Abstract
Background
Although non-stroke vertebral artery dissection (VAD) is diagnosed using MRI, detecting the subtle intravascular structure remains challenging. This study aimed to evaluate the validity of quantitative intravascular scanning based on novel zoomed high-resolution black blood (Z-HB) MRI for distinguishing VAD from other vessel pathologies.
Methods
Twenty-one patients with non-stroke VAD and 18 with symptomatic atherosclerotic plaques in their vertebral artery underwent Z-HB MRI and subsequent profile curve processing. Axial Z-HB imaging was obtained from dissected and normal segments in patients with VAD and atherosclerotic plaque in patients with ischemia. We investigated the qualitative categorization of the scanning patterns of the intravascular signals. We also evaluated the quantitative ability of each profile curve to discriminate multiple vessel pathologies by analyzing the receiver operating characteristics curves.
Results
Profile curve processing of 140 Z-HB images categorized the intravascular signal patterns into luminal, asymmetrical, and omega types. The asymmetrical type included both dissecting and atherosclerotic vessels, and the omega type included dissecting and normal vessels. In the asymmetrical type, quantitative evaluation successfully distinguished intramural hematomas of VAD from atherosclerotic plaque with an area under the curve of 0.80. The intimal flap of the VAD was distinguished from the blood flow artifact of the normal vessel with an area under the curve of 0.93 in the omega type.
Conclusions
A combination of novel Z-HB MRI and profile curve processing provided an ultra-high-resolution analysis of the intravascular structure of non-stroke VAD and successfully distinguished VAD from normal vessels or atherosclerotic plaques.
Keywords: Vertebral artery dissection, black-blood MRI, ultra-high resolution, profile curve, intimal flap
Introduction
Intracranial vertebral artery dissections (VADs) present with either subarachnoid hemorrhage, ischemic stroke, or pain only.1–3 Although the clinical course of pain-only (non-stroke) VAD is generally favorable, early detection, pain management, and blood pressure control are critical to prevent deterioration, including aneurysmal enlargement and rupture.4–7
In recent years, MRI has been more commonly used as a less invasive diagnostic tool for non-stroke VAD. 3 Although VAD is characterized by a change in the intravascular signals representing the intimal flap or intraluminal hematoma on MRI, these findings are not necessarily clear when examined early after dissection.1,2,8 This study aimed to evaluate the validity of our novel MRI technique named zoomed high-resolution black blood (Z-HB) MRI, which provides better contrast and higher-resolution images of the intravascular structure of the vertebral artery to properly detect and discriminate non-stroke VAD.
Methods
Patient selection and study design
Since June 2016, we have added our novel MRI sequence “Z-HB” to conventional MRA for patients suspected of having radiologically or clinically abnormal findings in their intracranial vertebral arteries. Between June 2016 and June 2019, patients with non-stroke VAD were diagnosed according to the conventional diagnostic criteria9,10 (Table 1). According to these criteria, some patients were diagnosed as having “definite VAD” at the initial MRA, while other patients were diagnosed as “probable VAD” at the first imaging, and the diagnosis of “definite VAD” was subsequently made based on chronological changes upon repeat imaging several days later. The patients underwent Z-HB when the conventional MRA revealed abnormal findings of their vertebral arteries, which could be responsible for their symptoms, at the attending physician’s discretion. Z-HB obtained on admission was analyzed, when some cases were diagnosed as “definite VAD” and others as “probable VAD.” The diagnosis of dissection was made using the conventional diagnostic criteria with conventional MRA alone, and Z-HB was used as a supporting tool for detecting intravascular abnormal structures. Patients were included when they presented with sudden headache, and Z-HB MRI was performed within 10 days from the onset. Separately, in the same period, patients who had atherosclerotic ischemic stroke according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria 11 owing to significant stenosis (>50% according to Warfarin-Aspirin Symptomatic Intracranial Disease criteria 12 ) of their vertebral arteries, and underwent Z-HB MRI for their atherosclerotic plaque on admission within 3 days from the onset, were analyzed for comparison of the intravascular findings. Since we wished to systematically correlate the vessel pathologies with specific symptoms, asymptomatic VADs and VADs presenting with subarachnoid hemorrhage, ischemic stroke, or mass effect were excluded. No pathology was confirmed in any lesion since none of the patients underwent any surgical procedure. Thus, 21 patients with non-stroke definite VAD and 18 patients with atherosclerotic plaque met the criteria for review.
Table 1.
Diagnostic criteria for cervicocephalic arterial dissection.
| Major criteria |
| 1. “Double lumen” or “intimal flap” demonstrated on either DSA, MRI, MRA, CTA, or duplex ultrasonography. |
| 2. “Pearl and string sign” or “string sign” demonstrated on DSA. |
| 3. Pathological confirmation on arterial dissection. |
| Minor criteria |
| 4. “Pearl sign” or “tapered occlusion” demonstrated on DSA. |
| 5. “Pearl and string sign,” “string sign” or “tapered occlusion” demonstrated on MRA. |
| 6. “Hyperintense intramural signal” (corresponding to intramural hematoma) demonstrated on T1-weighted MRI. |
| Additional criteria |
| 7. Change in the arterial shape demonstrated on either DSA, MRI, MRA, CTA, or duplex ultrasonography. |
| 8. No other causes of arterial abnormalities. |
DSA: digital subtraction angiography; MRI: magnetic resonance imaging; MRA: magnetic resonance angiography; CTA: computed tomography angiography.
