Abstract
Candida dubliniensis is a recently described Candida species associated with oral candidosis in human immunodeficiency virus (HIV)-infected and AIDS patients, from whom fluconazole-resistant clinical isolates have been previously recovered. Furthermore, derivatives exhibiting a stable fluconazole-resistant phenotype have been readily generated in vitro from fluconazole-susceptible isolates following exposure to the drug. In this study, fluconazole-resistant isolates accumulated up to 80% less [3H]fluconazole than susceptible isolates and also exhibited reduced susceptibility to the metabolic inhibitors 4-nitroquinoline-N-oxide and methotrexate. These findings suggested that C. dubliniensis may encode multidrug transporters similar to those encoded by the C. albicans MDR1, CDR1, and CDR2 genes (CaMDR1, CaCDR1, and CaCDR2, respectively). A C. dubliniensis homolog of CaMDR1, termed CdMDR1, was cloned; its nucleotide sequence was found to be 92% identical to the corresponding CaMDR1 sequence, while the predicted CdMDR1 protein was found to be 96% identical to the corresponding CaMDR1 protein. By PCR, C. dubliniensis was also found to encode homologs of CDR1 and CDR2, termed CdCDR1 and CdCDR2, respectively. Expression of CdMDR1 in a fluconazole-susceptible Δpdr5 null mutant of Saccharomyces cerevisiae conferred a fluconazole-resistant phenotype and resulted in a 75% decrease in accumulation of [3H]fluconazole. Northern analysis of fluconazole-susceptible and -resistant isolates of C. dubliniensis revealed that fluconazole resistance was associated with increased expression of CdMDR1 mRNA. In contrast, most studies showed that overexpression of CaCDR1 was associated with fluconazole resistance in C. albicans. Increased levels of the CdMdr1p protein were also detected in fluconazole-resistant isolates. Similar results were obtained with fluconazole-resistant derivatives of C. dubliniensis generated in vitro, some of which also exhibited increased levels of CdCDR1 mRNA and CdCdr1p protein. These results demonstrate that C. dubliniensis encodes multidrug transporters which mediate fluconazole resistance in clinical isolates and which can be rapidly mobilized, at least in vitro, on exposure to fluconazole.
The triazole antifungal drug fluconazole is commonly used to treat oral candidosis and since its introduction has proved effective in the treatment of oral yeast infections. However, recent studies have reported an increasing incidence of resistance to this compound among clinical isolates of Candida albicans from human immunodeficiency virus (HIV)-infected and AIDS patients (14, 15, 25, 31). Furthermore, some evidence suggests that since the introduction of fluconazole, the incidence of infections caused by non-C. albicans species of Candida, including C. glabrata and C. krusei, which are inherently less susceptible to fluconazole, has increased (14, 20, 22, 38, 42).
Candida dubliniensis is a recently described Candida species associated with oral candidosis in HIV-infected and AIDS patients, especially in those with recurrent infections. C. dubliniensis is phylogenetically closely related to C. albicans and has recently been shown to have a worldwide distribution (4, 18, 34–36). In a recent study of Irish subjects, C. dubliniensis was recovered from the oral cavities of 27% of HIV-infected individuals and 32% of AIDS patients presenting with symptoms of oral candidosis (6). The majority of C. dubliniensis clinical isolates tested to date are susceptible to fluconazole (MIC range, 0.125 to 1.0 μg/ml) and to other commonly used antifungal drugs, including ketoconazole, itraconazole, and amphotericin B (9, 17). Based on a limited study, Moran et al. (19) reported the occurrence of fluconazole resistance in 20% of oral isolates (MIC range, 8 to 32 μg/ml) of C. dubliniensis recovered from AIDS patients who had been treated previously with fluconazole. Furthermore, sequential exposure of fluconazole-susceptible clinical isolates of C. dubliniensis to increasing concentrations of fluconazole in agar medium resulted in the recovery of derivatives which expressed a stable fluconazole-resistant phenotype (MIC range, 16 to 64 μg/ml). It has been suggested that the ability of C. dubliniensis to rapidly develop resistance to fluconazole may contribute to its ability to successfully colonize the oral cavities of HIV-infected individuals who are receiving long-term therapy with this compound (19). Furthermore, this may, at least in part, explain the apparent recent emergence of this organism.
Several studies have demonstrated the importance of specific multidrug transporters in the development of fluconazole resistance in C. albicans. Sanglard et al. (30, 31) have shown that three C. albicans proteins, namely the ATP-binding cassette (ABC) transporters Cdr1p and Cdr2p, encoded by the CDR1 and CDR2 genes, respectively, and the major facilitator protein Mdr1p (also known as Benp), encoded by the MDR1 gene, play important roles in reducing the intracellular fluconazole content of fluconazole-resistant C. albicans isolates by a process of active drug efflux. White (39) has also demonstrated the importance of these proteins in fluconazole-resistant C. albicans. In addition, Löffler et al. (17), Sanglard et al. (29), and White (40) have characterized mutations in the cytochrome P-450 lanosterol 14α-demethylase enzyme, the intracellular target of fluconazole. Some of these mutations have been shown to reduce this protein’s affinity for fluconazole in resistant clinical isolates of C. albicans (29, 40).
The objectives of the present study were to investigate the mechanism(s) of fluconazole resistance in C. dubliniensis clinical isolates and in in vitro-generated fluconazole-resistant derivatives. Homologs of the C. albicans CDR1, CDR2, and MDR1 genes were identified in C. dubliniensis. (For maximum clarity, the C. albicans and C. dubliniensis genes, as well as their products, will be given the prefixes Ca and Cd, respectively.) Expression of these genes was examined in fluconazole-susceptible and -resistant clinical isolates of C. dubliniensis in order to characterize their role in fluconazole resistance in this organism.
MATERIALS AND METHODS
C. dubliniensis clinical isolates, in vitro-generated derivatives, and culture conditions.
C. dubliniensis isolates were routinely cultured on potato dextrose agar (Oxoid) medium, pH 5.6, at 37°C. For liquid culture, isolates were grown in yeast extract-peptone-dextrose (YPD) broth at 37°C in an orbital incubator (Gallencamp, Leicester, United Kingdom) at 200 rpm. Many of the C. dubliniensis clinical isolates and in vitro-generated fluconazole-resistant derivatives used in this study (Table 1) were previously described by Moran et al. (19). Also included in the study was the oral isolate CD72 and a series of nine in vitro-generated derivatives of the clinical C. dubliniensis isolate CD57, termed CD57C, CD57D, CD57E, CD57F, CD57G, CD57H, CD57I, CD57J, and CD57K (Table 1). C. dubliniensis CD72 was isolated from an oral swab specimen taken from the mid-dorsum of the tongue of an Irish AIDS patient without clinical symptoms indicative of oral candidosis who had previously been treated with fluconazole for oral candidosis. This specimen yielded 61 yeast colonies on potato dextrose agar, consisting of 52 colonies of C. dubliniensis and 9 colonies of Saccharomyces cerevisiae, which were identified as previously described (19). The derivative series CD57C to CD57K was generated in vitro as described by Moran et al. (19) and traces the origin of the fluconazole-resistant derivative CD57K, which was isolated from a plate containing 50 μg of fluconazole/ml. Initially, a fluconazole-susceptible colony of the C. dubliniensis clinical isolate CD57 (MIC, 0.5 μg/ml) was cultured on YPD agar medium containing 0.5 μg of fluconazole/ml for 48 h at 37°C. Subsequently, this colony was further subcultured on fresh YPD agar medium containing 0.5 μg of fluconazole/ml under the same conditions. This organism was then successively subcultured as described above, twice in each case (first [1°] and second [2°] subcultures), on YPD agar medium containing 1, 5, 10, or 25 μg of fluconazole/ml. The organism was finally subcultured on YPD medium containing 50 μg of fluconazole/ml, from which the derivative termed CD57K was recovered. Colonies were selected from the plates containing fluconazole (at the indicated concentrations) following incubation to obtain the series of derivatives termed CD57C to CD57K (Table 1) as follows: CD57C (1°; 0.5 μg/ml), CD57D (1°; 1.0 μg/ml), CD57E (1°; 5.0 μg/ml), CD57F (2°; 5.0 μg/ml), CD57G (1°; 10.0 μg/ml), CD57H (2°; 10.0 μg/ml), CD57I (1°; 25.0 μg/ml), CD57J (2°; 25.0 μg/ml), and CD57K (1°; 50.0 μg/ml). Series derivatives which exhibited an increase in the fluconazole MIC relative to the that for parental isolate CD57 (i.e., derivatives CD57F to CD57K), as determined by broth microdilution in RPMI–2% (wt/vol) glucose, were subcultured at least 10 times on fluconazole-free medium at 37°C for 48 h to determine if the fluconazole resistance phenotype was stable.
TABLE 1.
