Abstract
Purpose
Medical errors result in significant mortality and morbidity. The purpose of this study is to analyze skull-base errors at a single tertiary institution, identify common anatomic sites of errors, and offer strategies to reduce errors in this region.
Methods
A Neuroradiology Quality Assurance Database of radiologic errors was searched for attending physician computer tomography and magnetic resonance imaging errors in skull-base pathology from 2014 to 2020. Data were limited to CT and MRI reports. Errors were separated into four subcategories (tumor, trauma, vascular, and congenital) and further divided by relevant anatomic site.
Results
A total of 90 skull-based errors were identified. Most errors were perceptual (87%), with common study types including MRI Brain (39%) and CT Head (24%). Most common errors were tumors (55%), followed by trauma (24%), vascular (10%), and congenital (7%). Six anatomic sites were identified and encompassed over half of errors (58%): sella, occipital bone, cerebellopontine angle/internal auditory canal (CPA/IAC), foramen magnum and clivus, cavernous sinus, and dural venous sinus.
Summary
Most of the skull-base errors were perceptual. Placing a strong emphasis on both the pathology and closely examining its critical anatomic site (sella, occipital bone, CPA/IAC, foramen magnum and clivus, cavernous sinus, and dural venous sinus) could potentially reduce up to 60% of errors in these regions.
Keywords: Neuroradiology, radiologic errors, skull-base, quality improvement
Introduction
In the United States, medical errors result in significant mortality and morbidity with estimated deaths ranging from 98,000 1 to 250,000. 2 Medical errors contribute to unnecessary healthcare costs of $17 to $29 billion dollars annually. 1 There are many variables that predispose to radiologic errors, including type I heuristic thinking (fast quick thinking), cognitive biases (e.g., availability bias, framing bias, satisfaction of search bias), systematic errors (e.g., workplace interruptions, understaffing, software failure), longer shift length, and increased shift volume.3-6
Radiologic errors within Neuroradiology are reported to be in the 2–6% range,7–10 and can be categorized into perception and interpretation errors. For perception errors, the radiologist fails to identify the abnormality on initial examination which is evident on retrospective review. For interpretation errors, the radiologist correctly identifies the abnormality but misinterprets its significance. Perceptual errors make up 60–80% of radiologic errors.3–27 While there are qualitative articles discussing common blind spots in neurologic imaging, there is little research done on quantifying these areas in skull-base imaging. The skull-base is a challenging area that can be prone to errors given its complex anatomy, junction of multiple compartments, overlap of various structures, and being at the edge of the field of view. Furthermore, the most common studies are not ordered with a specific emphasis on the skull-base. Prior research in Head and Neck Neuroradiology has shown that errors occurred most often in studies that were not ordered for the evaluation of the head and neck region. 28 Awareness of these areas could permit more targeted search patterns that could potentially reduce errors. The purpose of this study is to describe radiologic errors identified involving the skull-base at a single tertiary academic center, define common anatomic blind spots, and offer potential strategies to decrease error rates.
Methods
Institutional review board approval was obtained for this retrospective study with a waiver of informed consent. A Neuroradiology Quality Assurance Database of missed cases from 20 attending physicians was searched for misses involving skull-base pathology from 2014 to 2020. Only computer tomography (CT) and magnetic resonance imaging (MRI) exams were included in the study. We collected data on specific missed pathologies involving the skull-base. Skull-base errors were defined as those involving the anterior, central, or posterior bony skull-base; any of its foramina or structures passing through the foramina; sella, parasellar space and cavernous sinus; sphenoid sinus walls; retroclival, cerebellopontine or cerebellomedullary space; internal auditory canal; craniocervical junction; transverse/sigmoid sinus; meninges or brain parenchyma abutting the bony skull-base where the potential surgical intervention would require involvement of a skull-base surgeon. We collected data on patient gender, patient age, radiologist post training experience, study type (CT Head, MRI Brain, CT Angiogram Head, CT Sinus/Face, CT Temporal Bone, Other), and if the error was clinically significant based on the American College of Radiology RADPEER system. 29 Misses were categorized as perceptual or interpretive.
Neuroradiology section and quality assurance process
Our Neuroradiology QA database includes only CT and MRI examinations, performed for all indications, and collected from a few different sources. As part of the QA process, each radiologist in our department is required to evaluate three randomly selected (computer-generated) studies on the days they are assigned to the clinical service, and assign a RADPEER score (1, 2A, 2B, 3A, or 3B) to each reviewed study. The American College of Radiology (ACR) RADPEER system is widely used, initially available in 2002, and with revisions in 2009 and 2016. 29 Additionally, in an effort to capture as many diagnostic errors as possible, all addended neuroradiology reports (approximately 1000 addendums per year) are reviewed. Most reports are addended for technical reasons (e.g., documentation of contrast or radiation dose, comparison to outside studies not available at the time of the original interpretation) and therefore automatically eliminated from the review process. Reports which contain potential diagnostic errors are flagged for further review. Approximately 90% of the cases that are flagged for our QA review are screened through the above two mechanisms. Additionally, half of all errors were obtained through the peer review system and the other half through evaluation of addended reports. This demonstrated the value of reviewing addended reports in our QA system. The remaining cases were obtained through various avenues, such as reports that are submitted by clinicians or radiologists because of disagreement with the original interpretation.
