Abstract
Acinic cell carcinoma (AciCC) is traditionally considered as a low-grade salivary gland carcinoma. However, a subset demonstrates high grade features with higher mortality rate and distant metastasis. In this large retrospective study of 117 cases, we aimed to establish a histologic grading scheme for AciCC. Adverse independent prognostic factors identified on multivariate analysis included older age, tumor necrosis, nuclear anaplasia, lymphovascular invasion, absence of tumor-associated lymphoid stroma, and high AJCC pT and pN stages. A 3-tiered grading scheme using four pathologic parameters (mitotic index, necrosis, tumor border, and fibrosis at the frankly invasive front) was subsequently applied. Compared to low/intermediate-grade, high-grade AciCC defined as mitotic index ≥5/10 high power fields and/or necrosis was an independently adverse prognostic factor. The 5-year overall survival was 50% in high-grade AciCCs, and 100% in low or intermediate-grade AciCCs. Compared with low- or intermediate-grade AciCC, high-grade tumors were associated with older age, larger tumor size, focal rather than diffuse zymogen granules, solid architecture, infiltrative tumor border, fibrosis at the frankly invasive front, lymphovascular invasion, perineural invasion, positive margin, high pT and pN stages. NR4A3 was a highly sensitive and specific immunohistochemical stain for diagnosing AciCC with a sensitivity and specificity of 96% and 93% respectively. In conclusion, although we proposed a two-tiered grading system for AciCC with high grade tumors defined by a mitotic count ≥5/10 high power fields and/or necrosis, more studies are needed to assess the prognostic value of intermediate grade. NR4A3 immunohistochemical stain is a useful diagnostic marker for AciCC.
Keywords: Acinic cell carcinoma, prognosis, NR4A3, histologic grade
Introduction
Acinic cell carcinoma (AciCC) is one of the common types of malignant salivary gland tumors, accounting for 11% of all salivary gland malignancies (1–3). The tumor is characterized by cells with acinic differentiation, containing zymogen granules, although various proportion of tumor cells with vacuolated, eosinophilic, and clear cell features are often also present (2, 3).
AciCC is traditionally considered as a low-grade salivary gland carcinoma with a reported 5-year overall survival (OS) of 83% to 97% and 5-year disease specific survival of 91% based on data on the Surveillance, Epidemiology, and End Results (SEER) and National Cancer Database (NCDB) (1, 4–6). In 1988, Stanley et al. first described high grade transformation (HGT, initially named as dedifferentiation) in AciCC (7). In the original paper, the authors defined HGT as “areas of dedifferentiated high-grade adenocarcinoma or undifferentiated carcinoma” in association with areas of classic low-grade AciCC. Stanley et al. and subsequent studies have shown that high-grade (HG) AciCC is associated with adverse outcome, including 40% to 56% risk of nodal metastasis, 50% to 75% risk of distant metastasis and 33% to 75% rate of disease-related mortality (7–12). However, the definition of HG AciCC is not consistent across these studies and no consistent histologic grading of AciCC has been proposed to date.
In 2019, a unique diagnostic fusion involving the upstream region of NR4A3 gene, which stands for Nuclear Receptor Subfamily 4 Group A member 3, was discovered in AciCCs (13). The fusion leads to enhancer hijacking and consistent overexpression of NR4A3 (NOR1) protein, which can be detected by immunohistochemistry (IHC) (13). Subsequently, nuclear expression of NR4A3 has been shown to be a highly sensitive and specific IHC for AciCC with a reported sensitivity of 82% to 100% and specificity of 97% to100% (13–17).
In this large single center retrospective study of 117 AciCCs, we conducted a detailed clinicopathologic review aiming to establish a histologic grading system for AciCC. Additionally, NR4A3 IHC was performed in 68 cases of AciCCs and 56 cases of non-AciCCs involving salivary gland to further confirm the utility of NR4A3 IHC in diagnosing this tumor.
Material and Methods
Study cohort and clinicopathologic review:
After obtaining Institutional Review Board approval from Memorial Sloan Kettering Cancer Center (MSKCC, New York, NY, United States), the pathology database was searched for cases of AciCC diagnosed between 1985 and 2020. All cases with H&E slides available were reviewed by two head and neck pathologists (BX and NK) to confirm the diagnosis according to the WHO classification (4th edition, 2017) (3) and collect detailed histopathologic features of each tumor. All tumors exhibited at least focal evidence of serous acinar differentiation in which tumor cells with zymogen granules were clearly present. Diagnostic mimickers, such as secretory carcinoma and/or mucoacinar carcinoma (18) were excluded. A total of 117 patients with a confirmed diagnosis of primary AciCC of the head and neck region were included in the study. Among them, 66 had resection at MSKCC; 47 had their pathology slides reviewed when patients were treated at our center, whereas the remaining 4 were personal pathology consultations.
The following clinicopathologic features were recorded for each case: age, sex, site of the primary tumor, tumor size, mitotic index, tumor necrosis, HGT, the presence and extent of zymogen granules within the tumor, nuclear anaplasia (defined as marked variation of nuclear size), the presence of microcystic and solid architecture, lymphovascular invasion, perineural invasion, growth pattern (being uninodular, multinodular, or infiltrative), fibrosis/desmoplastic reaction at the frankly invasive front, tumor-associated lymphoid stroma, margin status, AJCC 8th edition pT and pN stage, and treatment received. Given that AciCC is often associated with tumor-associated lymphoid stroma, only true positive lymph nodes with a definitive capsule, subcapsular sinus, and histiocytes within the sinusoid were considered as lymph node metastases (19).
Mitotic index was determined by counting 10 high-power fields (HPFs, 400X, total field size 2.4 mm2) with an Olympus microscope (U-DO model BX41, Olympus America Inc., Center Valley, PA, United States) in the areas of highest concentration of mitotic figures. HGT was determined at consensus using criteria defined by Stanley et al. (7) as areas of dedifferentiation into a high-grade adenocarcinoma or undifferentiated carcinoma.
