Abstract
Therapeutic options for severe infections caused by strains of oxacillin-resistant Staphylococcus aureus (ORSA) and coagulase-negative staphylococci (ORSE) are very limited. With the increasing resistance of such strains to aminoglycosides, rifampin, and currently available quinolone agents, as well as the recent documentation of increasing resistance of ORSA to vancomycin (VANCO), new treatment alternatives are imperative. The in vivo efficacy of trovafloxacin (TROVA), a new quinolone agent with excellent antistaphylococcal activity in vitro, against experimental endocarditis (IE) due to β-lactamase-producing ORSA and ORSE strains (ORSA and ORSE IE) was evaluated. TROVA (25 mg/kg of body weight intravenously [i.v.] twice daily [b.i.d.]) was compared to VANCO (20 mg/kg i.v. b.i.d.) and two regimens of ampicillin-sulbactam (AMP-SUL; 200 mg/kg intramuscularly [i.m.] three times a day [t.i.d.] and 20 mg/kg i.m. b.i.d.), with all agents given for 3 or 6 days. AMP-SUL was included as a comparative treatment regimen because of its proven efficacy against experimental ORSA and ORSE IE. For both ORSA and ORSE IE, TROVA, AMP-SUL, and VANCO each reduced staphylococcal densities in vegetations compared to untreated controls (P < 0.01). For ORSA IE, TROVA was the most rapidly bactericidal agent—although not to a statistically significant degree—correlating with its superior bactericidal effect in vitro compared to those of VANCO and AMP-SUL.
The staphylococci are the most common cause of nosocomial bloodstream infections worldwide, accounting for more than 40% of such events according to most studies (28, 31). Moreover, the staphylococci are frequent causes of community-acquired bacteremias, especially in certain patient populations (e.g., persons with diabetes mellitus and intravenous drug addicts) (19) and for certain infectious disease syndromes (e.g., prosthetic valve endocarditis [IE], addict-related IE, and hemodialysis vascular access site infections) (19). In addition, the staphylococci cause a variety of other serious, localized infections, such as wound infections, skin and soft-tissue abscesses, and intra-abdominal and retroperitoneal abscesses (19). Importantly, a substantial portion of cases of severe staphylococcal infections, both nosocomial and community acquired, are now being caused by strains which are resistant to the semisynthetic, antistaphylococcal penicillins (i.e., oxacillin-resistant strains (15, 25). Of note, many such strains of oxacillin-resistant Staphylococcus aureus (ORSA) and coagulase-negative staphylococci (ORSE) have evolved multidrug-resistant phenotypes, being variably resistant to selected aminoglycosides, rifampin, and/or currently available quinolone agents (21, 27, 30). For severe clinical infections caused by ORSA and ORSE strains, vancomycin remains the most pivotal parenteral agent. Of particular concern is the recent documentation of significant clinical infections due to ORSA strains with reduced susceptibility to vancomycin (VANCO) in vitro from diverse geographic areas in the United States, as well as from Japan (6, 7, 17). Use of vancomycin in such patients was associated with persistent or relapsing infection; these observations underscore the critical need for new antistaphylococcal antibiotics with potent activity against ORSA and ORSE strains.
The current study was designed to examine the in vivo efficacy against ORSA and ORSE of a new fluoroquinolone agent, trovafloxacin (TROVA), which possesses potent and broad-spectrum activity against both gram-negative and gram-positive organisms (13, 26). We have previously confirmed the in vivo efficacy of TROVA against experimental IE caused by vancomycin-resistant enterococcal strains with both vanA and vanB genotypes (2). The animal model examined (involving experimental IE) is a rigorous test of antimicrobial efficacy, featuring large in vivo inocula in cardiac vegetations (>106 to 107 CFU/g of tissue) (1, 3, 4, 18, 29).
(This study was presented in part at the 35th annual meeting of the Infectious Diseases Society of America, San Francisco, Calif., September 1997 [abstr. 268]).
MATERIALS AND METHODS
Bacterial strains.