All flagged studies are either reviewed by two attending neuroradiologists or during our quarterly QA conference, assigned a consensus RADPEER score, and entered in our QA Database. Study findings are correlated with histology or clinical follow-up, when available. The goal of our QA process is to identify as many errors as possible, allowing us to perform root cause analysis and devise interventional strategies to reduce the diagnostic errors. The goal of our efforts is not to capture every single error, which would be an impossible task requiring double reading of >40,000 CT and MRI exams per year. Our method of collecting cases has yielded a very narrow range of our section’s yearly diagnostic error rates of between 0.20 and 0.25% (years 2014–2020, internal records), indicating a consistent and reproducible process. Lastly, our Neuroradiology section does not currently support sub-specialized rotations such as head and neck, skull-base, brain or pediatric, and functions as a general neuroradiology practice.
Results
During the study period of 2014–2020, a total of 283,248 CT and MRI exams were interpreted by our Neuroradiology section. There were a total of 654 errors (0.23%), of which 90 (0.03%) involved the skull-base. 52% (N = 47) of patients were female and 48% (N = 43) male. Patient age range was 1– 91 years old, with an average age of 48 years. Attending Radiologist post training experience range at the end of the study period was 0.2– 46.7 years, with an average of 19 years. The majority of errors were perceptual (87%, N = 78). The average number of CT/MRI studies read during a shift was 43. The RADPEER scoring system showed that 98% of errors (N = 88) were clinically significant (a score of 2b or 3b), indicating a potential for change in clinical management or follow-up. The most common errors were identified on MRI Brain (39%, N = 35) and CT Head (24%, N = 22) exams. Please see Table 1. The majority of errors within CT studies were non-contrast (82%, N = 40) while those found in MRI were more often on contrast enhanced studies (71%, N = 29).
Table 1.
Demographics of skull-based errors.
| Total (N) | Percentage (%) | |
|---|---|---|
| Gender | ||
| Male | 43 | 48 |
| Female | 47 | 52 |
| Age group | ||
| Adult (>16 years old) | 75 | 83 |
| Pediatrics ≤16 years old) | 15 | 17 |
| Clinically significant | ||
| Yes | 88 | 98 |
| No | 2 | 2 |
| Exam type | ||
| MRI brain | 35 | 39 |
| CT head | 22 | 24 |
| CTA Head | 8 | 9 |
| CT sinus/Face | 8 | 9 |
| CT temporal bone | 7 | 8 |
| Other | 10 | 11 |
| Error type | ||
| Perceptual | 78 | 87 |
| Interpretative | 12 | 13 |
Errors were separated into one of five categories: tumor, trauma, vascular, congenital, and miscellaneous. Most errors could also be grouped into one of six anatomic sites (58%, N = 52). These included the sella (13%, N = 12), occipital bone (13%, N = 12), CPA/IAC (11%, N = 10), foramen magnum and clivus (8%, N = 7), cavernous sinus (7%, N = 6), and venous dural sinuses (6%, N = 5).
Greater than half of all errors were tumors (57%, N=51). Commonly missed tumors included meningioma (17%, N = 9), vestibular schwannoma (15%, N=8), sella/pituitary masses (14%, N = 7), and overcalling a normal pituitary gland (12%, N = 6). Please see Table 2. 55% of tumor-related errors (N = 28) involved the sella, CPA/IAC region, or cavernous sinus. The most common study type in these anatomic sites were MRI Brain (61%, N = 17) and CT Head (21%, N = 6). Overall, tumor-related errors occurred most often on MRI Brain (N = 30, 59%) and CT Head (N = 10, 20%).
Table 2.
Missed tumors in skull-based errors.
| Diagnosis | Location(s) | Total (N) | Percentage (%) |
|---|---|---|---|
| Meningioma | Cerebellopontine angle Posterior fossa Cavernous sinus Sphenoid bone |
9 | 17 |
| Schwannoma | Vestibular nerve Jugular foramen |
8 | 15 |
| Sella/pituitary mass | Sella/pituitary lesion | 7 | 14 |
| Overcalled normal pituitary or normal posttreatment changes | Pituitary gland | 6 | 12 |
| Intracranial metastasis abutting skull-base | Temporal lobe Cerebellum Hypoglossal nerve |
4 | 8 |
| Osseous metastasis | Mandibular condyle Occipital condyle Clivus |
3 | 6 |
| Lymphoma | Basal frontal lobe | 3 | 6 |
| Encephalocele/meningocele | Frontal ethmoid Middle cranial fossa |
2 | 4 |
| Masses without pathologic diagnosis | Cavernous sinus | 2 | 4 |
| Lipoma | Internal auditory canal | 1 | 2 |
| Myelomatous involvement | Cranial nerve V | 1 | 2 |
| Glomus jugulare | Temporal bone | 1 | 2 |
| Fibrous-osseous lesion | Roof/Lateral orbit | 1 | 2 |
| Cholesteatoma | External auditory canal | 1 | 2 |
| Craniopharyngioma | Suprasellar space | 1 | 2 |
| Squamous cell carcinoma | Petrous apex | 1 | 2 |
Trauma comprised the next largest category (22%, N=20). Most misses were fractures involving the occipital bone (65%, N = 13) with a fairly even distribution of misses in retroclival hematomas (20%, N = 4) and alar ligamentous injury (15%, N = 3). Please see Table 3. 70% of trauma-related errors (N = 14) involved the occipital bone or clivus. The most common study type in these anatomic sites were CT Head (71%, N = 10) and MRI C-Spine (21%, N = 3). Overall, trauma-related errors occurred most often on CT Head (N = 12, 60%), CT Sinus/Face (N = 3, 15%), and MRI C-Spine (N = 3, 15%).
