L.; Rasheed R. K.; Tambyrajah V. Novel Solvent Properties of Choline Chloride/Urea Mixtures. Chem. Commun. 2003, (1), 70–71. 10.1039/b210714g. [DOI] [PubMed] [Google Scholar]
- Nian Q.; Wang J.; Liu S.; Sun T.; Zheng S.; Zhang Y.; Tao Z.; Chen J. Aqueous Batteries Operated at −50 °C. Angew. Chem., Int. Ed. 2019, 58 (47), 16994–16999. 10.1002/anie.201908913. [DOI] [PubMed] [Google Scholar]
- Zhong Y.; Cai J.; Zhang L. N. A Review of Chitin Solvents and Their Dissolution Mechanisms. Chinese Journal of Polymer Science 2020 38:10 2020, 38 (10), 1047–1060. 10.1007/s10118-020-2459-x. [DOI] [Google Scholar]
- Zhang H.; Lang J.; Lan P.; Yang H.; Lu J.; Wang Z. Study on the Dissolution Mechanism of Cellulose by ChCl-Based Deep Eutectic Solvents. Materials 2020, 13 (2), 278. 10.3390/ma13020278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X.; Gu C. D.; Wang X. L.; Tu J. P. Endowing Manganese Oxide with Fast Adsorption Ability through Controlling the Manganese Carbonate Precursor Assembled in Ionic Liquid. J. Colloid Interface Sci. 2015, 438, 149–158. 10.1016/j.jcis.2014.09.029. [DOI] [PubMed] [Google Scholar]
- Wang K. Y.; Liu H. W.; Zhang S.; Ding D.; Cheng L.; Wang C. Selenidostannates and a Silver Selenidostannate Synthesized in Deep Eutectic Solvents: Crystal Structures and Thermochromic Study. Inorg. Chem. 2019, 58 (5), 2942–2953. 10.1021/acs.inorgchem.8b02610. [DOI] [PubMed] [Google Scholar]
- He B.; Li Y.; Zhang T.; Shi Y.; Li K.; Dai F.; Zhang R.; Liu R.; Zhang S. Synthesis of Porous and Highly Crystallinity Vanadium Phosphorus Oxide Catalysts by Multifunctional Biomass-Based Deep Eutectic Solvents. J. Phys. Chem. B 2020, 124 (18), 3743–3753. 10.1021/acs.jpcb.0c01564. [DOI] [PubMed] [Google Scholar]
- Liao J. H.; Wu P. C.; Bai Y. H. Eutectic Mixture of Choline Chloride/Urea as a Green Solvent in Synthesis of a Coordination Polymer: [Zn(O3PCH2CO2)] · NH4. Inorg. Chem. Commun. 2005, 8 (4), 390–392. 10.1016/j.inoche.2005.01.025. [DOI] [Google Scholar]
- Araujo C. F.; Coutinho J. A. P.; Nolasco M. M.; Parker S. F.; Ribeiro-Claro P. J. A.; Rudić S.; Soares B. I. G.; Vaz P. D. Inelastic Neutron Scattering Study of Reline: Shedding Light on the Hydrogen Bonding Network of Deep Eutectic Solvents. Phys. Chem. Chem. Phys. 2017, 19 (27), 17998–18009. 10.1039/C7CP01286A. [DOI] [PubMed] [Google Scholar]
- Hammond O. S.; Bowron D. T.; Edler K. J. Liquid Structure of the Choline Chloride-Urea Deep Eutectic Solvent (Reline) from Neutron Diffraction and Atomistic Modelling. Green Chem. 2016, 18 (9), 2736–2744. 10.1039/C5GC02914G. [DOI] [Google Scholar]
- Karimi M.; Hesaraki S.; Alizadeh M.; Kazemzadeh A. Synthesis of Calcium Phosphate Nanoparticles in Deep-Eutectic Choline Chloride-Urea Medium: Investigating the Role of Synthesis Temperature on Phase Characteristics and Physical Properties. Ceram. Int. 2016, 42 (2), 2780–2788. 10.1016/j.ceramint.2015.11.010. [DOI] [Google Scholar]
- Zhang C.; Xin B.; Chen T.; Ying H.; Li Z.; Hao J. Deep Eutectic Solvent Strategy Enables an Octahedral Ni-Co Precursor for Creating High-Performance NiCo2O4 Catalyst toward Oxygen Evolution Reaction. Green Energy & Environment 2022, 7, 1217–1227. 10.1016/j.gee.2021.01.017. [DOI] [Google Scholar]
- Crump M. R.; Bidinger S. L.; Pavinatto F. J.; Gong A. T.; Sweet R. M.; MacKenzie J. D. Sensorized Tissue Analogues Enabled by a 3D-Printed Conductive Organogel. NPJ. Flexible Electronics 2021, 5, 7. 10.1038/s41528-021-00104-0. [DOI] [Google Scholar]
- Lai C. W.; Yu S. S. 3D Printable Strain Sensors from Deep Eutectic Solvents and Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2020, 12 (30), 34235–34244. 10.1021/acsami.0c11152. [DOI] [PubMed] [Google Scholar]
- Cai L.; Chen G.; Tian J.; Su B.; He M. Three-Dimensional Printed Ultrahighly Sensitive Bioinspired Ionic Skin Based on Submicrometer-Scale Structures by Polymerization Shrinkage. Chem. Mater. 2021, 33 (6), 2072–2079. 10.1021/acs.chemmater.0c04581. [DOI] [Google Scholar]
- Cai L.; Chen G.; Su B.; He M. 3D Printing of Ultra-Tough, Self-Healing Transparent Conductive Elastomeric Sensors. Chem. Eng. J. 2021, 426, 130545. 10.1016/j.cej.2021.130545. [DOI] [Google Scholar]
- van Osch D. J. G. P.; Zubeir L. F.; van den Bruinhorst A.; Rocha M. A. A.; Kroon M. C. Hydrophobic Deep Eutectic Solvents as Water-Immiscible Extractants. Green Chem. 2015, 17 (9), 4518–4521. 10.1039/C5GC01451D. [DOI] [Google Scholar]
- Hoppe J.; Byzia E.; Szymańska M.; Drozd R.; Smiglak M. Acceleration of Lactose Hydrolysis Using Beta-Galactosidase and Deep Eutectic Solvents. Food Chem. 2022, 384, 132498. 10.1016/j.foodchem.2022.132498. [DOI] [PubMed] [Google Scholar]
- Ghaedi H.; Ayoub M.; Sufian S.; Shariff A. M.; Murshid G.; Hailegiorgis S. M.; Khan S. N. Density, Excess and Limiting Properties of (Water and Deep Eutectic Solvent) Systems at Temperatures from 293.15 to 343.15 K. J. Mol. Liq. 2017, 248, 378–390. 10.1016/j.molliq.2017.10.074. [DOI] [Google Scholar]
- Ghaedi H.; Ayoub M.; Sufian S.; Shariff A. M.; Lal B. The Study on Temperature Dependence of Viscosity and Surface Tension of Several Phosphonium-Based Deep Eutectic Solvents. J. Mol. Liq. 2017, 241, 500–510. 10.1016/j.molliq.2017.06.024. [DOI] [Google Scholar]
- Zhang H.; Lang J.; Lan P.; Yang H.; Lu J.; Wang Z. Study on the Dissolution Mechanism of Cellulose by ChCl-Based Deep Eutectic Solvents. Materials 2020, 13 (2), 278. 10.3390/ma13020278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranford S. W.; Buehler M. J. Selective Hydrogen Purification through Graphdiyne under Ambient Temperature and Pressure. Nanoscale 2012, 4 (15), 4587. 10.1039/c2nr30921a. [DOI] [PubMed] [Google Scholar]
- Keten S.; Buehler M. J. Asymptotic Strength Limit of Hydrogen Bond Assemblies in Proteins at Vanishing Pulling Rates. Phys. Rev. Lett. 2008, 100, 198301. 10.1103/PhysRevLett.100.198301. [DOI] [PubMed] [Google Scholar]
- Compton O. C.; Cranford S. W.; Putz K. W.; An Z.; Brinson L. C.; Buehler M. J.; Nguyen S. T. Tuning the Mechanical Properties of Graphene Oxide Paper and Its Associated Polymer Nanocomposites by Controlling Cooperative Intersheet Hydrogen Bonding. ACS Nano 2012, 6 (3), 2008–2019. 10.1021/nn202928w. [DOI] [PubMed] [Google Scholar]
- Beese A. M.; Sarkar S.; Nair A.; Naraghi M.; An Z.; Moravsky A.; Loutfy R. O.; Buehler M. J.; Nguyen S. T.; Espinosa H. D. Bio-Inspired Carbon Nanotube-Polymer Composite Yarns with Hydrogen Bond-Mediated Lateral Interactions. ACS Nano 2013, 7 (4), 3434–3446. 10.1021/nn400346r. [DOI] [PubMed] [Google Scholar]
- Keten S.; Buehler M. J. Geometric Confinement Governs the Rupture Strength of H-Bond Assemblies at a Critical Length Scale. Nano Lett. 2008, 8 (2), 743–748. 10.1021/nl0731670. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Yang Y.; Wang R.; Zhu Y.; Yang P.; Lin Z.; Wang Z.; Cong W. Efficient Extraction of Chitin from Crustacean Waste via a Novel Ternary Natural Deep Eutectic Solvents. Carbohydr. Polym. 2022, 286, 119281. 10.1016/j.carbpol.2022.119281. [DOI] [PubMed] [Google Scholar]
- Sharma M.; Mukesh C.; Mondal D.; Prasad K. Dissolution of α-Chitin in Deep Eutectic Solvents. RSC Adv. 2013, 3 (39), 18149–18155. 10.1039/c3ra43404d. [DOI] [Google Scholar]
- Li Y.; Wang J.; Liu X.; Zhang S. Towards a Molecular Understanding of Cellulose Dissolution in Ionic Liquids: Anion/Cation Effect, Synergistic Mechanism and Physicochemical Aspects. Chem. Sci. 2018, 9 (17), 4027–4043. 10.1039/C7SC05392D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.; Xin B.; Chen T.; Ying H.; Li Z.; Hao J. Deep Eutectic Solvent Strategy Enables an Octahedral Ni-Co Precursor for Creating High-Performance NiCo2O4 Catalyst toward Oxygen Evolution Reaction. Green Energy & Environment 2022, 7, 1217–1227. 10.1016/j.gee.2021.01.017. [DOI] [Google Scholar]
- Cai L.; Chen G.; Su B.; He M. 3D Printing of Ultra-Tough, Self-Healing Transparent Conductive Elastomeric Sensors. Chem. Eng. J. 2021, 426, 130545. 10.1016/j.cej.2021.130545. [DOI] [Google Scholar]
