Abstract
Various emerging technologies challenge existing governance processes to identify, assess, and manage risk. Though the existing risk-based paradigm has been essential for assessment of many chemical, biological, radiological, and nuclear technologies, a complementary approach may be warranted for the early-stage assessment and management challenges of high uncertainty technologies ranging from nanotechnology to synthetic biology to artificial intelligence, among many others. This paper argues for a risk governance approach that integrates quantitative experimental information alongside qualitative expert insight to characterize and balance the risks, benefits, costs, and societal implications of emerging technologies. Various articles in scholarly literature have highlighted differing points of how to address technological uncertainty, and this article builds upon such knowledge to explain how an emerging technology risk governance process should be driven by a multi-stakeholder effort, incorporate various disparate sources of information, review various endpoints and outcomes, and comparatively assess emerging technology performance against existing conventional products in a given application area. At least in the early stages of development when quantitative data for risk assessment remain incomplete or limited, such an approach can be valuable for policymakers and decision makers to evaluate the impact that such technologies may have upon human and environmental health.
Keywords: Synthetic biology, Biotechnology, Nanotechnology, Governance, Risk Assessment, Policy, Decision analysis, Regulations
Introduction
Emerging technologies, including the key enabling technologies, promise revolutionary benefits for humanity and the natural environment. However, some of them present uncertain risks along with uncertain or untested mechanisms for observation and monitoring. For example, the consequences of deploying certain applications deriving from nanotechnology or synthetic biology are yet very uncertain (especially if considering issues of biosafety and biosecurity) (König et al. 2016; Mukunda et al. 2009). Furthermore, as scientists and industry may not agree in either methods for assessing potential risks and consequences and data, various interpretations of the science may emerge, together with ambiguity and possible divergent perceptions of risks and benefits associated with the technologies (Renn et al. 2011; Falkner and Jaspers 2012). Next to technologies that have the capacity to fundamentally alter or even synthesize living organisms in complex socio-ecological systems and involve challenging issues of values and ethics, some emerging technologies may enhance applications of existing technologies involving new materials and processes (e.g., graphene or hydraulic fracturing) (Small et al. 2014; Linkov et al. 2014).
The pace of technology development is increasing, and will need regulators and other key stakeholders in industry and academia to continue to increase to meet increasing challenges to the status quo and to sustainability (Linkov et al. 2018). In part, this has led to public suspicion, sometimes mistrust, often unease, increasing vulnerability of objective valuations to misclaims made by interest groups and misguided individuals, tainting the well of public and consumer interest. As our world continues to develop technologically, so too must our ability to deal with a heterogeneity of knowledge and level of uncertainties (Scott-Fordsmand et al. 2014; Subramanian et al. 2014; Kuzma et al. 2008; Calvert and Martin 2009). Experts, policymakers, and regulators should design prospective, adaptive, and knowledge-based benefits and risks assessments and governance processes (Tait 2012).
Current practice relies upon risk assessment to quantify the risks of materials and technologies and upon management to control risks, typically by limiting exposure of humans and environmental receptors, are limited to acceptable levels. For mature and well-defined technologies, the current risk assessment/management approach has a long history of delivering valuable insight to regulators regarding how to establish best practices of policy and governance for various fields (Malloy et al. 2016; Seager et al. 2017; Shatkin 2008).
However, three features of the conventional approach hinder its effective application to emerging technologies. First, it typically requires substantial quantitative data regarding hazards, consequences, and exposure regarding the material or technology in question (Rycroft et al. 2018; Shatkin et al. 2016). Such data are often limited or unavailable due to the unique physical qualities of new materials as, for example, the unique and uncertain human health hazards that might occur within synthetic biology development (Epstein and Vermeire 2016). Second, it assumes that the potential consequences of using novel materials and technologies can be comprehensively cataloged (Hristozov et al. 2012, 2016). Emerging technologies such as synthetic biology and artificial intelligence intersect with complex biological, ecological, and sociotechnical systems, raising the specter of cascading effects and unpredictable outcomes. Given the limitations of current approaches to facilitate risk assessment of highly uncertain emerging technologies, a different approach is strongly desirable to balance development of innovative technologies with responsible use (see additional discussion for biotechnology in Vallero 2015). Finally, an innovation often challenges several policy areas that are used to operating in silos, whereas innovation may require more flexible, adaptive, and integrated approaches.
