Abstract
Introduction
Spontaneous intracranial hypotension (SIH) caused by a spinal cerebrospinal fluid (CSF) leak classically presents with orthostatic headache. Digital subtraction myelography (DSM) has a well-established diagnostic yield in the absence of extradural spinal collection. At our institution, DSM is followed by lateral decubitus CT myelogram (LDCTM) in the same decubitus position to increase diagnostic yield of the combined study. We evaluated the incremental diagnostic yield of LDCTM following negative DSM and reviewed patient outcomes.
Methods
Retrospective review of consecutive DSMs with subsequent LDCTM from April 2019 to March 2021 was performed. Combined reports were reviewed, and studies with positive DSMs were excluded. Of the exams with negative DSM, only studies with LDCTM reports identifying potential leak site were included. Interventions and follow-up clinical notes were reviewed to assess symptoms improvement following treatment.
Results
Of the 83 patients with negative DSMs, 11 (13.2%) had positive leak findings on LDCTMs, and 21 (25.3%) were equivocal. Of 11 positive LDCTMs, 6 leaks were nerve sheath tears (NSTs) and 5 were CSF-venous fistulas (CVFs). 10/11 (90.9%) had intervention and follow-up, with 9/10 (90%) having positive clinical outcome. Of the 21 equivocal LDCTM patients (19 CVFs and 2 NSTs), 15 (71.4%) had interventions and follow-up, with 3/15 (20.0%) with positive clinical outcomes.
Conclusion
LDCTM following negative DSM has an incremental diagnostic yield up to 38.6%, with up to 14.5% of positive patient outcomes following treatment. LDCTM should be considered after DSM to maximize diagnostic yield of the combined exam.
Keywords: Cerebrospinal fluid leak, spontaneous intracranial hypotension, digital subtraction myelography, CT myelography, cerebrospinal fluid-venous fistula
Introduction
Spontaneous intracranial hypotension (SIH) refers to low intracranial cerebrospinal fluid (CSF) volumes, caused by a dural tear, meningeal diverticulum/nerve sheath tear (NST), or CSF-venous fistula (CVF).1,2 SIH patients can have variable clinical presentation, most commonly orthostatic headache, but also cognitive impairment, movement disorders, vestibulocochlear dysfunction, cranial neuropathies, neck pain, and nausea. 3 The imaging workup for suspected SIH starts with a contrast enhanced brain MRI to assess for SIH signs and spine MRI to evaluate for extradural fluid collection.4–8 In the absence of a spinal longitudinal epidural collection (SLEC), further workup can entail a conventional CT myelography (CTM), dynamic CTM, MR myelography, or digital subtraction myelography (DSM) depending on the institutional preferences.2,6,9–12
DSM is a minimally invasive exam with a well-established diagnostic yield for CVFs in the absence of extradural spinal collection.7,9,11,13 Lateral decubitus CTM (LDCTM) and MR myelography have also been shown to localize the site of the leak in the absence of extradural collection.14,15 DSM does not preclude LDCTM following the procedure, although dependent contrast opacification after DSM may not be optimal compared to dedicated LDCTM performed immediately after contrast injection.
At our institution, we perform all DSMs in conjunction with LDCTM following DSM (usually within 10–30 min depending on CT scanner availability) in an attempt to increase diagnostic yield of the combined study. 6 We report incremental diagnostic yield of LDCTM immediately following negative DSM, and evaluate patient outcomes following leak treatment, which to our best knowledge has not been reported.
Methods
Inclusion criteria
Following institutional review board approval, we retrospectively identified a consecutive cohort of patients who underwent DSM with immediate subsequent LDCTM for evaluation of SIH from April 2019 to March 2021. The radiology reports for DSM/LDCTM exams were reviewed and the studies where DSM showed definite leak were excluded. Of the exams with negative DSM, corresponding LDCTM reports were reviewed for findings suspicious for the specific site of CSF leak, and only the studies that identified a specific site suspicious for a leak were included. Specifically, studies that showed only the presence of renal contrast as a suspicious finding were not included.