Susceptibility of C. dubliniensis clinical isolates and in vitro-generated derivatives to antifungal drugs and metabolic inhibitors
| Isolate or derivative | Source and/or commentsa | MIC (μg/ml)b
|
Reference(s) | |||
|---|---|---|---|---|---|---|
| Fluconazole | Itraconazole | Methotrexate | 4NQO | |||
| CM1 | Patient 1 | 0.5 | 0.03 | 64 | 1 | 19, 36 |
| CM2 | Patient 1 | 32 | 0.12 | >256 | 8 | 19, 36 |
| CD48-I | Patient 2 | 0.5 | 0.03 | 32 | 0.5 | 19 |
| CD48-II | Patient 2 | 0.5 | 0.03 | 32 | 0.5 | 19 |
| CD47-I | Patient 4 | 8 | 0.06 | 128 | 1 | 19 |
| CD47-IIa | Patient 4 | 8 | 0.12 | 128 | 1 | 19 |
| CD47-IIb | Patient 4 | 16 | 0.06 | 128 | 2 | 19 |
| CD72 | Patient 16 | 128 | 0.25 | >256 | 2 | This study |
| CD51-II | Patient 8; parental isolate | 0.25 | 0.015 | 32 | 0.5 | 19 |
| CD51-IIA | Flur derivative of CD51-II | 16 | 0.015 | >256 | 1 | 19 |
| CD51-IIB | Flur derivative of CD51-II | 64 | 0.03 | >256 | 4 | 19 |
| CD51-IIC | Flur derivative of CD51-II | 64 | 0.03 | >256 | 4 | 19 |
| CD57 | Patient 15; parental isolate | 0.5 | 0.12 | 32 | 0.5 | 19 |
| CD57A | Flur derivative of CD57 | 16 | 0.12 | 128 | 1 | 19 |
| CD57B | Flur derivative of CD57 | 32 | 0.25 | >256 | 4 | 19 |
| CD57C | Flus derivative of CD57 | 0.5 | 0.06 | 32 | 0.25 | This study |
| CD57D | Flus derivative of CD57 | 0.5 | 0.06 | 32 | 0.25 | This study |
| CD57E | Flus derivative of CD57 | 0.5 | 0.06 | 32 | 0.25 | This study |
| CD57F | Flur derivative of CD57 | 8 | 0.12 | 128 | 1 | This study |
| CD57G | Flur derivative of CD57 | 8 | 0.12 | 128 | 1 | This study |
| CD57H | Flur derivative of CD57 | 8 | 0.12 | 128 | 1 | This study |
| CD57I | Flur derivative of CD57 | 32 | 0.25 | >256 | 4 | This study |
| CD57J | Flur derivative of CD57 | 32 | 0.25 | >256 | 4 | This study |
| CD57K | Flur derivative of CD57 | 32 | 0.25 | >256 | 4 | This study |
Patient numbers refer to patients in Table 1 of reference 19. C. dubliniensis isolate CD72 is first described here (see Materials and Methods). All isolates are oral isolates from HIV-infected or AIDS patients who were previously treated with fluconazole for oral candidosis, with the exception of CD57, which was isolated from a high vaginal swab specimen from an HIV-negative patient. Flur, fluconazole resistant; Flus, fluconazole susceptible.
MICs were determined by the broth microdilution method as described in Materials and Methods.
Escherichia coli and S. cerevisiae strains, culture media, and growth conditions.
E. coli DH5α was used as the host strain for the phagemid pBluescript II KS(−) (Stratagene, La Jolla, Calif.) and its recombinant derivatives and was maintained on Luria-Bertani (LB) agar (27) containing ampicillin at 100 μg/ml. For liquid culture, E. coli DH5α harboring recombinant plasmids was routinely grown in LB broth containing 100 μg of ampicillin/ml in an orbital incubator (Gallencamp) at 37°C and 200 rpm. Transformation of E. coli DH5α and the identification of transformant derivatives harboring recombinant plasmids were carried out by standard protocols (27). E. coli LE392 and its lysogenic derivative LE392-P2 were used for the propagation of the bacteriophage lambda cloning vector EMBL3 and its recombinant derivatives, respectively, on LB medium supplemented with 10 mM MgSO4 and 0.2% (wt/vol) maltose as described by Sambrook et al. (27).
S. cerevisiae YKKB-13 (MATα ura3-52 lys2-801amber ade2-101ochre trp1-Δ63 his3-Δ200 leu2-1 Δpdr5::TRP1) (31) was routinely cultured on yeast nitrogen base (YNB; Difco) medium supplemented with uracil, lysine, adenine, tryptophan, and histidine (each at 50 mg/ml) and containing 2% (wt/vol) glucose (YNB–2% glucose) at 30°C. YKKB-13 is defective for the ABC transporter Sts1p (also known as Pdr5p) and is hypersusceptible to azole drugs (31).
Chemicals, enzymes, radioisotopes, and oligonucleotides.
Analytical-grade or molecular biology-grade chemicals were purchased from Sigma, BDH (Poole, Dorset, United Kingdom), or Boehringer Mannheim (Lewes, East Sussex, United Kingdom). Enzymes were purchased from the Promega Corporation (Madison, Wis.) or Boehringer Mannheim and used according to the manufacturer’s instructions. [α-32P]dATP (3,000 Ci mmol−1; 110 TBq mmol−1) was purchased from Amersham International Plc. (Little Chalfont, Buckinghamshire, United Kingdom). Fluconazole powder was a gift from Pfizer Pharmaceuticals (Sandwich, Kent, United Kingdom), itraconazole powder was a gift from Janssen Pharmaceuticals (Cork, Republic of Ireland), and amphotericin B was a gift from E. R. Squibb (Swords, Republic of Ireland). All other metabolic inhibitors used for the susceptibility testing of C. dubliniensis isolates and derivatives and S. cerevisiae YKKB-13 and its derivatives were purchased from Sigma, with the exception of terbinafine (Sandoz Pharma, Surrey, United Kingdom) and amorolfine chloride (Hoffman La Roche, Basel, Switzerland). Custom-synthesized oligonucleotides were purchased from Genosys Biotechnologies (Europe) Ltd. (Pampisford, Cambridgeshire, United Kingdom.)
Susceptibility testing procedures.
Fluconazole and itraconazole susceptibility testing of C. dubliniensis clinical isolates and their derivatives was carried out in 96-well microdilution plates (Corning-Costar) in RPMI 1640 medium (Sigma) supplemented with 2% (wt/vol) glucose (RPMI–2% glucose [26]) as described by Moran et al. (19). Amphotericin B susceptibility tests were carried out by the method of Rex et al. (24). Some C. dubliniensis isolates and derivatives were tested for susceptibility to metabolic inhibitors (concentration ranges are shown in parentheses) as follows: benomyl (0.5 to 256 μg/ml), 4-nitroquinoline-N-oxide (4NQO; 0.03 to 16 μg/ml), methotrexate (0.5 to 256 μg/ml), and cycloheximide (0.5 to 256 μg/ml) were tested in 96-well plates, with RPMI–2% glucose as the growth medium, essentially as described for the fluconazole susceptibility testing. Azole drug susceptibility testing of S. cerevisiae YKKB-13 and its derivatives was carried out by the broth microdilution method at 30°C in 96-well plates with YNB–2% glucose as the growth medium. The susceptibility of S. cerevisiae to other metabolic inhibitors was assayed by the method of Sanglard et al. on solid media by incorporating the inhibitors into 15 ml of YPD agar medium contained in 90-mm-diameter petri dishes to achieve the desired drug concentration (30). Yeast inocula for inhibitor susceptibility testing were prepared by harvesting cells by centrifugation from cultures grown for 18 h with shaking (180 rpm) at 30°C in YNB–2% glucose medium and resuspending them in 0.9% (wt/vol) NaCl at a cell density of 2 × 107 CFU/ml. Serial dilutions (1/10) of the suspensions were made in 0.9% (wt/vol) NaCl, and 5-μl volumes of each dilution were spotted onto inhibitor-containing agar plates which were subsequently incubated at 30°C for 24 h. The susceptibility of recombinant-plasmid-harboring S. cerevisiae YKKB-13 to each inhibitor was determined based on the highest dilution of the culture which could grow in the presence of the inhibitor as described previously by Sanglard et al. (30).
Accumulation of [3H]fluconazole in C. dubliniensis isolates.
Accumulation of [3H]fluconazole (Amersham) in C. dubliniensis and S. cerevisiae was assessed by the method of Sanglard et al. (31). All experiments were repeated on two separate occasions.
DNA isolation and Southern hybridization analysis.
Total genomic DNA of C. dubliniensis clinical isolates and derivatives was prepared from cells grown for 18 h in YPD broth cultures, as described by Gallagher et al. (8). Large-scale and small-scale E. coli plasmid DNA preparations were as described by Sambrook et al. (27). Restriction endonuclease-digested DNA was transferred to MagnaGraph nylon membranes (MSI, Westboro, Mass.) as described by Sullivan et al. (33), and hybridization reactions were carried out under high-stringency conditions with DNA probes labelled with [α-32P]dATP by random primer labelling in a rotary hybridization oven (Hybaid, Middlesex, United Kingdom) as described by Sullivan et al. (33).
Yeast chromosomes were prepared as described by Vazquez et al. (37) and separated in 1.3% (wt/vol) agarose gels by using the CHEF-Mapper system (Bio-Rad, Hercules, Calif.) as described by Sullivan et al. (36). Chromosome-sized DNA was transferred to nylon membranes for hybridization analysis by standard Southern blotting techniques (33).
Construction of a C. dubliniensis genomic DNA library.
High-molecular-mass total cellular DNA from C. dubliniensis CD36 was isolated as described by Bennett et al. (3) and was used for the construction of a lambda EMBL3 library. Sau3A-generated partial-digest products of C. dubliniensis CD36 DNA of greater than 10 kb in size were ligated with BamHI-generated prepared lambda bacteriophage replacement vector EMBL3 arms (Promega) and then packaged in vitro, using preprepared phage heads and tails (Promega), according to the manufacturer’s instructions. Following packaging, recombinant phage particles were propagated on the E. coli P2 lysogenic strain LE392-P2 as described by Sambrook et al. (27). A recombinant phage library containing 2 × 105 PFU was obtained.
Recombinant phages were propagated on E. coli LE392 to yield ∼600 to 700 PFU per plate for 10 90-mm-diameter petri dishes and were transferred from the plaques onto nitrocellulose membrane filters (Schleicher and Schuell, Dassel, Germany) by overlaying the plaques with the filters. These were then screened by plaque hybridization (27), using as a probe the α-32P-labelled C. albicans MDR1 gene contained on a 2.9-kb HindIII-BamHI fragment excised from plasmid p2002 (7). The genomic DNA of a recombinant EMBL3 phage, termed φCD1, which hybridized strongly with the probe was purified as described by Sambrook et al. (27). The cloned DNA insert of φCD1 was mapped with restriction endonucleases, and specific fragments were subcloned into pBluescript by conventional methods (27).
DNA sequencing.
DNA sequencing was performed by the dideoxy chain termination method of Sanger et al. (28), using an Applied Biosystems model 370A automated DNA sequencer. Sequencing reactions were carried out with an Applied Biosystems Prism dye terminator cycle sequencing reaction kit. Searches of the EMBL and GenBank databases for nucleotide and amino acid sequence similarities were performed with the BLAST family of computer programs (2).
PCR amplification of C. dubliniensis DNA sequences.