Risk governance for emerging technologies
In this context, it is worth considering the recommendations from the International Risk Governance Council, which describes that risk governance sits as the confluence of all analyses and actions relative to the development of a given technology (Renn 2005). This includes (i) framing the technology in the context of its possible deployment and applications, benefits, and risks for various stakeholders, (ii) assessing those benefits and risks (including assessment of perception and concerns), (iii) evaluating other aspects that decision makers will consider before making decisions, such as the existence of specific economic, political or societal interests, or also certain issues of national security or ideology, that must be considered, (iv) identifying various risk management options, which can be combined to establish a strategy for the development (or not) of the technology, and (v) communicating about risk and benefits. As will be described below, the advantages of such a risk governance approach for emerging technologies are driven by several key factors, including the following: the collaborative nature of such an approach amongst multiple pertinent stakeholders, its ability to integrate various sources of qualitative, semi-quantitative, and quantitative information to assess such technologies, and the various criteria of risk, cost, benefit, social implications, and other considerations that are inherently valuable to any such governance decision.
A comprehensive approach to potential risks involved in the development of emerging technologies requires a collaborative effort among different stakeholders, as the problem-solving capacities of the individual actors within government, industry, academia, and civil society are limited and often unequal to the major challenges of governing uncertain risks (Kuzma 2015). Therefore, there is a need to engage these stakeholder groups in a continuous dialogue and coordinate a profusion of roles, perspectives and goals in the process of the development and implementation of safe guidelines and good practices consistent with recent scientific advancements (Schmidt et al. 2009). Such guidance may arise in the form of formal legal requirements, such as new laws or regulatory instruments, or less formally via voluntary participation within multi-party codes of conduct.
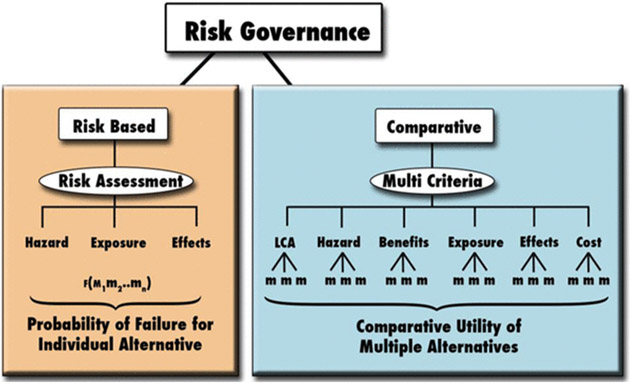
A comparative approach in risk governance is needed to address emerging technologies of this sort and to prove an environment that fosters responsible innovation (Renn et al. 2011; Linkov et al. 2013). This evolution in risk governance must overcome both institutional momentum and vested interests dedicated to the continuance of traditional approaches, a step outside our comfort zones effecting change in how we think about risk and its governance. Comparative risk governance differs from the conventional approach in several ways. First, it eschews a narrow focus on identifying and controlling quantifiable effects of new materials or technologies taken by conventional risk assessment and management (Canis et al. 2010). The approach should explicitly identify and address the trade-offs that must be made, by assessing the risks involved in a proposed new activity against other feasible alternatives, including safer designs that avoid or minimize risk by reducing the inherent hazard or exposure of the emerging material or technology itself. This idea is visually represented in Fig. 1, where disparate criteria such as cost, benefit, risk, and social utility are analyzed via relevant utility functions and then aggregated via a semi-quantitative metric. This is what the US chemical regulation aims to do when it pursues three policy objectives for assessing and regulating (i) the chemical effects on human health and the environment, (ii) the benefits of use and the availability of substitutes, and (iii) the effects on the economy and innovation. Further, the relation between trade-off analysis and sustainability has been considered in the US National Research Council report on Sustainability at the US Environmental Protection Agency (EPA) (NRC 2011).
Fig. 1.
Differentiation of a traditional ‘risk-based’ and a ‘comparative-based’ approach to risk policy and governance for emerging technologies
Second, recognizing that comprehensive quantitative data will be either unavailable or involve too much uncertainty to be reliable, governance should not require the collection of absolute measures of acceptable risk. Instead, governance should be based on collaboration between policymakers, regulators, industrial developers, workers, experts, and representatives of society from multiple disciplines, in a manner that establishes safe guidelines and best practices consistent with recent scientific advancements and expected new developments (Renn 2005; Trump et al. 2017; Kuiken et al. 2014). Such effort requires assessment across a material’s life cycle, including insight from laboratory researchers and workers involved in the initial production of a material who are particularly at risk, to safe containment and shipping, to consumer product safety, to proper end-of-life disposal.