The staphylococcal strains used in this investigation are clinical isolates which were kindly provided by H. F. Chambers, San Francisco, Calif. These strains have been characterized in detail elsewhere (8, 9). Strain 67-0 (wound isolate) is a β-lactamase-producing, heterotypic ORSA strain, which is virulent in the animal IE model (18). SE 220 (blood isolate) is a β-lactamase-producing homotypic ORSE strain (Staphylococcus haemolyticus) also used in previous animal IE studies (8, 29). Both strains are ciprofloxacin susceptible (MIC [105-CFU inoculum] for both is 0.25 μg/ml).
Antibiotics.
VANCO and ampicillin (AMP) were purchased from commercial sources. Sulbactam (SUL) and TROVA (as the prodrug [alatrofloxacin] for intravenous [i.v.] administration) were supplied by Pfizer Central Research (Groton, Conn.). For alatrofloxacin, 1 mg is equivalent to ∼0.80 mg of TROVA (32). For in vitro testing, stock solutions of each agent (1,000 μg/ml) were kept at −70°C until thawed on the day of study. For the in vitro studies, the parent compound, TROVA, was utilized. For use in animal treatments, antibiotics were reconstituted according to the manufacturers’ recommendations just prior to in vivo administrations.
Antibiotic susceptibility testing.
The MICs for the ORSA and ORSE strains of the study antibiotics (VANCO, AMP, SUL, and TROVA) were determined by a broth microdilution method (18, 29) in cation-supplemented Mueller-Hinton broth (CSMHB) plus 2% NaCl at a final inoculum of ∼5 × 105 CFU/ml. MICs were read after 24 h of incubation at 35°C as the lowest antibiotic concentrations yielding no visible growth. The ability of SUL to enhance the growth-inhibitory effects of AMP against the ORSA and ORSE strains was also studied by using the same microtiter system. The antibiotic concentration ranges for AMP and SUL were 0.125 to 128 and 0.062 to 64 μg/ml, respectively, to parallel the clinically available formulation of this drug (Unasyn) which provides a 2:1 drug ratio (18). Moreover, these microtiter antibiotic concentrations encompass those achieved in the experimental IE model using the AMP-SUL regimens in the current study (18, 29) (see below). An enhanced growth-inhibitory effect was defined as at least a fourfold decrease in the MIC of the AMP-SUL combination versus those of both single agents. The MIC studies outlined above have been previously reported for both the ORSA and ORSE strains (18, 29).
To determine the comparative in vitro bactericidal effects of AMP-SUL, VANCO, and TROVA, time-kill curves were generated. A final inoculum of ∼2 × 106 CFU of logarithmic-phase ORSA or ORSE cells was incorporated into either antibiotic-free or antibiotic-containing CSMHB (plus 2% NaCl). The final antibiotic concentrations utilized for VANCO and TROVA represented twice the MIC determined in the studies described above. For the AMP-SUL combination, the concentrations of each drug used represented twice the MIC for each agent determined in the combination drug microtiter study described above. After 0, 6, and 24 h of incubation (35°C), 100 μl from each growth tube was quantitatively cultured in CSMH agar plus 2% NaCl for an additional 48 h, and surviving CFU were counted. A decline in CFU per milliliter of ≥3 log10 units after 24 h of incubation (versus 0-h bacterial counts) was considered evidence of a bactericidal effect (18, 29).
Animal model of IE.
The rabbit model of IE was used to evaluate therapeutic efficacy in this study. New Zealand White rabbits underwent carotid artery-to-left ventricle catheterization as previously described (18, 29). Twenty-four hours later, animals were challenged i.v. with the 95% infective dose inoculum for each strain as previously determined (∼5 × 106 CFU). Twenty-four hours postchallenge, blood cultures were performed to document the induction of IE. Animals were then randomized to receive either no therapy or their first antibiotic treatment. The number of animals assigned to each treatment group was designed to achieve a statistical power of ≥80% at the P ≤ 0.05 level.
Serum antibiotic levels.