NR4A3 immunohistochemistry
Immunohistochemical stain for NR4A3 was performed on 68 AciCCs and a control group of 56 tumors (including 19 secretory carcinomas, 8 salivary duct carcinomas, 8 mucoepidermoid carcinomas, 5 adenoid cystic carcinomas, 4 myoepithelial carcinomas, 5 pleomorphic adenomas, 3 polymorphous adenocarcinomas/cribriform adenocarcinomas, 1 carcinoma of salivary gland, not otherwise specified, 1 clear cell carcinoma, 1 metastatic squamous cell carcinoma, and 1 metastatic EBV-positive nasopharyngeal carcinoma to an intra-parotid lymph node). The primary antibody for NR4A3 is a monoclonal antibody specific for an epitope mapping between amino acids 222 and 259 (clone: NOR-1 [H7], SC-393902, dilution: 1:100, Santa Cruz Biotechnology Inc., Heidelberg, Germany). A tumor was considered positive for NR4A3 when > 5% of tumor cells exhibited nuclear staining of any intensity. The percentage and intensity of NR4A3 IHC were additionally documented. The sensitivity and specificity of NR4A3 immunostain in diagnosing AciCC were calculated.
Clinical outcome and statistical analysis
Among the study cohort, 108 patients were followed at our center. The clinical outcome collected included overall survival (OS), disease specific survival (DSS), disease free survival (DFS) and distant metastasis free survival (DMFS). Follow up was calculated from the data of primary resection.
All statistics were performed using SPSS software v25.0 (IBM Corporation, Armonk, NY, USA). Univariate survival analysis was performed using the log rank test for categorical variables and the Cox proportional hazards model for continuous variable (e.g. age and tumor size) to determine the prognostic value of each clinicopathologic features. Subsequent multivariate analysis was conducted for DFS using the Cox proportional hazards model. Variables that were significant on univariate analyses were subjected to multivariate analyses. The clinicopathologic features of low or intermediate-grade and high-grade AciCC were compared using Fisher’s exact test for categorical variables and two-tailed Students’ t test for continuous variable. P values less than 0.05 were considered as statistically significant.
Results
Clinicopathologic characteristics
The clinical and pathologic features of the study cohort are summarized in Table 1. The median age of presentation was 52, with a wide age range from 11 to 88-year-old. Eight tumors (7%) occurred in patients who were 21-year-old or younger. There was a slight female predominance with a female: male ratio of 1.17:1. All tumors were located in the parotid gland.
Table 1.
Clinicopathologic characteristics of the acinic cell carcinoma (n=117).
| Clinical features | ||
|---|---|---|
|
| ||
| Sex | Female | 63 (54%) |
| Male | 54 (46%) | |
| Age, year, median (range) | 52 (11–88) | |
| Site of primary tumor | The parotid gland | 117 (100%) |
| Chemotherapy and radiation (n=106) | Radiation | 37 (35%) |
| Chemoradiation | 19 (18%) | |
|
| ||
| Pathologic features | ||
|
| ||
| Tumor size, cm, median (range) | 3.0 (0.1–14) | |
| Mitotic index (per 10 high power fields) | 0–1 | 70 (60%) |
| 2–4 | 13 (11%) | |
| ≥5 | 34 (29%) | |
| Nuclear anaplasia | 14 (12%) | |
| Tumor necrosis | 39 (33%) | |
| High grade transformation | 11 (9%) | |
| Zymogen granule | Focal | 20 (17%) |
| Diffuse | 95 (83%) | |
| Architecture | Solid | 103 (90%) |
| Microcystic | 99 (86%) | |
| Follicular | 3 (3%) | |
| Lympoepithelioma-like | 3 (3%) | |
| Lymphovascular invasion | 17 (15%) | |
| Perineural invasion | 35 (30%) | |
| Growth Pattern | Uninodular | 21 (18%) |
| Multinodular | 59 (51%) | |
| Infiltrative | 35 (30%) | |
| Fibrosis at the frankly invasive front | 62 (54%) | |
| Tumor associated lymphoid stroma | Absent | 42 (37%) |
| Focal/partial | 50 (44%) | |
| Diffuse | 23 (20%) | |
| Margin status | Negative | 69 (62%) |
| Positive | 42 (38%) | |
| AJCC 8th pT stage | pT1 | 36 (32%) |
| pT2 | 56 (49%) | |
| pT3 | 15 (13%) | |
| pT4 | 7 (6%) | |
| AJCC 8th pN stage | pNx/N0 | 106 (91%) |
| pN1/N2/N3 | 11 (9%) | |
Histologically, AciCCs contained various architectural patterns, with solid (n=103, 90%) and microcystic (n=99, 86%) being the most common. Follicular pattern was seen in three (3%) cases. Additionally, three (3%) high-grade AciCCs showed histologic features resembling lymphoepithelial carcinoma with tumor cells arranged as syncytial sheets in association with abundant reactive lymphocytic infiltrate (Figure 1). Papillocystic architecture was not seen in the entire study cohort.
Figure 1. Histologic features of acinic cell carcinoma (AciCC).