Current practices for emerging technologies must emphasize proactive and adaptive approaches to risk management and governance whenever risk assessment is hindered by limited availability of experimental data and the state of development (Oye 2012; Tait 2009; Trump 2017; Cummings et al. 2017). Comparative approaches driven by expert opinion and stakeholder engagement may help overcome at an early stage the limitations of quantitative risk assessment approaches through:
- an impacts analysis of technological substitution based on:
- a critical review of the risks potentially associated with an application of an emerging technology against a conventional technological application that it would replace,
- a review of how such a novel technology produces further economic, health, or social benefits and costs in lieu of the conventional alternative (Mohan et al. 2012),
- a review of the trade-offs between risks and between risks and opportunities, and an explicit and transparent communication about those trade-offs (Blaunstein et al. 2014; Yatsalo et al. 2016),
- considerations of other risk factors including social perception and the engagement of the public in an evaluation and decision-making process (Palma-Oliveira et al. 2017; Siegrist et al. 2007; Trump et al. 2015), as well as cost of development that may help or hinder continued research and maturation of the emerging technology, and
a participative and deliberate decision process to monitor risks and impacts of the new technology and integrate feedback into review of initial assessment (and subsequent management decisions) (Cummings and Kuzma 2017).
This is a realistic approach to reviewing the risks and benefits associated with an emerging and potentially disruptive technology in a manner that accounts for both physical (e.g., health and environment), economic, and social outcomes. The approach requires the willingness of the public and private actors and their engagement on knowledge-based adaptive assessment and decision processes where new expert judgment and stakeholder opinions data are analyzed and integrated as it arises (Linkov et al. 2011; Wood et al. in press). If necessary, best practices for technology governance would shift, based upon experimentation and testing and integrating feedback into revisions of the early decisions. Combining risk characterization with quantitative risk assessments require new techniques such as integrating narratives in scenario construction, using stakeholder engagement methods for calibrating expert judgments and applying recursive methods of data generation and analysis such as cross-balance impact analysis (Mandel and Marchant 2014).
Expert elicitation has been a valuable tool for potential environmental risks associated with nanotechnologies (Trump et al. in press). The National Research Council Red Book provides a risk framework for integrating empirical information with scientific judgment (NRC 1983; Small et al. 2014). Indeed, the U.S. has followed this framework for numerous comparative risk applications, including regulation of particulate matter, nuclear waste, and food safety. Uncertainty is particularly large when assessing the life cycles of the vast majority of chemical compounds (Csiszar et al. 2016; Malloy et al. 2016; Seager and Linkov 2008). Since risk is a function of both hazard and exposure, much of the uncertainty associated with new chemicals entering the marketplace is due to the paucity of reliable information regarding the toxicity, and even greater uncertainty about the frequency and extent of an individual’s contact with a specific compound given typical utilizations of that chemical (e.g., cosmetics, cleaning products, etc.) and individuals’ use patterns compared to the intended use (Grieger et al. 2009; Wilson and Schwarzman 2009; Ferson and Sentz 2016; Linkov et al. 2017).
Discussion
Such efforts to develop new approaches for governing risks involved in emerging technologies must adopt a holistic perspective of the elements of technology governance. Alongside analytical components of risk assessment, other elements should include active horizon scanning and anticipatory review of emerging technologies, methodological aspects of safe-by-design approaches, effective risk communication and engagement with publics on key issues regarding traditional technology risk (e.g., health implications), as well as non-traditional risk considerations (e.g., ethical/moral considerations, cost, social impact) (Gronvall 2018). This process should also work within the given framework of the jurisdiction at hand, where risk governance in the United States, European Union, and elsewhere must account for the unique institutional, political, and research environments that influence regulatory decision making and policymaking (Malsch et al. 2018).