The pharmacokinetics of VANCO, AMP, and SUL have been previously determined in this model of IE, and the determination was not repeated in this study (5, 18). The serum TROVA levels in infected animals with ORSA IE were determined. At 24 h postinfection, animals received either 10, 25, or 50 mg of TROVA per kg of body weight (as the prodrug) by i.v. bolus. Serum samples were obtained at 1, 2, 4, 6, and 24 h postdose for TROVA level determination by the agar diffusion method performed with antibiotic medium 11 (Difco Laboratories, Detroit, Mich.), with Bacillus subtilis (ATCC 6633) as the indicator strain (provided by Robert Polzer, Pfizer Central Research). The lower limit of detection for this assay was 0.1 μg of TROVA per ml.
Antibiotic regimens.
Animals received either no therapy (controls) or one of the following antibiotics: TROVA (25 mg/kg i.v., administered twice daily [b.i.d.] bid as the prodrug alatrofloxacin), VANCO (20 mg/kg i.v., administered b.i.d.), or AMP (200 mg/kg intramuscularly [i.m.], im, administered three times a day) plus SUL (20 mg/kg i.m., administered b.i.d.). Therapy was continued for either 3 or 6 days. The VANCO and AMP-plus-SUL regimens were based on prior pharmacokinetic and experimental IE efficacy studies in this laboratory (5, 18, 29). Thus, the peak serum AMP levels achieved with the above dose are ∼275 μg/ml (30 min postdose), with levels of ∼60 μg/ml at 60 min postdose (18). Importantly, this AMP regimen yields serum levels which exceed those concentrations known to effect an ∼50% in vitro saturation of penicillin-binding protein (PBP) 2a, the low-affinity PBP which determines the ORSA and ORSE phenotype (5, 11, 15, 18). In addition, the SUL dose regimen yields peak serum levels of >30 μg/ml (15 to 60 min postdose) (5), well exceeding the concentrations required for bactericidal synergy with AMP against both the ORSA and ORSE strains used in the present study. The TROVA regimen was based on both pharmacokinetic studies in this laboratory (see below) and prior studies of the efficacy of TROVA against experimental enterococcal IE performed in this laboratory (2).
Evaluation of efficacy.
For the assessment of treatment efficacy, all animals were sacrificed by i.v. sodium pentobarbital overdosage at least 24 h after the last drug dose to minimize antibiotic carryover effects in vivo. At the time of sacrifice, proper catheter placement across the aortic valve was confirmed, and only initially bacteremic animals with proper catheter placement and macroscopic vegetations on the aortic valve were further analyzed. All vegetations from a single animal were removed, weighed, homogenized, serially diluted, and quantitatively cultured. The serial dilution strategy further minimizes potential antibiotic carryover effects. For calculation of the mean bacterial densities per gram of vegetation, culture-negative vegetations were assigned a value based on vegetation weight and the lower limit of detection of CFU-per-gram levels (18, 29).
Statistical analyses.
The Fisher exact test was used for comparing proportional data, while the Kruskal-Wallis analysis of variance with correction for multiple comparisons was used for comparing differences between staphylococcal densities in vegetation.
RESULTS
Antibiotic susceptibilities.
The MICs of the study antibiotics for the ORSA and ORSE strains are shown in Table 1. As noted, both strains experienced synergistic growth inhibition by the combination of AMP and SUL, at achievable levels in serum for both antibiotics in this experimental model (5, 18). Both staphylococcal strains were susceptible to VANCO as well as TROVA (FDA-approved MIC breakpoint = 2 μg/ml). MICs of TROVA and VANCO were four- and eightfold lower, respectively, for the ORSE strain than for the ORSA strain.
TABLE 1.
In vitro MICs for ORSA and ORSE strains
| Agent(s) | MIC (μg/ml)
|
|
|---|---|---|
| ORSA | ORSE | |
| VANCO | 2 | 0.25 |
| AMP | >64 | >64 |
| SUL | >64 | >64 |
| AMP + SUL | 8/4a | 8/4a |
| TROVA | 1 | 0.25 |
MIC of AMP/MIC of SUL.