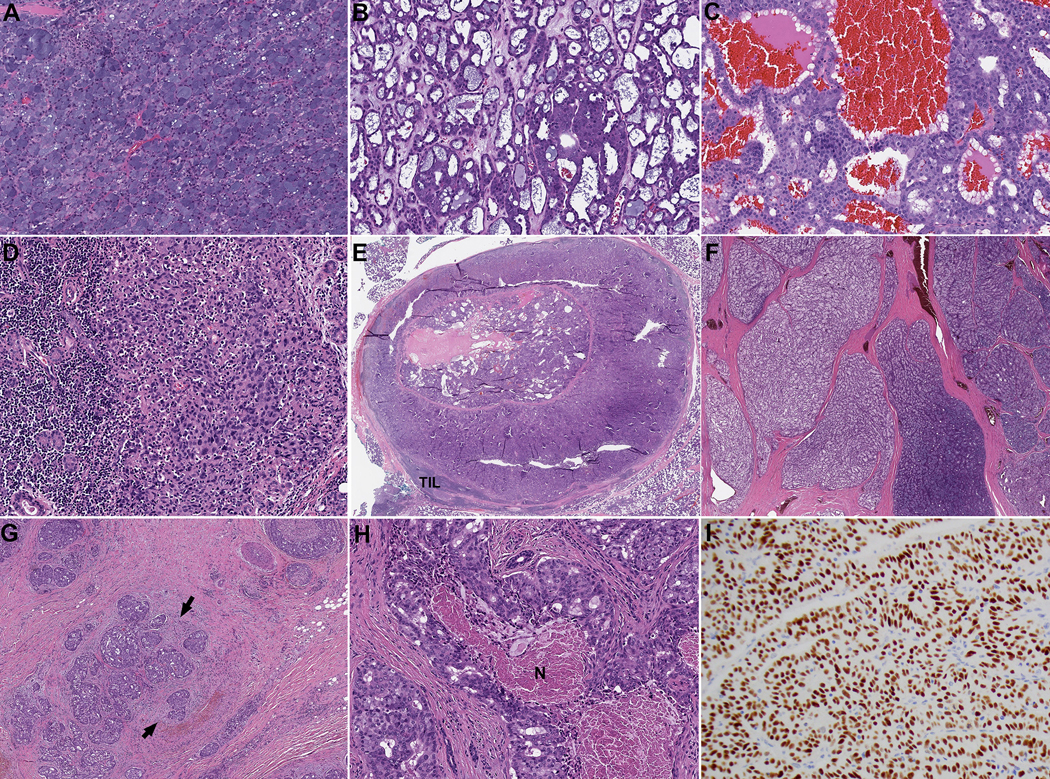
AciCC may contain solid (A), microcystic (B), follicular (C), and/or lymphoepithelioma-like (D) areas. The follicular pattern resembles thyroid follicles with centrally located eosinophilic material surrounded by a cuboidal layer of tumor cells. Peripheral scalloping may be seen. The lymphoepithelioma-like area is characterized by syncytial growth of tumor cells infiltrated and surrounded by reactive lymphocytes. (E-G) The growth patterns of AciCC include uninodular (E), multinodular (F) and infiltrative (G). Fibrosis and desmoplastic reaction at the frankly invasive front (arrows in G) may be seen. (H) Tumor necrosis (N) in AciCC. (I) An acinic cell carcinoma shows diffuse and strong (3+) immunopositivity of NR4A3.
Zymogen granules were identified in all AciCCs, being present focally (defined as identifiable zymogen granules within the tumor cells in ≤10% of total tumor volume) in 20 cases (17%) and diffusely (>10% of total tumor volume) in 95 cases (83%). High mitotic index of ≥5/10 HPFs (>4/2 mm2), nuclear anaplasia, and tumor necrosis were present in 34 (29%), 14 (12%), and 39 (33%) patients respectively. Eleven (9%) tumors were deemed to have HGT. The growth pattern was uninodular in 21 (18%), multinodular in 59 (51%), and infiltrative in 35 (30%) patients. In 62 (54%) patients, fibrosis or desmoplastic reaction was noted at the frankly invasive front. Tumor-associated lymphoid stroma was a common finding seen in 73 (63%) tumors either focally (defined as a few lymphoid aggregates within the tumor and/or at the tumor border) (n=50) or diffusely (defined as a complete band-like lymphoid infiltrate at the tumor border and/or diffuse lymphoid infiltrate within the tumor, with or without germinal centers) (n=23). The rate of lymphovascular invasion, perineural invasion, positive resection margin, and nodal metastasis was 15%, 35%, 38%, and 9% respectively.
NR4A3 immunohistochemistry
The results of NR4A3 immunohistochemical stain are shown in Table 2. NR4A3 was positive in 66 of 68 (97%) AciCCs tested. Among the positive cases, 59 AciCCs (87%) showed strong (3+) staining in at least 50% of tumor cells (Figure 1I). The median percentage of NR4A3-positive tumor cells was 90%. Two AciCCs (3%) with typical morphology and diffuse zymogen granules were negative for NR4A3 with either complete absence of staining (n=1) or weak (1+) nuclear staining in ≤5% tumor cells (n=1).
Table 2. NR4A3 immunohistochemistry in acinic cell carcinoma (AciCC) and non-AciCC.
PAC/CASG: polymorphous adenocarcinoma/cribriform adenocarcinoma of salivary gland, EBV: Epstein-Barr virus, NPC: nasopharyngeal carcinoma.
| AciCC | Non-AciCC | |
|---|---|---|
|
| ||
| NR4A3 positivity | 66/68 (97%) | 4/56 (7%) |
| Percentage of positive tumor cells, median (range) | 90 (0–100) | 0 (0–70) |
| Intensity | ||
| Negative (0) | 1 (1%) | 52 (93%) |
| Weak (1+) | 2 (3%) | 1 (2%) |
| Moderate (2+) | 2 (3%) | 2 (4%) |
| Strong (3+) | 63 (91%) | 1 (2%) |
|
| ||
| NR4A3 positivity in non-AciCCs | ||
|
| ||
| Secretory carcinoma | 1/19 (5%) | |
| Salivary duct carcinoma | 0/8 (0%) | |
| Mucoepidermoid carcinoma | 2/8 (20%) | |
| Adenoid cystic carcinoma | 0/5 (0%) | |
| Myoepithelial carcinoma | 0/5 (0%) | |
| Pleomorphic adenoma | 1/5 (20%) | |
| PAC/CASG | 0/3 (0%) | |
| Carcinoma, not otherwise specified | 0/1 (0%) | |
| Clear cell carcinoma | 0/1 (0%) | |
| Metastatic EBV-positive NPC | 0/1 (0%) | |
| Metastatic squamous cell carcinoma | 0/1 (0%) | |
Among the 56 non-AciCCs, 52 (93%) were completely negative for NR4A3. The four cases showing NR4A3 immunopositivity were 2/8 (20%) mucoepidermoid carcinoma with 70% tumor cells showing moderate staining in one case and 70% tumor cells showing strong nuclear staining in another; 1/5 (20%) pleomorphic adenoma containing 10% positive tumor cells (predominantly of myoepithelial origin) with moderate intensity; and 1/19 (5%) secretory carcinoma with 10% tumor cells demonstrating weak nuclear positivity. All other tumors tested were completely negative for NR4A3.
The sensitivity and specificity of NR4A3 in diagnosing AciCC were 97% and 93% respectively.
Clinical outcome and prognostic factors in AciCC
Follow up data were available in 108 patients with a median follow up of 42 months (range: 1–343 months). The 3-year, 5-year, 10-year OS, DSS, DFS, and DMFS are shown in Supplementary table 1. The 3-year, 5-year, and 10-year DSS were 88%, 85%, and 75% respectively.