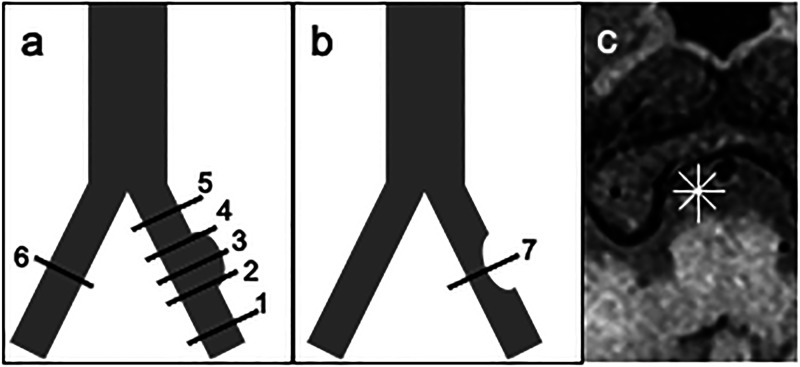
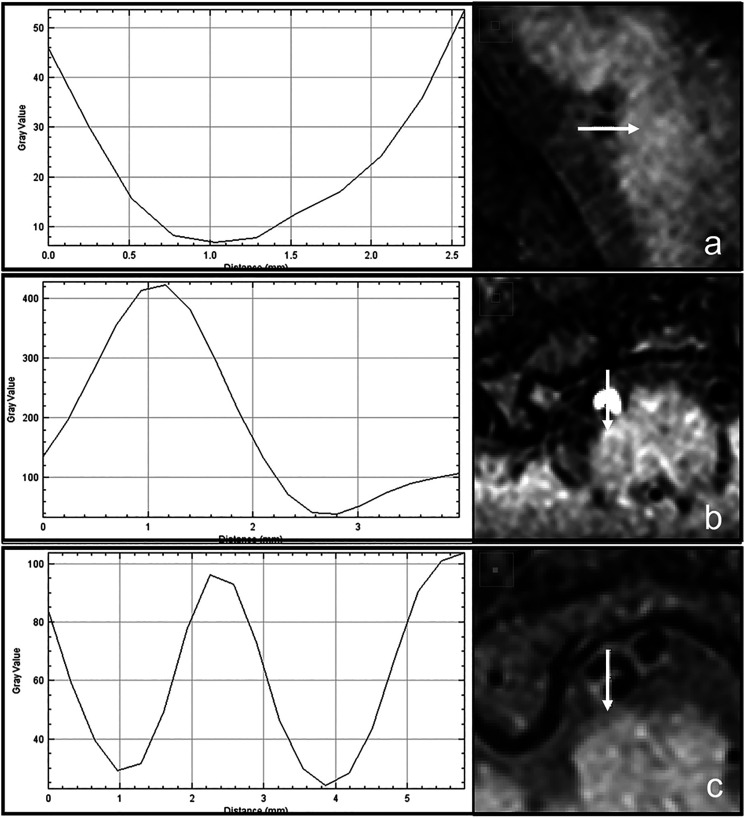
This is a single-center, retrospective cross-sectional study. All the patients underwent conventional MRA, T1WI, CTA, and Z-HB MRI. Schematic illustrations of the Z-HB imaging are shown in Figure 1. First, two independent observers (A. M. and H. M.) qualitatively evaluated the performance of Z-HB MRI in detecting intravascular structures of all the VAD pictures compared to conventional MRA and CTA. Thereafter, three axial images were obtained for the dissection segment, and another three for the normal segment as a within-subject control (Figure 1(a)). In the patients with atherosclerotic plaques, only a single axial image was obtained for the atherosclerotic segment, which is generally shorter than dissection, as a control against the eccentric lesion of the VAD (Figure 1(b)). In each axial image, four lines were drawn across the center of the vessel caliber at every 45° (Figure 1(c)). Signal intensity changes along the four lines were recorded using an image analysis software, and the graph with the most prominent signal intensity change was determined as the “profile curve” of the axial image. In general, the graph of the line that perpendicularly penetrated the intravascular structures was selected. Preliminary observation revealed that the profile curves could be categorized into three patterns: luminal, asymmetrical, and omega (𝜔). Examples of each profile curve type and the corresponding Z-HR image are shown in Figure 2. The luminal type was defined as one downward peak between the vessel walls at both ends. The asymmetrical type was defined as one upward and one downward peak, and the omega type as one upward peak sandwiched by two downward peaks. Each profile curve was evaluated and categorized into either of the three shape patterns by two independent observers (A. M. and H. M.) who were blinded to the patient and vessel characteristics. In case of discrepancy, consensus reading was employed. We subsequently investigated whether each qualitative pattern was associated with a vessel pathology and whether the quantitative assessment of the curve properly discriminates multiple vessel pathologies within a single profile curve pattern.