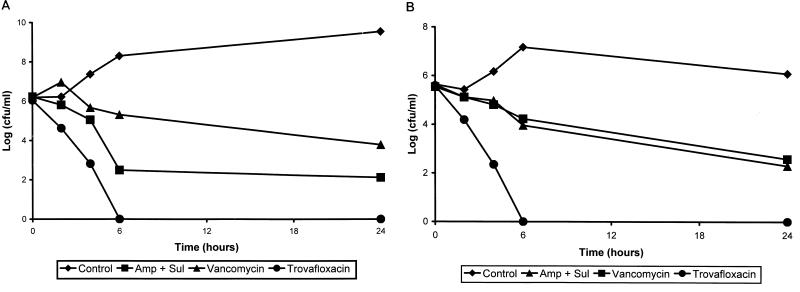
For the ORSA strain, TROVA and AMP-SUL exerted rapid bactericidal effects in vitro, with reductions in viable counts of 106 and 103.2 CFU/ml, respectively, by 6 h of incubation (Fig. 1A). In contrast, VANCO exerted a relatively slow bactericidal effect over the 24-h incubation period. TROVA exerted a rapid bactericidal effect against the ORSE strain (Fig. 1B).
FIG. 1.
Timed-kill curves of ORSA (A) and ORSE (B) strains. The concentrations (in micrograms per milliliter) of the antibiotics used (each is twice the MIC) are as follows: VANCO, 4; AMP, 16; SUL, 8; and TROVA, 2 for ORSA strains; and VANCO, 0.5; AMP, 16; SUL, 8; and TROVA, 0.5 for ORSE strains.
Serum antibiotic levels.
The elimination half-life of TROVA for the 25- and 50-mg/kg bolus doses was ∼2 h (Table 2). Peak TROVA levels achieved by the 25- and 50-mg/kg i.v. bolus doses were observed at 1 h postdose and exceeded the MICs for both the ORSA and ORSE strains by at least twofold. In contrast the 10-mg/kg TROVA dose achieved peak serum levels (at 1 h) that well exceeded the MIC for the ORSE strain but that exceeded the MIC for the ORSA strain by less than twofold. Moreover, the ratios of the area under the concentration-time curve to the MIC for the 25- and 50-mg/kg i.v. bolus doses (but not for the 10-mg/kg dose) were >7 for each study strain. Therefore, the 25 mg/kg dose was chosen for this study.
TABLE 2.
Pharmacokinetics of TROVA in rabbits with experimental IEa
| TROVA dose (mg/kg) | Peak serum level (μg/ml) | AUC (μg-hr/ml) | t1/2 (h) |
|---|---|---|---|
| 10 | 1.4 ± 0.8 | 3.2 ± 1.6 | 1.3 ± 0.3 |
| 25 | 2.5 ± 0.6 | 7.8 ± 1.4 | 2.1 ± 0.4 |
| 50 | 4.9 ± 1.6 | 17.5 ± 3.6 | 2.3 ± 0.6 |
Values are means ± standard deviations. AUC, area under the concentration-time curve; t1/2, elimination half-life.
Experimental IE.
For the ORSA strain, all three antibiotic regimens were very active in terms of significant reductions of intravegetation densities compared to untreated controls, after both 3 and 6 days of therapy. TROVA was the most rapidly bactericidal agent in vivo, with ORSA densities in vegetation following 3 days of therapy that were lower than those seen in animals receiving VANCO or AMP-SUL by ∼102.25 and ∼101.5 CFU/g, respectively (Table 3). However, these trends did not reach statistical significance. Relatively few vegetations were rendered culture negative by the three antibiotic regimens after 3 days of therapy. TROVA and AMP-SUL were the most active agents at day 6 of therapy in terms of sterilizing vegetations (63%), although this did not reach statistical significance compared to the VANCO regimen. (Table 3).
TABLE 3.