Thirty patients developed distant metastasis 2 to 154 months after the initial resection (mean 37 months). Six patients developed distant metastasis more than 5 years after the initial resection whereas two patients had distant metastasis after 10 years. The most common sites of metastasis were lung (n=24), bone (n=13), brain (n=2), and liver (n=2). The 3-year, 5-year, and 10-year DMFS were 77%, 74%, and 59% respectively.
Adverse prognostic clinicopathologic parameters for shorter OS, DSS, DFS, and DMFS identified on univariate survival analysis included large tumor size, high mitotic index ≥5/10 HPFs (>4/2 mm2), nuclear anaplasia, tumor necrosis, HGT, focal rather than diffuse zymogen granules, lymphovascular invasion, perineural invasion, infiltrative tumor border, fibrosis at tumor front, high AJCC pT and pN stage (Supplementary table 2). Additionally, older age predicted shortened OS, DSS, and DMFS. Positive margin was associated with decreased DFS and DMFS, and tumors without tumor-associated lymphoid stroma had reduced OS.
Independent prognostic factors identified on subsequent multivariate survival analysis using Cox proportional hazards model included tumor necrosis (hazard ratio HR=13.363, 95% confidence interval CI=1.498–119.160, p=0.020), marked nuclear anaplasia (HR=23.92, 95% CI=2.844–200.697, p=0.003), AJCC pT stage (HR=1.881, 95% CI=1.043–3.390, p=0.036), AJCC pN stage (HR=5.927, 95% CI=1.602–21.929, p=0.008), age (HR=0.061, 95% CI=1.020–1.103, p=0.003), and tumor associated lymphoid stroma (HR=0.222, 95% CI=0.080–0.613, p=0.004) for OS; as well as tumor necrosis (HR=8.696, 95% CI=1.623–47.619, p=0.012), lymphovascular invasion (HR=12.658, 95% CI=4.082–40.000, p<0.001), infiltrative tumor border (HR=3.876, 95% CI=1.004–14.925, p=0.049), and high AJCC pN stage (HR=7.576, 95% CI=2.694–21.277, p<0.001) for DFS (Table 3 and Figure 2). Multivariate analysis for DSS and DMFS was not performed due to insufficient events.
Table 3. Multivariate survival analysis for overall survival and disease-free survival.
Bold p values are significant p values.
| P value | Hazard ratio (95% confidence interval) | |
|---|---|---|
| Overall survival | ||
| Mitotic index | 0.206 | 1.271 (0.877–1.843) |
| Necrosis | 0.020 | 13.363 (1.498–119.160) |
| High grade transformation | 0.096 | 5.189 (0.748–35.990) |
| Zymogen granule | 0.065 | 3.700 (0.920–14.877) |
| Lymphovascular invasion | 0.961 | 0.969 (0.277–3.388) |
| Perineural invasion | 0.809 | 0.810 (0.145–4.507) |
| Tumor border | 0.504 | 1.333 (0.574–3.092) |
| Fibrosis at the frankly invasive front | 0.870 | 0.850 (0.122–5.942) |
| Nuclear anaplasia | 0.003 | 23.892 (2.844–200.697) |
| AJCC pT | 0.036 | 1.881 (1.043–3.390) |
| AJCC pN | 0.008 | 5.927 (1.602–21.929) |
| Age | 0.003 | 1.061 (1.020–1.103) |
| Size | 0.292 | 0.902 (0.744–1.093) |
| Tumor associated lymphoid stroma | 0.004 | 0.222 (0.080–0.613) |
| DFS | ||
| Mitotic index | 0.546 | 1.618 (0.340–7.7.07) |
| Necrosis | 0.012 | 8.696 (1.623–47.619) |
| High grade transformation | 0.211 | 2.629 (0.578–11.946) |
| Zymogen granule | 0.210 | 0.414 (0.104–1.645) |
| Lymphovascular invasion | <0.001 | 12.658 (4.082–40.000) |
| Perineural invasion | 0.694 | 1.263 (0.395–4.040) |
| Tumor border | 0.049 | 3.876 (1.004–14.925) |
| Fibrosis at the frankly invasive front | 0.871 | 1.114 (0.303–4.097) |
| Marked nuclear pleomorphism | 0.071 | 3.968 (0.889–17.857) |
| AJCC pT stage | 0.405 | 1.611 (0.525–4.944) |
| AJCC pN stage | <0.001 | 7.576 (2.694–21.277) |
| Age | 0.321 | 1.013 (0.987–1.041) |
| Size | 0.680 | 1.049 (0.759–1.196) |
| Margin status | 0.096 | 2.262 (0.865–5.914) |
Figure 2. Kaplan-Meier curves for overall survival (A), disease free survival (DFS) (B), and distant metastasis free survival according to the proposed grading system (C).