PCR amplification was performed in 100-μl reaction volumes containing 100 pmol each of a forward and reverse primer, 10 mM deoxynucleoside triphosphates (2.5 mM each), 2.5 mM MgCl2, 10 mM Tris (pH 9.0 at 25°C), 50 mM KCl, 0.1% (wt/vol) Triton X-100, 2.5 U of Taq DNA polymerase, and 100 ng of C. dubliniensis genomic DNA. PCRs were performed in a DNA thermal cycler (Perkin-Elmer, Norwalk, Conn.). Reactions were carried out with 35 cycles of denaturation for 1 min at 94°C, primer annealing for 1 min at 55°C, and extension for 2 min at 72°C; this was followed by a final incubation at 72°C for 10 min. For the amplification of the 5′ ends of the CdCDR1 and CdCDR2 genes, the primer sets CdCDR1F-CdCDR1R and CdCDR2F-CdCDR2R (with BamHI restriction sites at the 5′ ends) were designed (Table 2). For the amplification of the entire CdMDR1 gene, the primer set CdMDR1F-CdMDR1R (with HindIII restriction sites at the 5′ ends) (Table 2) was designed based on the nucleotide sequence of the C. dubliniensis CD36 gene determined in this study and the amplification reaction was carried out with a high-fidelity thermostable DNA polymerase (VentR; New England BioLabs, Beverly, Mass.). PCR products were cloned into pBluescript II KS(−) by conventional methods (27). Following digestion with HindIII, the CdMDR1 amplimer was cloned into the HindIII-cleaved S. cerevisiae expression vector plasmid pAAH5 to create recombinant plasmid pGM3. Plasmid pAAH5 contains a unique HindIII restriction site downstream of the S. cerevisiae ADC1 promoter, which allows constitutive expression of cloned sequences from this promoter region in S. cerevisiae (31). Both pAAH5 and pGM3 were used to transform S. cerevisiae YKKB-13 by standard protocols (31).
TABLE 2.
Nucleotide sequences of PCR primers used to amplify specific regions of C. dubliniensis DNA
| Primer | Sequence | Nucleotide coordinatesa | Restriction siteb | Reference |
|---|---|---|---|---|
| CDR1F | 5′-CGGATCCAAGATGAATCTAAATTAG-3′ | 25–43 | BamHI | 23 |
| CDR1R | 5′-CGGATCCTATTTATTTCTTCATGTTC-3′ | 239–257 | BamHI | 23 |
| CDR2F | 5′-CGGATCCGGGTGGATGCACTGGAC-3′ | 50–66 | BamHI | 30 |
| CDR2R | 5′-CGGATCCGCAGAATGGTGATCACC-3′ | 160–176 | BamHI | 30 |
| CdMDR1F | 5′-AAAAGCTTATGCATTACAGATTTTTAAGAG-3′ | 1–22 | HindIII | This study |
| CdMDR1R | 5′-AAAAGCTTCTATTTAGCATATTTCGATCTT-3′ | 1653–1674 | HindIII | This study |
Nucleotide coordinates of the C. albicans or C. dubliniensis gene (where position +1 corresponds to the first base of the ATG translational start codon) on which the nucleotide sequence of the primer is designed. The primer sets CDR1F-CDR1R and CDR2F-CDR2R were designed based on the nucleotide sequence of the C. albicans CDR1 and CDR2 genes, respectively (GenBank accession no. X77589 [CDR1] and U63812 [CDR2]). The CdMDR1F-CdMDR1R primer set was designed based on the nucleotide sequence of the C. dubliniensis CdMDR1 gene determined in this study (Fig. 4).
Restriction endonuclease recognition sequences included in the primer sequences are underlined.
RNA extraction and Northern analysis.
RNA was extracted from C. dubliniensis cultures grown to mid-exponential phase (optical density at 600 nm, 0.6) in 50-ml volumes of YPD broth at 37°C with shaking at 200 rpm in an orbital incubator (Gallenkamp). Extractions were carried out by the glass bead disruption method described by Hube et al. (13). To remove contaminating DNA, 2 volumes of 6 M LiCl was added to each RNA sample, and after incubation of the solutions at −20°C for at least 2 h, they were centrifuged at 11,600 × g. Pelleted RNA was resuspended in diethyl pyrocarbonate-treated water (∼150 μl), and 20-μg quantities, in 5- to 10-μl volumes, were used for electrophoresis in 1.2% (wt/vol) agarose gels containing 6% (vol/vol) formaldehyde as described by Hube et al. (13). RNA was transferred onto MagnaGraph nylon membranes by capillary transfer in 20× SSC buffer (3 M NaCl, 0.3 M trisodium citrate). The RNA was fixed by baking the membranes for 30 min at 80°C followed by UV cross-linking in a Bio-Rad UV cross-linker. Hybridization reactions were performed with a dextran sulfate-containing hybridization solution at 42°C by the method of Sanglard et al. (30). The membranes were then exposed to BioMax MS film (Eastman Kodak Company, Rochester, N.Y.) for 24 to 72 h. All membranes were hybridized with a probe homologous to the C. albicans TEF3 gene, consisting of a 0.7-kb EcoRI-PstI fragment from plasmid pDC1, as described by Hube et al. (13). Relative levels of mRNA expression were measured by using an imaging densitometer (Bio-Rad model GS-670) to scan the hybridization signal intensity on autoradiograms, with the signal intensity of TEF3 mRNA being employed as a loading control.
Extraction and Western blotting of proteins from C. dubliniensis.
Crude protein extracts were prepared from C. dubliniensis isolates and derivatives grown in YNB broth containing 2% (wt/vol) glucose to mid-exponential phase. A 2-ml volume of each culture was harvested by centrifugation at 5,000 × g for 5 min, and each portion of cells was resuspended in 1 ml of sterile distilled H2O. Cells were lysed by the addition of 150 μl of 1.85 M NaOH–7.5% (vol/vol) β-mercaptoethanol and then incubated on ice for 10 min. Protein was precipitated by the addition of 150 μl of ice-cold 50% (vol/vol) trichloroacetic acid and incubation on ice for 10 min; this was followed by centrifugation at 10,000 × g for 5 min at 4°C. Each sample was resuspended in 100 μl of sample buffer (40 mM Tris-HCl, 8 M urea, 5% [wt/vol] sodium dodecyl sulfate, 0.1 mM EDTA, 1% [vol/vol] β-mercaptoethanol, and 0.1 mg of bromophenol blue per ml), incubated for 15 min at 37°C, and then centrifuged as described above to remove cell debris. For electrophoresis, 10-μl volumes (each containing approximately 20 μg of protein) of each sample were loaded on sodium dodecyl sulfate–10% (wt/vol) polyacrylamide gels and electrophoresed in a Mini-PROTEAN II electrophoresis cell (Bio-Rad). Following electrophoresis, proteins were transferred to nitrocellulose membranes by Western blotting, using the Bio-Rad Mini Trans-blot electrophoretic transfer cell in accordance with the manufacturer’s instructions. Immunodetection of proteins was carried out with polyclonal rabbit sera raised against purified glutathione S-transferase-fused N-terminal regions of the C. albicans Mdr1p, Cdr1p, and Cdr2p proteins (28a). Antibody-protein complexes were detected with horseradish peroxidase-conjugated anti-rabbit sera (Sigma). Signals were developed by using the Supersignal chemiluminescent substrate (Pierce). Membranes were exposed to X-ray film (Fuji, Tokyo, Japan) for documentation.
Nucleotide sequence accession number.
The sequence of CdMDR1 has been deposited in the EMBL nucleotide sequence database under accession no. AJ227752.
RESULTS
Susceptibility testing of C. dubliniensis isolates.
All of the C. dubliniensis clinical isolates listed in Table 1, except CD72, were tested previously for their susceptibilities to the azole antifungal drugs fluconazole and itraconazole and the polyene antifungal drug amphotericin B (19) (Table 1). The clinical isolate CM2 from patient no. 1 and the CD47 series of isolates from patient no. 4 all displayed reduced susceptibility to fluconazole (MICs, 8 to 32 μg/ml) (Table 1). No cross-resistance to itraconazole or amphotericin B was observed for these isolates. The C. dubliniensis clinical isolate CD72, first described in this study, was found to display the highest level of fluconazole resistance (MIC, 128 μg/ml) of all the clinical isolates tested here or previously but was not cross resistant to itraconazole or amphotericin B (Table 1). The fluconazole-resistant C. dubliniensis derivatives previously described by Moran et al. (19) (Table 1) also showed reduced susceptibility to fluconazole compared to their respective parental isolates (MICs, 16 to 64 μg/ml) (Table 1). These derivatives were originally generated by culturing their respective fluconazole-susceptible parental isolates successively on agar media containing increasing concentrations of fluconazole. To investigate the development of fluconazole resistance in C. dubliniensis more closely, the fluconazole-susceptible clinical isolate CD57 was cultured on YPD agar containing progressively increasing concentrations of fluconazole (0.5 to 50 μg/ml). The derivative series generated, CD57C to CD57K (Table 1), follows the development of altered levels of fluconazole susceptibility, from the susceptible parental isolate (CD57) through the final fluconazole-resistant derivative, CD57K. The derivatives CD57C to CD57E were found to exhibit the same fluconazole susceptibility as their parental isolate, CD57 (MIC, 0.5 μg/ml); derivatives CD57F to CD57H each exhibited a fluconazole MIC of 8 μg/ml, and derivatives CD57I-K exhibited a fluconazole MIC of 32 μg/ml. No cross-resistance to itraconazole was exhibited by any of the derivative series CD57C to CD57K (Table 1).
Further susceptibility tests were carried out with methotrexate, 4NQO, cycloheximide, and benomyl. Fluconazole-susceptible isolates and derivatives had methotrexate MICs of 32 to 64 μg/ml, whereas isolates with reduced susceptibility to fluconazole (MICs, ≥8 μg/ml) had MICs which were up to fourfold higher (128 to >256 μg/ml) (Table 1). Similarly, resistance to fluconazole was also associated with reduced susceptibility to 4NQO. Isolates with fluconazole MICs of ≥8 μg/ml had 4NQO MICs which were four- to eightfold higher than those for fluconazole-susceptible isolates and derivatives (Table 1). The MICs of cycloheximide were >256 μg/ml for all of the clinical isolates and derivatives tested, with the exception of the clinical isolates CD48-I and CD48-II, each of which had a cycloheximide MIC of 128 μg/ml, and the fluconazole-susceptible parental isolate CD51-II, which had a cycloheximide MIC of 64 μg/ml. Benomyl MICs were found to range between 16 to 32 μg/ml for all the isolates and derivatives tested. No correlation between fluconazole resistance and increased benomyl or cycloheximide MICs was found. These data suggested that fluconazole resistance in C. dubliniensis is associated with cross-resistance to the structurally unrelated compounds methotrexate and 4NQO and therefore would indicate a multidrug-resistant phenotype.