Figure 1.
Methodology of axial image sampling from dissecting and atherosclerotic vertebral arteries. (a) A schematic drawing of the VAD axial image sampling. VAD axial imaging is obtained at the proximal, middle, and distal points of the dissecting segment (2, 3, and 4). Normal vessel imaging as a control is obtained in the ipsilateral vertebral artery (1, 5) and contralateral vertebral artery (6). (b) A schematic drawing of the axial image sampled from an atherosclerotic vertebral artery (7). (c) Radiating lines are set at every 45° on axial imaging to obtain the most characteristic profile curve. VAD, vertebral artery dissection.
Figure 2.
Creation and categorization of the profile curve across the center of the vessel. Arrows indicate the location and direction of signal intensity scanning. (a) Luminal type, (b) asymmetrical type, (c) omega type.
Definite dissection
Presence of one or more of the major criteria, or presence of one or more of the minor criteria and both the additional criteria.
Probable dissection
Presence of one or more of the minor criteria.
Radiological evaluation
Three-dimensional CTA protocol was set at 120 kV, auto mA, 0.75 s/rotation, and helical pitch of 0.637. The injection rate of the contrast media varied by weight into 300 mgI/kg. MRI (TOF and Z-HR) was conducted using a 3.0T-MR system (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) with a 32-channel head coil. TOF-MRA images were used for the diagnosis of VAD. The three-dimensional two slabs TOF-MRA protocol was TR, 21.0 ms; TE, 3.69 ms; FOV, 180 mm; averages, 1; FA, 11°; matrix, 256 × 320; slice thickness, 0.55 mm; and NEX, 1. The Z-HB protocol was a three-dimensional-variable flip angle Turbo Spin Echo sequence with 600/31/2 (repetition time/echo time/excitation); FOV, 90 mm × 180 mm; thickness, 0.6 mm; imaging range, 40 mm; matrix, 122 × 256 with a square pixel of 0.4 mm × 0.4 mm; fat suppression (+); and acquisition time, 4 min 32 s for the Z-HB imaging. Since the inner volume excitation called “ZOOMit” selectively excites the defined imaging range, imaging slices can be arbitrarily changed while eliminating the aliasing effect. Moreover, by using “ZOOMit,” the smaller imaging range can reduce the scan time by a synergistic effect of decreasing the number of phase encoding and avoiding oversampling, thereby preventing g-factor-associated SNR reduction compared to that of parallel imaging. 13 Short axis images were processed to obtain Z-HB images using a workstation (Ziostation version 2.1; Ziosoft, Inc, Redwood City, California). A signal intensity profile curve was drawn by scanning the intravascular structures along the axis using Image-J software (https://imagej.nih.gov/ij/index.html), which has been widely used for CT and MR image processing. 14
Statistical analysis
Quantitative variables are expressed as the mean ± standard deviation or the median value and interquartile range (interquartile range, 25th–75th percentile) as appropriate. Associations between the categorical variables were analyzed using the chi-square or Fisher’s exact test. The normality of the data was evaluated using the Shapiro–Wilk test. Non-normally distributed continuous variables were analyzed using the Mann–Whitney U test. The Cohenk κ value was calculated to quantify the level of agreement concerning the categorization of the profile curve, which was interpreted as poor (<0.20), fair (0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80), or almost perfect (0.81–1.0). 15 In the quantitative analysis of the profile curve, a receiver operating characteristic curve was drawn to evaluate the discriminative ability and the optimal cut-off value of the intravascular signal intensity values. The area under the curve was calculated to determine the accuracy of the discrimination and was interpreted as good (0.9–1.0), moderate (0.7–0.9), or poor (0.5–0.7). The optimal cut-off value for discrimination was determined by identifying the nearest point to the upper-left corner of the graph (sensitivity, 1.0; 1-specificity, 0.0) 16 . P-values of less than 0.05 were considered statistically significant. All the statistical analyses were performed using SPSS version 25.0 (IBM Corp, Armonk, New York, USA).
Results
Patients’ Characteristics
The characteristics of the patients with VAD and atherosclerotic ischemic stroke are summarized in Table 2. Patients in the VAD group were significantly younger than those in the atherosclerotic group (p < .001, Mann–Whitney U test). Furthermore, comorbidities including hypertension (p = .001, chi-square test) and diabetes mellitus (p = .005, Fisher’s exact test) were less prevalent in the VAD group. Dissection of the vertebral artery in one patient extended to the basilar artery. The diameter of the contralateral vertebral artery was large enough for evaluation as a normal control segment in all the patients with VAD. The diagnosis of VAD on admission when Z-HB was obtained was “definite” in 15 patients and “probable” in six patients. Among the 15 definite cases, 14 patients were diagnosed with “double lumen or intimal flap” as major criteria in the one-point basis, while one patient was diagnosed based on the combination of meeting the minor criteria in the previous image and change in arterial shape upon repeat imaging (see representative case presentation below). All the six probable cases on admission were confirmed as “definite” upon repeat imaging several days later.