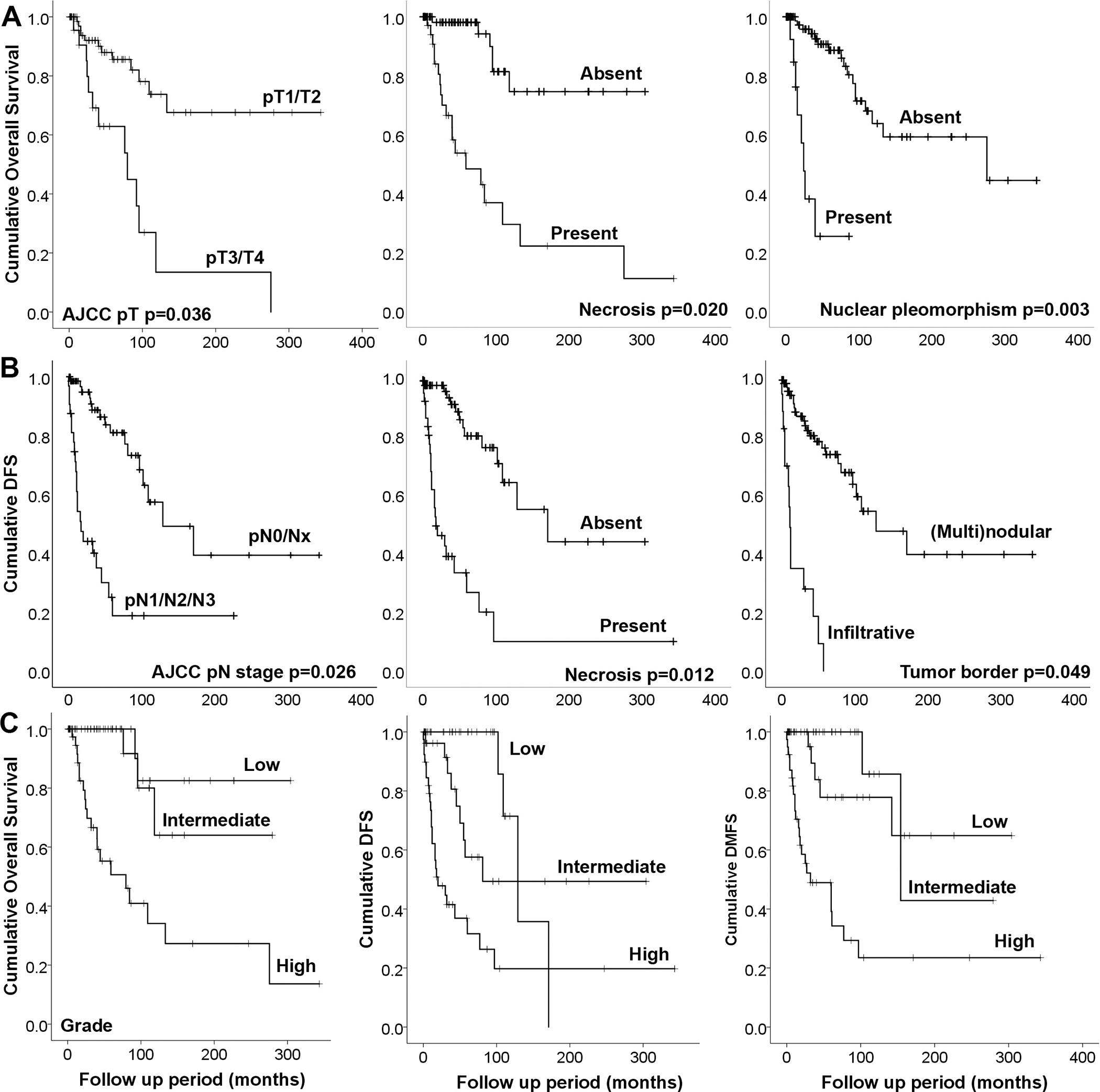
DFS: disease free survival, DMFS: distant metastasis free survival. P Values in panels A and B are obtained from multivariate survival analysis using Cox proportional hazards model.
Proposed grading system for acinic cell carcinoma and its performance in predicting outcome
Based on the prognostic histologic features identified on univariate and multivariate survival analyses, we herein applied a grading system for AciCC using a combination of four histologic features: mitotic index, tumor necrosis, fibrosis at the frankly invasive front, and tumor borders. Although significant on univariate and multivariate survival analysis, marked nuclear anaplasia was a rare histologic feature that was only seen in tumors with necrosis, and was therefore excluded from the grading system.
In brief, AciCC was considered as low-grade when it had a mitotic index of 0–1/10 HPFs (0–1/ 2 mm2), uninodular or multinodular growth pattern, absence of tumor necrosis, and absence of fibrosis/desmoplastic reaction at the frankly invasive front. Intermediate-grade AciCCs had at least one of the following features: mitotic index of 2–4/10 HPFs (2–4/2 mm2), infiltrative tumor borders, and/or fibrosis at the frankly invasive front. Lastly, high-grade AciCC was defined as those with either a mitotic index of ≥5/10 high power fields (>4/2 mm2) or tumor necrosis.
A total of 115 AciCCs were graded based on this grading system. Among these, 45 (39%) AciCCs were considered as low grade, 27 (24%) as intermediate grade, and 43 (37%) as high-grade. The prognostic significance of this grading system in predicting outcomes (DSS, DFS, and DMFS) on univariate and multivariate survival analysis when adjusted for AJCC pT stage, pN stage, margin status, perineural invasion and lymphovascular invasion are shown in Table 4 and the Kaplan-Meier curves are presented in Figure 2. The prognosis of low grade and intermediate grade did not differ significantly on univariate and multivariate survival analysis for OS, DFS, and DMFS. High grade AciCC was an independent adverse prognostic factor for OS (HR=6.132, 95% CI=1.254–29.991, p=0.025), DFS (HR=4.979, 95% CI=1.332–18.616, p=0.017), and DMFS (HR=6.646, 95% CI=1.381–31.881, p=0.018). The 5-year OS, DSS, DFS, and DMFS were 100%, 100%, 80%, 90% for low or intermediate grade AciCC, and 50%, 50%, 37% and 48% for high grade AciCC (Supplementary table 1).
Table 4. Prognostic values of the proposed grading system for acinic cell carcinoma.
The multivariate analysis was performed using Cox proportional hazards models adjusted for AJCC pT stage, pN stage, margin status, perineural invasion and lymphovascular invasion. Bold p values are significant p values.
| OS | DFS | DMFS | ||||
|---|---|---|---|---|---|---|
| P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | |
|
| ||||||
| Univariate analysis | ||||||
| Low | Reference | |||||
| Intermediate | 0.705 | 0.708 (0.118–4.243) | 0.075 | 2.922 (0.897–9.514) | 0.202 | 2.916 (0.564–15.089) |
| High | 0.002 | 6.867 (2.034–23.186) | <0.001 | 9.058 (3.130–26.209) | <0.001 | 14.494 (3.405–61.705) |
| Multivariate analysis | ||||||
| Low | Reference | |||||
| Intermediate | 0.696 | 0.670 (0.090–5.006) | 0.377 | 1.843 (0.475–7.154) | 0.762 | 1.302 (0.237–7.155) |
| High | 0.025 | 6.132 (1.254–29.991) | 0.017 | 4.979 (1.332–18.616) | 0.018 | 6.646 (1.386–31.881) |
When comparing with low or intermediate grade AciCC, HG tumors were associated with older age at presentation, larger tumor size, focal rather than diffuse zymogen granules, solid architecture, lymphovascular invasion, perineural invasion, infiltrative tumor borders, fibrosis at the frankly invasive front, positive resection margin, and high AJCC pT and pN stage (p<0.05, Table 5).