Accumulation of [3H]fluconazole in fluconazole-susceptible and -resistant cells.
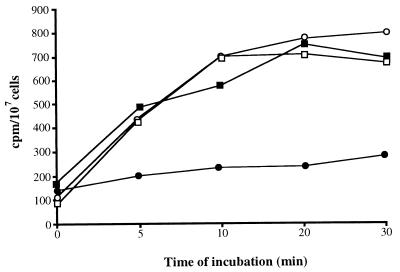
To determine if alterations in cellular permeability to fluconazole could be responsible for fluconazole resistance in C. dubliniensis isolates and derivatives, cells were incubated in the presence of [3H]fluconazole and the intracellular contents of this compound in fluconazole-susceptible and -resistant cells were determined. The clinical isolates CM1 (fluconazole MIC, 0.5 μg/ml) and CM2 (fluconazole MIC, 32 μg/ml) were recovered from the same AIDS patient on two separate occasions, and by using DNA fingerprinting it was shown previously that the two isolates were different strains (Table 1) (19). These isolates were exposed to [3H]fluconazole and examined at different time intervals (Fig. 1). The two isolates were found to differ with regard to fluconazole accumulation. The fluconazole-resistant isolate CM2 was found to accumulate 75% less [3H]fluconazole than the susceptible isolate CM1 following a 20-min incubation (Fig. 1). To determine if this was an active, energy-dependent process, both isolates were exposed to a subinhibitory concentration of sodium azide (NaN3; 0.01 mM), which affects the generation of ATP. NaN3 had little effect on the fluconazole accumulation level of the fluconazole-susceptible isolate CM1; however, CM2 was found to accumulate approximately three times more [3H]fluconazole in the presence of NaN3 than in its absence. These findings indicated that the reduced level of fluconazole accumulation observed in the fluconazole-resistant isolate CM2 was the result of an active, energy-dependent process, similar to that in fluconazole-resistant C. albicans isolates described previously by Sanglard et al. (31).
FIG. 1.
Accumulation of [3H]fluconazole by the C. dubliniensis oral isolates CM1 (fluconazole susceptible [MIC, 0.5 μg/ml]) (▪) and CM2 (fluconazole resistant [MIC, 32 μg/ml]) (•), which were recovered on two successive occasions from the same AIDS patient (Table 1) following treatment with fluconazole. Previous DNA fingerprinting studies demonstrated that the two isolates were different strains (19). Accumulation of [3H]fluconazole in the presence of 0.01 mM NaN3 was also examined in CM1 (□) and CM2 (○).
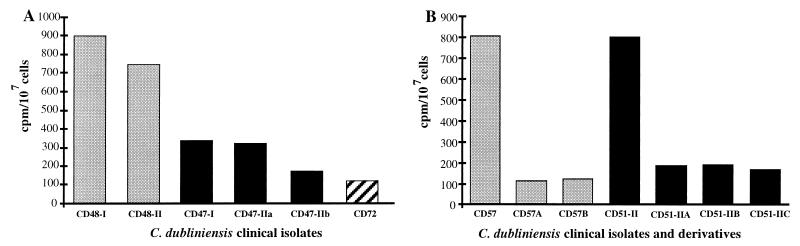
The accumulation of [3H]fluconazole in the remaining clinical isolates and in the in vitro-generated derivatives was also examined (Table 1); however, these isolates were examined only at a single time point, following a 20-min exposure to [3H]fluconazole (Fig. 2). The fluconazole-resistant clinical isolates all showed lower levels of accumulation of [3H]fluconazole than fluconazole-susceptible isolates (Fig. 2). The fluconazole-susceptible clinical isolates CM1, CD48-I, and CD48-II (MICs, 0.5 to 1 μg/ml) gave an average of 800 cpm/107 cells following a 20-min incubation in the presence of [3H]fluconazole. Two isolates from patient no. 4 (CD47-1 and CD47-IIa), which were recovered on two separate occasions and which each had a fluconazole MIC of 8 μg/ml, accumulated approximately 50% less [3H]fluconazole than the susceptible isolates, whereas isolate CD47-IIb from the same patient, with an MIC of 16 μg/ml, accumulated almost 80% less [3H]fluconazole than the susceptible isolates (Fig. 2A). The fluconazole-resistant in vitro-generated derivatives, including derivatives CD57F to CD57K generated in this study, were also found to accumulate up to 80% less fluconazole than their respective fluconazole-susceptible parental isolates, indicating that a similar mechanism(s) may have been responsible for fluconazole resistance in these derivatives and the clinical isolates described above (Fig. 2B).
FIG. 2.
Accumulation of [3H]fluconazole by fluconazole-susceptible and -resistant clinical isolates of C. dubliniensis and in vitro-generated fluconazole-resistant derivatives. Accumulation levels were determined following a 20-min incubation in the presence of [3H]fluconazole. Shown are levels of accumulation of [3H]fluconazole by C. dubliniensis clinical isolates (A) and by the fluconazole-susceptible clinical isolates C. dubliniensis CD57 and CD51-II and their in vitro-generated fluconazole-resistant derivatives CD57A and CD57B and CD51-IIA, CD51-IIB, and CD57-IIC, respectively (B).
Identification of multidrug resistance genes in C. dubliniensis.
To determine if specific multidrug resistance genes could be responsible for fluconazole resistance in the C. dubliniensis clinical isolates and in the vitro-generated fluconazole-resistant derivatives, it was decided to investigate whether genes encoding multidrug transporters, homologous to those present in C. albicans, were present in C. dubliniensis (7, 23, 30). Two pairs of oligonucleotide primers, one of which was complimentary to sequences at the 5′ end of the C. albicans CDR1 gene and the other of which was complimentary to sequences at the 5′ end of the CDR2 multidrug resistance genes, were designed (Table 2); the 5′ end of these genes were previously shown to contain the largest amount of nucleotide sequence divergence (23, 30). Following PCR amplification with template DNA from C. dubliniensis CD36, the CDR1F-CDR1R and CDR2F-CDR2R primer sets in each case yielded single amplimers of approximately 230 and 130 bp, respectively. The nucleotide sequences of the amplimers obtained with the CDR1F-CDR1R and CDR2F-CDR2R primer sets were found to be 91 and 98% identical to the corresponding sequences of the C. albicans CDR1 and CDR2 genes, respectively. These findings suggested that C. dubliniensis encodes homologs of the C. albicans CDR1 and CDR2 multidrug resistance genes, termed CdCDR1 and CdCDR2, respectively.
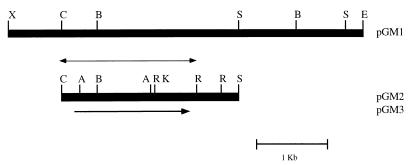
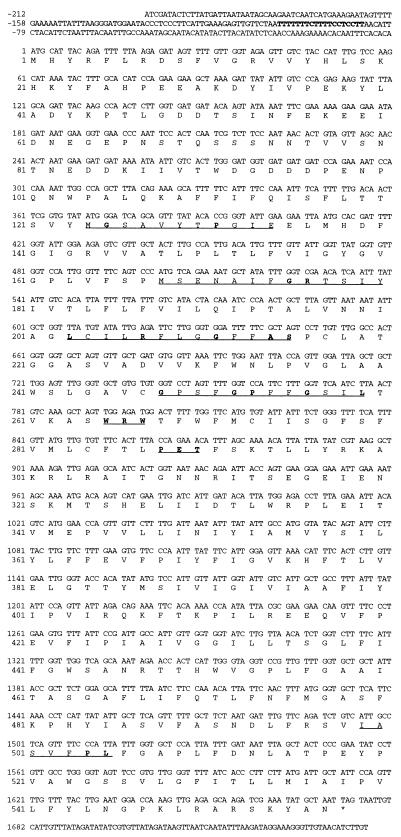
In an attempt to identify a homolog of the C. albicans MDR1 gene in C. dubliniensis, a library of C. dubliniensis genomic DNA cloned in the lambda replacement vector EMBL3 was screened by plaque hybridization with a radioactively labelled probe consisting of the entire C. albicans MDR1 gene. Five reactive plaques were identified, and the phage from the plaque which gave the strongest hybridization signal was chosen for further study and termed φCD1. Phage φCD1 was found to contain a cloned DNA insert of approximately 20 kb, and Southern hybridization analysis of restriction endonuclease-generated fragments of φCD1 DNA with the C. albicans MDR1 gene as a probe identified a strongly hybridizing XbaI-EcoRI insert DNA fragment of 5 kb. This fragment was cloned into pBluescript, and the resulting plasmid was termed pGM1 (Fig. 3). Further restriction endonuclease mapping studies and Southern hybridization analysis with the C. albicans MDR1 gene identified a 2.6-kb ClaI-SpeI fragment within the cloned DNA of pGM1; this was also subcloned in pBluescript to yield plasmid pGM2 (Fig. 3). To identify an open reading frame (ORF), approximately 2 kb of the ClaI-SpeI fragment of pGM2 was sequenced on both strands, corresponding to the region between the ClaI site and the RsaI site, as shown in Fig. 3. Computer analysis of the 1,967-bp ClaI-RsaI fragment of pGM2 revealed the presence of one significant ORF of 1,815 bp with two potential ATG start codons at nucleotide positions −141 and +1 (numbering the sequence in the 5′-to-3′ direction from the first base [+1] of the proposed translation start codon [Fig. 4]). The size of the protein encoded by CdMDR1, as determined in Western blotting experiments, and comparison with the corresponding sequence of CaMDR1 suggested that the actual coding sequence starts at position +1, as shown in Fig. 4. This proposed start codon is preceded by a putative promoter region in the 5′ flanking sequence, including a CT block at nucleotide positions −104 to −86 and an adenine residue at position −3. Although a number of TA-rich regions were present, none matched the transcription initiation consensus TATAA (Fig. 4).
FIG. 3.