Lateral decubitus digital subtraction myelography/lateral decubitus CT myelogram techniques
Our technique for DSM has been previously described. 6 Each DSM/LDCTM combined exam is referred to one side down DSM followed by the same position LDCTM on the same day. The combined study is repeated the next day on the opposite side due to a limit of intrathecal iodine amount per day. That is, one patient will usually undergo two DSM/LDCTM combined exams: right-side down on day#1 and left-side down on day#2.
Each exam was performed by one of the eleven fellowship-trained neuroradiologists within our spine practice that focuses on CSF leaks, with neuroradiology experience ranging from 1 to 25 years. Following each DSM exam in the fluoroscopy suite, the patients were kept in the same lateral decubitus position and moved to CT suite while strictly maintaining that same decubitus position. Entire spine CT myelogram was then performed in the same lateral decubitus position in a single scan pass from the skull base through the sacrum with the patient’s arms raised by the head. Most LDCTMs were performed under Somatom Definition FLASH scanner (Siemens, Erlangen, Germany) in dual energy mode at the following setting: kVp: 100/140, reference mAs: 230/178, rotation time: 1 s, pitch: 0.9. Axial reconstructions were made at 0.75 mm and 3 mm in soft tissue and bone kernels for both 100 kVp and 140 kVp scans. Axial 0.75 mm 50 keV reconstructions were also made. In larger patients with abdominal size larger than 45 cm diameter, single energy scan was performed since 100 kVp scan quality can be suboptimal. The single energy mode setting is: kVp: 120, reference mAs: 260, rotation time: 1 s, pitch: 0.9. The interpreting neuroradiologist was also the proceduralist performing the DSM, and thus DSM/LDCTM combined exam was interpreted by the same neuroradiologist. The LDCTM images were scrutinized under multiplanar reformats using Visage PACS software (Visage Imaging, Inc, San Diego, CA).
Data extraction
Each DSM and LDCTM report was reviewed by two fellowship-trained neuroradiologists who routinely perform DSM/LDCTM, one with 2 years, and one with 4 years of neuroradiology experience. Both findings and impression sections of the reports were reviewed. DSM was categorized as positive or negative for identification of CSF leak site. LDCTM findings were categorized as positive, equivocal, or negative.
“Positive” finding was recorded per report if the interpreting radiologist unequivocally stated the finding, for example, “right L3 NST or findings consistent with a left T9 CVF.” Whereas “equivocal” finding was recorded in cases of variable degree of certainty as interpreted by the radiologist performing the procedure, for example, “findings suspicious/equivocal/concerning for a left T4 CVF.”
For studies that met the inclusion criteria, that is, ones with negative DSM and positive or equivocal LDCTM findings in the radiology report, electronic medical records were reviewed for subsequent intervention and clinical follow-up to assess clinical response to the intervention. Positive clinical outcomes were defined as significant symptoms improvement for at least 3 months or resolution of symptoms.
Patient demographic information and time interval (minutes) between each DSM and subsequent LDCTM were recorded.
Results
Out of 349 DSM exams, 9 exams were excluded: 6 were nondiagnostic, 1 had no subsequent LDCTM, 1 was a very suboptimal study investigating extradural fluid collection seen on MRI, and 1 showed a very complex intradural fluid collection favoring postoperative multiloculated arachnoiditis. Therefore, a total of 340 combined DSM + LDCTM exams from 150 patients were included in the final study cohort. 24 patients had more than one set of DSM/LDCTM exams. 12 patients had only 1 day of DSM/LDCTM, either right or left-side down, and did not undergo the second day opposite side decubitus exam, either because a definite leak was localized on day one, or the second day exam was canceled due to the patient being unable/unwilling to undergo the 2nd day exam.
Of these 340 DSM/LDCTM combined exams, 67 (19.7%) DSMs were positive for a localized CSF leak and 273 (80.3%) DSMs were negative.