Table 5. Clinicopathologic features according to proposed grading system of AciCC.
P values were obtained using Fisher’s exact test for categorical variables and two-tailed Student’s t test for continuous variables. Bold p values are significant p values.
| Low or intermediate grade (n=72) | High grade (n=43) | P values | ||
|---|---|---|---|---|
| Female: male ratio | 38:34 (1.12:1) | 23:20 (1.15:1) | 1.000 | |
| Age, years, median (range) | 46 (11–80) | 62 (19–88) | <0.001 | |
| Tumor size, cm, median (range) | 2.5 (0.1–9.5) | 3.0 (1.5–13.5) | 0.003 | |
| Mitotic index ≥5/10 HPFs | 0 (0%) | 34 (79%) | <0.001 | |
| Tumor necrosis | 0 (0%) | 39 (91%) | <0.001 | |
| Marked nuclear pleomorphism | 0 (0%) | 14 (33%) | <0.001 | |
| High grade transformation | 0 (0%) | 11 (26%) | <0.001 | |
| Diffuse zymogen granules | 71 (99%) | 24 (56%) | <0.001 | |
| Solid architecture | 61 (85%) | 42 (98%) | 0.030 | |
| Microcystic architecture | 62 (86%) | 37 (86%) | 1.000 | |
| Lymphovascular invasion | 2 (2.8%) | 15 (35%) | <0.001 | |
| Perineural invasion | 6 (8%) | 29 (67%) | <0.001 | |
| Infiltrative tumor border | 6 (8.3%) | 29 (67%) | <0.001 | |
| Fibrosis at the frankly invasive front | 24 (33%) | 38 (88%) | <0.001 | |
| Tumor associated lymphoid stroma | Absent | 22 (31%) | 20 (47%) | 0.055 |
| Focal/partial | 31 (43%) | 19 (44%) | ||
| Diffuse | 19 (26%) | 4 (9%) | ||
| Positive margin | 19 (28%) | 23 (55%) | 0.008 | |
| AJCC pT stage | pT1/T2 | 62 (89%) | 28 (67%) | 0.007 |
| pT3/T4 | 8 (11%) | 14 (33%) | ||
| AJCC pN stage | pNx/N0 | 69 (96%) | 35 (81%) | 0.019 |
| pN1/N2/N3 | 3 (4%) | 8 (19%) | ||
In patients with a negative resection margin (R0), high grade AciCC remained to be a significant adverse prognostic factor for OS, DFS, and DMFS (log rank test, p<0.001) compared with low or intermediate-grade AciCC.
Discussion
AciCC has an annual incidence of 1.3 cases per 1 million population (2, 20). Given the rarity of this salivary gland carcinoma, large-scale studies are relatively uncommon. Moreover, studies predating the characterization of salivary secretory carcinoma in 2010 (21), may have included cases of secretory carcinoma since this tumor was often classified as AciCC prior to 2010. Notably, the papillary-cystic pattern initially described in AciCC was not seen in any of the cases in the current study, and it likely represents a histologic pattern of secretory carcinoma rather than AciCC. Registry (e.g. SEER and NCDB)-based studies reported a 5-year OS of 83% to 97% (1, 4, 6) and 10-year OS of 94% (1), although these data may also be contaminated by the misdiagnosed SC as AciCC. Center-based retrospective cohort studies demonstrated similar outcomes of AciCC: the 5-year OS ranged from 87% to 90% (22–25), and the 10-year OS was 83% (25). In our study, the 5-year and 10-year OS was 80% and 58% respectively. The relatively worse outcome as well as the higher frequency of lymphovascular invasion, perineural invasion, positive resection margin, and nodal metastasis in the current study might be attributed to the selection bias towards patients with more aggressive/advanced disease at our tertiary cancer center. Regardless, these survival data confirmed that AciCC generally has an excellent outcome and a low mortality, supporting the traditional view that regards AciCC as an overall low-grade salivary gland carcinoma (26).
While most AciCCs follow an indolent clinical course, a subset of tumors which has been often characterized by HG features can be aggressive and are associated with high risk of distant metastasis and high mortality rate (7–12, 25, 27, 28). However, the definition of HG AciCC has not been consistent among the reported studies.
Gomez et al. have used proliferative features and defined HG AciCC as tumors with necrosis or a mitotic index of ≥2/10 HPFs, and showed that HG AciCCs were associated with a decreased DFS and OS in a study of 35 AciCCs (25). Schwarz et al. described HG AciCC as tumors with a Ki67 proliferation index > 5%, nuclear anaplasia, and high nuclear: cytoplasmic ratio, and found that high grade histology was associated with shortened RFS in 40 cases of AciCCs (27).
Moreover, HGT (defined as “areas of dedifferentiated high-grade adenocarcinoma or undifferentiated carcinoma”) was shown to impart a 73% to 75% disease specific mortality rate, and a decreased overall survival (mean 40 months compared with 125 months in AciCC without HGT) (7,8,9,11). While some pathologists use the term “HGT” to describe undifferentiated tumors that shows histologic progression from a low grade AciCC with acinar differentiation into an undifferentiated HG tumor, others would use the term HGT interchangeably with high grade to describe tumors with necrosis and increased mitotic activity (10,11). Lastly, there are several studies that did not provide a clear definition of HGT or HG AciCC and have also shown that HGT or HG AciCC was associated with high risk of distant metastasis (12, 28) or decreased survival (29).
Since the criteria used for grading are inconsistent and often subjective and because some conventional AciCCs still behave aggressively, there is a need for a more robust histologic grading system in daily clinical practice. Therefore, we aimed to establish a prognostically relevant and reproducible grading system of AciCC in the current study. Using the significant prognostic histologic features identified on univariate and multivariate survival analysis, we classified AciCC as low, intermediate, and high grade. AciCC was considered as low grade when it had ≤1/10 HPFs (≤1/2 mm2) and lacked necrosis and tumor fibrosis and infiltrative front, intermediate grade when it showed 2–4 mitoses/10 HPFs (2–4/ 2 mm2), or infiltrative tumor borders, and/or fibrosis at the frankly invasive front and high-grade when it had ≥5/10 HPFs (>4/2 mm2) or tumor necrosis. When comparing low to intermediate grade AciCCs, they seemed to behave in a similar fashion and did not differ significantly on univariate and multivariate survival analysis. Thus, we propose utilizing a 2-tiered grading system, defining high grade as tumor showing a mitotic index ≥5/10 HPFs (>4/2 mm2) or tumor necrosis. Further larger studies are needed to assess the significance of intermediate grade AciCC.