Restriction map of CdMDR1-encoding DNA from C. dubliniensis CD36. The black rectangular boxes represent C. dubliniensis genomic DNA. The upper part of the figure shows the 5-kb CdMDR1-encoding EcoRI-XbaI fragment subcloned from recombinant phage φCD1 into vector plasmid pBluescript, yielding recombinant plasmid pGM1. The lower part of the figure shows the 2.6-kb CdMDR1-encoding SpeI-ClaI fragment of pGM1 subcloned into pBluescript, yielding recombinant plasmid pGM2. The thin double-arrowed line represents the 2-kb fragment of pGM2 insert DNA which was sequenced. The single-arrowed line, indicating the position of and showing the direction of transcription of the 1,674-bp ORF encoding the CdMDR1 gene, represents the region which was PCR amplified from pGM2 insert DNA, using a high-fidelity proofreading polymerase, and subcloned into the S. cerevisiae expression vector plasmid pAAH5, yielding recombinant plasmid pGM3. Restriction endonuclease cleavage sites are abbreviated as follows: A, AccI; B, BstxI; C, ClaI; E, EcoRI; K, KpnI; R, RsaI; S, SpeI; and X, XbaI.
FIG. 4.
Nucleotide sequence and deduced amino acid sequence of the C. dubliniensis CdMDR1 gene. Nucleotide sequences are numbered in the 5′-to-3′ direction from the first base (+1) of the ATG translation start codon. Amino acid sequences are numbered from the initial methionine. A putative CT block is shown in boldface at nucleotide positions −104 to −86. Amino acid residues which are underlined show the positions of motifs typical of proteins within the MFS of transporter proteins, and residues shown in boldface are those which match the consensus motif as described by Paulsen et al. (21). These correspond to motif D2 (residues 124 to 134), motif A (residues 168 to 180), motif B (residues 203 to 215), motif C (residues 248 to 259), and motif G (residues 499 to 505). Also underlined are the WRW and PET motifs, at residues 265 to 267 and residues 288 to 290, respectively. The WRW and PET motifs correspond to highly conserved regions in related MFS proteins from S. cerevisiae, although their functions are unknown (11).
This ORF, termed CdMDR1, has the capacity to encode a protein of 557 amino acids with a predicted molecular weight of 62.2 kDa and a pI of 6.4 (Fig. 4). A hydropathy plot generated by the method of Kyte and Doolittle (16) indicates that the structure of the predicted protein encoded by CdMDR1, termed CdMdr1p, is very similar to that of the corresponding C. albicans protein, CaMdr1p, consisting of two halves, each with six putative transmembrane hydrophobic domains, typical of the 12-transmembrane segment (12-TMS) family of drug export proteins within the major facilitator superfamily (MFS) of transporters (11, 21). Also in common with the corresponding C. albicans Mdr1p protein is the presence of a hydrophilic stretch of amino acids near the N terminus.
The C. dubliniensis and C. albicans MDR1 genes are highly homologous, being 92% identical at the nucleotide sequence level, as determined with the CLUSTAL sequence alignment computer program (12). CdMdr1p, at 557 amino acids in length, is 7 amino acids shorter than CaMdr1p (7). Alignment of the amino acid sequences of the two proteins shows that they are highly homologous, being 96.2% identical. Much of the divergence occurs within the hydrophilic N terminus. CaMdr1p contains an asparagine-rich region from amino acid residues 81 to 87 which is partly absent in CdMdr1p (Fig. 5). Most of the remaining amino acid substitutions in CdMdr1p are conservative in nature, the two proteins being 98.7% similar. Interestingly, CdMDR1, like CaMDR1, does not encode a CUG codon.
FIG. 5.
Alignment of the N-terminal amino acid sequence of the Mdr1p protein encoded by the C. albicans MDR1 gene and the corresponding amino acid sequence of the CdMdr1p protein, encoded by the C. dubliniensis CdMDR1 gene, generated with the CLUSTAL sequence alignment program (12). Asterisks indicate identical residues, dots represent similar residues, and colons represent dissimilar residues. Dashes indicate gaps created to obtain alignment.
A number of motifs, described by Paulsen et al. (21), which are conserved within the 12-TMS family of drug export proteins can be identified in the CdMdr1p and CaMdr1p amino acid sequences. Motifs A (CdMdr1p amino acid residues 168 to 180, G x L a D r x G r K x x l, where residues shown in uppercase type are present in at least 70% of aligned sequences analyzed by Paulsen et al. [21] and those shown in lower case are present in approximately 50% of the analyzed sequences) and B (CdMdr1p amino acid residues 203 to 215, l x x x R x x q G g a s) are common throughout the MFS and are believed to play a critical structural role (Fig. 4). Motif A is poorly conserved; however, motif B can be clearly identified in both proteins. Motif C (CdMdr1p amino acid residues 248 to 259, g x x x G P x x G G x l), which is specific for drug transporters within the MFS, is well conserved and may play a role in drug binding or transport. Motifs D2 (CdMdr1p amino acid residues 124 to 134, l g x x x x x P v x P) and G (CdMdr1p amino acid residues 248 to 259; G x x x G P L) are specific to the 12-TMS transporters and are partly conserved. Also present are the WRW and PET motifs at amino acid residues 265 to 267 and 288 to 290, respectively, as described by Goffeau et al. (11). These motifs, found preceding and just after the sixth transmembrane span, respectively, were identified as highly conserved regions in related MFS proteins from S. cerevisiae, although their function is unknown (11).
Southern hybridization analysis of EcoRI-XbaI restriction endonuclease-digested C. dubliniensis CD36 genomic DNA, with the CdMDR1 gene, localized the CdMDR1 gene to a single 5-kb fragment, identical in size to the fragment isolated from the recombinant phage φCD1 (data not shown). Further analysis of ClaI-KpnI-digested CD36 genomic DNA identified a band of approximately 1.4 kb, similar in size to the ClaI-KpnI region of pGM2 as shown in Fig. 3, and a band of 5 kb corresponding to the 3′ end of the gene and its flanking sequences. Southern hybridization analysis of chromosome-sized DNA molecules from CD36 separated by pulsed-field gel electrophoresis with the CdMDR1 gene as a probe showed that the CdMDR1 gene was located on a chromosome of approximately 1.3 Mb in size, which is similar in size to C. albicans chromosome no. 6, which has been reported as the chromosomal location of CaMDR1 (7).
Expression of the C. dubliniensis CdMDR1 gene in S. cerevisiae.
Sanglard et al. (31) demonstrated that expression of the C. albicans MDR1 gene in an azole-susceptible S. cerevisiae strain led to the expression of a fluconazole-resistant phenotype. In the present study, similar experiments were carried out with the CdMDR1 structural gene, using the S. cerevisiae Δpdr5 mutant strain YKKB-13. The S. cerevisiae PDR5 gene is a functional homolog of the C. albicans CDR1 gene, and in S. cerevisiae YKKB-13 the PDR5 deletion renders the organism hypersusceptible to fluconazole. The entire CdMDR1 gene was amplified from CD36, using a high-fidelity thermostable DNA polymerase, with the primer set CdMDR1F-CdMDR1R (Table 2). A single amplification product was obtained, which was cloned into the S. cerevisiae expression vector plasmid pAAH5 via the HindIII restriction endonuclease cleavage sites within the primer sequences, yielding the plasmid pGM3 (Fig. 3). The plasmid pAAH5 contains the promoter for the S. cerevisiae ADC1 gene, which allows for constitutive expression of genes when cloned into this vector in S. cerevisiae. A representative transformant of YKKB-13 harboring pGM3, termed YGM3, was tested for susceptibility to fluconazole and was found to have a fluconazole MIC of 128 μg/ml, whereas the fluconazole MIC for a transformant of YKKB-13 bearing only the vector plasmid pAAH5 (termed YP5) was only 2 μg/ml. No differences in the MICs of itraconazole and ketoconazole were found for YGM3 and YP5. Examination of the fluconazole accumulation levels in YGM3 and YP5 showed that YGM3 accumulated approximately 75% less [3H]fluconazole than YP5. These findings illustrate that CdMDR1 mediated expression of a fluconazole resistance phenotype in S. cerevisiae.
To assess whether CdMDR1 could confer a multidrug resistance phenotype on YGM3, susceptibility to a number of unrelated compounds which are known multidrug transporter substrates was tested. Susceptibility tests were carried out on YPD agar medium as described in Materials and Methods. Transformant YGM3 was found to be less susceptible than YP5 to benomyl, brefeldin A, cerulenin, cycloheximide, fluphenazine, 4-NQO, 1,10-phenanthroline, sulfometuron methyl, and terbinafine (Table 3). This range of substrates is similar to that which has been described for the C. albicans Mdr1p transporter. Reduced susceptibility to amorolfine was also noted in YGM3, which has not been described previously as a substrate for the C. albicans Mdr1p transporter (7, 30).
TABLE 3.
Susceptibility of the S. cerevisiae transformants YP5, harboring vector plasmid pAAH5, and YGM3, harboring pGM3, to metabolic inhibitors
| Inhibitor (concn)a | Susceptibility ofb:
|
|
|---|---|---|
| YP5 | YGM3 | |
| None | 4 | 4 |
| Amorolfine (0.05) | — | 2 |
| Benomyl (50) | 0 | 4 |
| Brefeldin A (25) | 2 | 4 |
| Cerulenin (0.5) | — | 4 |
| Cycloheximide (0.025) | 1 | 4 |
| Fluphenazine (10) | — | 3 |
| 4NQO (0.5) | — | 4 |
| 1,10-Phenanthroline (20) | — | 4 |
| Rhodamine 6G (5) | 0 | 0 |
| Sulfometuron methyl (50) | 1 | 4 |
Parenthetical values are concentrations of inhibitor (in micrograms per milliliter) incorporated into YPD agar medium.
Values refer to the growth on YPD agar of colonies from inocula prepared at various dilutions as described in Materials and Methods. 4, growth at 10−4 dilution; 3, growth at 10−3 dilution; 2, growth at 10−2 dilution; 1, growth at 10−1 dilution; 0, growth at 10−0 (undiluted culture); —, no growth at 10−0, as determined by the method of Sanglard et al. (30).
Analysis of multidrug resistance gene expression in C. dubliniensis.