Table 2.
Baseline characteristics of patients who underwent zoomed high-resolution black blood MRI for their vertebral artery dissection and atherosclerosis.
| Variable (n = 39) | Vertebral artery pathology | p value | |
| Dissection (n = 21) | Atherosclerosis (n = 18) | ||
| Median age (yrs) | 50 (39–81) | 77 (65–94) | <.001* |
| Women | 9 (43) | 8 (44) | .80 |
| Hypertension | 9 (43) | 17 (94) | .001* |
| Diabetes mellitus | 1 (5) | 8 (44) | .005* |
| Hyperlipidemia | 8 (38) | 6 (33) | .76 |
Note: Variables showing significant difference by univariate analysis (p < 0.05) are indicated by asterisk (*). Data are presented as the median (interquartile range), or as the number of patients (%).
Categorization of the profile curve shape of Z-HB imaging
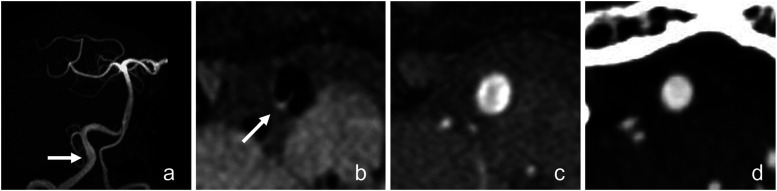
According to qualitative evaluation by two independent observers, the performance of Z-HB to detect intravascular structures in VAD was higher than that of conventional MRA or CTA generally because the hyperintense intravascular structures were well contrasted to the hypointense black-blood to the observers’ eyes. An example of higher performance of Z-HB imaging in detecting intimal flap is shown in Figure 3.
Figure 3.
Images obtained from a 74-year-old man who presented with a sudden headache. (a) TOF-MRA shows focal dilatation of the right vertebral artery (arrow). (b) Z-HB axial image of the dilated segment of the vertebral artery. The intimal flap is clearly visualized as a hyperintense line (arrow). TOF-MRA (c) and CTA (d) axial image of the dilated segment shows no intravascular signal change based on the observer’s eyes. Z-HB, zoomed high-resolution black blood.
Among the 144 axial Z-HB images obtained, two normal segment control images were unavailable owing to extradural extension of the dissection. Moreover, two images of the dissecting segment were considered inappropriate owing to insufficient suppression of the blood flow and were thus excluded. Thus, the profile curves were obtained from 63, 63, and 18 axial images of dissecting vessels, normal control vessels, and atherosclerotic vessels, respectively, which were qualitatively categorized into three different types; luminal, asymmetrical, and omega (𝜔). The Cohen κ value qualifying the level of interrater agreement was 0.917, indicating almost perfect degree of agreement. The association of each profile curve type with the vessel pathology is shown in Table 3. Most luminal types were observed in normal control segments. The asymmetrical type was observed in dissecting and atherosclerotic vessels, but never in normal control segments. The omega type was observed in both dissecting vessels and normal control segments, but never in atherosclerotic vessels.
Table 3.
Association of the profile curve types and vessel pathology.
| Profile curve type | Vessel pathology (n = 144) | ||
| Normal segment (n = 63) | Dissection (n = 63) | Atherosclerosis (n = 18) | |
| Luminal | 38 | 8 | 3 |
| Asymmetrical | 0 | 25 | 15 |
| Omega | 23 | 28 | 0 |
Note: Two vessels in the normal segment and two in the dissection are uncategorizable. Data are presented as the number of vessels.
Discrimination of the intravascular structure of dissecting vessels from other pathologies: Quantitative evaluation of Z-HB imaging
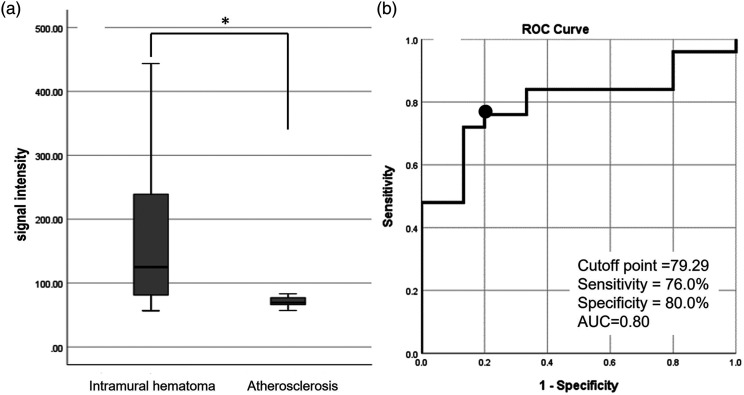
The profile curves of the Z-HB imaging were further quantitatively analyzed to assess whether the different vessel pathologies were properly discriminated in the asymmetrical and omega types. In the asymmetrical type, the signal intensity of the intravascular structure over the blood flow intensity was calculated by subtracting the absolute signal intensity value of the downward peak from that of the upward peak. As shown in Figure 4(a), the median signal intensity of the dissecting vessel was 125.9 (interquartile range, 77.0–242.2), which was significantly higher than that of the atherosclerotic plaque (median, 69.5 (interquartile range, 63.7–77.0)) (p = 0.001). Figure 4(b) shows the analysis of the receiver operating characteristics curve for the signal intensity of the intravascular structure to properly discriminate the VAD vessel and the atherosclerotic vessel. The optimal cut-off value of the signal intensity was 79.29, with a sensitivity of 76.0% and specificity of 80.0%. The area under the curve was 0.80, suggesting moderate accuracy for discrimination.