In our study, HG AciCC was associated with shortened OS, DSS, DFS, and DMFS, and was an independent adverse prognostic factor when adjusted for other risk factors, including stage, margin status, perineural invasion and lymphovascular invasion. The 5-year OS and DMFS were 50% and 48% in HG AciCC, compared with 100% and 90% in tumors of low or intermediate grade. Clearly, the grading scheme proposed in the current study is a useful tool to risk-stratify patients with AciCC allowing more appropriate clinical follow up and management. In our study, 11 tumors (9%) meet the original criteria of HGT defined by Stanley et al. In contrast, the rate of HG AciCC defined using the proposed grading system was higher, being 37%. HGT was a significant prognostic factor for OS, DSS, DFS, and DMFS on univariate analysis, but failed to reach statistical significance on multivariate analysis. Given the low frequency and lack of independent prognostic value of HGT, we argue that the proposed grading system for HG tumors might be superior to HGT in prognosticating AciCC. It also is a more objective system since it relies on mitotic count and tumor necrosis, two parameters that are less prone to interobserver variability than other histologic features such as nuclear anaplasia and nuclear: cytoplasmic ratio.
In addition to predicting prognosis, several previous studies have shown that HGT or HG AciCC was associated with older age (9, 10), high T stage (6), nodal metastasis (6, 9), lymphovascular invasion (10), positive margin (6), and large tumor size (6). Such associations were all confirmed by the current study. Additionally, HG tumors often had focal (rather than diffuse) zymogen granules, solid architecture, infiltrative (rather than uninodular/multinodular) tumor border, and fibrosis at the frankly invasive front.
Adverse prognostic factors other than high grade have also been reported including male gender (24), high T stage (24, 29, 30), nodal metastasis (28), extranodal extension (24), facial nerve deficiency (30), (gross) perineural invasion (23, 29), and positive margin (30). However, most of these studies included a small cohort of AciCC, ranging from 32 to 71 patients. There are only two studies that have reported results of multivariate survival analysis. One is from China, by Fang et al., which identified nodal metastasis and HG histology as independent adverse prognostic factors for DMFS in 144 patients with parotid AciCC (28). The other study from South Korea, by Park et al., reported TNM stage as the only independent prognostic factor for survival in a cohort of 59 parotid AciCC (24). Our study was one of the largest cohorts of AciCC from North America. We herein identified necrosis, nuclear anaplasia, larger tumor size, older age and advanced AJCC pT and pN stage as independent adverse predictor for decreased OS; as well as high stage, necrosis, lymphovascular invasion, infiltrative tumor border and high AJCC pN stage as adverse predictor for shortened DMFS.
Beside histologic grade, we also assessed the utility of NR4A3 IHC in diagnosing AciCC, which can be especially helpful in cases of AciCC with focal zymogen granules or HGT/HG features. In 2019, a characteristics fusion involving NR4A3 gene was identified in AciCC (13, 31). The fusion results in enhancer hijacking and subsequent overexpression of NR4A3 protein, which can be detected by NR4A3 (NOR1) IHC. Several recent studies have shown that NR4A3 was a highly sensitive and specific IHC marker for AciCC in surgical (13, 14, 16) and cytologic specimens (15, 17). Using a slightly variable threshold of any to 5% nuclear immunopositivity, the sensitivity and specificity of NR4A3 in diagnosing AciCC were 82% to 100%, and 97 to 100% respectively. Similarly, we reported a sensitivity of 96% and specificity of 93%. Rarely, non-AciCC tumors show NR4A3 nuclear immunopositivity and often in a focal and weak fashion. In the current study, only 7% of non-AciCC tumors expressed NR3A3 and these included a small percentage of secretory carcinoma, mucoepidermoid carcinoma, polymorphous adenocarcinoma and pleomorphic adenoma. The latter two entities were also shown to express NR4A3 reported by Wong et al. (15).
In conclusion, we herein identified necrosis, nuclear anaplasia, large tumor size, older age, lymphovascular invasion, infiltrative border, advanced AJCC pT and PN stage as independent adverse prognostic factors in a large retrospective cohort of 117 AciCCs. While we attempted to classify AciCC into low-, intermediate-, and high-grade using mitotic index, necrosis, fibrosis at the frankly invasive front, and infiltrative border, we found that low and intermediate grade tumors behaved in a similar fashion. Therefore, we propose to use a two-tiered grading scheme, with HG AciCC characterized by a mitotic index of ≥5/10HPFs and/or necrosis. Larger studies are needed to assess the 3-tiered grading system and the significance of intermediate-grade AciCC. In addition, we found that NR4A3 is a highly sensitive and specific IHC marker for AciCC supporting its utility as a diagnostic marker for AciCC.
Supplementary Material
Footnotes
Conflicts of Interest and Source of Funding:
The authors have disclosed that they have no significant relationships with, or financial interest in any commercial companies pertaining to this article. Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/ National Cancer Institute under award number P30CA008748.