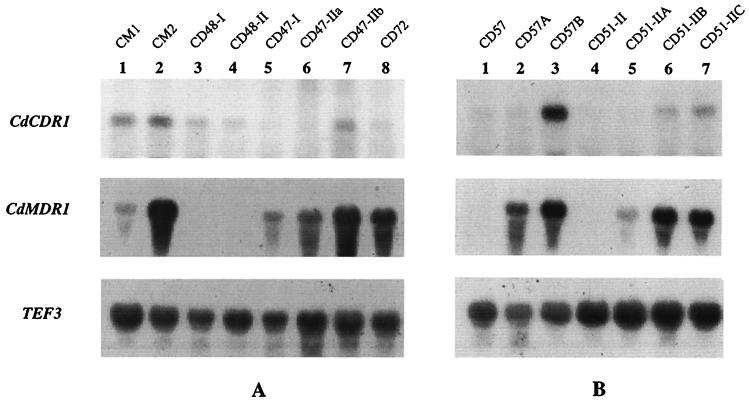
To determine if the fluconazole-resistant clinical isolates and in vitro-generated derivatives of C. dubliniensis exhibited increased expression of the CdCDR1, CdCDR2, or CdMDR1 gene, Northern blot analysis of total cellular RNA was performed. For analysis of CdCDR1 and CdCDR2 expression, the cloned PCR amplimers from C. dubliniensis were used as probes. For analysis of CdMDR1 expression, a 1-kb AccI fragment of the CdMDR1 gene from pGM2 (Fig. 3) was used as a probe. In loading control experiments, RNA was probed with a portion of the C. albicans gene encoding translation elongation factor 3 (TEF3) (13). TEF3 signals were detected from all C. dubliniensis clinical isolates and derivatives tested. The fluconazole-resistant C. dubliniensis derivatives CD57A and CD57B were both found to express increased levels of CdMDR1 mRNA compared to their fluconazole-susceptible parental isolate, CD57 (Fig. 6). Increased levels of CdCDR1 expression were also detected in CD57B (Fig. 6). The fluconazole-resistant C. dubliniensis derivatives CD51-IIA, CD51-IIB, and CD51-IIC also overexpressed CdMDR1 mRNA compared to their fluconazole-susceptible parent, CD51-II (Fig. 6). The derivative series CD57C to CD57K was also examined, and the derivatives CD57C, CD57D, and CD57E, which are fluconazole susceptible (MIC, 0.5 μg/ml), were found to have low or undetectable levels of expression of CdCDR1 and CdMDR1 mRNA. In contrast, the fluconazole-resistant derivatives CD57F, CD57G, and CD57H (MIC, 8 μg/ml) were found to express increased levels of CdMDR1 and CdCDR1 mRNAs, whereas the fluconazole-resistant derivatives CD57I, CD57J, and CD57K (MIC, 32 μg/ml) were found to express four- to fivefold-higher levels of CdMDR1 mRNA than derivatives CD57F to CD57H.
FIG. 6.
Northern analysis of total RNA isolated from C. dubliniensis clinical isolates and in vitro-generated derivatives. For analysis of CdCDR1 expression, the cloned PCR amplimer from C. dubliniensis CD36 was used as a probe. A 1-kb AccI fragment from pGM2 (Fig. 3) was used to probe for CdMDR1 expression. A 0.7-kb EcoRI-PstI fragment from pDC1 encoding a portion of the C. albicans TEF3 gene was used to probe TEF3 expression. Expression of TEF3 was used to control for RNA loading. (A) Total RNA isolated from fluconazole-susceptible and -resistant clinical isolates of C. dubliniensis. (B) Total RNA isolated from fluconazole-susceptible parental isolates and fluconazole-resistant in vitro-generated derivatives.
Northern analysis of clinical isolates showed that low levels of CdMDR1 mRNA were detected in the fluconazole-susceptible isolate CM1 (Table 1); however, CdMDR1 was expressed at approximately 15-fold-higher levels in the fluconazole-resistant isolate CM2, which was recovered from the same patient as CM1 (Fig. 6). CM2 also expressed two times more CdCDR1 mRNA than CM1. In the fluconazole-susceptible clinical isolates CD48-I and CD48-II, the levels of expression of CdCDR1 and CdMDR1 mRNAs were almost undetectable. However, in the CD47 series of isolates from patient no. 4 (Table 1), increased levels of CdMDR1 mRNA were observed. CD47-I and CD47-IIa (fluconazole MIC, 8 μg/ml) both showed relatively high-level expression of this gene, while CD47-IIb (fluconazole MIC, 16 μg/ml) expressed a twofold-higher level (Fig. 6). Some expression of CdCDR1 mRNA was also detected in C. dubliniensis isolate CD47-IIb. CD72, with a fluconazole MIC of 128 μg/ml, also expressed higher levels of CdMDR1 mRNA than the fluconazole-susceptible isolates (Fig. 6). Despite the high fluconazole MIC of CD72, no expression of CdCDR1 was detected. All of the C. dubliniensis clinical isolates and in vitro-generated derivatives were also examined for expression of CdCDR2 mRNA; however, no signals for this gene were detected.
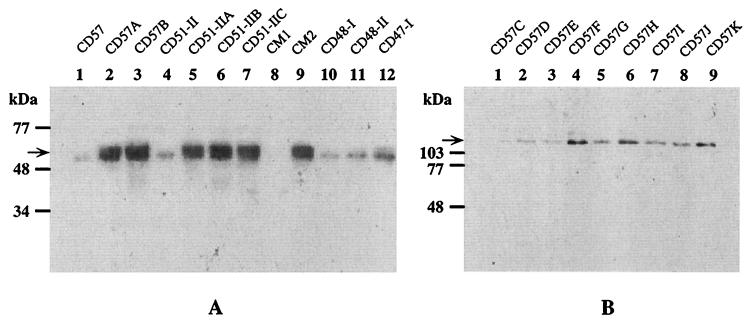
Expression of the translation products of the C. dubliniensis CdMDR1, CdCDR1, and CdCDR2 genes in clinical isolates and in vitro-generated derivatives was also investigated by Western immunoblotting. Due to the high degrees of homology between CaMDR1 and CdMDR1 and between CaCDR1 and CdCDR1 detected in this study it was predicted that rabbit polyclonal antisera raised against the N-terminal fragments of C. albicans Mdr1p, Cdr1p, and Cdr2p would recognize the corresponding C. dubliniensis proteins. Using these antisera, low levels of CdCdr1p were detected in isolates CD57 and CD57A, which also expressed low levels of CdCDR1 mRNA. However, increased protein levels were expressed by CD57B, which also expressed higher levels of CdCDR1 mRNA. Interestingly, in the C. dubliniensis clinical isolate CD51-II and its fluconazole-resistant derivatives CD51-IIA, CD51-IIB, and CD51-IIC, although CdCDR1 mRNA was detectable, no CdCdr1p was detected by immunoblotting. Increased expression of CdCdr1p was also detected in the in vitro-generated fluconazole-resistant derivatives CD57F, CD57G, CD57H, CD57I, CD57J, and CD57K (Fig. 7), which expressed higher levels of CdCDR1 mRNA than the fluconazole-susceptible derivatives from the same series, including CD57C, CD57D, and CD57E. CdCdr1p expression was also detected in the C. dubliniensis clinical isolates. CM2 expressed a twofold-higher level of CdCdr1p than CM1. CD47-I and CD47-IIa both expressed CdCdr1p; however, no CdCdr1p was detected in clinical isolate CD47-IIb from the same patient, despite the observation that noticeable levels of CdCDR1 mRNA were expressed by this isolate.
FIG. 7.
Western immunoblots of protein extracts from C. dubliniensis clinical isolates and in vitro-generated derivatives with polyclonal rabbit sera raised against the N-terminal regions of C. albicans Mdr1p and Cdr1p. (A) Immunodetection of CdMdr1p in Western-blotted protein extracts from C. dubliniensis isolates and derivatives. The arrow on the left of the panel shows the position of CdMdr1p. A slightly lower-molecular-mass protein reacted with the anti-Mdr1p antiserum used and was detectable as a noticeably weaker band in Western blots of extracts from the majority of isolates and derivatives tested. (B) Immunodetection of CdCdr1p in Western-blotted protein extracts of the in vitro-generated derivative series CD57C to CD57K. The positions of molecular size reference markers are indicated on the left of the panels. Arrows indicate the position of CdCdr1p.
CdMdr1p expression studies revealed that CdMdr1p was not detected in any of the fluconazole-susceptible isolates or derivatives in which no expression of CdMDR1 mRNA was detected (Fig. 7). However, in fluconazole-resistant clinical isolates and in fluconazole-resistant in vitro-generated derivatives, all of which expressed CdMDR1 mRNA, increased levels of CdMdr1p were detected (Fig. 7). No expression of CdCdr2p was detected by Western immunoblotting in any of the clinical isolates or in vitro-generated derivatives, which is consistent with the absence of CdCDR2 mRNA in these isolates.
DISCUSSION
We previously reported the recovery from AIDS patients of oral C. dubliniensis isolates with reduced susceptibility to fluconazole and the rapid generation of stable fluconazole-resistant derivatives of them in vitro (19). The purpose of the present study was to investigate the molecular basis of this resistance phenotype in these isolates and derivatives. In addition to confirming their reduced susceptibility to fluconazole, we have also shown that these isolates and derivatives exhibit decreased susceptibility to methotrexate and 4NQO, suggesting a multidrug resistance phenotype. Analysis of [3H]fluconazole accumulation by these organisms indicated that fluconazole-resistant isolates and in vitro-generated derivatives with fluconazole MICs of ≥16 μg/ml accumulated up to 80% less drug than fluconazole-susceptible isolates. Clinical isolates with intermediate levels of fluconazole susceptibility (i.e., an MIC of 8 μg/ml) accumulated approximately 50% less [3H]fluconazole than susceptible isolates, indicating a good correlation between susceptibility and accumulation levels. When accumulation levels were examined in the presence of NaN3, the fluconazole-resistant isolate CM2 showed a marked increase in the accumulation of [3H]fluconazole, indicating that the fluconazole-resistant phenotype is the result of an active, energy-dependent process. A similar resistance phenotype has been demonstrated to be associated with overexpression of the multidrug transporter genes CDR1, CDR2, and MDR1 in C. albicans (1, 30, 31, 39). These findings and the multidrug resistance phenotype exhibited by fluconazole-resistant C. dubliniensis prompted us to examine C. dubliniensis for the presence of homologs of these genes.