Table 3.
Missed traumas in skull-based errors.
| Diagnosis | Location(s) | Total (N) | Percentage (%) |
|---|---|---|---|
| Fracture | Carotid canal Occipital bone Mastoid bone Sphenoid sinus |
13 | 65 |
| Hematoma | Retroclival | 4 | 20 |
| Ligamentous injury | Alar ligament | 3 | 15 |
The next category was vascular errors (10%, N = 9). The most commonly missed vascular finding was dural venous thrombosis (56%, N = 5), particularly the sigmoid sinus (see Table 4). The most common study type in this anatomic site were evenly distributed between MRI Brain (40%, N = 2), CTA Head (20%, N = 1), CTA Neck (20%, N = 1), and MRI C-Spine (20%, N = 1). Overall, vascular-related errors occurred most often on CTA Head (33%, N = 3) and MRI Brain (33%, N = 3).
Table 4.
Missed vascular findings in skull-based errors.
| Diagnosis | Location(s) | Total (N) | Percentage (%) |
|---|---|---|---|
| Dural venous sinuses | Transverse/sigmoid sinus thrombosis | 5 | 56 |
| Aneurysm | Cavernous ICA | 1 | 11 |
| Fistula | Cavernous sinus-carotid | 1 | 11 |
| Aberrant ICA | Carotid canal | 1 | 11 |
| AICA compression of CN VII | AICA root entry zone | 1 | 11 |
Finally, for congenital findings (7%, N = 7), the most commonly missed finding was Chiari I malformation (29%, N = 2) and an enlarged vestibular aqueduct (29%, N = 2). Please see Table 5. 29% of congenital-related errors (N = 2) involved the foramen magnum. The most common study type in this anatomic site were CT C-Spine (50%, N = 1) and MRI Brain (50%, N = 1). Overall, congenital-related errors occurred most often on CT Temporal Bone (43%, N = 3), MRI Brain (29%, N = 2), and CT C-Spine (29%, N = 2).
Table 5.
Missed congenital findings in skull-based errors.
| Diagnosis | Location(s) | Total (N) | Percentage (%) |
|---|---|---|---|
| Chiari 1 malformation | Foramen magnum | 2 | 29 |
| Large vestibular aqueduct | Vestibular aqueduct | 2 | 29 |
| Temporal bone abnormalities | Temporal bone | 1 | 14 |
| Rathke’s cleft cyst | Sella | 1 | 14 |
| Achondroplasia, severe spinal canal stenosis | Craniocervical junction | 1 | 14 |
Of note, the miscellaneous category contained only three errors including bilateral otospongiosis, sphenoid bone dehiscence, and tegmen tympani dehiscence. Given such few errors in the miscellaneous category, they are included here for completeness sake but are not included in the discussion.
Discussion
In our review of skull-base errors, we identified six vital anatomic sites that contained the majority of misses involving all subcategories of errors (tumors, trauma, vascular findings, and congenital findings). Furthermore, each subcategory had specific anatomic sites that most often involved the relevant pathology. Recognizing this allows for an enhanced search pattern to reduce the probability of future errors.
For tumor-related errors, 55% of errors involved the sella, CPA/IAC region, or cavernous sinus. In the sella, a common error involved overcalls of lesions in a normal-appearing pituitary gland (Figure 1). This may be attributable to lack of experience regarding the normal appearance of the pituitary gland particularly in young and middle-aged adults.30–32 Additionally, pituitary/sella masses can be difficult to see on the most commonly ordered study, CT Head non-contrast (Figure 2), as they are often obtained for different indications and appear similar in density to surrounding structures. In the CPA/IAC region, the most missed tumor was schwannoma. The cases missed in our study were incidental findings identified on exams that were not dedicated IAC studies, often subtle despite the presence of contrast (Figure 3). Finally, in the cavernous sinus, the most missed tumor was meningioma (Figures 4 and 5). A pitfall in imaging diagnosis of meningioma includes its T1 and T2 iso-intensity—this can often make them difficult to detect on non-contrast MRI studies. Given that the majority of MRI studies are ordered for various indications and are most often non-contrast enhanced studies, it is particularly important to closely evaluate these regions even in the absence of contrast.
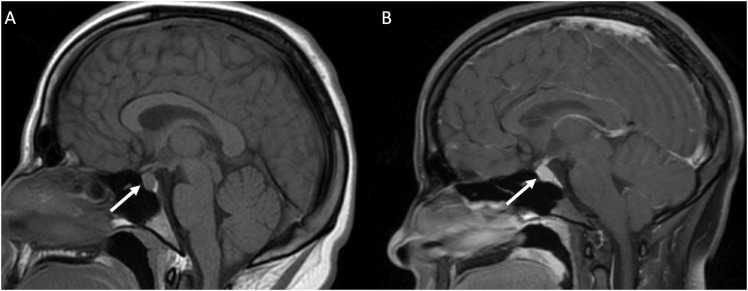
Figure 1.