References
- 1.Patel NR, Sanghvi S, Khan MN, et al. Demographic trends and disease-specific survival in salivary acinic cell carcinoma: an analysis of 1129 cases. Laryngoscope. 2014;124:172–178. [DOI] [PubMed] [Google Scholar]
- 2.Vander Poorten V, Triantafyllou A, Thompson LD, et al. Salivary acinic cell carcinoma: reappraisal and update. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies. 2016;273:3511–3531. [DOI] [PubMed] [Google Scholar]
- 3.El-Naggar AK, Chan JKC, Grandis JR, et al. World Health Organization Classification of Tumours: pathology and genetics of head and neck tumours (4th edition). Lyon: International Agency for Research on Cancer (IARC); 2017. [Google Scholar]
- 4.Hoffman HT, Karnell LH, Robinson RA, et al. National Cancer Data Base report on cancer of the head and neck: acinic cell carcinoma. Head Neck. 1999;21:297–309. [DOI] [PubMed] [Google Scholar]
- 5.Yibulayin F, Feng L, Wang M, et al. Head & neck acinar cell carcinoma: a population-based study using the seer registry. BMC Cancer. 2020;20:631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scherl C, Kato MG, Erkul E, et al. Outcomes and prognostic factors for parotid acinic cell Carcinoma: A National Cancer Database study of 2362 cases. Oral Oncol. 2018;82:53–60. [DOI] [PubMed] [Google Scholar]
- 7.Stanley RJ, Weiland LH, Olsen KD, et al. Dedifferentiated acinic cell (acinous) carcinoma of the parotid gland. Otolaryngol Head Neck Surg. 1988;98:155–161. [DOI] [PubMed] [Google Scholar]
- 8.Skalova A, Sima R, Vanecek T, et al. Acinic cell carcinoma with high-grade transformation: a report of 9 cases with immunohistochemical study and analysis of TP53 and HER-2/neu genes. Am J Surg Pathol. 2009;33:1137–1145. [DOI] [PubMed] [Google Scholar]
- 9.Chiosea SI, Griffith C, Assaad A, et al. The profile of acinic cell carcinoma after recognition of mammary analog secretory carcinoma. Am J Surg Pathol. 2012;36:343–350. [DOI] [PubMed] [Google Scholar]
- 10.Chintakuntlawar AV, Shon W, Erickson-Johnson M, et al. High-grade transformation of acinic cell carcinoma: an inadequately treated entity? Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121:542–549 e541. [DOI] [PubMed] [Google Scholar]
- 11.Thompson LD, Aslam MN, Stall JN, et al. Clinicopathologic and Immunophenotypic Characterization of 25 Cases of Acinic Cell Carcinoma with High-Grade Transformation. Head Neck Pathol. 2016;10:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yue LE, Samankan S, Liu X, et al. Ten patients with high-grade transformation of acinic cell carcinomas: Expression profiling of beta-catenin and cyclin D1 is useful. Pathol Res Pract. 2020;216:152767. [DOI] [PubMed] [Google Scholar]
- 13.Haller F, Bieg M, Will R, et al. Enhancer hijacking activates oncogenic transcription factor NR4A3 in acinic cell carcinomas of the salivary glands. Nat Commun. 2019;10:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haller F, Skalova A, Ihrler S, et al. Nuclear NR4A3 Immunostaining Is a Specific and Sensitive Novel Marker for Acinic Cell Carcinoma of the Salivary Glands. Am J Surg Pathol. 2019;43:1264–1272. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen L, Chopra S, Laskar DB, et al. NOR-1 distinguishes acinic cell carcinoma from its mimics on fine-needle aspiration biopsy specimens. Hum Pathol. 2020;102:1–6. [DOI] [PubMed] [Google Scholar]
- 16.Wong KS, Marino-Enriquez A, Hornick JL, et al. NR4A3 Immunohistochemistry Reliably Discriminates Acinic Cell Carcinoma from Mimics. Head Neck Pathol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skaugen JM, Seethala RR, Chiosea SI, et al. Evaluation of NR4A3 immunohistochemistry (IHC) and fluorescence in situ hybridization and comparison with DOG1 IHC for FNA diagnosis of acinic cell carcinoma. Cancer Cytopathol. 2021;129:104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bundele M, Weinreb I, Xu B, et al. Mucoacinar Carcinoma: A Rare Variant of Mucoepidermoid Carcinoma. Am J Surg Pathol. 2021;45:1028–1037. [DOI] [PubMed] [Google Scholar]
- 19.Kurian EM, Miller R, McLean-Holden AL, et al. Low Molecular Weight Cytokeratin Immunostaining for Extrafollicular Reticulum Cells is an Effective Means of Separating Salivary Gland Tumor-Associated Lymphoid Proliferation from True Lymph Node Involvement. Head Neck Pathol. 2020;14:593–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavaliere M, De Luca P, Scarpa A, et al. Acinic cell carcinoma of the parotid gland: from pathogenesis to management: a literature review. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies. 2020;277:2673–2679. [DOI] [PubMed] [Google Scholar]
- 21.Skalova A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34:599–608. [DOI] [PubMed] [Google Scholar]
- 22.Lewis JE, Olsen KD, Weiland LH. Acinic cell carcinoma. Clinicopathologic review. Cancer. 1991;67:172–179. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Su M, Yang Y, et al. Prognostic Factors Associated With Decreased Survival in Patients With Acinic Cell Carcinoma of the Parotid Gland. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2017;75:416–422. [DOI] [PubMed] [Google Scholar]
- 24.Park YM, Yoon SO, Kim JH, et al. Comprehensive Analysis of Clinicopathologic Factors Predictive of an Unfavorable Prognosis in Patients With Acinic Cell Carcinoma of the Parotid Gland. Clinical and experimental otorhinolaryngology. 2021;14:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez DR, Katabi N, Zhung J, et al. Clinical and pathologic prognostic features in acinic cell carcinoma of the parotid gland. Cancer. 2009;115:2128–2137. [DOI] [PubMed] [Google Scholar]
- 26.Barnes EL, Eveson JW, Reichart P, et al. World Health Organization Classification of Tumours: pathology and genetics of head and neck tumours. Lyon: International Agency for Research on Cancer (IARC); 2005. [Google Scholar]
- 27.Schwarz S, Zenk J, Muller M, et al. The many faces of acinic cell carcinomas of the salivary glands: a study of 40 cases relating histological and immunohistological subtypes to clinical parameters and prognosis. Histopathology. 2012;61:395–408. [DOI] [PubMed] [Google Scholar]
- 28.Fang Q, Wu J, Du W, et al. Predictors of distant metastasis in parotid acinic cell carcinoma. BMC Cancer. 2019;19:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ali SA, Kovatch KJ, Yousif J, et al. Predictors of distant metastasis in acinic cell carcinoma of the parotid gland. World journal of clinical oncology. 2020;11:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sideris A, Rao A, Maher N, et al. Acinic cell carcinoma of the salivary gland in the adult and paediatric population: a survival analysis. ANZ journal of surgery. 2020. [DOI] [PubMed] [Google Scholar]
- 31.Lee DY, Brayer KJ, Mitani Y, et al. Oncogenic Orphan Nuclear Receptor NR4A3 Interacts and Cooperates with MYB in Acinic Cell Carcinoma. Cancers. 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