Out of 273/340 (80.3%) negative DSMs, 11/273 (4.0%) had a positive finding for a CSF leak on the subsequent LDCTM, and 23/273 (8.4%) had equivocal findings. Two LDCTMs had positive findings and one LDCTM had equivocal findings outside the field of view (FOV) on the preceding DSM. We do not routinely include spine segment below needle placement on the DSM as the contrast preferentially runs cephalad due to patient positioning on a wedge and table head-down tilt. 6 Therefore, the DSM would not be able to evaluate the sacrum. 16
Patient demographics, LDCTM findings, subsequent interventions, and clinical follow-up if applicable are summarized in Table 1 (positive findings are highlighted in bold).
Table 1.
Patient demographics, CTM findings, subsequent interventions, and clinical follow-up. Positive findings are highlighted in bold. R: right; L: left; FOV: field of view; NST: nerve sheath tear; CVF: CSF-venous fistula; BP: blood patch; h/a: headache.
| Gender (M/F) | Age | Side down | CTM findings | Renal contrast | Time b/w DSM-CT (min) | Intervention (BP, ligation, embolization) | F/U after intervention (days) | Symptom improvement |
|---|---|---|---|---|---|---|---|---|
| M | 52 | R | R L3 NST | no | 23 | Targeted BP with fibrin | 32 | Resolution of h/a |
| M | 37 | R | R T10 and T11 NST | no | 20 | Targeted BP with fibrin (had dynamic CTM with similar findings) | 58 |
Resolution of h/a
Rebound intracranial hypertension |
| M | 57 | R | R T11 CVF | no | 18 | R T11 + L T8 ligation | 102 | Resolution of h/a |
| M | 49 | L | L L2 CVF | yes | 15 | Embolization after targeted BPs | 52 |
Transient 3 weeks improvement after BPs,
Stable improvement after same level embolization |
| F | 34 | R | R T7 NST | yes | 20 | BP no fibrin | 316 |
Resolved h/a
Rebound intracranial hypertension |
| M | 43 | R |
Outside FOV, puncture at L3-4;
R S1 NST |
yes | 20 | Targeted BP with fibrin | 150 | Improved h/a |
| M | 58 | R |
Outside FOV, puncture at L3-4;
L4-5 extradural contrast, CVF at surgery |
yes | 21 | Targeted BP with fibrin | 159 | Transient improvement 3 months after BP, lost to follow-up after laminectomy |
| M | 61 | L | L T7 NST | yes | 14 | Targeted BP with fibrin | 371 | Resolved h/a |
| M | 50 | L | L T9 CVF | yes | 12 | Venous embolization | 62 | Resolved h/a |
| F | 58 | L | L T9 CVF | yes | 30 | Venous embolization | 57 | No significant improvement |
| F | 35 | L | L NST T10 and T11 | yes | 18 | no | n/a | n/a |
| F | 46 | R | R T4 and T10 CVF | yes | 11 | R T4 + T10 ligation | 557 | 1-year improvement, h/a recurred |
| F | 36 | R | R T3 CVF | yes | 11 | Multifocal BP no fibrin | 21 | no significant improvement |
| M | 69 | R | R T4-6 CVF | yes | 19 | BP interlaminar T4-5, T7-8 | 365 | Essentially resolved |
| F | 56 | L | L T4 CVF | no | 42 | BP no fibrin | 40 | Transient improvement 2 weeks |
| F | 58 | L | Outside FOV, puncture at L1-2; R L2-3 CVF |