(a) MRI non-contrast obtained for dizziness and headache in a 19 year old student. The pituitary gland (white arrow) measured up to 9 mm in craniocaudal dimension and a dedicated MRI Sella/Pituitary protocol was recommended. (b) Dynamic contrast enhanced MRI demonstrated normal homogenous enhancement of the pituitary gland and stalk (white arrow). In young adults, a prominent pituitary gland is a normal finding.
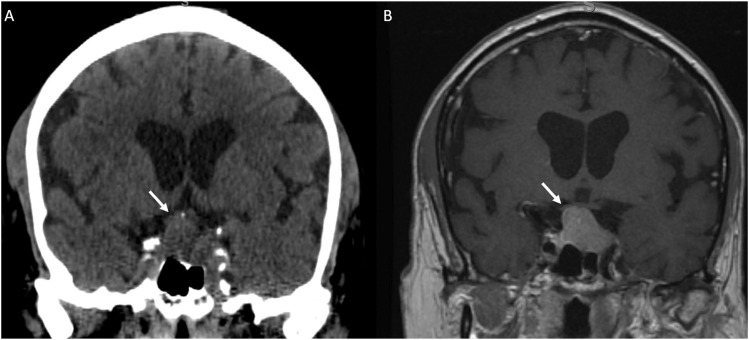
Figure 2.
(a) CT Head non-contrast obtained for trauma and was negative for acute findings. The pituitary mass (white arrow) was not mentioned and is similar in density to the surrounding brain parenchyma. (b) MRI with contrast obtained 1 week later demonstrated a large enhancing pituitary macroadenoma (white arrow).
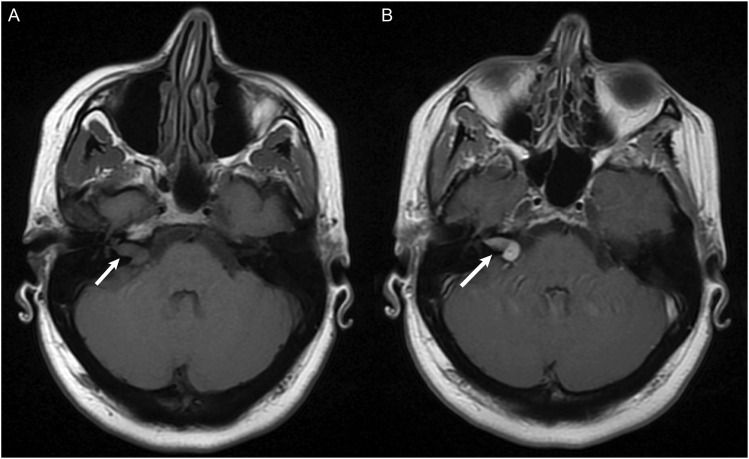
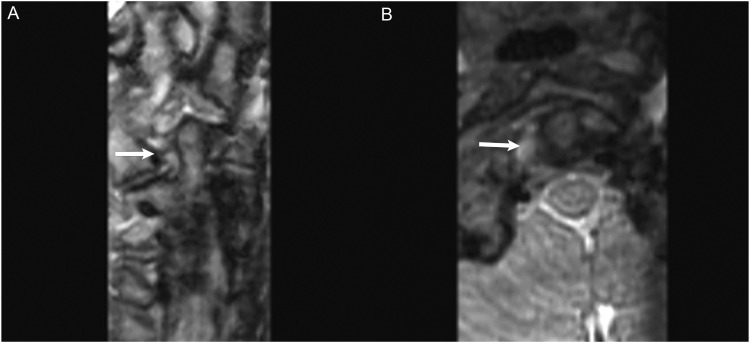
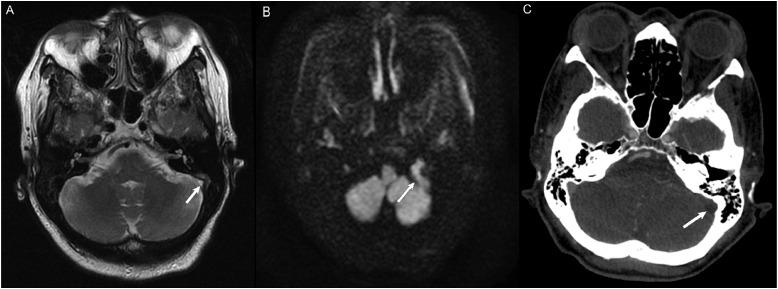
Figure 3.
MRI without and with contrast obtained to evaluate for dural sinus thrombosis and read as a negative study. (a) Non-contrast images demonstrated a right intracanalicular mass extending to the cerebellopontine angle (white arrow). (b) Contrast demonstrated uniform enhancement of the mass (white arrow), consistent with a vestibular schwannoma.