Ultimately, a risk governance approach for emerging technologies will assist with the risk-based approaches utilized by regulators and other risk assessors by accounting for a broad view of comparative assessment of emerging and conventional technologies (Tervonen et al. 2009). The approach will help with early-stage guidance for emerging technologies like synthetic biology by generating information about expert perceptions of technological risk, benefit, time to development, ethics, cost, and various other considerations that all influence how a technology may assist with economic, medical, environmental, and social wellbeing (Bates et al. 2015). Such an approach inherently requires a collaborative effort between various stakeholders for an emerging technology’s governance, where input from industry, workers, academia, government, non-governmental institutions, and civil society at large will not only help evaluate the benefits and risks of an uncertain technology, but also address public wariness to adopt and utilize such technologies as they enter the marketplace.
This approach may open new opportunities to improve public trust on regulation as informed guidance. A significant dividend of the approach is to facilitate an anticipatory and adaptive style of governance for emerging technologies, where governments would be increasingly able to perceive the impacts and applications of enabling and emerging technologies on the horizon, while iteratively improving risk assessment for such technologies as quantitative guidance becomes available (Mandel and Marchant 2014; Trump et al. 2017). This approach is expected to offer a broader set of evidence-based considerations than traditional risk assessment/management, supporting democratic decision making on governing the emerging technologies.
Acknowledgements
The authors were participants in the “Risk Policy Forum on Key Enabling Technologies” hosted by the Society for Risk Analysis, and Ca Foscari University, Venice, Italy, on March 2017. This workshop and paper were part of a SRA New Initiative Project. The authors thank the conference attendees and panelists that drove discussions, as well as Emily Wells for her editorial assistance, and George Shephard for his figure design assistance. The views expressed within this paper are solely the opinions of the authors, and are not necessarily representative of their organizational affiliations.
References
- Bates M, Grieger KD, Trump BD, Keisler JM, Plourde KJ, Linkov I (2015) Emerging technologies for environmental remediation: integrating data and judgment. Environ Sci Technol 50:349–358 [DOI] [PubMed] [Google Scholar]
- Blaunstein R, Trump B, Linkov I (2014) Nanotechnology risk management: an insurance industry perspective. In: Hull M, Bowman D (eds) Nanotechnology environmental health and safety: risks, regulation, and management, 2nd edn. Elsevier, Oxford, pp 247–263 [Google Scholar]
- Calvert J, Martin P (2009) The role of social scientists in synthetic biology. EMBO Rep 10(3):201–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canis L, Linkov I, Seager TP (2010) Application of stochastic multiattribute analysis to assessment of single walled carbon nanotube synthesis processes. Environ Sci Technol 44(22):8704–8711 [DOI] [PubMed] [Google Scholar]
- Csiszar SA, Meyer DE, Dionisio KL, Egeghy P, Isaacs KK, Price PS et al. (2016) Conceptual framework to extend life cycle assessment using near-field human exposure modeling and high-throughput tools for chemicals. Environ Sci Technol 50(21):11922–11934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings CL, Kuzma J (2017) Societal risk evaluation scheme (SRES): scenario-based multi-criteria evaluation of synthetic biology applications. PLoS ONE 12:e0168564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings CL, Lin SH, Trump BD (2017) Public perceptions of climate geoengineering: a systematic review of the literature. Clim Res 73(3):247–264 [Google Scholar]
- Epstein MM, Vermeire T (2016) Scientific opinion on risk assessment of synthetic biology. Trends Biotechnol 34(8):601–603 [DOI] [PubMed] [Google Scholar]
- Falkner R, Jaspers N (2012) Regulating nanotechnologies: risk, uncertainty and the global governance gap. Global Environ Politics 12(1):30–55 [Google Scholar]