- Pollet P.; Davey E. A.; Ureña-Benavides E. E.; Eckert C. A.; Liotta C. L. Solvents for Sustainable Chemical Processes. Green Chem. 2014, 16 (3), 1034–1055. 10.1039/C3GC42302F. [DOI] [Google Scholar]
- Ravula S.; Larm N. E.; Mottaleb M. A.; Heitz M. P.; Baker G. A. Vapor Pressure Mapping of Ionic Liquids and Low-Volatility Fluids Using Graded Isothermal Thermogravimetric Analysis. ChemEngineering 2019, 3 (2), 42. 10.3390/chemengineering3020042. [DOI] [Google Scholar]
- Cvjetko Bubalo M.; Ćurko N.; Tomašević M.; Kovačević Ganić K.; Radojcic Redovnikovic I. Green Extraction of Grape Skin Phenolics by Using Deep Eutectic Solvents. Food Chem. 2016, 200, 159–166. 10.1016/j.foodchem.2016.01.040. [DOI] [PubMed] [Google Scholar]
- Bi W.; Tian M.; Row K. H. Evaluation of Alcohol-Based Deep Eutectic Solvent in Extraction and Determination of Flavonoids with Response Surface Methodology Optimization. J. Chromatogr A 2013, 1285, 22–30. 10.1016/j.chroma.2013.02.041. [DOI] [PubMed] [Google Scholar]
- Ge X.; Gu C.; Wang X.; Tu J. Deep Eutectic Solvents (DESs)-Derived Advanced Functional Materials for Energy and Environmental Applications: Challenges, Opportunities, and Future Vision. J. Mater. Chem. A 2017, 5 (18), 8209–8229. 10.1039/C7TA01659J. [DOI] [Google Scholar]
- Wang S.; Gao Q.; Wang J. Thermodynamic Analysis of Decomposition of Thiourea and Thiourea Oxides. J. Phys. Chem. B 2005, 109 (36), 17281–17289. 10.1021/jp051620v. [DOI] [PubMed] [Google Scholar]
- Mat Hussin S. A.; Varanusupakul P.; Shahabuddin S.; Yih Hui B.; Mohamad S. Synthesis and Characterization of Green Menthol-Based Low Transition Temperature Mixture with Tunable Thermophysical Properties as Hydrophobic Low Viscosity Solvent. J. Mol. Liq. 2020, 308, 113015. 10.1016/j.molliq.2020.113015. [DOI] [Google Scholar]
- Brahma B.; Narzary R.; Baruah D. C. Acetamide for Latent Heat Storage: Thermal Stability and Metal Corrosivity with Varying Thermal Cycles. Renew Energy 2020, 145, 1932–1940. 10.1016/j.renene.2019.07.109. [DOI] [Google Scholar]
- Zhang Q.; De Oliveira Vigier K.; Royer S.; Jérôme F. Deep Eutectic Solvents: Syntheses, Properties and Applications. Chem. Soc. Rev. 2012, 41 (21), 7108–7146. 10.1039/c2cs35178a. [DOI] [PubMed] [Google Scholar]
- Alonso D. A.; Baeza A.; Chinchilla R.; Guillena G.; Pastor I. M.; Ramón D. J. Deep Eutectic Solvents: The Organic Reaction Medium of the Century. Eur. J. Org. Chem. 2016, 2016 (4), 612–632. 10.1002/ejoc.201501197. [DOI] [Google Scholar]
- Makoś P.; Słupek E.; Gȩbicki J. Hydrophobic Deep Eutectic Solvents in Microextraction Techniques-A Review. Microchem. J. 2020, 152, 104384. 10.1016/j.microc.2019.104384. [DOI] [Google Scholar]
- Cao J.; Su E. Hydrophobic Deep Eutectic Solvents: The New Generation of Green Solvents for Diversified and Colorful Applications in Green Chemistry. J. Clean Prod 2021, 314, 127965. 10.1016/j.jclepro.2021.127965. [DOI] [Google Scholar]
- Florindo C.; Romero L.; Rintoul I.; Branco L. C.; Marrucho I. M. From Phase Change Materials to Green Solvents: Hydrophobic Low Viscous Fatty Acid-Based Deep Eutectic Solvents. ACS Sustain Chem. Eng. 2018, 6 (3), 3888–3895. 10.1021/acssuschemeng.7b04235. [DOI] [Google Scholar]
- Zhang Y.; Ni S.; Wu R.; Fu Y.; Qin M.; Willför S.; Xu C. Green Fractionation Approaches for Isolation of Biopolymers and the Critical Technical Challenges. Ind. Crops Prod 2022, 177, 114451. 10.1016/j.indcrop.2021.114451. [DOI] [Google Scholar]
- Niemczak M.ł; Rzemieniecki T.; Sobiech Łu.; Skrzypczak G.; Praczyk T.; Pernak J. Influence of the Alkyl Chain Length on the Physicochemical Properties and Biological Activity in a Homologous Series of Dichlorprop-Based Herbicidal Ionic Liquids. J. Mol. Liq. 2019, 276, 431–440. 10.1016/j.molliq.2018.12.013. [DOI] [Google Scholar]
- Maugeri Z.; Domínguez De María P. Novel Choline-Chloride-Based Deep-Eutectic-Solvents with Renewable Hydrogen Bond Donors: Levulinic Acid and Sugar-Based Polyols. RSC Adv. 2012, 2 (2), 421–425. 10.1039/C1RA00630D. [DOI] [Google Scholar]
- Jangir A. K.; Nain A. K.; Kuperkar K. Insight into Structural Properties and Molecular Interactions of Maline (Choline Chloride + Malonic Acid) and 1, 4- Butanediol Based Pseudo-Binary Mixture: A Thermophysical, Spectral, and Simulation Portrayal. J. Mol. Liq. 2021, 334, 116050. 10.1016/j.molliq.2021.116050. [DOI] [Google Scholar]
- van Osch D. J. G. P.; Zubeir L. F.; van den Bruinhorst A.; Rocha M. A. A.; Kroon M. C. Hydrophobic Deep Eutectic Solvents as Water-Immiscible Extractants. Green Chem. 2015, 17 (9), 4518–4521. 10.1039/C5GC01451D. [DOI] [Google Scholar]
- Liu Y. T.; Chen Y. A.; Xing Y. J. Synthesis and Characterization of Novel Ternary Deep Eutectic Solvents. Chin. Chem. Lett. 2014, 25 (1), 104–106. 10.1016/j.cclet.2013.09.004. [DOI] [Google Scholar]
- Kadhom M. A.; Abdullah G. H.; Al-Bayati N. Studying Two Series of Ternary Deep Eutectic Solvents (Choline Chloride-Urea-Glycerol) and (Choline Chloride-Malic Acid-Glycerol), Synthesis and Characterizations. Arab J. Sci. Eng. 2017, 42 (4), 1579–1589. 10.1007/s13369-017-2431-4. [DOI] [Google Scholar]
- Ghaedi H.; Ayoub M.; Sufian S.; Hailegiorgis S. M.; Murshid G.; Khan S. N. Thermal Stability Analysis, Experimental Conductivity and PH of Phosphonium-Based Deep Eutectic Solvents and Their Prediction by a New Empirical Equation. J. Chem. Thermodyn 2018, 116, 50–60. 10.1016/j.jct.2017.08.029. [DOI] [Google Scholar]
- Florindo C.; McIntosh A. J. S.; Welton T.; Branco L. C.; Marrucho I. M. A Closer Look into Deep Eutectic Solvents: Exploring Intermolecular Interactions Using Solvatochromic Probes. Phys. Chem. Chem. Phys. 2018, 20 (1), 206–213. 10.1039/C7CP06471C. [DOI] [PubMed] [Google Scholar]
- Tan Y. T.; Ngoh G. C.; Chua A. S. M. Evaluation of Fractionation and Delignification Efficiencies of Deep Eutectic Solvents on Oil Palm Empty Fruit Bunch. Ind. Crops Prod 2018, 123, 271–277. 10.1016/j.indcrop.2018.06.091. [DOI] [Google Scholar]
- Dwamena A. K.; Raynie D. E. Solvatochromic Parameters of Deep Eutectic Solvents: Effect of Different Carboxylic Acids as Hydrogen Bond Donor. J. Chem. Eng. Data 2020, 65 (2), 640–646. 10.1021/acs.jced.9b00872. [DOI] [Google Scholar]
- Zhang H.; Wang Y.; Xu K.; Li N.; Wen Q.; Yang Q.; Zhou Y. Ternary and Binary Deep Eutectic Solvents as a Novel Extraction Medium for Protein Partitioning. Analytical Methods 2016, 8 (46), 8196–8207. 10.1039/C6AY01860B. [DOI] [Google Scholar]
- Xu P.; Zheng G. W.; Zong M. H.; Li N.; Lou W. Y. Recent Progress on Deep Eutectic Solvents in Biocatalysis. Bioresour Bioprocess 2017, 4 (1), 1–18. 10.1186/s40643-017-0165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Achkar T.; Greige-Gerges H.; Fourmentin S. Basics and Properties of Deep Eutectic Solvents: A Review. Environ. Chem. Lett. 2021, 19 (4), 3397–3408. 10.1007/s10311-021-01225-8. [DOI] [Google Scholar]
- Cui Y.; Li C.; Yin J.; Li S.; Jia Y.; Bao M. Design, Synthesis and Properties of Acidic Deep Eutectic Solvents Based on Choline Chloride. J. Mol. Liq. 2017, 236, 338–343. 10.1016/j.molliq.2017.04.052. [DOI] [Google Scholar]
- Liu F.; Xue Z.; Zhao X.; Mou H.; He J.; Mu T. Catalytic Deep Eutectic Solvents for Highly Efficient Conversion of Cellulose to Gluconic Acid with Gluconic Acid Self-Precipitation Separation. Chem. Comm 2018, 54 (48), 6140–6143. 10.1039/C8CC03798A. [DOI] [PubMed] [Google Scholar]
- Florindo C.; Romero L.; Rintoul I.; Branco L. C.; Marrucho I. M. From Phase Change Materials to Green Solvents: Hydrophobic Low Viscous Fatty Acid-Based Deep Eutectic Solvents. ACS Sustain Chem. Eng. 2018, 6 (3), 3888–3895. 10.1021/acssuschemeng.7b04235. [DOI] [Google Scholar]
- Lemaoui T.; Darwish A. S.; Attoui A.; Abu Hatab F.; Hammoudi N. E. H.; Benguerba Y.; Vega L. F.; Alnashef I. M. Predicting the Density and Viscosity of Hydrophobic Eutectic Solvents: Towards the Development of Sustainable Solvents. Green Chem. 2020, 22 (23), 8511–8530. 10.1039/D0GC03077E. [DOI] [Google Scholar]