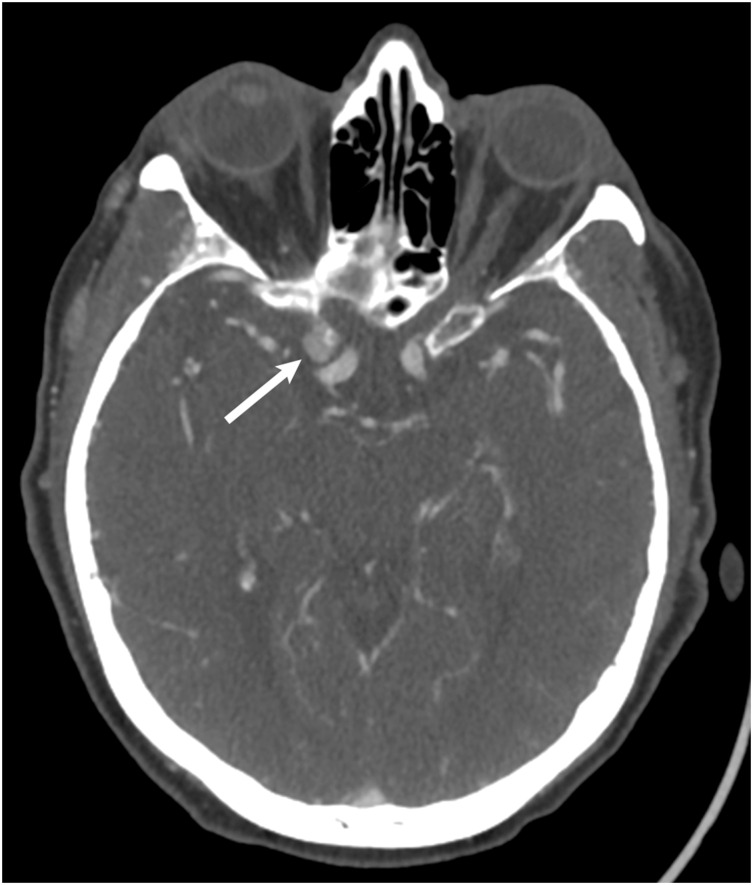
Figure 4.
CT Angiography of the Head to evaluate for stroke. In the region of the right cavernous sinus, there is a 0.9 cm rounded anterior clinoid meningioma (white arrow). This was originally called a supraclinoid internal carotid artery aneurysm.
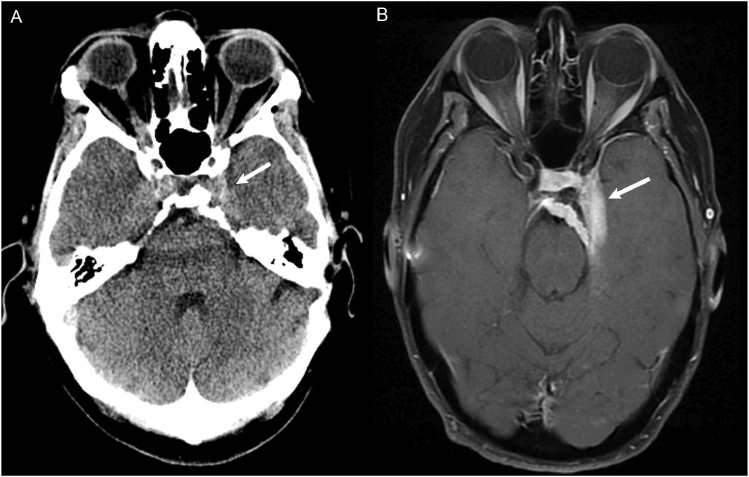
Figure 5.
(a) CT Head non-contrast obtained for chronic headache, originally read as a negative study. In retrospect, there is soft tissue thickening at the left cavernous sinus (white arrow) with possible adjacent hyperostosis and retroclival extension. (b) MRI with contrast 4 years later demonstrated an extra-axial dural based enhancing meningioma involving the left cavernous sinus with extension to the suprasellar cistern and sella (white arrow).
For trauma-related errors, 70% of errors involved the occipital bone or clivus (Figure 6). It is important to note that all misses pertaining to occipital bone fractures occurred on CT Head studies. Additionally, alar ligamentous injuries were included in this anatomic site given the strong association with type III occipital condyle fractures. MRI is vital in detecting ligamentous injury (Figure 7). Use of 3D images in addition to sagittal and coronal reformats can assist in the sensitivity in detecting these findings. All errors pertaining to the clivus in this subcategory were retroclival hematomas. Analysis for the presence of retroclival hematoma should include sagittal planes to follow the tectorial membrane as it extends from the craniocervical junction and adheres closely to the clivus (Figure 8).
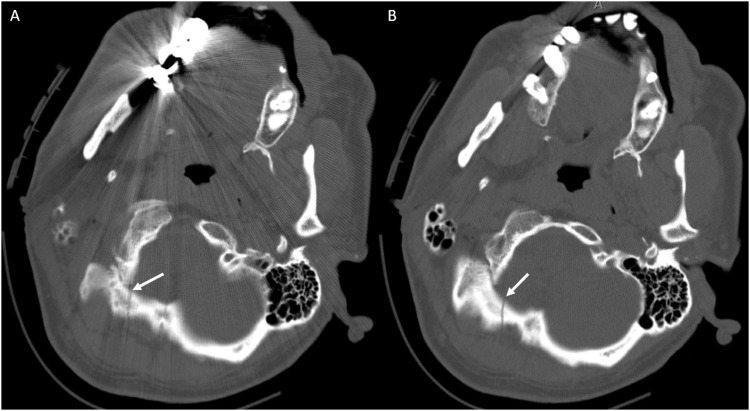
Figure 6.