yes | 22 | BP no fibrin | 145 | Transient improvement 1 week |
| F | 36 | L | L T2 CVF | yes | 30 | Targeted BP with fibrin | 14 | Transient improvement 1 week |
| F | 48 | L | Bilat C7-T1 CVF | no | 18 | BP interlaminar C7-T1 | 97 | Transient improvement 5 days |
| F | 63 | R | L3 CVF | yes | 11 | Targeted BP with fibrin | 7 | Transient improvement 3 days |
| F | 28 | L | L T12 CVF | yes | 25 | Targeted BP with fibrin | 112 | Transient improvement 3 days |
| F | 28 | R | R T11 CVF | yes | 10 | Targeted BP with fibrin | 112 | Transient improvement 3 days |
| F | 52 | R | R L4 CVF | yes | 25 | Root packing | 131 | No improvement |
| F | 35 | R | R T1, T9, T11, T12, L1, L2 NST | yes | 16 | Targeted BP with fibrin | 348 | No improvement |
| F | 63 | L | L T10, L T12 CVF | yes | 12 | BP interlaminar T10-11 | 12 | No improvement |
| F | 50 | R | R T11 CVF | yes | 12 | Targeted BP with fibrin | 425 | No improvement |
| F | 26 | L | L T11 CVF | no | 17 | No | n/a | n/a |
| F | 17 | L | L T11 CVF | no | 20 | No | n/a | n/a |
| M | 51 | L | L T4 CVF | no | 36 | No | n/a | n/a |
| F | 63 | L | L T1 and T11 CVF | no | 13 | No (more definitive leak on the other side) | n/a | n/a |
| M | 61 | R | R T6, T12, L2 CVF | yes | 13 | No (more definitive leak on the other side) | n/a | n/a |
| F | 58 | R | R T12, L2 CVF | no | 11 | No (more definitive leak on the other side) | n/a | n/a |
| F | 65 | R | R T2 CVF | yes | 24 | BP no fibrin | 438 | Significantly improved |
| F | 86 | R | R T7 NST | yes | 40 | BP no fibrin | n/a | n/a |
| F | 44 | R | R T8 CVF | yes | 25 | BP interlaminar T8-9 | n/a | n/a |
Positive LDCTM cases
Of the 83 DSM negative patients, 11 (13.3%) had positive CSF leaks on LDCTM. Six (54.5%) were NST and 5 (45.5%) were CSF-venous fistulas (CVF). In these 11 cases, LDCTM was obtained 19.2 min (mean) after DSM, range 12–30 min; 3 were females (27.3%); mean age 48.5 years, range 34–61 years.
An example of positive LDCTM findings for a CSF-venous fistula following a negative DSM is shown in Figure 1. An example of positive LDDCTM findings for a lateral CSF leak is shown in Figure 2.
- ⋄ 5 NST patients underwent targeted blood/fibrin patch with resolution or significantly improved symptoms (one also had a dynamic CTM after DSM with similar findings).
- ○ two of these patients developed rebound intracranial hypertension which were medically managed.
⋄ 1 NST patient did not undergo any intervention and was lost to follow-up.
⋄ 2 CVF patients underwent venous embolization with significantly improved or resolved symptoms.
⋄ 1 CVF patient had resolution of symptoms after laminectomy and nerve root ligation together with a ligation of a contralateral diverticulum.
⋄ 1 CVF patient had transient 3-month improvement after targeted blood patches, and then underwent definitive treatment with laminectomy, but was subsequently lost to follow-up.
⋄ 1 CVF patient had persistent symptoms despite catheter venous embolization.