- Ferson S, Sentz K (2016) Epistemic uncertainty in agent-based modeling. In: 7th international workshop on reliable engineering computing [Google Scholar]
- Grieger K, Hansen SF, Baun A (2009) The known unknowns of nanomaterials: describing and characterizing uncertainty within environmental, health and safety risks. Nanotoxicology 3:222–233 [Google Scholar]
- Gronvall GK (2018) Safety, security, and serving the public interest in synthetic biology. J Ind Microbiol Biotechnol 21:1–4 [DOI] [PubMed] [Google Scholar]
- Hristozov DR, Gottardo S, Critto A, Marcomini A (2012) Risk assessment of engineered nanomaterials: a review of available data and approaches from a regulatory perspective. Nanotoxicology 6(8):880–898 [DOI] [PubMed] [Google Scholar]
- Hristozov D, Gottardo S, Semenzin E, Oomen A, Bos P, Peijnenburg W et al. (2016) Frameworks and tools for risk assessment of manufactured nanomaterials. Environ Int 95:36–53 [DOI] [PubMed] [Google Scholar]
- König H, Frank D, Heil R, Coenen C (2016) Synthetic biology’s multiple dimensions of benefits and risks: implications for governance and policies. In: Boldt J (ed) Synthetic biology. Springer Fachmedien, Wiesbaden, pp 217–232 [Google Scholar]
- Kuiken T, Dana G, Oye K, Rejeski D (2014) Shaping ecological risk research for synthetic biology. J Environ Stud Sci 4(3):191–199 [Google Scholar]
- Kuzma J (2015) Translational governance research for synthetic biology. J Responsible Innov 2(1):109–112 [Google Scholar]
- Kuzma J, Paradise J, Ramachandran G, Kim J, Kokotovich A, Wolf SM (2008) An integrated approach to oversight assessment for emerging technologies. Risk Anal 28(5):1197–1220 [DOI] [PubMed] [Google Scholar]
- Linkov I, Bates ME, Canis LJ, Seager TP, Keisler JM (2011) A decision-directed approach for prioritizing research into the impact of nanomaterials on the environment and human health. Nat Nanotechnol 6(12):784. [DOI] [PubMed] [Google Scholar]
- Linkov I, Bates ME, Trump BD, Seager TP, Chappell MA, Keisler JM (2013) For nanotechnology decisions, use decision analysis. Nano Today 8(1):5–10 [Google Scholar]
- Linkov I, Trump B, Jin D, Mazurczak M, Schreurs M (2014) A decision-analytic approach to predict state regulation of hydraulic fracturing. Environ Sci Eur 26(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkov I, Trump BD, Wender BA, Seager TP, Kennedy AJ, Keisler JM (2017) Integrate life-cycle assessment and risk analysis results, not methods. Nat Nanotechnol 12(8):740–743 [DOI] [PubMed] [Google Scholar]
- Linkov I, Trump BD, Poinsatte-Jones K, Florin MV (2018) Governance strategies for a sustainable digital world. Sustainability 10(2):440 [Google Scholar]
- Malloy T, Trump BD, Linkov I (2016) Risk-based and prevention-based governance for emerging materials. Environ Sci Technol 50:6822–6824 [DOI] [PubMed] [Google Scholar]
- Malsch I, Mullins M, Semenzin E, Zabeo A, Hristozov D, Marcomini A (2018) Decision support for international agreements regulating nanomaterials. NanoEthics 12(1):39–54 [Google Scholar]
- Mandel G, Marchant GE (2014) The living regulatory challenges of synthetic biology. Iowa L Rev 100:155 [Google Scholar]
- Mohan M, Trump BD, Bates ME, Monica JC Jr, Linkov I (2012) Integrating legal liabilities in nanomanufacturing risk management. Environ Sci Technol 46(15):7955–7962 [DOI] [PubMed] [Google Scholar]
- Mukunda G, Oye KA, Mohr SC (2009) What rough beast? Synthetic biology, uncertainty, and the future of biosecurity. Politics Life Sci 28(2):2–26 [DOI] [PubMed] [Google Scholar]
- National Research Council (1983) Risk assessment in the federal government: managing the process. National Academies Press, Washington, DC: [PubMed] [Google Scholar]
- National Research Council (2011) Sustainability and the U.S. EPA. The National Academies Press, Washington, DC. 10.17226/13152 [DOI] [Google Scholar]
- Oye KA (2012) Proactive and adaptive governance of emerging risks: the case of DNA synthesis and synthetic biology. International Risk Governance Council, Geneva [Google Scholar]
- Palma-Oliveira JM, Trump BD, Wood MD, Linkov I (2017) Community-driven hypothesis testing: a solution for the tragedy of the anticommons. Risk Anal 38:620–634 [DOI] [PubMed] [Google Scholar]
- Renn O (2005) White paper on risk governance: towards an integrative approach. International Risk Governance Council, Geneva [Google Scholar]