CT Head non-contrast obtained for trauma. (a) Non-displaced right occipital bone fracture (white arrow) that was not reported. Note that evaluation at this particular level was challenging given streak artifact from dental fillings. (b) The same CT on a different slice allowed superior identification of the right occipital bone fracture (white arrow), which is often best identified on axial images.
Figure 7.
MRI Cervical Spine obtained after fall off trampoline onto neck. (a) Coronal CUBE images demonstrated edema of the right alar ligament (white arrow) with fluid tracking in the right C1–C2 facet joint space. (b) Axial CUBE images demonstrated edema/fluid between the right-side of C1 and odontoid process. Findings are compatible with alar ligamentous injury.
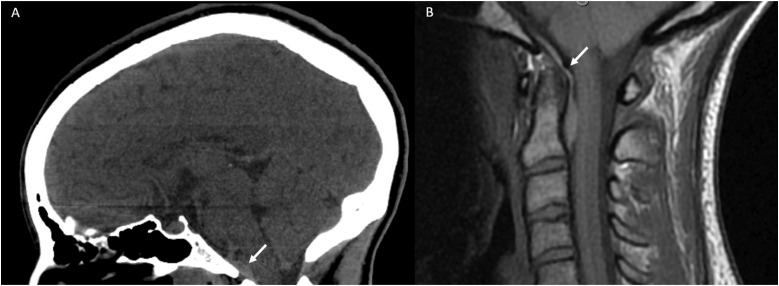
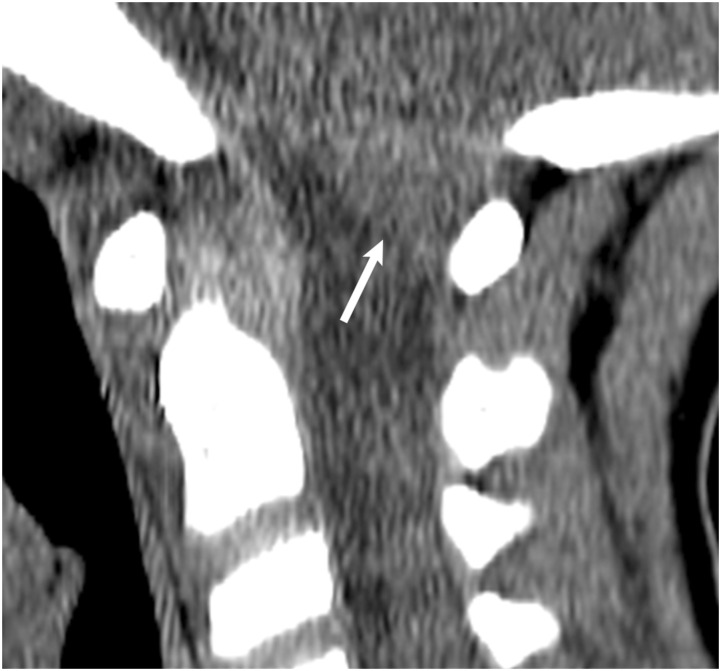
Figure 8.
(a) CT Head non-contrast obtained for trauma. Although multiple other injuries were present, a subtle lens shaped hyperdensity seen in the retroclival region (white arrow) was not mentioned. It is best seen on the sagittal view. (b) MRI C-Spine obtained the same day demonstrated a retroclival hematoma (white arrow) clearly identified on the T1 sagittal sequence.
For vascular-related errors, 56% of errors involved the dural venous sinus and pertained to thrombosis. Of note, 50% of these errors were present on MRI studies not dedicated to evaluating the dural sinuses. On MRI, analysis for the normal flow void which implies patency should be performed on conventional and DWI (Figure 9) weighted sequences, 33 particularly if there is a nearby occipital bone fracture. This finding can often be seen in the evaluation of a routine CT angiogram of the head and neck, when present. Despite the arterial phase of contrast, the dural venous sinuses are almost always well-opacified as well (Figure 9). For congenital-related errors, 29% of errors involved the foramen magnum and pertained to Chiari I malformations. Of note, errors occurred on both CT C-Spine and MR C-Spine studies. Evaluation in the sagittal plane (Figure 10) is important as tonsillar descent is more readily assessed. Incorporating sagittal images in the radiologist’s search pattern is how Chiari malformations can be diagnosed, especially when the diagnosis has not already been established.
Figure 9.
(a) MRA of the Head and Neck to evaluate for stroke without evidence of acute ischemic infarct. Axial T2 sequence demonstrated abnormal T2 signal within the left sigmoid sinus extending to the internal jugular vein (white arrow), concerning for slow flow versus thrombus. CT Venogram was recommended but not obtained. (b) Diffusion weighted imaging demonstrated restricted diffusion in the left sigmoid sinus extending to the internal jugular vein, increasing suspicion for thrombus. (c) CT Angiogram of the Head and Neck to evaluate for stroke 3 months after initial MRA. There is non-opacification of the left sigmoid sinus (white arrow) compared to the opacified right sigmoid sinus, consistent with dural venous thrombosis.
Figure 10.