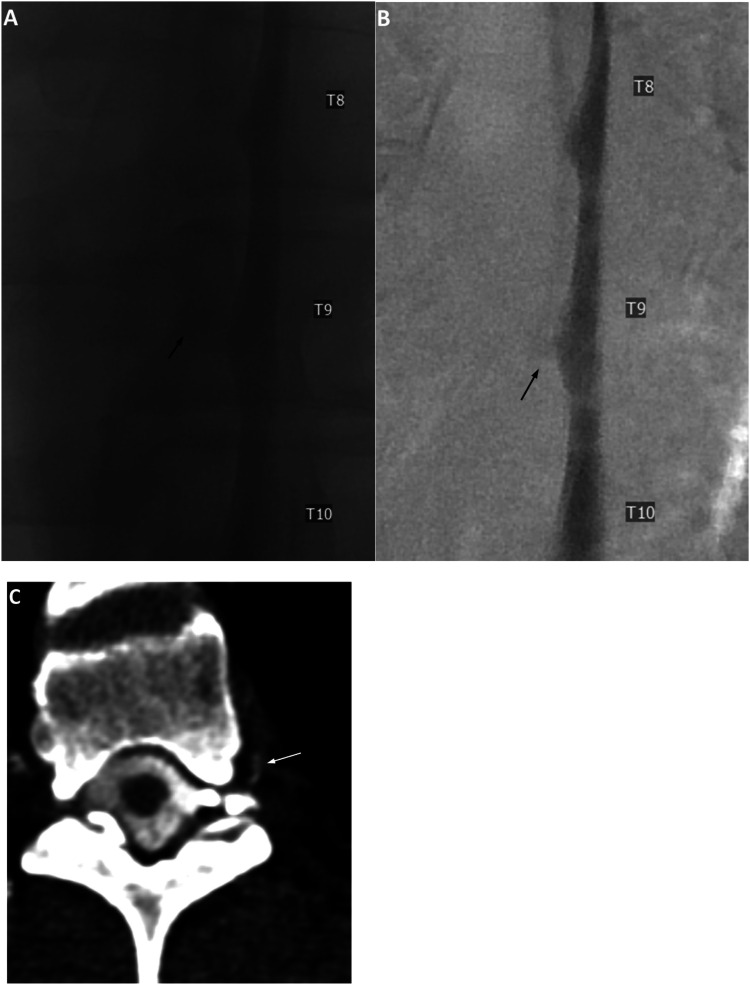
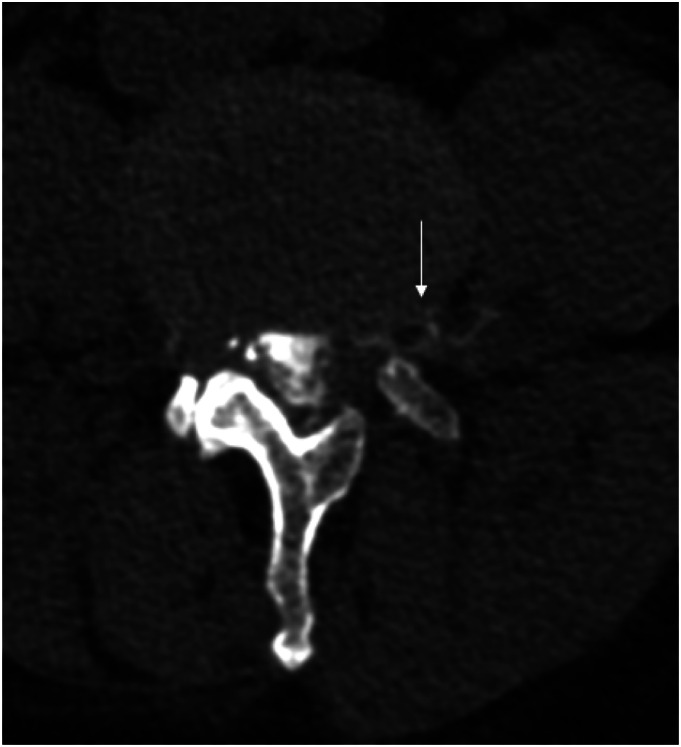
Figure 1.
Unsubtracted (a) and subtracted (b) left lateral decubitus digital subtraction myelogram images show only an ill-defined diverticulum at the left T9 level (black arrows). Subsequent CTM in the same lateral decubitus position after approximately 12 min following LDDSM (c, reformatted axial image) shows a hyperdense paraspinal vein at this level (white arrow), consistent with a CSF-Venous fistula. Renal contrast was present (not shown). Patient underwent venous embolization with resolution of postural headache.
Figure 2.
Right lateral decubitus CTM image immediately following right lateral decubitus DSM shows a non-dependent, contralateral left L4-L5 lateral CSF leak (arrow).