Figure 4.
Quantitative analysis of the signal intensity of the intravascular structure over that of the blood flow signal in the asymmetrical-type profile curve. (a) Box-and-whisker plot of the distribution of the signal intensity of the intravascular structure in VAD and atherosclerotic ischemic stroke. (b) Receiver operating characteristic curve of the signal intensity to discriminate VAD and atherosclerotic ischemic stroke. A signal intensity cut-off value of 79.29 (black dot) shows a sensitivity of 76.0% and specificity of 80.0%, with an area under the curve of 0.80. VAD, vertebral artery dissection.
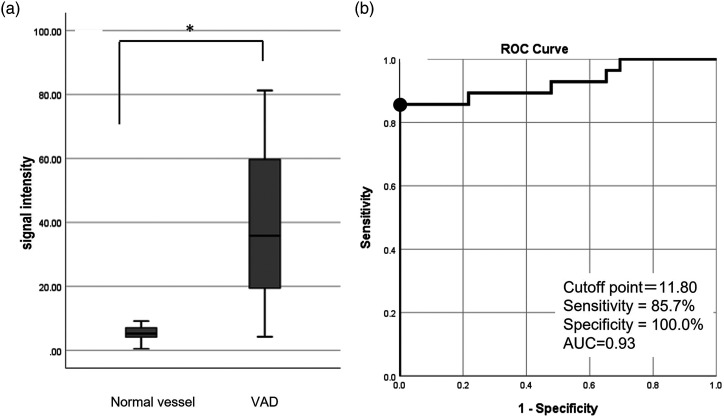
In the omega type, the signal intensity of the intravascular structure over the luminal flow intensity was calculated by subtracting the absolute signal intensity value of the lower value of the two downward peaks from that of the upward peak. As shown in Figure 5(a), the signal intensity of the dissecting vessel was 35.8 (18.7–60.4) (median (interquartile range)), which was significantly higher than that of the normal segment (5.2 (4.1–7.1)) by the Mann–Whitney U test (p < 0.001). Figure 5(b) shows the analysis of the receiver operating characteristics curve for the signal intensity of the intravascular structure to properly discriminate the dissecting vessel from the normal segment. The optimal cut-off value of the signal intensity was 11.80, with a sensitivity of 85.7% and specificity of 100%. The area under the curve was 0.93, suggesting moderate accuracy for discrimination.
Figure 5.
Quantitative analysis of the signal intensity of the intravascular structure over that of the blood flow signal in the omega-type profile curve. (a) Box-and-whisker plot of the distribution of the signal intensity of the intravascular structure in VAD and normal vessels. (b) Receiver operating characteristic curve of the signal intensity to discriminate VAD and normal vessels. A signal intensity cut-off value of 11.80 (black dot) shows a sensitivity of 85.7% and specificity of 100%, with an area under the curve of 0.93. VAD, vertebral artery dissection.
Representative case presentation
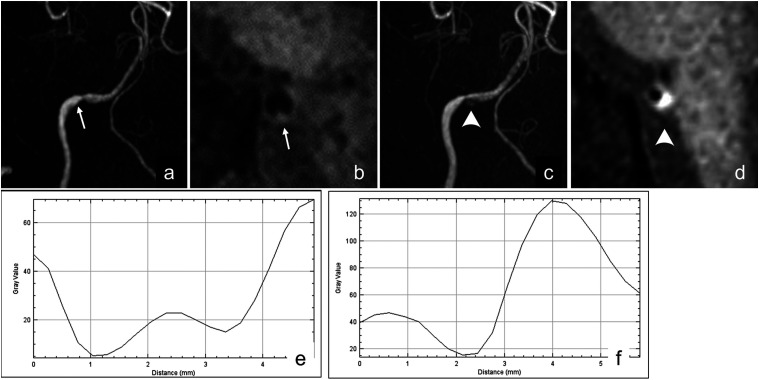
A 54-year-old man presented with sudden occipitalgia and was referred to our clinic. His head MRA showed mild stenosis of the right vertebral artery (Figure 6(a)). However, the patient was sent home because it did not appear as a dissection upon Z-HB imaging to the physician’s eyes (Figure 6(b)). Seven days later, he visited our clinic again for a deteriorating headache. Repeat MRA showed progressive stenosis and intramural hematoma (arrowheads) in his right vertebral artery (Figures 6(c) and (d)). He was diagnosed with non-stroke right VAD and admitted for pain and blood pressure control. His initial Z-HB imaging was retrospectively evaluated using a profile curve across the vessel, revealing the omega-type pattern (Figure 6(e)). By quantitative analysis, the signal intensity of the intravascular structure over the blood flow signal was 17.74, which was higher than the threshold mentioned above between the VAD and normal vessels. We presumed that his VAD commenced with intimal flap generation that progressed into intramural hematoma with a signal intensity of 111.00 in the pseudolumen (Figure 6(f)).