- Cardellini F.; Tiecco M.; Germani R.; Cardinali G.; Corte L.; Roscini L.; Spreti N. Novel Zwitterionic Deep Eutectic Solvents from Trimethylglycine and Carboxylic Acids: Characterization of Their Properties and Their Toxicity. RSC Adv. 2014, 4 (99), 55990–56002. 10.1039/C4RA10628H. [DOI] [Google Scholar]
- Siongco K. R.; Leron R. B.; Li M. H. Densities, Refractive Indices, and Viscosities of N,N-Diethylethanol Ammonium Chloride-Glycerol or -Ethylene Glycol Deep Eutectic Solvents and Their Aqueous Solutions. J. Chem. Thermodyn 2013, 65, 65–72. 10.1016/j.jct.2013.05.041. [DOI] [Google Scholar]
- Tak S. S.; Kundu D. A-Priori Modelling of Density of Deep Eutectic Solvent with Cohesion Based Cubic Equation of State. Chem. Thermodyn Therm Anal 2022, 5, 100026. 10.1016/j.ctta.2021.100026. [DOI] [Google Scholar]
- Abdollahzadeh M.; Khosravi M.; Hajipour Khire Masjidi B.; Samimi Behbahan A.; Bagherzadeh A.; Shahkar A.; Tat Shahdost F. Estimating the Density of Deep Eutectic Solvents Applying Supervised Machine Learning Techniques. Sci. Rep 2022, 12 (1), 4954. 10.1038/s41598-022-08842-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X. J.; Yu L. Y.; Wang Y. X.; Wu K. J.; He C. H. Comprehensive Prediction of Densities for Deep Eutectic Solvents: A New Bonding-Group Interaction Contribution Scheme. Ind. Eng. Chem. Res. 2021, 60 (35), 13127–13139. 10.1021/acs.iecr.1c02260. [DOI] [Google Scholar]
- Shahbaz K.; Baroutian S.; Mjalli F. S.; Hashim M. A.; Alnashef I. M. Densities of Ammonium and Phosphonium Based Deep Eutectic Solvents: Prediction Using Artificial Intelligence and Group Contribution Techniques. Thermochim. Acta 2012, 527, 59–66. 10.1016/j.tca.2011.10.010. [DOI] [Google Scholar]
- Shahbaz K.; Mjalli F. S.; Hashim M. A.; AlNashef I. M. Prediction of the Surface Tension of Deep Eutectic Solvents. Fluid Phase Equilib. 2012, 319, 48–54. 10.1016/j.fluid.2012.01.025. [DOI] [Google Scholar]
- Shahbaz K.; Mjalli F. S.; Hashim M. A.; Alnashef I. M. Prediction of Deep Eutectic Solvents Densities at Different Temperatures. Thermochim. Acta 2011, 515 (1–2), 67–72. 10.1016/j.tca.2010.12.022. [DOI] [Google Scholar]
- Makoś P.; Słupek E.; Gȩbicki J. Hydrophobic Deep Eutectic Solvents in Microextraction Techniques-A Review. Microchem. J. 2020, 152, 104384. 10.1016/j.microc.2019.104384. [DOI] [Google Scholar]
- Liu P.; Hao J. W.; Mo L. P.; Zhang Z. H. Recent Advances in the Application of Deep Eutectic Solvents as Sustainable Media as Well as Catalysts in Organic Reactions. RSC Adv. 2015, 5 (60), 48675–48704. 10.1039/C5RA05746A. [DOI] [Google Scholar]
- Hoppe J.; Drozd R.; Byzia E.; Smiglak M. Deep Eutectic Solvents Based on Choline Cation - Physicochemical Properties and Influence on Enzymatic Reaction with β-Galactosidase. Int. J. Biol. Macromol. 2019, 136, 296–304. 10.1016/j.ijbiomac.2019.06.027. [DOI] [PubMed] [Google Scholar]
- Mjalli F. S. Mass Connectivity Index-Based Density Prediction of Deep Eutectic Solvents. Fluid Phase Equilib. 2016, 409, 312–317. 10.1016/j.fluid.2015.09.053. [DOI] [Google Scholar]
- Haghbakhsh R.; Keshtkar M.; Shariati A.; Raeissi S. A Comprehensive Experimental and Modeling Study on CO2 Solubilities in the Deep Eutectic Solvent Based on Choline Chloride and Butane-1,2-Diol. Fluid Phase Equilib. 2022, 561, 113535. 10.1016/j.fluid.2022.113535. [DOI] [Google Scholar]
- Lemaoui T.; Hammoudi N. E. H.; Alnashef I. M.; Balsamo M.; Erto A.; Ernst B.; Benguerba Y. Quantitative Structure Properties Relationship for Deep Eutectic Solvents Using Sσ-Profile as Molecular Descriptors. J. Mol. Liq. 2020, 309, 113165. 10.1016/j.molliq.2020.113165. [DOI] [Google Scholar]
- Roosta A.; Haghbakhsh R.; Duarte A. R. C.; Raeissi S. Machine Learning Coupled with Group Contribution for Predicting the Density of Deep Eutectic Solvents. Fluid Phase Equilib. 2023, 565, 113672. 10.1016/j.fluid.2022.113672. [DOI] [Google Scholar]
- Alkhatib I. I. I.; Bahamon D.; Llovell F.; Abu-Zahra M. R. M.; Vega L. F. Perspectives and Guidelines on Thermodynamic Modelling of Deep Eutectic Solvents. J. Mol. Liq. 2020, 298, 112183. 10.1016/j.molliq.2019.112183. [DOI] [Google Scholar]
- Nasrifar K.; Moshfeghian M. A Saturated Liquid Density Equation in Conjunction with the Predictive-Soave-Redlich-Kwong Equation of State for Pure Refrigerants and LNG Multicomponent Systems. Fluid Phase Equilib. 1998, 153 (2), 231–242. 10.1016/S0378-3812(98)00422-1. [DOI] [Google Scholar]
- Ji W. R.; Lempe D. A. Density Improvement of the SRK Equation of State. Fluid Phase Equilib. 1997, 130 (1–2), 49–63. 10.1016/S0378-3812(96)03190-1. [DOI] [Google Scholar]
- Valderrama J. O.; Zarricueta K. A Simple and Generalized Model for Predicting the Density of Ionic Liquids. Fluid Phase Equilib. 2009, 275 (2), 145–151. 10.1016/j.fluid.2008.10.002. [DOI] [Google Scholar]
- Lemaoui T.; Darwish A. S.; Hammoudi N. E. H.; Abu Hatab F.; Attoui A.; Alnashef I. M.; Benguerba Y. Prediction of Electrical Conductivity of Deep Eutectic Solvents Using COSMO-RS Sigma Profiles as Molecular Descriptors: A Quantitative Structure-Property Relationship Study. Ind. Eng. Chem. Res. 2020, 59 (29), 13343–13354. 10.1021/acs.iecr.0c02542. [DOI] [Google Scholar]
- Senthil Muthu Kumar T.; Senthil Kumar K.; Rajini N.; Siengchin S.; Ayrilmis N.; Varada Rajulu A. A Comprehensive Review of Electrospun Nanofibers: Food and Packaging Perspective. Compos B Eng. 2019, 175, 107074. 10.1016/j.compositesb.2019.107074. [DOI] [Google Scholar]
- Topuz F.; Satilmis B.; Uyar T. Electrospinning of Uniform Nanofibers of Polymers of Intrinsic Microporosity (PIM-1): The Influence of Solution Conductivity and Relative Humidity. Polymer 2019, 178, 121610. 10.1016/j.polymer.2019.121610. [DOI] [Google Scholar]
- Reuter D.; Binder C.; Lunkenheimer P.; Loidl A. Ionic Conductivity of Deep Eutectic Solvents: The Role of Orientational Dynamics and Glassy Freezing. Phys. Chem. Chem. Phys. 2019, 21 (13), 6801–6809. 10.1039/C9CP00742C. [DOI] [PubMed] [Google Scholar]
- Koi Z. K.; Yahya W. Z. N.; Kurnia K. A. Prediction of Ionic Conductivity of Imidazolium-Based Ionic Liquids at Different Temperatures Using Multiple Linear Regression and Support Vector Machine Algorithms. New J. Chem. 2021, 45 (39), 18584–18597. 10.1039/D1NJ01831K. [DOI] [Google Scholar]
- Nancarrow P.; Al-Othman A.; Mital D. K.; Döpking S. Comprehensive Analysis and Correlation of Ionic Liquid Conductivity Data for Energy Applications. Energy 2021, 220, 119761. 10.1016/j.energy.2021.119761. [DOI] [Google Scholar]
- Balos V.; Imoto S.; Netz R. R.; Bonn M.; Bonthuis D. J.; Nagata Y.; Hunger J. Macroscopic Conductivity of Aqueous Electrolyte Solutions Scales with Ultrafast Microscopic Ion Motions. Nat. Comm. 2020, 11 (1), 1611. 10.1038/s41467-020-15450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroso I. M.; Paiva A.; Reis R. L.; Duarte A. R. C. Natural Deep Eutectic Solvents from Choline Chloride and Betaine - Physicochemical Properties. J. Mol. Liq. 2017, 241, 654–661. 10.1016/j.molliq.2017.06.051. [DOI] [Google Scholar]
- Agieienko V.; Buchner R. A Comprehensive Study of Density, Viscosity, and Electrical Conductivity of (Choline Chloride + Glycerol) Deep Eutectic Solvent and Its Mixtures with Dimethyl Sulfoxide. J. Chem. Eng. Data 2021, 66 (1), 780–792. 10.1021/acs.jced.0c00869. [DOI] [Google Scholar]
- Agieienko V.; Buchner R. Variation of Density, Viscosity, and Electrical Conductivity of the Deep Eutectic Solvent Reline, Composed of Choline Chloride and Urea at a Molar Ratio of 1:2, Mixed with Dimethylsulfoxide as a Cosolvent. J. Chem. Eng. Data 2020, 65 (4), 1900–1910. 10.1021/acs.jced.9b01105. [DOI] [Google Scholar]
- Boublia A.; Lemaoui T.; Abu Hatab F.; Darwish A. S.; Banat F.; Benguerba Y.; AlNashef I. M. Molecular-Based Artificial Neural Network for Predicting the Electrical Conductivity of Deep Eutectic Solvents. J. Mol. Liq. 2022, 366, 120225. 10.1016/j.molliq.2022.120225. [DOI] [Google Scholar]