CT Cervical Spine of a pediatric patient after motor vehicle accident and read as negative for trauma. Sagittal images on soft tissue window demonstrated extension of the cerebellar tonsils below the foramen magnum by 11 mm (white arrow) and compatible with a Chiari I malformation.
There are additional methods that can be implemented to reduce errors including increased education initiatives such as through comprehensive anatomic specific review articles, 34 attending multidisciplinary conferences (tumor board), following up on radiologic-pathologic correlation, root cause analysis of common institutional errors, attending mortality and morbidity (M&M) subspecialty conferences, and developing a supportive culture to discuss errors.5,16 Limitations of our study included a single institution with an overall low rate of misses given the number of exams read. Some anatomic sites of interest, such as the foramen magnum, only included as few as two errors. Finally, the common anatomic sites to include errors may vary between different Neuroradiology groups. However, we strongly believe that these anatomic locations should be part of every radiologists’ search pattern.
Conclusion
We have presented the most commonly missed findings at the skull-base in our retrospective review of radiologic misses. We emphasized the importance of routine incorporation of the most common anatomic blind spots in the search pattern for commonly missed diagnoses within the tumor, trauma, vascular, and congenital categories.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Stephen Vong https://orcid.org/0000-0002-3275-6916
References
- 1.Institute of Medicine (US) Committee on Quality of Health Care in America. To Err is Human: Building a Safer Health System. Kohn LT Corrigan JM Donaldson MS, editors. Washington (DC): National Academies Press (US); 2000. PMID: 25077248. [PubMed] [Google Scholar]
- 2.Makary MA, Daniel M. Medical error-the third leading cause of death in the US. BMJ 2016; 353: i2139. DOI: 10.1136/bmj.i2139 [DOI] [PubMed] [Google Scholar]
- 3.Itri JN, Tappouni RR, McEachern RO, et al. Fundamentals of diagnostic error in imaging. Radiographics 2018; 38(6): 1845–1865. DOI: 10.1148/rg.2018180021 [DOI] [PubMed] [Google Scholar]
- 4.Itri JN, Patel SH. Heuristics and cognitive error in medical imaging. AJR Am J Roentgenol 2018; 210(5): 1097–1105. DOI: 10.2214/AJR.17.18907 [DOI] [PubMed] [Google Scholar]
- 5.Busby LP, Courtier JL, Glastonbury CM. Bias in radiology: the how and why of misses and misinterpretations. Radiographics 2018; 38(1): 236–247. DOI: 10.1148/rg.2018170107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanna TN, Lamoureux C, Krupinski EA, et al. Effect of shift, schedule, and volume on interpretive accuracy: a retrospective analysis of 2.9 million radiologic examinations. Radiology 2018; 287(1): 205–212. DOI: 10.1148/radiol.2017170555 [DOI] [PubMed] [Google Scholar]
- 7.Verdoorn JT, Hunt CH, Luetmer MT, et al. Increasing neuroradiology exam volumes on-call do not result in increased major discrepancies in primary reads performed by residents. Open Neuroimag J 2014; 8: 11–15. DOI: 10.2174/1874440001408010011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viertel VG, Babiarz LS, Carone M, et al. Quality control in neuroradiology: impact of trainees on discrepancy rates. AJNR Am J Neuroradiol 2012; 33(6): 1032–1036. DOI: 10.3174/ajnr.A2933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babiarz LS, Yousem DM. Quality control in neuroradiology: discrepancies in image interpretation among academic neuroradiologists. AJNR Am J Neuroradiol 2012; 33(1): 37–42. DOI: 10.3174/ajnr.A2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lian K, Bharatha A, Aviv RI, et al. Interpretation errors in CT angiography of the head and neck and the benefit of double reading. AJNR Am J Neuroradiol 2011; 32(11): 2132–2135. DOI: 10.3174/ajnr.A2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samei E, Krupsinski E. The handbook of medical image perception and techniques. Cambridge University Press: Cambridge, UK. 2010. [Google Scholar]
- 12.Berlin L. Radiologic errors, past, present and future. Diagnosis (Berl) 2014; 1(1): 79–84. DOI: 10.1515/dx-2013-0012 [DOI] [PubMed] [Google Scholar]
- 13.Donald JJ, Barnard SA. Common patterns in 558 diagnostic radiology errors. J Med Imaging Radiat Oncol 2012; 56(2): 173–178. DOI: 10.1111/j.1754-9485.2012.02348.x [DOI] [PubMed] [Google Scholar]
- 14.Kundel HL. Perception errors in chest radiography. Semin Respir Med 1989; 10(3): 203–210. [Google Scholar]
- 15.Quekel LG, Kessels AG, Goei R, et al. Miss rate of lung cancer on the chest radiograph in clinical practice. Chest 1999; 115(3): 720–724. DOI: 10.1378/chest.115.3.720 [DOI] [PubMed] [Google Scholar]