Figure 6.
Images obtained from a 54-year-old man who presented with a sudden headache. (a) TOF-MRA at the onset shows mild focal stenosis on his right vertebral artery (arrow). (b) Z-HB imaging of the stenotic segment shows an irregular vessel wall (arrow), but no intravascular structure is confirmed based on of the observer’s eyes. (c) TOF-MRA 7 days after the onset shows intramural hyperintensity (arrowheads). (d) Z-HB imaging of the stenotic segment shows eccentric hyperintensity (arrowheads). (e) Retrospectively, the profile curve of the Z-HB imaging obtained at onset is created and analyzed. The profile curve is categorized into omega type, with a signal intensity of the intravascular structure over that of the blood flow signal of 17.74, suggesting the presence of an intimal flap in VAD. f) The profile curve of the Z-HB imaging obtained 7 days after the onset is analyzed. The profile curve is categorized into the asymmetrical type, with the signal intensity of the intravascular structure over the blood flow signal being 111.00, suggesting the presence of intramural hematoma in VAD. VAD, vertebral artery dissection; Z-HB, zoomed high-resolution black blood.
Discussion
Although the clinical course of non-stroke pain-only VAD is generally favorable, early detection, pain management, and blood pressure control are proposed because some non-stroke VADs become symptomatic by enlargement or rupture.3,17–19 Instead of catheter angiography, MR imaging has been more common as a less invasive alternative diagnostic tool to detect non-stroke VAD. However, early detection of non-stroke VAD is not straightforward for several reasons. First, unlike ruptured VAD with acute laceration of the adventitia, caliber changes of early non-stroke VAD are minimal.1,2,8 Without drastic aneurysmal dilatation or severe stenotic changes, observers must diagnose VAD from a sole subtle intravascular signal change. Second, intramural hematoma, which is characteristic of VAD and is depicted as an extreme hyperintensity on T1-weighted imaging, is not yet formed at the early stage of dissection.20–22 Intimal flap, another intravascular finding of VAD, is sometimes too thin to be detected. Therefore, higher-resolution imaging than conventional 3.0-T MR imaging has been awaited to detect a subtle intravascular change in early non-stroke VAD. Some previous reports suggested the validity of a blood-suppression technique in offering better contrast to the intravascular structures, 23 while others provided zoomed high-resolution imaging for minute intracranial structures, 24 both of which are available in our novel Z-HB.
In this study, we combined the zoomed technique and profile curve processing of the intravascular structure of VAD. The zoomed technique enabled virtually ultra-high-resolution imaging of the small vessel area by narrowing the FOV. 25 Profile curve processing enabled both qualitative and quantitative evaluation of the intravascular structure. More specifically, a qualitative evaluation of the profile curve shape detects the presence of an intravascular structure, which if present, categorizes whether the structure is located concentrically (omega type) or eccentrically (asymmetrical type). Multiple pathologies potentially included in the same profile curve pattern were discriminated using quantitative analysis of the intravascular structure referencing the signal intensity of the blood flow. Qualitative evaluation of VAD has been mentioned in previous literature using 3.0-T MRI.26–28 However, we propose virtual ultra-high resolution imaging and profile curve processing to detect subtle intravascular structures that would be missed through the subjective eyes of observers. Although previous studies have also reported on quantitative analysis, most of them evaluated the signal intensity ratio to an adjacent muscle, for example, sternocleidomastoid muscle.8,29 This methodology requires a larger FOV to include the vertebral artery and the adjacent muscle, failing to obtain high-resolution imaging that would have been available through a limited FOV. Indeed, the resolution of our zoomed technique was 0.4 mm/pixel (2.5 dot pixel/mm), which was twice as high as that of the conventional method using adjacent muscles as a control (0.8 mm/pixel (1.25 dot pixel/mm)). 29
Quantitative evaluation of the profile curve in our study may provide insight into unsolved concerns in terms of MR imaging for unruptured VAD. First, since pain-only VAD lacks focal neurological deficits, the distinction from nonspecific headache has been challenging. Upon quantitative analysis of the omega-type curve, the thin intimal flap as a subtle intravascular signal change in the acute stage could be discriminated from a signal artifact of the normal vessel caused by phase incoherence in the center of the vessel caliber, 30 as shown in our representative case presentation. Second, when posterior circulation ischemic stroke is associated with significant stenosis of the vertebral artery, determining whether it is attributed to VAD or atherosclerotic stenosis is crucial because the indication of antiplatelet therapy depends on the underlying pathologies.4–6 Upon quantitative analysis of asymmetrical-type curves, the intravascular eccentric structure could be categorized into intramural hematoma of VAD or atherosclerotic plaque. Third, our quantitative analysis methodology of virtual ultra-high-resolution imaging may aid in determining the exact range of the dissection. Some VADs must undergo endovascular or surgical trapping when they deteriorate 19 ; however, surgical exploration has occasionally observed dissection to extend beyond the range of abnormal angiographic findings. 23 Our methodology may be sensitive enough to evaluate the exact range of the dissection, which is especially important when critical branches, including the posterior inferior cerebellar artery or anterior spinal artery, are adjacent.