- Bagh F. S. G.; Shahbaz K.; Mjalli F. S.; AlNashef I. M.; Hashim M. A. Electrical Conductivity of Ammonium and Phosphonium Based Deep Eutectic Solvents: Measurements and Artificial Intelligence-Based Prediction. Fluid Phase Equilib. 2013, 356, 30–37. 10.1016/j.fluid.2013.07.012. [DOI] [Google Scholar]
- Adeyemi I.; Abu-Zahra M. R. M.; AlNashef I. M. Physicochemical Properties of Alkanolamine-Choline Chloride Deep Eutectic Solvents: Measurements, Group Contribution and Artificial Intelligence Prediction Techniques. J. Mol. Liq. 2018, 256, 581–590. 10.1016/j.molliq.2018.02.085. [DOI] [Google Scholar]
- Weiß N.; Schmidt C. H.; Thielemann G.; Heid E.; Schröder C.; Spange S. The Physical Significance of the Kamlet-Taft Π* Parameter of Ionic Liquids. Phys. Chem. Chem. Phys. 2021, 23 (2), 1616–1626. 10.1039/D0CP04989A. [DOI] [PubMed] [Google Scholar]
- Afonso J.; Mezzetta A.; Marrucho I. M.; Guazzelli L. History Repeats Itself Again: Will the Mistakes of the Past for ILs Be Repeated for DESs? From Being Considered Ionic Liquids to Becoming Their Alternative: The Unbalanced Turn of Deep Eutectic Solvents. Green Chem. 2023, 25 (1), 59–105. 10.1039/D2GC03198A. [DOI] [Google Scholar]
- Rodrigues L. A.; Cardeira M.; Leonardo I. C.; Gaspar F. B.; Radojčić Redovniković I.; Duarte A. R. C.; Paiva A.; Matias A. A. Deep Eutectic Systems from Betaine and Polyols - Physicochemical and Toxicological Properties. J. Mol. Liq. 2021, 335, 116201. 10.1016/j.molliq.2021.116201. [DOI] [Google Scholar]
- Xu P.; Zheng G. W.; Zong M. H.; Li N.; Lou W. Y. Recent Progress on Deep Eutectic Solvents in Biocatalysis. Bioresour Bioprocess 2017, 4 (1), 1–18. 10.1186/s40643-017-0165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y.; Witkamp G. J.; Verpoorte R.; Choi Y. H. Tailoring Properties of Natural Deep Eutectic Solvents with Water to Facilitate Their Applications. Food Chem. 2015, 187, 14–19. 10.1016/j.foodchem.2015.03.123. [DOI] [PubMed] [Google Scholar]
- Haghbakhsh R.; Taherzadeh M.; Duarte A. R. C.; Raeissi S. A General Model for the Surface Tensions of Deep Eutectic Solvents. J. Mol. Liq. 2020, 307, 112972. 10.1016/j.molliq.2020.112972. [DOI] [Google Scholar]
- Chen Y.; Chen W.; Fu L.; Yang Y.; Wang Y.; Hu X.; Wang F.; Mu T. Surface Tension of 50 Deep Eutectic Solvents: Effect of Hydrogen-Bonding Donors, Hydrogen-Bonding Acceptors, Other Solvents, and Temperature. Ind. Eng. Chem. Res. 2019, 58 (28), 12741–12750. 10.1021/acs.iecr.9b00867. [DOI] [Google Scholar]
- Tang R.; Darragh M.; Orme C. A.; Guan X.; Hoyer J. R.; Nancollas G. H. Control of Biomineralization Dynamics by Interfacial Energies. Angew. Chem. 2005, 117 (24), 3764–3768. 10.1002/ange.200500153. [DOI] [PubMed] [Google Scholar]
- Rudloff J.; Cölfen H. Superstructures of Temporarily Stabilized Nanocrystalline CaCO3 Particles: Morphological Control via Water Surface Tension Variation. Langmuir 2004, 20 (3), 991–996. 10.1021/la0358217. [DOI] [PubMed] [Google Scholar]
- Bassett D. C.; McKee M. D.; Barralet J. E. The Role of the Air-Liquid Interface in Protein-Mediated Biomineralization of Calcium Carbonate. Cryst. Growth Des 2011, 11 (3), 803–810. 10.1021/cg101437h. [DOI] [Google Scholar]
- Sanchez-Fernandez A.; Moody G. L.; Murfin L. C.; Arnold T.; Jackson A. J.; King S. M.; Lewis S. E.; Edler K. J. Self-Assembly and Surface Behaviour of Pure and Mixed Zwitterionic Amphiphiles in a Deep Eutectic Solvent. Soft Matter 2018, 14 (26), 5525–5536. 10.1039/C8SM00755A. [DOI] [PubMed] [Google Scholar]
- Bryant S. J.; Christofferson A. J.; Greaves T. L.; McConville C. F.; Bryant G.; Elbourne A. Bulk and Interfacial Nanostructure and Properties in Deep Eutectic Solvents: Current Perspectives and Future Directions. J. Colloid Interface Sci. 2022, 608, 2430–2454. 10.1016/j.jcis.2021.10.163. [DOI] [PubMed] [Google Scholar]
- McDonald S.; Murphy T.; Imberti S.; Warr G. G.; Atkin R. Amphiphilically Nanostructured Deep Eutectic Solvents. J. Phys. Chem. Lett. 2018, 9, 3922–3927. 10.1021/acs.jpclett.8b01720. [DOI] [PubMed] [Google Scholar]
- Buzolic J. J.; Li H.; Aman Z. M.; Warr G. G.; Atkin R. Self-Assembled Nanostructure Induced in Deep Eutectic Solvents via an Amphiphilic Hydrogen Bond Donor. J. Colloid Interface Sci. 2022, 616, 121–128. 10.1016/j.jcis.2022.02.029. [DOI] [PubMed] [Google Scholar]
- Yamauchi N.; Yachi T.; Kurumada K. One-Pot Spontaneous Formation of Submicron Hexane-Dispersible Silica Particles with the Aid of Amphiphilic Reaction Solvent. Colloids Surf. A 2018, 553, 253–258. 10.1016/j.colsurfa.2018.05.053. [DOI] [Google Scholar]
- Nisticò R.; Scalarone D.; Magnacca G. Sol-Gel Chemistry, Templating and Spin-Coating Deposition: A Combined Approach to Control in a Simple Way the Porosity of Inorganic Thin Films/Coatings. Microporous Mesoporous Mater. 2017, 248, 18–29. 10.1016/j.micromeso.2017.04.017. [DOI] [Google Scholar]
- Rauner N.; Meuris M.; Dech S.; Godde J.; Tiller J. C. Urease-Induced Calcification of Segmented Polymer Hydrogels - A Step towards Artificial Biomineralization. Acta Biomater 2014, 10 (9), 3942–3951. 10.1016/j.actbio.2014.05.021. [DOI] [PubMed] [Google Scholar]
- Gilmore M.; Swadzba-Kwasny M.; Holbrey J. D. Thermal Properties of Choline Chloride/Urea System Studied under Moisture-Free Atmosphere. J. Chem. Eng. Data 2019, 64 (12), 5248–5255. 10.1021/acs.jced.9b00474. [DOI] [Google Scholar]
- Ma C.; Laaksonen A.; Liu C.; Lu X.; Ji X. The Peculiar Effect of Water on Ionic Liquids and Deep Eutectic Solvents. Chem. Soc. Rev. 2018, 47 (23), 8685–8720. 10.1039/C8CS00325D. [DOI] [PubMed] [Google Scholar]
- Hammond O. S.; Bowron D. T.; Edler K. J. The Effect of Water upon Deep Eutectic Solvent Nanostructure: An Unusual Transition from Ionic Mixture to Aqueous Solution. Angew. Chem., Int. Ed. 2017, 56 (33), 9782–9785. 10.1002/anie.201702486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke C. J.; Tu W. C.; Levers O.; Bröhl A.; Hallett J. P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118 (2), 747–800. 10.1021/acs.chemrev.7b00571. [DOI] [PubMed] [Google Scholar]
- Hessel V.; Tran N. N.; Asrami M. R.; Tran Q. D.; Van Duc Long N.; Escribà-Gelonch M.; Tejada J. O.; Linke S.; Sundmacher K. Sustainability of Green Solvents - Review and Perspective. Green Chem. 2022, 24 (2), 410–437. 10.1039/D1GC03662A. [DOI] [Google Scholar]
- Nejrotti S.; Antenucci A.; Pontremoli C.; Gontrani L.; Barbero N.; Carbone M.; Bonomo M. Critical Assessment of the Sustainability of Deep Eutectic Solvents: A Case Study on Six Choline Chloride-Based Mixtures. ACS Omega 2022, 7 (51), 47449–47461. 10.1021/acsomega.2c06140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Q.; Chen J. X.; Tang Y. L.; Wang J.; Yang Z. Assessing the Toxicity and Biodegradability of Deep Eutectic Solvents. Chemosphere 2015, 132, 63–69. 10.1016/j.chemosphere.2015.02.061. [DOI] [PubMed] [Google Scholar]
- Gracia-Barberán S.; Leal-Duaso A.; Pires E. Are Deep Eutectic Solvents a Real Alternative to Ionic Liquids in Metal-Catalysed Reactions?. Curr. Opin Green Sustain Chem. 2022, 35, 100610. 10.1016/j.cogsc.2022.100610. [DOI] [Google Scholar]
- Dietz C. H. J. T.; Creemers J. T.; Meuleman M. A.; Held C.; Sadowski G.; Van Sint Annaland M.; Gallucci F.; Kroon M. C. Determination of the Total Vapor Pressure of Hydrophobic Deep Eutectic Solvents: Experiments and Perturbed-Chain Statistical Associating Fluid Theory Modeling. ACS Sustain Chem. Eng. 2019, 7 (4), 4047–4057. 10.1021/acssuschemeng.8b05449. [DOI] [Google Scholar]
- Chan V. B. S.; Johnstone M. B.; Wheeler A. P.; Mount A. S. Chitin Facilitated Mineralization in the Eastern Oyster. Front Mar Sci. 2018, 5, 347. 10.3389/fmars.2018.00347. [DOI] [Google Scholar]
- Ehrlich H. Chitin and Collagen as Universal and Alternative Templates in Biomineralization. International Geology Reviews 2010, 52, 661–699. 10.1080/00206811003679521. [DOI] [Google Scholar]