Antimicrobial efficacy against ORSA IE
| Drug(s) | ORSA density in vegetation (log10CFU/g) ± SD and P value vs controls on day:
|
No. of vegetations made culture-negative/total no. of vegetations (%) on day:
|
||||
|---|---|---|---|---|---|---|
| 3
|
6
|
3 | 6 | |||
| Density (no. sacrificed) | P | Density (no. sacrificed) | P | |||
| None (controls) | 8.56 ± 0.52 (12) | 8.16 ± 1.7 (3) | 0/12 (0) | 0/3 (0) | ||
| AMP + SUL | 4.97 ± 3.8 (10) | <0.01 | 3.46 ± 2.8 (8) | <0.01 | 2/10 (20) | 5/8 (63) |
| TROVA | 3.35 ± 1.8 (10) | <0.001 | 2.7 ± 1.5 (11) | <0.01 | 3/10 (30) | 7/11 (63) |
| VANCO | 5.6 ± 3.7 (13) | <0.05 | 4.0 ± 2.8 (11) | NSa | 0/13 (0) | 3/11 (27) |
NS, not significant.
For the ORSE strain (Table 4), all three antibiotic regimens were equally effective at significantly reducing intravegetation staphylococcal densities after both 3 and 6 days of therapy, compared to untreated controls. Similarly, all three antibiotic regimens were equally effective in rendering most vegetations culture negative after both 3 and 6 days of therapy (Table 4).
TABLE 4.
Antimicrobial efficacy against experimental ORSE IE
| Drug(s) | ORSE density in vegetation (log10CFU/g) ± SD and P value vs controls on day:
|
No. of vegetations made culture- negative/total no. of vegetations (%) on day:
|
||||
|---|---|---|---|---|---|---|
| 3
|
6
|
3 | 6 | |||
| Density (no. sacrificed) | P | Density (no. sacrificed) | P | |||
| None (controls) | 7.08 ± 0.96 (12) | 6.5 ± 0.86 (9) | 0/12 (0) | 0/9 (0) | ||
| AMP + SUL | 3.23 ± 1.61 (13) | <0.001 | 2.8 ± 2.17 (13) | <0.005 | 8/13 (63) | 9/13 (71) |
| TROVA | 2.10 ± 0.11 (13) | <0.0001 | 2.36 ± 0.5 (12) | <0.005 | 11/13 (87) | 8/12 (66) |
| VANCO | 2.40 ± 0.45 (12) | <0.001 | 2.0 ± 0.0 (12) | <0.001 | 8/12 (66) | 12/12 (100) |
DISCUSSION
Over the past decade, the antimicrobial therapy of serious staphylococcal infections (e.g., IE) has been extensively studied (10, 21, 23). The use of quinolone antibiotics for human IE was addressed by several groups, predominantly in terms of providing a potential oral treatment regimen for this serious infection. These clinical trials documented the excellent efficacy of such quinolone-based regimens (12, 16). However, over the past decade, a number of important trends in antimicrobial resistance observed in both ORSA and ORSE strains have made the evaluation of newer antimicrobial regimens for invasive staphylococcal infections mandatory. For example, in prosthetic valve IE, the vast majority of cases are caused by ORSE strains (21). Moreover, increasing numbers of native valve IE cases are being caused by ORSA strains (24). In addition, many ORSA and ORSE strains exhibit multidrug resistance, especially to the aminoglycosides. Of paramount importance, despite the near-uniform susceptibility of ORSA and ORSE strains to VANCO in vitro, numerous reports have confirmed the often slow and suboptimal treatment outcomes by using VANCO in single-drug or combination therapy regimens (24). Lastly, the recent isolation of ORSA strains, from broad geographic areas, with intermediate susceptibilities to VANCO (MICs of 4 to 8 μg/ml) in patients failing VANCO-based regimens (6, 7, 17) underscores the critical necessity for newer antistaphylococcal therapies.
TROVA is a new fluoroquinolone agent with excellent and broad-spectrum activity against both gram-positive and gram-negative organisms (13, 26). A number of investigators have compared the in vitro antibacterial activities of TROVA with those of other quinolones and have confirmed that TROVA was significantly more potent than standard, clinically available quinolones (e.g., ciprofloxacin) against many gram-positive organisms, including pneumococci, group A streptococci, and quinolone-susceptible or quinolone-resistant staphylococci (13, 26). In addition, recent pharmacokinetic investigations of tissue (using positron emission tomography) have documented the excellent penetration of TROVA into most clinically relevant tissues, including the heart tissue (14). Furthermore, recent studies of diverse experimental animal models have provided in vivo evidence of the clinical potential of TROVA against serious human infections (2, 22). The current study was designed to evaluate the therapeutic efficacy of TROVA against experimental IE due to both ORSA and ORSE strains (ORSA and ORSE IE), in comparison to two regimens which have proven effectiveness in this model (VANCO and AMP-SUL) (18, 29).