By PCR, we identified sequences in C. dubliniensis which are homologous to the C. albicans CDR1 and CDR2 genes; furthermore, we have cloned a gene from a library of C. dubliniensis genomic DNA, termed CdMDR1, which is highly homologous to CaMDR1. The C. dubliniensis MDR1 gene represents the first complete protein-encoding ORF to be determined from this species. Although highly similar to its counterpart in C. albicans, CdMDR1 exhibits divergence (8%) at the nucleotide sequence level, which is further supporting evidence that C. dubliniensis represents a unique taxon within the genus Candida (10, 36). At the amino acid sequence level, CdMdr1p is highly homologous to CaMdr1p, the proteins being 96.2% identical. Most of the differences in amino acid sequence are present in the hydrophilic N-terminal region, which is not thought to form a transmembrane loop in the 12-TMS family of drug export proteins (7, 21). The possible function of this region and its role, if any, in drug efflux have not been examined for CaMdr1p. Both proteins have a predicted topology consistent with that of the 12-TMS family of MFS drug exporters, and a number of amino acid sequence motifs specific for this family of proteins are present within the two proteins (Fig. 4), providing evidence that they are involved in drug efflux.
To demonstrate that CdMDR1 can confer resistance to fluconazole, the gene was cloned into the S. cerevisiae expression vector pAAH5, yielding plasmid pGM3 (Fig. 3). pGM3 was transformed into S. cerevisiae YKKB-13, which is fluconazole susceptible due to the deletion of the gene encoding the ABC transporter Pdr5p. Transformants harboring pGM3 exhibited a fluconazole MIC of 128 μg/ml and accumulated 75% less [3H]fluconazole than YKKB-13 transformants harboring only the vector (MIC, 2.0 μg/ml). No difference in itraconazole or ketoconazole susceptibility was observed for the two types of transformants. S. cerevisiae harboring pGM3 also exhibited reduced susceptibility to a number of other compounds, including 4NQO, which suggests that CdMDR1 may be responsible for the multidrug-resistant phenotype of fluconazole-resistant C. dubliniensis isolates and their derivatives.
Involvement of the C. dubliniensis MDR1, CDR1, and CDR2 genes in the fluconazole resistance of clinical isolates and in vitro-generated derivatives was examined by probing for their expression in Northern and Western blots with polyclonal sera raised against the N-terminal regions of CaMdr1p, CaCdr1p, and CaCdr2p. All of the C. dubliniensis clinical isolates with reduced susceptibility to fluconazole were found to express increased levels of CdMDR1 mRNA and CdMdr1p, whereas in fluconazole-susceptible isolates, the levels of expression of CdMDR1 mRNA and CdMdr1p were low or absent (Table 4). These findings directly reflect previous findings with the C. albicans MDR1 gene, which was shown to confer a fluconazole-specific azole resistance phenotype when expressed in S. cerevisiae (31). Interestingly, CdCdr1p was not detected in CD47-IIb, which showed significant levels of CdCDR1 mRNA. Isolate CD72 was found to have a fluconazole MIC of 128 μg/ml; however, it expressed CdMDR1 mRNA at levels similar to those of clinical isolates with fluconazole MICs of 16 to 32 μg/ml. In addition, CD72 expressed very low levels of CdCDR1 mRNA. White (40), Löffler et al. (17), and Sanglard et al. (29) have all reported that mutations in the cytochrome P-450 lanosterol 14α-demethylase enzyme can be associated with fluconazole resistance. We have not yet examined the possibility that similar mutations are involved in fluconazole resistance in the C. dubliniensis clinical isolates studied here, but it is possible that a mutation(s) in the cytochrome P-450 lanosterol 14α-demethylase enzyme could contribute to the fluconazole-resistant phenotype, particularly in CD72. It is also possible that an additional multidrug transporter is involved.
TABLE 4.
Summary of resistance mechanisms observed in fluconazole-resistant C. dubliniensis isolates and derivatives
| Isolate or derivative | Relative (fold) increase in MIC of:
|
Relative (fold) increase in mRNA ofa:
|
||
|---|---|---|---|---|
| Fluconazole | Itraconazole | CdMDR1 | CdCDR1 | |
| CM2 | 64 | 4 | 15 | 2 |
| CD47-Ib | 16 | 1 | 9 | 1 |
| CD47-IIab | 16 | 2 | 10 | 1 |
| CD47-IIbb | 32 | 1 | 25 | 3c |
| CD72b | 256 | 4 | 20 | 1 |
| CD51-IIA | 64 | 1 | 5 | 1 |
| CD51-IIB | 256 | 2 | 16 | 2c |
| CD51-IIC | 256 | 2 | 15 | 2c |
| CD57A | 32 | 1 | 14 | 1 |
| CD57B | 64 | 2 | 17 | 5 |
| CD57F | 16 | 2 | 4 | 2 |
| CD57G | 16 | 2 | 5 | 2 |
| CD57H | 16 | 2 | 4 | 2 |
| CD57I | 64 | 4 | 20 | 4 |
| CD57J | 64 | 4 | 20 | 4 |
| CD57K | 64 | 4 | 25 | 4 |
Determined by laser densitometry of Northern blot mRNA signals on autoradiograms.
Baseline fluconazole and itraconazole MICs of 0.5 and 0.06 μg/ml, respectively, were chosen.
Increased expression of CDR1 mRNA was not correlated with increased levels of CdCdr1p.
Although only a relatively small number of fluconazole-resistant C. dubliniensis and C. albicans isolates have been examined so far, the patterns of expression of the CaMDR1 and CaCDR1 genes and their homologs in C. dubliniensis appear to differ in the two species. Two recent studies reported that increased expression of CDR1 was the primary mechanism of fluconazole resistance in C. albicans clinical isolates (1, 31), while it appears that increased expression of CdMDR1 is the main mechanism involved in C. dubliniensis clinical isolates. Expression of CDR2 has also been reported in some fluconazole-resistant C. albicans isolates; however, no expression of CdCDR2 mRNA or CdCdr2p was detected in the C. dubliniensis isolates and derivatives described here.
Examination of the CdMDR1 mRNA expression levels in the in vitro-generated fluconazole-resistant derivatives recovered from the fluconazole-susceptible isolates CD51-II and CD57 yielded results similar to those obtained for the fluconazole-resistant clinical isolates (Table 4). All of the fluconazole-resistant derivatives were found to overexpress the CdMDR1 gene and CdMdr1p, whereas very low levels of expression were observed with the isogenic fluconazole-susceptible parental isolates. Three separate events could be identified during the development of fluconazole resistance in this series of derivatives (CD57C to CD57K), the first two being increases in expression of CdMDR1 and CdCDR1 mRNAs, correlating with an increase in the fluconazole MIC to 8 μg/ml, and the second, occurring subsequently, being further overexpression of CdMDR1, associated with a fluconazole MIC of 32 μg/ml (Table 4). White (39) reported a similar series of events, in a series of clinical isolates of C. albicans from a single patient, in which a number of separate events, including overexpression of the CDR1 and CaMDR1 genes, led to the development of fluconazole resistance.
By passage of a susceptible isolate in fluconazole-containing broth cultures, Albertson et al. (1) isolated a fluconazole-resistant mutant of C. albicans which was found to express increased levels of the CaMDR1 gene. Calvet et al. (5) also isolated unstable fluconazole-resistant mutants of C. albicans by similar means, although no involvement of the MDR1 or CDR1 gene was observed. In our experience, exposure of C. albicans to fluconazole in agar medium did not lead to a stable change in fluconazole susceptibility, and others have also reported difficulties in using solid media for this purpose (5). However, fluconazole-resistant derivatives of susceptible C. dubliniensis isolates which overexpress CaMDR1 and, in some cases, CdCDR1 mRNA can be readily generated on fluconazole-containing agar medium. The fluconazole-resistant phenotype of these derivatives is stable in the absence of fluconazole; it appears to be due to a heritable genetic change(s) rather than to transient stress-activated transcription of multidrug transporter genes. The genomic DNA fingerprinting and karyotype profiles of some in vitro-generated fluconazole-resistant derivatives of C. dubliniensis have been found to differ from those of their fluconazole-susceptible parental isolates (19). It is possible that alterations of sequences flanking drug transporter genes or trans-acting factors influence their rates or regulation of transcription (41).
The ability of C. dubliniensis to rapidly develop fluconazole resistance in vitro may have implications for antifungal resistance in vivo. If the development of fluconazole resistance in C. dubliniensis in vitro correlates with the development of fluconazole resistance in vivo, it may prove to be a useful model system for studying the mechanisms involved in the development of fluconazole resistance in a clinical context. It is still unknown why C. dubliniensis has emerged, apparently, only in recent years. However, the appearance of C. dubliniensis shortly after the widespread introduction of fluconazole for the treatment of oral candidosis in HIV-infected and AIDS patients, particularly in patients with recurrent infection, may be correlated. Perhaps the ability of C. dubliniensis to rapidly switch on expression of the CdMDR1 gene enables this organism to persist in the oral cavities of patients undergoing fluconazole therapy. However, it is important to note that not all HIV-infected and AIDS patients undergoing fluconazole therapy who are colonized with C. dubliniensis yield fluconazole-resistant isolates, a situation similar to that observed with C. albicans (15, 24). The development of fluconazole resistance may depend on the dosage of drug administered, the duration of therapy, or the immune status of the patient, as has been observed in the case of C. albicans infection (15, 25). In addition, few investigators have examined how Candida species respond to fluconazole exposure in vivo, and although we observed stable changes in C. dubliniensis multidrug resistance gene expression in vitro, it is not known whether exposure to fluconazole in vivo could lead to changes in multidrug resistance gene expression. Schoofs et al. (32) demonstrated that in vivo populations of C. albicans can consist of a large number of subtypes which differ in their relative susceptibility to antifungal agents, and the authors suggested that exposure to fluconazole in vivo could lead to selection of such fluconazole-resistant subtypes. Clearly, in vivo Candida populations, including those of C. dubliniensis, may have the ability to respond in a dynamic fashion to antifungal therapy, a response which may involve changes in gene expression or the relative abundance of yeast species in the population. To determine the extent and nature of fluconazole resistance in populations of C. dubliniensis, epidemiological studies are currently continuing to follow the progress of C. dubliniensis-colonized patients who are undergoing fluconazole therapy.