- Nair A. K.; Gautieri A.; Chang S. W.; Buehler M. J. Molecular Mechanics of Mineralized Collagen Fibrils in Bone. Nat. Comm. 2013, 4, 1724. 10.1038/ncomms2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich H.; Deutzmann R.; Brunner E.; Cappellini E.; Koon H.; Solazzo C.; Yang Y.; Ashford D.; Thomas-Oates J.; Lubeck M.; Baessmann C.; Langrock T.; Hoffmann R.; Wörheide G.; Reitner J.; Simon P.; Tsurkan M.; Ereskovsky A. v.; Kurek D.; Bazhenov V. v.; Hunoldt S.; Mertig M.; Vyalikh D. v.; Molodtsov S. L.; Kummer K.; Worch H.; Smetacek V.; Collins M. J. Mineralization of the Metre-Long Biosilica Structures of Glass Sponges Is Templated on Hydroxylated Collagen. Nat. Chem. 2010, 2 (12), 1084–1088. 10.1038/nchem.899. [DOI] [PubMed] [Google Scholar]
- Levi-Kalisman Y.; Falini G.; Addadi L.; Weiner S. Structure of the Nacreous Organic Matrix of a Bivalve Mollusk Shell Examined in the Hydrated State Using Cryo-TEM. J. Struct Biol. 2001, 135 (1), 8–17. 10.1006/jsbi.2001.4372. [DOI] [PubMed] [Google Scholar]
- Pouget E.; Dujardin E.; Cavalier A.; Moreac A.; Valéry C.; Marchi-Artzner V.; Weiss T.; Renault A.; Paternostre M.; Artzner F. Hierarchical Architectures by Synergy between Dynamical Template Self-Assembly and Biomineralization. Nat. Mater. 2007, 6 (6), 434–439. 10.1038/nmat1912. [DOI] [PubMed] [Google Scholar]
- Wittig N. K.; Birkedal H. Bone Hierarchical Structure: Spatial Variation across Length Scales. Acta Crystallogr. 2022, 78, 305–311. 10.1107/S2052520622001524. [DOI] [PubMed] [Google Scholar]
- Sen D.; Buehler M. J. Structural Hierarchies Define Toughness and Defect-Tolerance despite Simple and Mechanically Inferior Brittle Building Blocks. Sci. Rep. 2011, 1 (1), 35. 10.1038/srep00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakaki A.; Shimizu K.; Oda M.; Sakamoto T.; Nishimura T.; Kato T. Biomineralization-Inspired Synthesis of Functional Organic/Inorganic Hybrid Materials: Organic Molecular Control of Self-Organization of Hybrids. Org. Biomol Chem. 2015, 13 (4), 974–989. 10.1039/C4OB01796J. [DOI] [PubMed] [Google Scholar]
- Pillai C. K. S.; Paul W.; Sharma C. P. Chitin and Chitosan Polymers: Chemistry, Solubility and Fiber Formation. Prog. Polym. Sci. 2009, 34 (7), 641–678. 10.1016/j.progpolymsci.2009.04.001. [DOI] [Google Scholar]
- Zargar V.; Asghari M.; Dashti A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng. Reviews 2015, 2 (3), 204–226. 10.1002/cben.201400025. [DOI] [Google Scholar]
- Kurita K. Controlled Functionalization of the Polysaccharide Chitin. Prog. Polym. Sci. 2001, 26 (9), 1921–1971. 10.1016/S0079-6700(01)00007-7. [DOI] [Google Scholar]
- Bisht M.; Macário I. P. E.; Neves M. C.; Pereira J. L.; Pandey S.; Rogers R. D.; Coutinho J. A. P.; Ventura S. P. M. Enhanced Dissolution of Chitin Using Acidic Deep Eutectic Solvents: A Sustainable and Simple Approach to Extract Chitin from Crayfish Shell Wastes as Alternative Feedstocks. ACS Sustain Chem. Eng. 2021, 9 (48), 16073–16081. 10.1021/acssuschemeng.1c04255. [DOI] [Google Scholar]
- Li Z.; Li M. C.; Liu C.; Liu X.; Lu Y.; Zhou G.; Liu C.; Mei C. Microwave-Assisted Deep Eutectic Solvent Extraction of Chitin from Crayfish Shell Wastes for 3D Printable Inks. Ind. Crops Prod 2023, 194, 116325. 10.1016/j.indcrop.2023.116325. [DOI] [Google Scholar]
- Khajavian M.; Vatanpour V.; Castro-Muñoz R.; Boczkaj G. Chitin and Derivative Chitosan-Based Structures — Preparation Strategies Aided by Deep Eutectic Solvents: A Review. Carbohydr. Polym. 2022, 275, 118702. 10.1016/j.carbpol.2021.118702. [DOI] [PubMed] [Google Scholar]
- Saravana P. S.; Ho T. C.; Chae S. J.; Cho Y. J.; Park J. S.; Lee H. J.; Chun B. S. Deep Eutectic Solvent-Based Extraction and Fabrication of Chitin Films from Crustacean Waste. Carbohydr. Polym. 2018, 195, 622–630. 10.1016/j.carbpol.2018.05.018. [DOI] [PubMed] [Google Scholar]
- Li Z.; Liu C.; Hong S.; Lian H.; Mei C.; Lee J.; Wu Q.; Hubbe M. A.; Li M.-C. Recent Advances in Extraction and Processing of Chitin Using Deep Eutectic Solvents. Chem. Eng. J. 2022, 446, 136953. 10.1016/j.cej.2022.136953. [DOI] [Google Scholar]
- Kuo D.; Nishimura T.; Kajiyama S.; Kato T. Bioinspired Environmentally Friendly Amorphous CaCO3-Based Transparent Composites Comprising Cellulose Nanofibers. ACS Omega 2018, 3 (10), 12722–12729. 10.1021/acsomega.8b02014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y.; Colfen H.; Xiong R. Organized Mineralized Cellulose Nanostructures for Biomedical Applications. J. Mater. Chem. B 2023, 11 (24), 5321–5349. 10.1039/D2TB02611B. [DOI] [PubMed] [Google Scholar]
- Matsumura S.; Kajiyama S.; Nishimura T.; Kato T. Formation of Helically Structured Chitin/CaCO3 Hybrids through an Approach Inspired by the Biomineralization Processes of Crustacean Cuticles. Small 2015, 11 (38), 5127–5133. 10.1002/smll.201501083. [DOI] [PubMed] [Google Scholar]
- Richardson J. J.; Tardy B. L.; Guo J.; Liang K.; Rojas O. J.; Ejima H. Continuous Metal-Organic Framework Biomineralization on Cellulose Nanocrystals: Extrusion of Functional Composite Filaments. ACS Sustain Chem. Eng. 2019, 7 (6), 6287–6294. 10.1021/acssuschemeng.8b06713. [DOI] [Google Scholar]
- Guan Q.-F.; Yang H.-B.; Han Z.-M.; Ling Z.-C.; Yu S.-H. An All-Natural Bioinspired Structural Material for Plastic Replacement. Nat. Commun. 2020, 11 (1), 1–7. 10.1038/s41467-020-19174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdanowicz M.; Wilpiszewska K.; Spychaj T. Deep Eutectic Solvents for Polysaccharides Processing. A Review. Carbohydr. Polym. 2018, 200, 361–380. 10.1016/j.carbpol.2018.07.078. [DOI] [PubMed] [Google Scholar]
- Almeida R. O.; Maloney T. C.; Gamelas J. A. F. Production of Functionalized Nanocelluloses from Different Sources Using Deep Eutectic Solvents and Their Applications. Ind. Crops Prod 2023, 199, 116583. 10.1016/j.indcrop.2023.116583. [DOI] [Google Scholar]
- Wang H.; Li J.; Yu X.; Yan G.; Tang X.; Sun Y.; Zeng X.; Lin L. Cellulose Nanocrystalline Hydrogel Based on a Choline Chloride Deep Eutectic Solvent as Wearable Strain Sensor for Human Motion. Carbohydr. Polym. 2021, 255, 117443. 10.1016/j.carbpol.2020.117443. [DOI] [PubMed] [Google Scholar]
- Chen Y. L.; Zhang X.; You T. T.; Xu F. Deep Eutectic Solvents (DESs) for Cellulose Dissolution: A Mini-Review. Cellulose 2019, 26 (1), 205–213. 10.1007/s10570-018-2130-7. [DOI] [Google Scholar]
- Zhong Y.; Wu J.; Kang H.; Liu R. Choline Hydroxide Based Deep Eutectic Solvent for Dissolving Cellulose. Green Chem. 2022, 24 (6), 2464–2475. 10.1039/D1GC04130D. [DOI] [Google Scholar]
- Sirviö J. A. Fabrication of Regenerated Cellulose Nanoparticles by Mechanical Disintegration of Cellulose after Dissolution and Regeneration from a Deep Eutectic Solvent. J. Mater. Chem. A 2019, 7 (2), 755–763. 10.1039/C8TA09959F. [DOI] [Google Scholar]
- Li P.; Sirviö J. A.; Haapala A.; Liimatainen H. Cellulose Nanofibrils from Nonderivatizing Urea-Based Deep Eutectic Solvent Pretreatments. ACS Appl. Mater. Interfaces 2017, 9 (3), 2846–2855. 10.1021/acsami.6b13625. [DOI] [PubMed] [Google Scholar]
- Ling S.; Qin Z.; Huang W.; Cao S.; Kaplan D. L.; Buehler M. J. Design and Function of Biomimetic Multilayer Water Purification Membranes. Sci. Adv. 2017, 3 (4), e1601939. 10.1126/sciadv.1601939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debari M. K.; King C. I.; Altgold T. A.; Abbott R. D. Silk Fibroin as a Green Material. ACS Biomater Sci. Eng. 2021, 7 (8), 3530–3544. 10.1021/acsbiomaterials.1c00493. [DOI] [PubMed] [Google Scholar]
- Anastas P.; Eghbali N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. 10.1039/B918763B. [DOI] [PubMed] [Google Scholar]
- Zimmerman J. B.; Anastas P. T.; Erythropel H. C.; Leitner W. Designing for a Green Chemistry Future. Science 2020, 367, 397–400. 10.1126/science.aay3060. [DOI] [PubMed] [Google Scholar]