- 16.Patel SH, Stanton CL, Miller SG, et al. Risk factors for perceptual-versus-interpretative errors in diagnostic neuroradiology. AJNR Am J Neuroradiol 2019; 40(8): 1252–1256. DOI: 10.3174/ajnr.A6125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenkrantz AB, Bansal NK. Diagnostic errors in abdominopelvic CT interpretation: characterization based on report addenda. Abdom Radiol (NY) 2016; 41(9): 1793–1799. DOI: 10.1007/s00261-016-0741-8 [DOI] [PubMed] [Google Scholar]
- 18.Lauritzen PM, Andersen JG, Stokke MV, et al. Radiologist-initiated double reading of abdominal CT: retrospective analysis of the clinical importance of changes to radiology reports. BMJ Qual Saf 2016; 25(8): 595–603. DOI: 10.1136/bmjqs-2015-004536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brigham LR, Mansouri M, Abujudeh HH. Journal club: radiology report addenda: a self-report approach to error identification, quantification, and classification. AJR Am J Roentgenol 2015; 205(6): 1230–1239. DOI: 10.2214/AJR.15.14891 [DOI] [PubMed] [Google Scholar]
- 20.Kim YW, Mansfield LT. Fool me twice: delayed diagnoses in radiology with emphasis on perpetuated errors. AJR Am J Roentgenol 2014; 202(3): 465–470. DOI: 10.2214/AJR.13.11493 [DOI] [PubMed] [Google Scholar]
- 21.Balthazar P, Konstantopoulos C, Wick CA, et al. Trainees may add value to patient care by decreasing addendum utilization in radiology reports. AJR Am J Roentgenol 2017; 209(5): 976–981. DOI: 10.2214/AJR.17.18339 [DOI] [PubMed] [Google Scholar]
- 22.Itri JN, Kang HC, Krishnan S, et al. Using focused missed-case conferences to reduce discrepancies in musculoskeletal studies interpreted by residents on call. AJR Am J Roentgenol 2011; 197(4): W696–W705. DOI: 10.2214/AJR.11.6962 [DOI] [PubMed] [Google Scholar]
- 23.Abujudeh HH, Boland GW, Kaewlai R, et al. Abdominal and pelvic computed tomography (CT) interpretation: discrepancy rates among experienced radiologists. Eur Radiol 2010; 20(8): 1952–1957. DOI: 10.1007/s00330-010-1763-1 [DOI] [PubMed] [Google Scholar]
- 24.Briggs GM, Flynn PA, Worthington M, et al. The role of specialist neuroradiology second opinion reporting: is there added value? Clin Radiol 2008; 63(7): 791–795. DOI: 10.1016/j.crad.2007.12.002 [DOI] [PubMed] [Google Scholar]
- 25.Ruchman RB, Jaeger J, Wiggins EF, et al. Preliminary radiology resident interpretations versus final attending radiologist interpretations and the impact on patient care in a community hospital. AJR Am J Roentgenol 2007; 189(3): 523–526. DOI: 10.2214/AJR.07.2307 [DOI] [PubMed] [Google Scholar]
- 26.Loevner LA, Sonners AI, Schulman BJ, et al. Reinterpretation of cross-sectional images in patients with head and neck cancer in the setting of a multidisciplinary cancer center. AJNR Am J Neuroradiol 2002; 23(10): 1622–1626. [PMC free article] [PubMed] [Google Scholar]
- 27.Fitzgerald R. Error in radiology. Clin Radiol 2001; 56(12): 938–946. DOI: 10.1053/crad.2001.0858 [DOI] [PubMed] [Google Scholar]
- 28.Ferguson A, Assadsangabi R, Chang J, et al. Analysis of misses in imaging of head and neck pathology by attending neuroradiologists at a single tertiary academic medical centre. Clin Radiol 2021; 76(10): 786.e9–786.e13. DOI: 10.1016/j.crad.2021.06.011 [DOI] [PubMed] [Google Scholar]
- 29.Goldberg-Stein S, Frigini LA, Long S, et al. ACR RADPEER committee white paper with 2016 updates: revised scoring system, new classifications, self-review, and subspecialized reports. J Am Coll Radiol 2017; 14(8): 1080–1086. DOI: 10.1016/j.jacr.2017.03.023 [DOI] [PubMed] [Google Scholar]
- 30.Satogami N, Miki Y, Koyama T, et al. Normal pituitary stalk: high-resolution MR imaging at 3T. AJNR Am J Neuroradiol 2010; 31(2): 355–359. DOI: 10.3174/ajnr.A1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Côté M, Salzman KL, Sorour M, et al. Normal dimensions of the posterior pituitary bright spot on magnetic resonance imaging. J Neurosurg 2014; 120(2): 357–362. DOI: 10.3171/2013.11.JNS131320 [DOI] [PubMed] [Google Scholar]
- 32.Bonneville F, Cattin F, Marsot-Dupuch K, et al. T1 signal hyperintensity in the sellar region: spectrum of findings. Radiographics 2006; 26(1): 93–113. DOI: 10.1148/rg.261055045 [DOI] [PubMed] [Google Scholar]
- 33.Chang YM, Kuhn AL, Porbandarwala N, et al. Unilateral nonvisualization of a transverse dural sinus on phase-contrast MRV: frequency and differentiation from sinus thrombosis on noncontrast MRI. AJNR Am J Neuroradiol 2020; 41(1): 115–121. DOI: 10.3174/ajnr.A6337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lubomirsky B, Jenner ZB, Jude MB, et al. Sellar, suprasellar, and parasellar masses: Imaging features and neurosurgical approaches. Neuroradiol J 2021. 19714009211055195. DOI: 10.1177/19714009211055195 [DOI] [PMC free article] [PubMed] [Google Scholar]