The present investigation confirmed the excellent in vivo efficacy of TROVA against both ORSA and ORSE experimental IE. Against ORSA IE, TROVA was clearly the most rapidly bactericidal agent tested and was the most active agent against the tested strain for rendering vegetations culture negative over the 6-day treatment period (∼50% versus ∼40% for AMP-SUL regimens and ∼18% for VANCO regimens). These in vivo findings paralleled those showing the rapid and complete killing of the ORSA strain in vitro within 6 h of incubation with TROVA. Against ORSE IE, all three antibiotic regimens were quite active at reducing vegetation bacterial densities over the 6-day treatment period. The observed superior activity of VANCO against ORSE IE compared to its activity against ORSA IE undoubtedly reflects the eightfold-lower in vitro MIC of VANCO for the ORSE strain than for the ORSA strain. Of note, against both ORSA and ORSE IE, the AMP-SUL regimen was effective, confirming several previous experiences with this combination against experimental staphylococcal IE (18, 29). Of interest, Kaatz et al. (20) recently studied the efficacy of TROVA by using a similar model of S. aureus IE caused by oxacillin-susceptible and ORSA strains. Although the dose regimens of TROVA and VANCO used were different from those in the present study and although the duration of therapy selected was shorter than that in the present study (4 versus 6 days), these investigators documented microbiologic outcomes which parallel our own observations. Thus, for the ORSA strain in their study, both TROVA and VANCO were highly efficacious in eradicating ORSA from cardiac vegetations, sterilizing nearly all lesions over the 4-day therapy course. These high rates of vegetation sterilization for ORSA IE treated with either TROVA or VANCO (100 and 98%, respectively) compared to those from the present study (63 and 27%, respectively) may well relate to the substantially lower MICs of both study drugs in the former investigation. Accordingly, the TROVA and VANCO MICs for the ORSA strain in the study of Kaatz et al. were 20-fold and 5-fold lower, respectively, than the MICs for the ORSA strain utilized in the present study. Moreover, the peak achievable levels of TROVA in serum seen in the previous study at 1 h postdose (20) were substantially higher than those that we observed, despite a lower overall TROVA dose. These differences in achievable levels in infected rabbits in the two studies may well reflect intrinsic differences in the virulences of the infecting strains between these investigations, and the subsequent impact of these differences on drug clearances.
In summary, TROVA exhibited excellent in vivo activity against β-lactamase-producing strains of ORSA and ORSE in a severe and rigorous model of invasive staphylococcal infection. This agent thus provides a potentially important advance in antimicrobial therapy, and further clinical evaluation of this agent is justified. Moreover, should this agent prove to be effective against ORSA strains with reduced susceptibility to VANCO in vivo (6, 7, 17), it would substantially broaden the therapeutic arsenal against multidrug-resistant staphylococci.
ACKNOWLEDGMENT
This study was supported by a research grant from Pfizer Pharmaceuticals, Inc., New York, N.Y.