- Bai C.; Wei Q.; Ren X. Selective Extraction of Collagen Peptides with High Purity from Cod Skins by Deep Eutectic Solvents. ACS Sustainable Chem. Eng. 2017, 5 (8), 7220–7227. 10.1021/acssuschemeng.7b01439. [DOI] [Google Scholar]
- Hu Y.; Liu L.; Yu J.; Wang Z.; Fan Y. Preparation of Natural Multicompatible Silk Nanofibers by Green Deep Eutectic Solvent Treatment. ACS Sustainable Chem. Eng. 2020, 8, 4499–4510. 10.1021/acssuschemeng.9b07668. [DOI] [Google Scholar]
- Cui F.; Han W.; Ge J.; Wu X.; Kim H.; Ding B. Electrospinning: A Versatile Strategy for Mimicking Natural Creatures. Composites Communications 2018, 10, 175–185. 10.1016/j.coco.2018.10.001. [DOI] [Google Scholar]
- Rong K.; Wei J.; Wang Y.; Liu J.; Qiao Z. A.; Fang Y.; Dong S. Deep Eutectic Solvent Assisted Zero-Waste Electrospinning of Lignin Fiber Aerogels. Green Chem. 2021, 23 (16), 6065–6075. 10.1039/D1GC01872H. [DOI] [Google Scholar]
- Khatri M.; Khatri Z.; El-Ghazali S.; Hussain N.; Qureshi U. A.; Kobayashi S.; Ahmed F.; Kim I. S. Zein Nanofibers via Deep Eutectic Solvent Electrospinning: Tunable Morphology with Super Hydrophilic Properties. Sci. Rep. 2020, 10 (1), 1–11. 10.1038/s41598-020-72337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zastrow M. 3D Printing Gets Bigger, Faster and Stronger. Nature 2020, 578 (7793), 20–23. 10.1038/d41586-020-00271-6. [DOI] [PubMed] [Google Scholar]
- Shahrubudin N.; Lee T. C.; Ramlan R. An Overview on 3D Printing Technology: Technological, Materials, and Applications. Procedia Manuf 2019, 35, 1286–1296. 10.1016/j.promfg.2019.06.089. [DOI] [Google Scholar]
- Chang T. J.; Vaut L.; Voss M.; Ilchenko O.; Nielsen L. H.; Boisen A.; Hwu E. te. Micro and Nanoscale 3D Printing Using Optical Pickup Unit from a Gaming Console. Commun. Phys. 2021, 4 (1), 1–8. 10.1038/s42005-021-00532-4. [DOI] [Google Scholar]
- Hengsteler J.; Mandal B.; van Nisselroy C.; Lau G. P. S.; Schlotter T.; Zambelli T.; Momotenko D. Bringing Electrochemical Three-Dimensional Printing to the Nanoscale. Nano Lett. 2021, 21 (21), 9093–9101. 10.1021/acs.nanolett.1c02847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana J. A.; Olvera D.; Fuller S. M.; Kelly J. P.; Graeve O. A.; Schwarz E. M.; Kates S. L.; Awad H. A. 3D Printing of Composite Calcium Phosphate and Collagen Scaffolds for Bone Regeneration. Biomaterials 2014, 35 (13), 4026–4034. 10.1016/j.biomaterials.2014.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milazzo M.; Fitzpatrick V.; Owens C. E.; Carraretto I. M.; McKinley G. H.; Kaplan D. L.; Buehler M. J. 3D Printability of Silk/Hydroxyapatite Composites for Microprosthetic Applications. ACS Biomater Sci. Eng. 2023, 9, 1285. 10.1021/acsbiomaterials.2c01357. [DOI] [PubMed] [Google Scholar]
- Wen X.; Zhang B.; Wang W.; Ye F.; Yue S.; Guo H.; Gao G.; Zhao Y.; Fang Q.; Nguyen C.; Zhang X.; Bao J.; Robinson J. T.; Ajayan P. M.; Lou J. 3D-Printed Silica with Nanoscale Resolution. Nat. Mater. 2021, 20 (11), 1506–1511. 10.1038/s41563-021-01111-2. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Zhang F.; Yan Z.; Ma Q.; Li X.; Huang Y.; Rogers J. A. Printing, Folding and Assembly Methods for Forming 3D Mesostructures in Advanced Materials. Nat. Rev. Mater. 2017, 2 (4), 1–17. 10.1038/natrevmats.2017.19. [DOI] [Google Scholar]
- Fernandes M. C.; Aizenberg J.; Weaver J. C.; Bertoldi K. Mechanically Robust Lattices Inspired by Deep-Sea Glass Sponges. Nat. Mater. 2021, 20 (2), 237–241. 10.1038/s41563-020-0798-1. [DOI] [PubMed] [Google Scholar]
- Robson Brown K.; Bacheva D.; Trask R. S. The Structural Efficiency of the Sea Sponge Euplectella Aspergillum Skeleton: Bio-Inspiration for 3D Printed Architectures. J. R Soc. Interface 2019, 16 (154), 20180965. 10.1098/rsif.2018.0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.; Xue J.; Li T.; Zhai D.; Yu X.; Huan Z.; Wu C. 3D Printing of Sponge Spicules-Inspired Flexible Bioceramic-Based Scaffolds. Biofabrication 2022, 14 (3), 035009. 10.1088/1758-5090/ac66ff. [DOI] [PubMed] [Google Scholar]
- Dimas L. S.; Bratzel G. H.; Eylon I.; Buehler M. J. Tough Composites Inspired by Mineralized Natural Materials: Computation, 3D Printing, and Testing. Adv. Funct Mater. 2013, 23 (36), 4629–4638. 10.1002/adfm.201300215. [DOI] [Google Scholar]
- Libonati F.; Gu G. X.; Qin Z.; Vergani L.; Buehler M. J. Bone-Inspired Materials by Design: Toughness Amplification Observed Using 3D Printing and Testing. Adv. Eng. Mater. 2016, 18 (8), 1354–1363. 10.1002/adem.201600143. [DOI] [Google Scholar]
- Kim J.; Yoon Y.; Kim S. K.; Park S.; Song W.; Myung S.; Jung H.-K.; Lee S. S.; Yoon D. H.; An K.-S. Chemically Stabilized and Functionalized 2D-MXene with Deep Eutectic Solvents as Versatile Dispersion Medium. Adv. Funct Mater. 2021, 31 (13), 2008722. 10.1002/adfm.202008722. [DOI] [Google Scholar]
- Kasprzak D.; Mayorga-Martinez C. C.; Alduhaish O.; Pumera M. Wearable and Flexible All-Solid-State Supercapacitor Based on MXene and Chitin. Energy Technology 2023, 11, 2201103. 10.1002/ente.202201103. [DOI] [Google Scholar]
- Meng J.; Wang Y.; Zhou Y.; Chen J.; Wei X.; Ni R.; Liu Z.; Xu F. A Composite Consisting of a Deep Eutectic Solvent and Dispersed Magnetic Metal-Organic Framework (Type UiO-66-NH2) for Solid-Phase Extraction of RNA. Microchimica Acta 2020, 187 (1), 1–9. 10.1007/s00604-019-4040-2. [DOI] [PubMed] [Google Scholar]
- Tanizaki Y.; Maeda Y.; Sasaki Y.; Ogawa H.; Mori H. Deep Eutectic Silsesquioxane Hybrids with Quaternary Ammonium/Urea Derivatives: Synthesis and Physicochemical and Ion-Conductive Properties. Mater. Today Chem. 2021, 20, 100455. 10.1016/j.mtchem.2021.100455. [DOI] [Google Scholar]
- Moura H. M.; Unterlass M. M. Biogenic Metal Oxides. Biomimetics 2020, 5 (2), 29. 10.3390/biomimetics5020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J.; Song F.; Yin X. W.; Zhang Z. L.; Liu Y.; Wang X. L.; Wang Y. Z. Cellulose Nanocrystal-Templated Synthesis of Mesoporous TiO2 with Dominantly Exposed (001) Facets for Efficient Catalysis. ACS Sustain Chem. Eng. 2017, 5 (5), 3721–3725. 10.1021/acssuschemeng.7b00341. [DOI] [Google Scholar]
- Siwińska-Ciesielczyk K.; Świgoń D.; Rychtowski P.; Moszyński D.; Zgoła-Grześkowiak A.; Jesionowski T. The Performance of Multicomponent Oxide Systems Based on TiO2, ZrO2 and SiO2 in the Photocatalytic Degradation of Rhodamine B: Mechanism and Kinetic Studies. Colloids Surf. A 2020, 586, 124272. 10.1016/j.colsurfa.2019.124272. [DOI] [Google Scholar]
- Cheng Y.; Chan K. H.; Wang X. Q.; Ding T.; Li T.; Lu X.; Ho G. W. Direct-Ink-Write 3D Printing of Hydrogels into Biomimetic Soft Robots. ACS Nano 2019, 13 (11), 13176–13184. 10.1021/acsnano.9b06144. [DOI] [PubMed] [Google Scholar]
- Huang Z. L.; Wu B. P.; Wen Q.; Yang T. X.; Yang Z. Deep Eutectic Solvents Can Be Viable Enzyme Activators and Stabilizers. J. Chem. Technol. Biotechnol. 2014, 89 (12), 1975–1981. 10.1002/jctb.4285. [DOI] [Google Scholar]
- Milano F.; Giotta L.; Guascito M. R.; Agostiano A.; Sblendorio S.; Valli L.; Perna F. M.; Cicco L.; Trotta M.; Capriati V. Functional Enzymes in Nonaqueous Environment: The Case of Photosynthetic Reaction Centers in Deep Eutectic Solvents. ACS Sustain Chem. Eng. 2017, 5 (9), 7768–7776. 10.1021/acssuschemeng.7b01270. [DOI] [Google Scholar]
- Monhemi H.; Housaindokht M. R.; Moosavi-Movahedi A. A.; Bozorgmehr M. R. How a Protein Can Remain Stable in a Solvent with High Content of Urea: Insights from Molecular Dynamics Simulation of Candida Antarctica Lipase B in Urea : Choline Chloride Deep Eutectic Solvent. Phys. Chem. Chem. Phys. 2014, 16 (28), 14882–14893. 10.1039/c4cp00503a. [DOI] [PubMed] [Google Scholar]
- Cao J.; Wu R.; Zhu F.; Dong Q.; Su E. Enzymes in Nearly Anhydrous Deep Eutectic Solvents: Insight into the Biocompatibility and Thermal Stability. Enzyme Microb Technol. 2022, 157, 110022. 10.1016/j.enzmictec.2022.110022. [DOI] [PubMed] [Google Scholar]