Thus, out of 11 patients with a positive CSF leak on a subsequent same side down LDCTM in a setting of negative DSM, 10/11 (90.9%) had interventions and clinical follow-up, with resolved or improved symptoms in 9 out of these 10 patients (90.0%), for a total of 9/83 (10.8%) of additional positive clinical outcomes based on LDCTM findings in a setting of a preceding negative DSM.
Equivocal LDCTM cases
Of the 83 DSM negative patients, 23 equivocal LDCTM findings were in 21 patients including two patients with bilateral equivocal CVF (one patient—with equivocal bilateral C7-T1 CVFs, both suspected on just left-side exam and counted as one finding; one patient—with two equivocal CVFs on both sides LDCTMs—left T12 and right T11).
Out of these 21 patients, 19 (90.5%) were equivocal for CVFs and 2 (9.5%) for NSTs. LDCTM was obtained 20.1 min (mean) after DSM, range 10–42 min; 19 were females (90.5%); mean age 50.5 years, range 17–86 years. Example of equivocal LDCTM findings for a CSF-venous fistula following a negative DSM is shown in Figure 3.
- ⋄ Both equivocal NSTs underwent targeted blood patches:
- ⋄ 1—without improvement at 1-year follow-up.
- ⋄ 1—lost to follow-up.
- ⋄ 13 of the 21 equivocal CVFs were treated with targeted and multilevel blood patches with the following results
- ⋄ 2—sustained improvement/essentially resolved headache, both at greater than a year follow-up.
- ⋄ 10—no significant or minimal transient improvement (less than 2 weeks).
- ⋄ 1—no follow-up.
- ⋄ 2 of the 21 equivocal CVF patients underwent surgery:
- ⋄ 1—with nerve root ligation and symptoms improvement for a year with subsequent symptoms recurrence.
- ⋄ 1—with nerve root packing, but persistent symptoms at a 4.5-month follow-up.
⋄ 3 of the 21 equivocal CVFs had more definitive leaks on the contralateral side and did not undergo treatment for equivocal findings.
⋄ 3 equivocal CVFs did not undergo intervention.
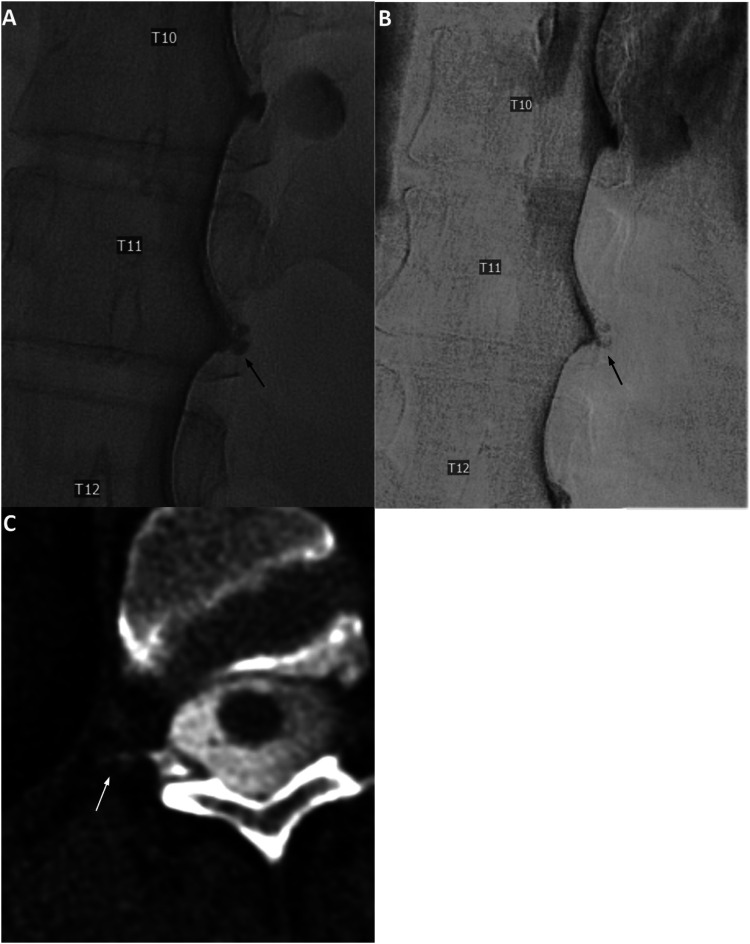
Figure 3.