- Renn O, Klinke A, van Asselt M (2011) Coping with complexity, uncertainty, and ambiguity in risk governance: a synthesis. Ambio 40:231–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rycroft T, Trump B, Poinsatte-Jones K, Linkov I (2018) Nanotoxicology and nanomedicine: making development decisions in an evolving governance environment. J Nanopart Res 20(2):52 [Google Scholar]
- Schmidt M, Kelle A, Ganguli-Mitra A, de Vriend H (eds) (2009) Synthetic biology: the technoscience and its societal consequences. Springer, New York [Google Scholar]
- Scott-Fordsmand JJ, Pozzi-Mucelli S, Tran L, Aschberger K, Sabella S, Vogel U et al. (2014) A unified framework for nanosafety is needed. Nano Today 9(5):546–549 [Google Scholar]
- Seager TP, Linkov I (2008) Coupling multicriteria decision analysis and life cycle assessment for nanomaterials. J Ind Ecol 12(3):282–285 [Google Scholar]
- Seager TP, Trump BD, Poinsatte-Jones K, Linkov I (2017) Why life cycle assessment does not work for synthetic biology. Environ Sci Technol. 10.1021/acs.est.7b01604 [DOI] [PubMed] [Google Scholar]
- Shatkin JA (2008) Informing environmental decision making by combining life cycle assessment and risk analysis. J Ind Ecol 12(3):278–281 [Google Scholar]
- Shatkin JA, Ong KJ, Beaudrie C, Clippinger AJ, Hendren CO, Haber LT et al. (2016) Advancing risk analysis for nanoscale materials: report from an international workshop on the role of alternative testing strategies for advancement. Risk Anal 36(8):1520–1537 [DOI] [PubMed] [Google Scholar]
- Siegrist M, Keller C, Kastenholz H, Frey S, Wiek A (2007) Laypeople’s and experts’ perception of nanotechnology hazards. Risk Anal 27(1):59–69 [DOI] [PubMed] [Google Scholar]
- Small M, Stern PC, Bomberg E, Christopherson SM, Goldstein BD, Israel AL, Jackson RB, Krupnick A, Mauter MS, Nash J, North DW, Olmstead SM, Prakash A, Rabe B, Richardson N, Tierney S, Webler T, Wong-Parodi G, Zielinska B (2014) Risks and risk governance in unconventional shale gas development. Environ Sci Technol 48:8289–8297 [DOI] [PubMed] [Google Scholar]
- Subramanian V, Semenzin E, Hristozov D, Marcomini A, Linkov I (2014) Sustainable nanotechnology: defining, measuring and teaching. Nano Today 9(1):6–9 [Google Scholar]
- Tait J (2009) Governing synthetic biology: processes and outcomes. In: Schmidt M (ed) Synthetic biology. Springer, Dordrecht, pp 141–154 [Google Scholar]
- Tait J (2012) Adaptive governance of synthetic biology. EMBO Rep 13(7):579–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervonen T, Linkov I, Figueira JR, Steevens J, Chappell M, Merad M (2009) Risk-based classification system of nanomaterials. J Nanopart Res 11(4):757–766 [Google Scholar]
- Trump BD (2017) Synthetic biology regulation and governance: lessons from TAPIC for the United States, European Union, and Singapore. Health Policy 121(11):1139–1146 [DOI] [PubMed] [Google Scholar]
- Trump BD, Linkov F, Edwards RP, Linkov I (2015) Not a Humbug: the evolution of patient-centred medical decision-making. Evid Based Med 20(6):193–197 [DOI] [PubMed] [Google Scholar]
- Trump BD, Cummings C, Kuzma J, Linkov I (2017) A decision analytic model to guide early-stage government regulatory action: applications for synthetic biology. Regul Gov. 10.1111/rego.12142 [DOI] [Google Scholar]
- Trump BD, Hristozov D, Malloy T, Linkov I (in press) Risk associated with engineered nanomaterials: different tools for different ways to govern. Nano Today [Google Scholar]
- Vallero D (2015) Environmental biotechnology: a biosystems approach. Academic Press, Amsterdam [Google Scholar]
- Wilson MP, Schwarzman MR (2009) Toward a new US chemicals policy: rebuilding the foundation to advance new science, green chemistry, and environmental health. Environ Health Perspect 117(8):1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MD, Plourde K, Larkin S, Egeghy PE, Williams AJ, Zemba V, Linkov I, Vallero DA (in press) Advances on a decision analytic approach to exposure-based chemical prioritization. Risk Anal [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsalo B, Gritsyuk S, Sullivan T, Trump B, Linkov I (2016) Multi-criteria risk management with the use of DecernsMCDA: methods and case studies. Environ Syst Decis 36(3):266–276 [Google Scholar]