- Taklimi S. M.; Divsalar A.; Ghalandari B.; Ding X.; Di Gioia M. L.; Omar K. A.; Saboury A. A. Effects of Deep Eutectic Solvents on the Activity and Stability of Enzymes. J. Mol. Liq. 2023, 377, 121562. 10.1016/j.molliq.2023.121562. [DOI] [Google Scholar]
- Yadav N.; Venkatesu P. Current Understanding and Insights towards Protein Stabilization and Activation in Deep Eutectic Solvents as Sustainable Solvent Media. Phys. Chem. Chem. Phys. 2022, 24 (22), 13474–13509. 10.1039/D2CP00084A. [DOI] [PubMed] [Google Scholar]
- Pätzold M.; Siebenhaller S.; Kara S.; Liese A.; Syldatk C.; Holtmann D. Deep Eutectic Solvents as Efficient Solvents in Biocatalysis. Trends Biotechnol 2019, 37 (9), 943–959. 10.1016/j.tibtech.2019.03.007. [DOI] [PubMed] [Google Scholar]
- Huang Z. L.; Wu B. P.; Wen Q.; Yang T. X.; Yang Z. Deep Eutectic Solvents Can Be Viable Enzyme Activators and Stabilizers. J. Chem. Technol. Biotechnol. 2014, 89 (12), 1975–1981. 10.1002/jctb.4285. [DOI] [Google Scholar]
- Niu L.; Jiao K.; Qi Y.; Yiu C. K. Y.; Ryou H.; Arola D. D.; Chen J.; Breschi L.; Pashley D. H.; Tay F. R. Infiltration of Silica Inside Fibrillar Collagen. Angew. Chem. 2011, 123 (49), 11892–11895. 10.1002/ange.201105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkić L. M.; Cepanec I.; Pavelić S. K.; Pavelić K. Biological and Therapeutic Effects of Ortho-Silicic Acid and Some Ortho-Silicic Acid-Releasing Compounds: New Perspectives for Therapy. Nutr Metab 2013, 10 (1), 1–12. 10.1186/1743-7075-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panic M.; Cvjetko Bubalo M.; Radojcic Redovnikovic I. Designing a biocatalytic process involving deep eutectic solvents. J. Chem. Technol. Biotechnol. 2021, 96, 14–30. 10.1002/jctb.6545. [DOI] [Google Scholar]
- Nguyen M. K.; Nguyen V. H.; Natarajan A. K.; Huang Y.; Ryssy J.; Shen B.; Kuzyk A. Ultrathin Silica Coating of DNA Origami Nanostructures. Chem. Mater. 2020, 32 (15), 6657–6665. 10.1021/acs.chemmater.0c02111. [DOI] [Google Scholar]
- Kuzyk A.; Jungmann R.; Acuna G. P.; Liu N. DNA Origami Route for Nanophotonics. ACS Photonics 2018, 5, 1151–1163. 10.1021/acsphotonics.7b01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Jing X.; Liu P.; Pan M.; Liu Z.; Dai X.; Lin J.; Li Q.; Wang F.; Yang S.; Wang L.; Fan C. DNA Framework-Encoded Mineralization of Calcium Phosphate. Chem. 2020, 6 (2), 472–485. 10.1016/j.chempr.2019.12.003. [DOI] [Google Scholar]
- Shang Y.; Li N.; Liu S.; Wang L.; Wang Z. G.; Zhang Z.; Ding B. Site-Specific Synthesis of Silica Nanostructures on DNA Origami Templates. Adv. Mater. 2020, 32 (21), 2000294. 10.1002/adma.202000294. [DOI] [PubMed] [Google Scholar]
- Athanasiadou D.; Carneiro K. M. M. DNA Nanostructures as Templates for Biomineralization. Nat. Rev. Chem. 2021, 5, 93–108. 10.1038/s41570-020-00242-5. [DOI] [PubMed] [Google Scholar]
- Hong F.; Zhang F.; Liu Y.; Yan H. DNA Origami: Scaffolds for Creating Higher Order Structures. Chem. Rev. 2017, 117 (20), 12584–12640. 10.1021/acs.chemrev.6b00825. [DOI] [PubMed] [Google Scholar]
- Dey S.; Fan C.; Gothelf K. v.; Li J.; Lin C.; Liu L.; Liu N.; Nijenhuis M. A. D.; Saccà B.; Simmel F. C.; Yan H.; Zhan P. DNA Origami. Nat. Rev. Methods Primers 2021, 1 (1), 13. 10.1038/s43586-020-00009-8. [DOI] [Google Scholar]
- Nangreave J.; Han D.; Liu Y.; Yan H. DNA Origami: A History and Current Perspective. Curr. Opin Chem. Biol. 2010, 14 (5), 608–615. 10.1016/j.cbpa.2010.06.182. [DOI] [PubMed] [Google Scholar]
- Saccà B.; Niemeyer C. M. DNA Origami: The Art of Folding DNA. Angew. Chem., Int. Ed. 2012, 51, 58–66. 10.1002/anie.201105846. [DOI] [PubMed] [Google Scholar]
- Shen M.-j.; Jiao K.; Wang C.-y.; Ehrlich H.; Wan M.-c.; Hao D.-x.; Li J.; Wan Q.-q.; Tonggu L.; Yan J.-f.; Wang K.-y.; Ma Y.-x.; Chen J.-h.; Tay F. R.; Niu L.-n. Extracellular DNA: A Missing Link in the Pathogenesis of Ectopic Mineralization. Adv. Sci. 2022, 9, 2103693. 10.1002/advs.202103693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Z.; Fujimi T. J.; Nakamura M.; Konishi T.; Yoshimura H.; Aizawa M. Development of a,b-Plane-Oriented Hydroxyapatite Ceramics as Models for Living Bones and Their Cell Adhesion Behavior. Acta Biomater. 2013, 9 (5), 6732–6740. 10.1016/j.actbio.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Londoño-Restrepo S. M.; Jeronimo-Cruz R.; Millán-Malo B. M.; Rivera-Muñoz E. M.; Rodriguez-García M. E. Effect of the Nano Crystal Size on the X-Ray Diffraction Patterns of Biogenic Hydroxyapatite from Human, Bovine, and Porcine Bones OPEN. Sci. Rep 2019, 9, 5915. 10.1038/s41598-019-42269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.; Zhang M.; Song J.; Weber S.; Liu X.; Fan C.; Wu Y. Fine Customization of Calcium Phosphate Nanostructures with Site-Specific Modification by DNA Templated Mineralization. ACS Nano 2021, 15 (1), 1555–1565. 10.1021/acsnano.0c08998. [DOI] [PubMed] [Google Scholar]
- Ding H.; Pan H.; Xu X.; Tang R. Toward a Detailed Understanding of Magnesium Ions on Hydroxyapatite Crystallization Inhibition. Cryst. Growth Des 2014, 14 (2), 763–769. 10.1021/cg401619s. [DOI] [Google Scholar]
- Liu X.; Zhang F.; Jing X.; Pan M.; Liu P.; Li W.; Zhu B.; Li J.; Chen H.; Wang L.; Lin J.; Liu Y.; Zhao D.; Yan H.; Fan C. Complex Silica Composite Nanomaterials Templated with DNA Origami. Nature 2018, 559, 593–598. 10.1038/s41586-018-0332-7. [DOI] [PubMed] [Google Scholar]
- Meyers M. A.; Mckittrick J.; Chen P.-Y. Structural Biological Materials: Critical Mechanics-Materials Connections. Science 2013, 339, 773–779. 10.1126/science.1220854. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Phipps M. L.; Werner J. H.; Chakraborty S.; Martinez J. S. DNA Templated Metal Nanoclusters: From Emergent Properties to Unique Applications. Acc. Chem. Res. 2018, 51 (11), 2756–2763. 10.1021/acs.accounts.8b00366. [DOI] [PubMed] [Google Scholar]
- Gállego I.; Grover M. A.; Hud N. v. Folding and Imaging of DNA Nanostructures in Anhydrous and Hydrated Deep-Eutectic Solvents. Angew. Chem., Int. Ed. 2015, 54 (23), 6765–6769. 10.1002/anie.201412354. [DOI] [PubMed] [Google Scholar]
- Unterlass M. M. Geomimetics and Extreme Biomimetics Inspired by Hydrothermal Systems—What Can We Learn from Nature for Materials Synthesis?. Biomimetics 2017, 2 (2), 8. 10.3390/biomimetics2020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrenko I.; Summers A. P.; Simon P.; Zółtowska-Aksamitowska S.; Motylenko M.; Schimpf C.; Rafaja D.; Roth F.; Kummer K.; Brendler E.; Pokrovsky O. S.; Galli R.; Wysokowski M.; Meissner H.; Niederschlag E.; Joseph Y.; Molodtsov S.; Ereskovsky A.; Sivkov V.; Nekipelov S.; Petrova O.; Volkova O.; Bertau M.; Kraft M.; Rogalev A.; Kopani M.; Jesionowski T.; Ehrlich H. Extreme Biomimetics: Preservation of Molecular Detail in Centimeter-Scale Samples of Biological Meshes Laid down by Sponges. Sci. Adv. 2019, 5 (10), eaax280 10.1126/sciadv.aax2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich H.Extreme Biomimetics; Springer International Publishing: Cham, 2016. [Google Scholar]
- Tsurkan D.; Simon P.; Schimpf C.; Motylenko M.; Rafaja D.; Roth F.; Inosov D. S.; Makarova A. A.; Stepniak I.; Petrenko I.; Springer A.; Langer E.; Kulbakov A. A.; Avdeev M.; Stefankiewicz A. R.; Heimler K.; Kononchuk O.; Hippmann S.; Kaiser D.; Viehweger C.; Rogoll A.; Voronkina A.; Kovalchuk V.; Bazhenov V. v.; Galli R.; Rahimi-Nasrabadi M.; Molodtsov S. L.; Rahimi P.; Falahi S.; Joseph Y.; Vogt C.; Vyalikh D. v.; Bertau M.; Ehrlich H. Extreme Biomimetics: Designing of the First Nanostructured 3D Spongin-Atacamite Composite and Its Application. Adv. Mater. 2021, 33 (30), 2101682. 10.1002/adma.202101682. [DOI] [PubMed] [Google Scholar]
- Wysokowski M.; Motylenko M.; Beyer J.; Makarova A.; Stöcker H.; Walter J.; Galli R.; Kaiser S.; Vyalikh D.; Bazhenov V. v.; Petrenko I.; Stelling A. L.; Molodtsov S. L.; Stawski D.; Kurzydłowski K. J.; Langer E.; Tsurkan M. v.; Jesionowski T.; Heitmann J.; Meyer D. C.; Ehrlich H. Extreme Biomimetic Approach for Developing Novel Chitin-GeO2 Nanocomposites with Photoluminescent Properties. Nano Res. 2015, 8 (7), 2288–2301. 10.1007/s12274-015-0739-5. [DOI] [Google Scholar]