REFERENCES
- 1.Bayer A S, Crowell D, Bradley D, Yih J, Norman D C. Differential antimicrobial pharmacokinetics and pharmacodynamics in right-sided versus left-sided vegetations in experimental Pseudomonas aeruginosa endocarditis. J Infect Dis. 1988;158:355–359. doi: 10.1093/infdis/158.2.355. [DOI] [PubMed] [Google Scholar]
- 2.Bayer A S, Li C, Kim E, Ing M. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Efficacy of trovafloxacin (CP-99219) in the therapy of experimental endocarditis due to drug-resistant enterococci, abstr. B2; p. 21. [Google Scholar]
- 3.Bayer A S, Norman D C. Valve-site specific pathogenetic differences between right-sided and left-sided bacterial endocarditis. Chest. 1990;98:200–205. doi: 10.1378/chest.98.1.200. [DOI] [PubMed] [Google Scholar]
- 4.Bayer A S, Norman D C, Chiu C Y, Nast C. Pathogenetic effects of neutropenia, monocytopenia and steroid treatment in experimental Pseudomonas endocarditis. Chemotherapy. 1989;35:278–288. doi: 10.1159/000238683. [DOI] [PubMed] [Google Scholar]
- 5.Bayer A S, Tu J. Chemoprophylactic efficacy against experimental endocarditis caused by β-lactamase-producing, aminoglycoside-resistant enterococci is associated with prolonged serum inhibitory activity. Antimicrob Agents Chemother. 1990;34:1068–1074. doi: 10.1128/aac.34.6.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Staphylococcus aureus with reduced susceptibility to vancomycin—United States, 1997. Morbid Mortal Weekly Rep. 1997;46:765–766. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Update: Staphylococcus aureus with reduced susceptibility to vancomycin—United States, 1997. Morbid Mortal Weekly Rep. 1997;46:813–815. [PubMed] [Google Scholar]
- 8.Chambers H F. Coagulase-negative staphylococci resistant to β-lactam antibiotics in vivo produce penicillin-binding protein 2a. Antimicrob Agents Chemother. 1987;31:1919–1924. doi: 10.1128/aac.31.12.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers H F, Archer G L, Matsuhashi M. Low-level methicillin resistance in strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1989;33:424–428. doi: 10.1128/aac.33.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers H F, Miller T, Newman M D. Right-sided Staphylococcus aureus endocarditis in intravenous drug addicts: two-week combination therapy. Ann Intern Med. 1988;109:619–624. doi: 10.7326/0003-4819-109-8-619. [DOI] [PubMed] [Google Scholar]
- 11.Chambers H F, Sachedeva M. Binding of β-lactam antibiotics to penicillin-binding proteins in methicillinn-resistant Staphylococcus aureus. J Infect Dis. 1990;161:1170–1176. doi: 10.1093/infdis/161.6.1170. [DOI] [PubMed] [Google Scholar]
- 12.Dworkin R J, Lee B L, Sande M A, Chambers H F. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampin. Lancet. 1989;ii:1071–1073. doi: 10.1016/s0140-6736(89)91083-0. [DOI] [PubMed] [Google Scholar]
- 13.Eliopoulos G M, Klimm K, Eliopoulos C T, Ferraro M J, Moellering R C., Jr In vitro activity of CP-99,219, a new fluoroquinolone, against clinical isolates of gram-positive bacteria. Antimicrob Agents Chemother. 1993;37:366–370. doi: 10.1128/aac.37.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischman A J, Babich J W, Alpert N M, Vincent J, Wilkinson R A, Callahan R J, et al. Pharmacokinetics of 18F-labeled trovafloxacin in normal and Escherichia coli-infected rats and rabbits studied with positron emission tomography. Clin Microbiol Infect. 1996;3:63–72. doi: 10.1111/j.1469-0691.1997.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 15.Hackbarth C J, Chambers H F. Methicillin-resistant staphylococci: detection methods and treatment of infections. Antimicrob Agents Chemother. 1989;33:995–999. doi: 10.1128/aac.33.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heldman A W, Hartert T V, Ray S C, Daoud E G, Kowalski T E, Pompili V J, et al. Oral antibiotic treatment of right-sided staphylococcal endocarditis in injection drug users: prospective randomized comparison with parenteral therapy. Am J Med. 1996;101:68–76. doi: 10.1016/s0002-9343(96)00070-8. [DOI] [PubMed] [Google Scholar]