Unsubtracted (a) and subtracted (b) right lateral decubitus digital subtraction myelogram images show prominent diverticulum at the right T11 level (black arrows). Subsequent CTM in the same lateral decubitus position after approximately 12 min following LDDSM (c, reformatted axial image) shows a suggestion of subtle lateral contrast extension at this level (white arrow), which was described as “indeterminant, but suspicious” for CSF-Venous fistula. Renal contrast was present (not shown). Patient underwent targeted blood patch with fibrin glue, without improvement of postural headache.
Thus, out of 21 equivocal CSF leak patients on subsequent LDCTM in a setting of negative preceding DSM, 15/21 (71.4%) had interventions and clinical follow-up, with improved symptoms for at least a year in 3/15 (20.0%) patients, with one patient having recurrence of symptoms in a year after nerve root ligation for a CVF. 12/15 (80.0%) patients had no/very transient minimal symptomatic improvement. Therefore, there was 3/83 (3.6%) incremental positive clinical outcomes following treatment of equivocal LDCTM findings in a setting of a preceding negative DSM.
Comparison of clinical outcomes between positive and equivocal CSF leak cohorts who underwent intervention based only on LDCTM findings and who had subsequent clinical follow-up is summarized in Table 2.
Table 2.
Comparison of positive clinical outcomes between positive and equivocal CSF leak cohorts, who had intervention based only on LDCTM findings after a negative DSM, and who had subsequent clinical follow-up.
| Positive clinical outcomes | No positive clinical outcomes | |
|---|---|---|
| Positive CSF leak with intervention and follow-up | 9/10 (90.0%) | 1/10 (10.0%) |
| Equivocal CSF leak with intervention and follow-up | 3/15 (20.0%) | 12/15 (80.0%) |
| Total incremental positive clinical outcomes for positive and equivocal CSF leak | 12/83 (14.5%) |
Discussion
Our largest to date retrospective review of LDCTMs following DSM demonstrates incremental diagnostic yield of LDCTM of up to 38.6% in a setting of preceding negative DSM. In the cohort of patients who had intervention based solely on LDCTM findings with a preceding negative LDDSM and who had clinical follow-up, total incremental positive clinical outcomes were seen in 14.5% of cases. This is a significant number of patients who would benefit from a complementary LDCTM, particularly at a large CSF dynamics center investigating CSF leaks with multiple imaging modalities.
To our best knowledge, there are currently no studies specifically evaluating incremental diagnostic yield and clinical outcomes of LDCTMs following negative DSMs. Previous review of 5 patients by Kranz et al. in 2019 14 showed the utility of LDCTM for CSF leak localization, performed 10–15 min after completion of fluoroscopic imaging for dynamic myelogram, based on abnormality previously seen on the nondecubitus CTM. CVF may be more conspicuous through increased contrast flow on the dependent side, which would account for higher sensitivity of the LDCTM over the routine CTM. The study does not specify if the leak was also visible on the preceding fluoroscopic myelogram. While a recent study of 22 patients by Mamlouk et al. 17 demonstrated the efficacy of dedicated LDCTM (without preceding DSM) with intrathecal access on the CT table and immediate CT scan (11/22 (50%) cases positive for a CVF), these patients did not have a previous DSM and therefore the incremental diagnostic yield of these immediate LDCTMs cannot be estimated.