- Wysokowski M.; Jesionowski T.; Ehrlich H. Biosilica as a Source for Inspiration in Biological Materials Science. Am. Mineral. 2018, 103 (5), 665–691. 10.2138/am-2018-6429. [DOI] [Google Scholar]
- Uchida M.; Kang S.; Reichhardt C.; Harlen K.; Douglas T. The Ferritin Superfamily: Supramolecular Templates for Materials Synthesis. Biochimica et Biophysica Acta 2010, 1800 (8), 834–845. 10.1016/j.bbagen.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre D.; Bottger L. H.; Matzanke B. F.; Schuler D. Intracellular Magnetite Biomineralization in Bacteria Proceeds by a Distinct Pathway Involving Membrane-Bound Ferritin and an Iron(II) Species. Angew. Chem., Int. Ed. 2007, 46 (44), 8495–8499. 10.1002/anie.200700927. [DOI] [PubMed] [Google Scholar]
- Ehrlich H.; Luczak M.; Ziganshin R.; Mikšík I.; Wysokowski M.; Simon P.; Baranowska-Bosiacka I.; Kupnicka P.; Ereskovsky A.; Galli R.; Dyshlovoy S.; Fischer J.; Tabachnick K. R.; Petrenko I.; Jesionowski T.; Lubkowska A.; Figlerowicz M.; Ivanenko V. N.; Summers A. P. Arrested in Glass: Actin within Sophisticated Architectures of Biosilica in Sponges. Adv. Sci. 2022, 9, 2105059. 10.1002/advs.202105059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Schloßmacher U.; Wiens M.; Batel R.; Schröder H. C.; Müller W. E. G. Silicateins, Silicatein Interactors and Cellular Interplay in Sponge Skeletogenesis: Formation of Glass Fiber-like Spicules. FEBS J. 2012, 279 (10), 1721–1736. 10.1111/j.1742-4658.2012.08533.x. [DOI] [PubMed] [Google Scholar]
- Shimizu K.; Cha J.; Stucky G. D.; Morse D. E. Silicatein α: Cathepsin L-like Protein in Sponge Biosilica. Proc. Natl. Acad. Sci. U. S. A. 1998, 95 (11), 6234–6238. 10.1073/pnas.95.11.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutchey R. L.; Morse D. E. Silicatein and the Translation of Its Molecular Mechanism of Biosilicification into Low Temperature Nanomaterial Synthesis. Chem. Rev. 2008, 108 (11), 4915–4934. 10.1021/cr078256b. [DOI] [PubMed] [Google Scholar]
- Shimizu K.; Amano T.; Bari M. R.; Weaver J. C.; Arima J.; Mori N. Glassin, a Histidine-Rich Protein from the Siliceous Skeletal System of the Marine Sponge Euplectella, Directs Silica Polycondensation. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 (37), 11449–11454. 10.1073/pnas.1506968112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi M.; Kobayashi H.; Amano T.; Sakate Y.; Bito T.; Arima J.; Shimizu K. Identification of the Domains Involved in Promotion of Silica Formation in Glassin, a Protein Occluded in Hexactinellid Sponge Biosilica, for Development of a Tag for Purification and Immobilization of Recombinant Proteins. Marine Biotechnology 2020, 22 (6), 739–747. 10.1007/s10126-020-09967-2. [DOI] [PubMed] [Google Scholar]
- Ehrlich H.; Wysokowski M.; Jesionowski T. The Philosophy of Extreme Biomimetics. Sustainable Materials and Technologies 2022, 32, e00447 10.1016/j.susmat.2022.e00447. [DOI] [Google Scholar]
- Xu H.; Kong Y.; Peng J.; Wang W.; Li B. Mechanism of Deep Eutectic Solvent Delignification: Insights from Molecular Dynamics Simulations. ACS Sustain Chem. Eng. 2021, 9 (20), 7101–7111. 10.1021/acssuschemeng.1c01260. [DOI] [Google Scholar]
- Smirnov M. A.; Sokolova M. P.; Tolmachev D. A.; Vorobiov V. K.; Kasatkin I. A.; Smirnov N. N.; Klaving A. V.; Bobrova N. V.; Lukasheva N. V.; Yakimansky A. V. Green Method for Preparation of Cellulose Nanocrystals Using Deep Eutectic Solvent. Cellulose 2020, 27 (8), 4305–4317. 10.1007/s10570-020-03100-1. [DOI] [Google Scholar]
- Alizadeh V.; Kirchner B. Molecular Level Insight into the Solvation of Cellulose in Deep Eutectic Solvents. J. Chem. Phys. 2021, 155 (8), 084501. 10.1063/5.0058333. [DOI] [PubMed] [Google Scholar]
- Kumari P.; Kumari M.; Kashyap H. K. How Pure and Hydrated Reline Deep Eutectic Solvents Affect the Conformation and Stability of Lysozyme: Insights from Atomistic Molecular Dynamics Simulations. J. Phys. Chem. B 2020, 124 (52), 11919–11927. 10.1021/acs.jpcb.0c09873. [DOI] [PubMed] [Google Scholar]
- Butler K. T.; Davies D. W.; Cartwright H.; Isayev O.; Walsh A. Machine Learning for Molecular and Materials Science. Nature 2018, 559, 547–555. 10.1038/s41586-018-0337-2. [DOI] [PubMed] [Google Scholar]
- Guo K.; Yang Z.; Yu C. H.; Buehler M. J. Artificial Intelligence and Machine Learning in Design of Mechanical Materials. Mater. Horiz 2021, 8 (4), 1153–1172. 10.1039/D0MH01451F. [DOI] [PubMed] [Google Scholar]
- Shen S. C.-y.; Peña Fernández M.; Tozzi G.; Buehler M. J. Deep Learning Approach to Assess Damage Mechanics of Bone Tissue. J. Mech Behav Biomed Mater. 2021, 123, 104761. 10.1016/j.jmbbm.2021.104761. [DOI] [PubMed] [Google Scholar]
- Kerner J.; Dogan A.; von Recum H. Machine Learning and Big Data Provide Crucial Insight for Future Biomaterials Discovery and Research. Acta Biomater 2021, 130, 54–65. 10.1016/j.actbio.2021.05.053. [DOI] [PubMed] [Google Scholar]
- Hakimi O.; Krallinger M.; Ginebra M. P. Time to Kick-Start Text Mining for Biomaterials. Nature Reviews Materials 2020 5:8 2020, 5 (8), 553–556. 10.1038/s41578-020-0215-z. [DOI] [Google Scholar]
- Suwardi A.; Wang F.; Xue K.; Han M.-Y.; Teo P.; Wang P.; Wang S.; Liu Y.; Ye E.; Li Z.; Loh X. J. Machine Learning-Driven Biomaterials Evolution. Adv. Mater. 2022, 34 (1), 2102703. 10.1002/adma.202102703. [DOI] [PubMed] [Google Scholar]
- Lew A. J.; Yu C. H.; Hsu Y. C.; Buehler M. J. Deep Learning Model to Predict Fracture Mechanisms of Graphene. npj 2D Materi. Appl. 2021, 5 (1), 1–8. 10.1038/s41699-021-00228-x. [DOI] [Google Scholar]
- Shen S. C.; Buehler M. J. Nature-Inspired Architected Materials Using Unsupervised Deep Learning. Commun. Eng. 2022, 1 (1), 37. 10.1038/s44172-022-00037-0. [DOI] [Google Scholar]
- Lew A. J.; Jin K.; Buehler M. J. Designing Architected Materials for Mechanical Compression via Simulation, Deep Learning, and Experimentation. npj Comput. Mater. 2023, 9, 80. 10.1038/s41524-023-01036-1. [DOI] [Google Scholar]
- Buehler M. J. FieldPerceiver: Domain Agnostic Transformer Model to Predict Multiscale Physical Fields and Nonlinear Material Properties through Neural Ologs. Mater. Today 2022, 57, 9–25. 10.1016/j.mattod.2022.05.020. [DOI] [Google Scholar]
- Lew A. J.; Buehler M. J. Single-Shot Forward and Inverse Hierarchical Architected Materials Design for Nonlinear Mechanical Properties Using an Attention-Diffusion Model. Mater. Today 2023, 64, 10–20. 10.1016/j.mattod.2023.03.007. [DOI] [Google Scholar]
- Khare E.; Yu C. H.; Gonzalez Obeso C.; Milazzo M.; Kaplan D. L.; Buehler M. J. Discovering Design Principles of Collagen Molecular Stability Using a Genetic Algorithm, Deep Learning, and Experimental Validation. Proc. Natl. Acad. Sci. U. S. A. 2022, 119 (40), e2209524119 10.1073/pnas.2209524119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L. Y.; Ren G. P.; Hou X. J.; Wu K. J.; He Y. Transition State Theory-Inspired Neural Network for Estimating the Viscosity of Deep Eutectic Solvents. ACS Cent Sci. 2022, 8 (7), 983–995. 10.1021/acscentsci.2c00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D.; Zhou F.; Mu W.; Ling C.; Mu T.; Yu G.; Li R. Deep Insights into the Viscosity of Deep Eutectic Solvents by an XGBoost-Based Model plus SHapley Additive ExPlanation. Phys. Chem. Chem. Phys. 2022, 24 (42), 26029–26036. 10.1039/D2CP03423A. [DOI] [PubMed] [Google Scholar]
- Hsu Y. C.; Yu C. H.; Buehler M. J. Tuning Mechanical Properties in Polycrystalline Solids Using a Deep Generative Framework. Adv. Eng. Mater. 2021, 23 (4), 2001339. 10.1002/adem.202001339. [DOI] [Google Scholar]
- Luu R. K.; Wysokowski M.; Buehler M. J. Generative Discovery of de Novo Chemical Designs Using Diffusion Modeling and Transformer Deep Neural Networks with Application to Deep Eutectic Solvents. Appl. Phys. Lett. 2023, 122 (23), 234103. 10.1063/5.0155890. [DOI] [Google Scholar]