This study has several limitations. First, this is a retrospective study in a single institution, and the sample size was relatively small. Second, owing to the lack of sample specimen investigation, pre-defined vessel pathologies could contaminate one another although meeting the radiological criteria. Third, one of the concerns is that an omega sign as abnormal finding was observed in 23/63 normal arterial segments. Although we successfully discriminated it from that in arterial dissections by quantitative analysis, this may qualitatively confuse the observers’ eyes. Further development of black-blood MRI technique would be warranted to reduce the signal artifact at the center of the vessel caliber. Fourth, because profile curve processing and quantitative measurement currently takes time, clinical decision-making based on this methodology may not necessarily be available promptly at the time of patients’ presentation. However, our methodology that combines novel Z-HB and profile curve processing elaborately evaluates the intravascular structure of non-stroke VAD in detail by providing objective and measurable signal data, which could be applied to telemedicine and unmanned diagnosis using artificial intelligence in the future.
Conclusions
A combination of novel Z-HB MR imaging and profile curve processing depicted the intravascular structure of non-stroke VAD in detail and successfully discriminated the intimal flap and intramural hematoma from the normal vessel and atherosclerotic plaque, respectively.
Acknowledgements
The authors thank Dr. Yukio Miki, Chairman of Department of Diagnostic Radiology, Osaka Metropolitan University, Osaka, Japan, Dr. Shoji Matsumoto, Professor of Department of Stroke Neurology, Fujita Medical School, Aichi, Japan, and Dr. Yoshito Ichiba, Research & collaboration scientist of Siemens Healthcare K.K., Tokyo, Japan, for their helpful comments and critical reading on our manuscript. We also thank Editage (www.editage.com) for English language editing.
Appendix.
Abbreviations
- VAD
vertebral artery dissection
- Z-HB
zoomed high-resolution black blood
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author’s note: This work was done in Department of Radiology and Neurosurgery, Mominoki Hospital, 6-1, Tsukanohara, Kochi-city, Kochi, 780-0952 Japan.
Data availability: Any underlying research materials related to our paper including data can be assessed.
ORCID iD
Hitoshi Fukuda https://orcid.org/0000-0003-3803-8242
References
- 1.Yoshimoto Y, Wakai S. Unruptured intracranial vertebral artery dissection: clinical course and serial radiographic imagings. Stroke 1997; 28: 370–374. [DOI] [PubMed] [Google Scholar]
- 2.Hosoya T, Adachi M, Yamaguchi K, et al. Clinical and neuroradiological features of intracranial vertebrobasilar artery dissection. Stroke 1999; 30: 1083–1090. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi N, Murayama Y, Yuki I, et al. Natural course of dissecting vertebrobasilar artery aneurysms without stroke. AJNR Am J Neuroradiol 2014; 35: 1371–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke 2011; 42: 227–276. [DOI] [PubMed] [Google Scholar]
- 5.Dreier JP, Lurtzing F, Kappmeier M, et al. Delayed occlusion after internal carotid artery dissection under heparin. Cerebrovasc Dis 2004; 18: 296–303. [DOI] [PubMed] [Google Scholar]
- 6.Kai Y, Nishi T, Watanabe M, et al. Strategy for treating unruptured vertebral artery dissecting aneurysms. Neurosurgery 2011; 69: 1085–1091. [DOI] [PubMed] [Google Scholar]
- 7.Metso TM, Metso AJ, Helenius J, et al. Prognosis and safety of anticoagulation in intracranial artery dissections in adults. Stroke 2007; 38: 1837–1842. [DOI] [PubMed] [Google Scholar]
- 8.Yun SY, Heo YJ, Jeong HW, et al. Spontaneous intracranial vertebral artery dissection with acute ischemic stroke: High-resolution magnetic resonance imaging findings. Neuroradiol J 2018; 31: 262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato S, Toyoda K, Matsuoka H, et al. Isolated anterior cerebral artery territory infarction: dissection as an etiological mechanism. Cerebrovasc Dis 2010; 29: 170–177. [DOI] [PubMed] [Google Scholar]
- 10.Minematsu K, Matsuoka H, Kasuya J. Cervicocephalic arterial dissection in Japan: analysis of 454 patients in the spontaneous cervicocephalic arterial dissection study I (SCADS-I) (abstract). Stroke 2008; 39: 566. [Google Scholar]