- 17.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover F C. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 18.Hirano L, Bayer A S. β-lactam–β-lactamase-inhibitor combinations are active in experimental endocarditis caused by β-lactamase-producing oxacillin-resistant staphylococci. Antimicrob Agents Chemother. 1991;35:685–690. doi: 10.1128/aac.35.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ing M B, Baddour L, Bayer A S. Staphylococcal bacteremia and infective endocarditis—pathogenesis, diagnosis and complications. In: Archer G, Crossley K, editors. Staphylococci and staphylococcal diseases. New York, N.Y: Churchill-Livingstone Publishers; 1997. pp. 331–354. [Google Scholar]
- 20.Kaatz G W, Seo S M, Aeschlimann J R, Houlihan H H, Mercier R-C, Rybak M J. Efficacy of trovafloxacin against experimental Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1998;42:254–256. doi: 10.1128/aac.42.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karchmer A W, Archer G L, Dismukes W E. Staphylococcus epidermidis causing prosthetic valve endocarditis—microbiologic and clinical observations as guides to therapy. Ann Intern Med. 1983;98:447–455. doi: 10.7326/0003-4819-98-4-447. [DOI] [PubMed] [Google Scholar]
- 22.Kim Y S, Liu Q, Chow L L, Täuber M G. Trovafloxacin in treatment of rabbits with experimental meningitis caused by high-level penicillin-resistant Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:1186–1189. doi: 10.1128/aac.41.5.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korzeniowski O, Sande M A The National Collaborative Endocarditis Study Group. Combination antimicrobial therapy for Staphylococcus aureus endocarditis in patients addicted to parenteral drugs and in nonaddicts: a prospective study. Ann Intern Med. 1982;97:496–503. doi: 10.7326/0003-4819-97-4-496. [DOI] [PubMed] [Google Scholar]
- 24.Levine D P, Fromm B S, Reddy B R. Slow response to vancomycin or vancomycin plus rifampin in therapy among patients with methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115:674–680. doi: 10.7326/0003-4819-115-9-674. [DOI] [PubMed] [Google Scholar]
- 25.Massanari R M, Pfaller M A, Wakesfield D S, Hammons G T, McNut L A, Woolson R E, et al. Implications of acquired oxacillin resistance in the management and control of Staphylococcus aureus infections. J Infect Dis. 1988;158:702–709. doi: 10.1093/infdis/158.4.702. [DOI] [PubMed] [Google Scholar]
- 26.Neu H C, Chin N-X. In vitro activity of the new fluoroquinolone CP-99,219. Antimicrob Agents Chemother. 1994;38:2615–2622. doi: 10.1128/aac.38.11.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piercy E A, Barbaro D, Luby J P, Mackowiak P A. Ciprofloxacin for methicillin-resistant Staphylococcus aureus infections. Antimicrob Agents Chemother. 1989;33:128–130. doi: 10.1128/aac.33.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pittet D, Wenzel R P. Nosocomial blood-stream infections—secular trends in rates, mortality and contribution to total hospital deaths. Arch Intern Med. 1995;155:1177–1184. doi: 10.1001/archinte.155.11.1177. [DOI] [PubMed] [Google Scholar]
- 29.Ramos M C, Ing M, Kim E, Witt M D, Bayer A S. Ampicillin-sulbactam is effective in prevention and therapy of experimental endocarditis caused by β-lactam-producing coagulase-negative staphylococci. Antimicrob Agents Chemother. 1996;40:97–101. doi: 10.1128/aac.40.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders, C. C., W. E. Sanders, Jr., and K. S. Thomson. 1995. Fluoroquinolone-resistance in staphylococci—new challenges. Eur. J. Clin. Microbiol. Infect. Dis. 14(Suppl.):6–11. [PubMed]
- 31.Sidebottom D G, Freeman J, Platt R, Epstein M F, Goldmann D A. Fifteen-year experience with bloodstream isolates of coagulase-negative staphylococci. J Clin Microbiol. 1988;26:713–718. doi: 10.1128/jcm.26.4.713-718.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vincent, J., J. Venitz, R. Teng, B. A. Baris, S. A. Willavize, R. J. Polzer, and H. L. Friedman. 1997. Pharmacokinetics and safety of trovafloxacin in healthy male volunteers following administration of single intravenous doses of the prodrug, alatrofloxacin. J. Antimicrob. Chemother. 39(Suppl.):75–80. [DOI] [PubMed]