ACKNOWLEDGMENTS
Work performed in the laboratory of D.C.C. was supported by a grant from the Wellcome Trust (no. 047204). G.P.M. was supported by the School of Dental Science and Dublin Dental Hospital, Trinity College Dublin, and by a short-term EMBO fellowship (no. ASTF 8887). D.S. was supported by a grant from the Swiss Research National Foundation.
We thank B. B. Magee for the gift of plasmid p2002 and B. Hube for plasmid pDC1.
REFERENCES
- 1.Albertson G D, Niimi M, Cannon R D, Jenkinson H F. Multiple efflux mechanisms are involved in Candida albicans fluconazole resistance. Antimicrob Agents Chemother. 1996;40:2835–2841. doi: 10.1128/aac.40.12.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Bennett D E, McCreary C E, Coleman D C. Genetic characterisation of a phospholipase C gene from Candida albicans: presence of homologous sequences in Candida species other than C. albicans. Microbiology. 1998;144:55–72. doi: 10.1099/00221287-144-1-55. [DOI] [PubMed] [Google Scholar]
- 4.Boerlin P, Boerlin-Petzold F, Durussel C, Addo M, Pagani J-L, Chave J-P, Bille J. Cluster of oral atypical Candida albicans isolates in a group of human immunodeficiency virus-positive drug users. J Clin Microbiol. 1995;33:1129–1135. doi: 10.1128/jcm.33.5.1129-1135.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calvet H M, Yeaman M R, Filler S G. Reversible fluconazole resistance in Candida albicans: a potential in vitro model. Antimicrob Agents Chemother. 1997;41:535–539. doi: 10.1128/aac.41.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman D C, Sullivan D J, Bennett D E, Henman M C, Moran G P, Barry H J, Shanley D B. The emergence of Candida dubliniensis: a novel Candida species associated with oral candidiasis in individuals infected with HIV and with AIDS. AIDS. 1997;11:557–567. doi: 10.1097/00002030-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Fling M E, Kopf J, Tamarkin A, Gorman J A, Smith H A, Koltin Y. Analysis of a Candida albicans gene that encodes a novel mechanism for resistance to benomyl and methotrexate. Mol Gen Genet. 1991;227:318–329. doi: 10.1007/BF00259685. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher P J, Bennett D E, Henman M C, Russell R J, Flint S R, Shanley D B, Coleman D C. Reduced azole susceptibility of Candida albicans from HIV-positive patients and a derivative exhibiting colony morphology variation. J Gen Microbiol. 1992;138:1901–1911. doi: 10.1099/00221287-138-9-1901. [DOI] [PubMed] [Google Scholar]
- 9.Genetics Computer Group. Program manual for the GCG package, version 8.0, September 1994. Madison, Wis: Genetics Computer Group; 1994. [Google Scholar]
- 10.Gilfillan G D, Sullivan D J, Haynes K, Parkinson T, Coleman D C, Gow N A R. Candida dubliniensis: phylogeny and putative virulence factors. Microbiology. 1998;144:829–838. doi: 10.1099/00221287-144-4-829. [DOI] [PubMed] [Google Scholar]
- 11.Goffeau A, Park J, Paulsen I T, Jonniaux J-L, Dinh T, Mordant P, Saier M H., Jr Multidrug-resistant transport proteins in yeast: complete inventory and phylogenetic characterisation of yeast open reading frames within the major facilitator superfamily. Yeast. 1997;13:43–54. doi: 10.1002/(SICI)1097-0061(199701)13:1<43::AID-YEA56>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 12.Higgins D G, Sharp P M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1998;73:237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- 13.Hube B, Monod M, Schofield D A, Brown A J P, Gow N A R. Expression of seven members of the gene family encoding secretory aspartyl proteinases in Candida albicans. Mol Microbiol. 1994;14:87–99. doi: 10.1111/j.1365-2958.1994.tb01269.x. [DOI] [PubMed] [Google Scholar]
- 14.Johnson E M, Warnock D W. Azole drug resistance in yeasts. J Antimicrob Chemother. 1995;36:751–755. doi: 10.1093/jac/36.5.751. [DOI] [PubMed] [Google Scholar]
- 15.Klepser M E, Ernst E J, Pfaller M A. Update on antifungal resistance. Trends Microbiol. 1997;5:372–375. doi: 10.1016/S0966-842X(97)01108-6. [DOI] [PubMed] [Google Scholar]
- 16.Kyte J, Doolittle R. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 17.Löffler J, Kelly S L, Hebart H, Schumacher U, Lass-Flörl C, Einsele H. Molecular analysis of cyp51 from fluconazole resistant Candida albicans isolates. FEMS Microbiol Lett. 1997;151:263–268. doi: 10.1111/j.1574-6968.1997.tb12580.x. [DOI] [PubMed] [Google Scholar]
- 18.McCullough M, Ross B, Reade P. Characterization of genetically distinct subgroup of Candida albicans strains isolated from oral cavities of patients infected with human immunodeficiency virus. J Clin Microbiol. 1995;33:696–700. doi: 10.1128/jcm.33.3.696-700.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moran G P, Sullivan D J, Henman M C, McCreary C E, Harrington B J, Shanley D B, Coleman D C. Antifungal drug susceptibilities of oral Candida dubliniensis isolates from human immunodeficiency virus (HIV)-infected and non-HIV-infected subjects and generation of stable fluconazole-resistant derivatives in vitro. Antimicrob Agents Chemother. 1997;41:617–623. doi: 10.1128/aac.41.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen M H, Peacock J E, Jr, Morris A J, Tanner D C, Nguyen M L, Snydman D R, Wagener M M, Rinaldi M G, Yu V L. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am J Med. 1996;100:617–623. doi: 10.1016/s0002-9343(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 21.Paulsen I T, Brown M H, Skurray R A. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfaller, M. A. 1996. Nosocomial candidiasis: emerging species, reservoirs, and modes of transmission. Clin. Infect. Dis. 22(Suppl. 2):S89–S94. [DOI] [PubMed]
- 23.Prasad R, De Wergifosse P, Goffeau A, Balzi E. Molecular cloning and characterisation of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr Genet. 1995;27:320–329. doi: 10.1007/BF00352101. [DOI] [PubMed] [Google Scholar]
- 24.Rex J H, Cooper C R, Jr, Merz W G, Galgiani J N, Anaissie E J. Detection of amphotericin B-resistant Candida isolates in a broth-based system. Antimicrob Agents Chemother. 1995;39:906–909. doi: 10.1128/aac.39.4.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rex J H, Rinaldi M G, Pfaller M A. Resistance of Candida species to fluconazole. Antimicrob Agents Chemother. 1995;39:1–8. doi: 10.1128/aac.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Tudela J L, Martinez-Suárez J V. Defining conditions for microbroth antifungal susceptibility tests: influence of RPMI and RPMI-2% glucose on the selection of endpoint criteria. J Antimicrob Chemother. 1995;35:739–749. doi: 10.1093/jac/35.6.739. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 28.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Sanglard, D. Unpublished data.
- 29.Sanglard D, Ischer F, Kogmans L, Bille J. Amino acid substitutions in the cytochrome P-450 lanosterol 14α-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob Agents Chemother. 1998;42:241–253. doi: 10.1128/aac.42.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanglard D, Ischer F, Monod M, Bille J. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterisation of CDR2, a new multidrug ABC transporter gene. Microbiology. 1997;143:405–416. doi: 10.1099/00221287-143-2-405. [DOI] [PubMed] [Google Scholar]
- 31.Sanglard D, Kuchler K, Ischer F, Pagani J-L, Monod M, Bille J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother. 1995;39:2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoofs A, Odds F C, Colebunders R, Ieven M, Wouters L, Goosens H. Isolation of Candida species on media with and without added fluconazole reveals high variability in relative growth susceptibility phenotypes. Antimicrob Agents Chemother. 1997;41:1625–1635. doi: 10.1128/aac.41.8.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan D, Bennett D, Henman M, Harwood P, Flint S, Mulcahy F, Shanley D, Coleman D. Oligonucleotide fingerprinting of isolates of Candida species other than C. albicans and of atypical Candida species from human immunodeficiency virus-positive and AIDS patients. J Clin Microbiol. 1993;31:2124–2133. doi: 10.1128/jcm.31.8.2124-2133.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sullivan D, Haynes K, Bille J, Boerlin P, Rodero L, Lloyd S, Henman M, Coleman D. Widespread geographic distribution of oral Candida dubliniensis strains in human immunodeficiency virus-infected individuals. J Clin Microbiol. 1997;35:960–964. doi: 10.1128/jcm.35.4.960-964.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sullivan D J, Coleman D C. Candida dubliniensis: characteristics and identification. J Clin Microbiol. 1998;36:329–334. doi: 10.1128/jcm.36.2.329-334.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sullivan D J, Westerneng T J, Haynes K A, Bennett D E, Coleman D C. Candida dubliniensis sp. nov.: phenotypic and molecular characterisation of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology. 1995;141:1507–1521. doi: 10.1099/13500872-141-7-1507. [DOI] [PubMed] [Google Scholar]
- 37.Vazquez J A, Beckley A, Sobel J D, Zervos M J. Comparison of restriction enzyme analysis and pulsed-field gradient gel electrophoresis as typing systems for Candida albicans. J Clin Microbiol. 1991;29:962–967. doi: 10.1128/jcm.29.5.962-967.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warnock D W. Azole drug resistance in Candida species. J Med Microbiol. 1992;37:225–226. doi: 10.1099/00222615-37-4-225. [DOI] [PubMed] [Google Scholar]
- 39.White T C. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1997;41:1482–1487. doi: 10.1128/aac.41.7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White T C. Presence of an R467K amino acid substitution and loss of allelic variation correlate with an azole-resistant lanosterol 14α demethylase in Candida albicans. Antimicrob Agents Chemother. 1997;41:1488–1494. doi: 10.1128/aac.41.7.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wickes B L, Petter R. Genomic variation in C. albicans. Curr Top Med Mycol. 1996;7:71–86. [PubMed] [Google Scholar]
- 42.Wingard J R, Merz W G, Rinaldi M G, Johnson T R, Karp J E, Saral R. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N Engl J Med. 1991;325:1274–1277. doi: 10.1056/NEJM199110313251803. [DOI] [PubMed] [Google Scholar]