- 11.Adams HP,, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 12.The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Study Group . Prognosis of patients with symptomatic vertebral or basilar artery stenosis. The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Study Group. Stroke 1998; 29: 1389–1392. [DOI] [PubMed] [Google Scholar]
- 13.Mitsouras D, Mulkern RV, Rybicki FJ. Strategies for inner volume 3D fast spin echo magnetic resonance imaging using nonselective refocusing radio frequency pulses. Med Phys 2006; 33: 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneider CA, Rasband WS, Eliceiri KW. NIH image to Image J: 25 years of image analysis. Nat Methods 2012; 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibrahim GM, Weidauer S, Macdonald RL. Interobserver variability in the interpretation of computed tomography following aneurysmal subarachnoid hemorrhage. J Neurosurg 2011; 115: 1191–1196. [DOI] [PubMed] [Google Scholar]
- 16.Akobeng AK. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr 2007; 96: 644–647. [DOI] [PubMed] [Google Scholar]
- 17.Mizutani T. Natural course of intracranial arterial dissections. J Neurosurg 2011; 114: 1037–1044. [DOI] [PubMed] [Google Scholar]
- 18.Ahn SS, Kim BM, Suh SH, et al. Spontaneous symptomatic intracranial vertebrobasilar dissection: Initial and follow-up imaging findings. Radiology 2012; 264: 196–202. [DOI] [PubMed] [Google Scholar]
- 19.Naito I, Iwai T, Sasaki T. Management of intracranial vertebral artery dissections initially presenting without subarachnoid hemorrhage. Neurosurgery 2002; 51: 930–937. [DOI] [PubMed] [Google Scholar]
- 20.Tsukahara T, Minematsu K. Overview of spontaneous cervicocephalic arterial dissection in Japan. Acta Neurochir Suppl 2010; Supp 107: 35–40. [DOI] [PubMed] [Google Scholar]
- 21.Iwama T, Andoh T, Sakai N, et al. Dissecting and fusiform aneurysms of vertebro-basilar systems. MR imaging. Neuroradiology 1990; 32: 272–279. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Lou X, Li Y, et al. Imaging investigation of intracranial arterial dissecting aneurysms by using 3T high-resolution MRI and DSA: From the interventional neuroradiologists’ view. Acta Neurochir (Wien) 2014; 156: 515–525. [DOI] [PubMed] [Google Scholar]
- 23.Arai D, Satow T, Komuro T, et al. Evaluation of the arterial wall in vertebrobasilar artery dissection using high-resolution magnetic resonance vessel wall imaging. J Stroke Cerebrovasc Dis 2016; 25: 1444–1450. [DOI] [PubMed] [Google Scholar]
- 24.Schulze M, Reimann K, Seeger A, et al. Improvement in imaging common temporal bone pathologies at 3T MRI: small structures benefit from a small field of view. Clin Radiol 2017; 72: 267.e1–e267. [DOI] [PubMed] [Google Scholar]
- 25.Blasche M, Riffel P, Lichy M. TimTX TrueShape and syngo ZOOMit: Technical and practical aspects. Magnetom Flash 2012; 1: 74–84. https://cdn0.scrvt.com/39b415fb07de4d9656c7b516d8e2d907/1800000000118051/a06d5db36c83/TimTX_TrueShape_and_syngo_Zoomit_Aspects_1800000000118051.pdf (accessed 10 January 2022. [Google Scholar]
- 26.Matsukawa H, Shinoda M, Fujii M, et al. Differences in vertebrobasilar artery morphology between spontaneous intradural vertebral artery dissections with and without subarachnoid hemorrhage. Cerebrovasc Dis 2012; 34: 393–399. [DOI] [PubMed] [Google Scholar]
- 27.Ahn JY, Han IB, Kim TG, et al. Endovascular treatment of intracranial vertebral artery dissections with stent placement or stent-assisted coiling. AJNR Am J Neuroradiol 2006; 27: 1514–1520. [PMC free article] [PubMed] [Google Scholar]
- 28.Mandell DM, Mossa-Basha M, Qiao Y, et al. Intracranial vessel wall MRI: Principles and expert consensus recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol 2017; 38: 218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saba L, Yuan C, Hatsukami TS, et al. Carotid artery wall imaging: Perspective and guidelines from the ASNR vessel wall imaging study group and expert consensus recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol 2018; 39: E9–E31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Provenzale JM, Sarikaya B, Hacein-Bey L, et al. Causes of misinterpretation of cross-sectional imaging studies for dissection of the craniocervical arteries. AJR Am J Roentgenol 2011; 196: 45–52. [DOI] [PubMed] [Google Scholar]