Our study also highlights an apparent benefit of LDCTM following DSM—evaluation of the levels outside the field of view on the preceding DSM. Given limitations of craniocaudal extent of the biplane fluoroscopy especially in taller patients, upper cervical and lower lumbar levels are essentially always excluded from the field of view on the DSM. In addition, since the intrathecal contrast preferentially flows cephalad due to patient positioning on a wedge and/or table head-down tilt, spine segments below the needle access would not opacify. In our case series 2 positive and 1 equivocal CSF leaks were found on LDCTM below the needle placement outside the field of view on the preceding DSM, with positive patient outcomes after intervention in two positive cases. The thecal sac is essentially always adequately opacified through lumbar-sacral region on subsequent LDCTM due to several factors: the patient is taken off the wedge, table is flattened and sometimes even tilted slightly feet-down to help redistribute intrathecal contrast. Additionally, there is extra time for intrathecal contrast to redistribute while the patient is being transferred to CT in a relatively flat lateral decubitus position compared to tilted head-down for DSM.
Given the possible intermittent nature of CVFs, 13 LDCTMs add another time point and increase temporal resolution of the study, potentially registering/highlighting CVF in its active/more conspicuous phase. Finally, the presence of early excreted renal contrast on CTMs, to our knowledge only seen in SIH population, can assist proceduralists in more confident identification of CSF leaks, particularly CSF-venous fistulas. 18 Of our 11 patients with positive CTM after negative DSM, we saw renal contrast in 8 patients (72.7%). More work is needed in future studies to examine the link between early renal contrast and CSF leak characteristics.
In addition to the retrospective design, our study has several limitations. First, we only recorded results documented by the performing proceduralists in the report, without reevaluating their findings. This was however a deliberate aspect of the study design, as the subsequent intervention was or was not pursued based on the original report. We did not perform objective measurements of intrathecal contrast density in positive, negative, or equivalent cases. In addition, our clinical follow-up was very variable and inconsistent due to the retrospective nature of the study, with a total of 9 patients lost to follow-up. We also performed DSM in end inspiration, while resisted inspiration is a possible tool that was not used in our study. 19 Finally, CSF pressures were not recorded, which is in part due to technical limitations. Patients are positioned with a bump under their hips and would therefore falsely decrease CSF pressure. Additionally, prior work has shown that patients with SIH can have a normal CSF pressure. 20
It is also difficult to objectively quantify additional benefit of the complementary LDCTM that helps to interpret preceding DSM. For example, CT images help evaluate morphology of the nerve root sleeve diverticula, which can look suspicious on DSM if filling intermittently or incompletely due to larger size and limited time. Future studies from the centers performing high volume of combined DSMs/LDCTMs with more consistent follow-up after treatment would be useful for evaluation of incremental diagnostic yield of LDCTMs following negative DSMs and long-term patient outcomes after treatment.
Conclusion
LDCTM following negative DSM has an incremental diagnostic yield up to 38.6%, with up to 14.5% of positive patient outcomes following treatment. LDCTM should be considered after DSM to maximize diagnostic yield of the combined exam.
Appendix.
Abbreviations
- CVF
CSF-venous fistula
- FOV
Field of view
- LDCTM
Lateral decubitus CT myelogram
- DSM
Lateral decubitus digital subtraction myelogram
- NST
Nerve sheath tear
- SIH
Spontaneous intracranial hypotension
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Ian T Mark https://orcid.org/0000-0002-4036-2992
John C Benson https://orcid.org/0000-0002-4038-5422
Ajay A Madhavan https://orcid.org/0000-0003-1794-4502
Jared T Verdoorn https://orcid.org/0000-0002-1592-1182
References
- 1.Callen AL, Timpone VM, Schwertner A, et al. Algorithmic multimodality approach to diagnosis and treatment of spinal CSF leak and venous fistula in patients with spontaneous intracranial hypotension. AJR Am J Roentgenol 2022; 219(2): 292–301. [published Online First: 20220309]. DOI: 10.2214/ajr.22.27485 [DOI] [PubMed] [Google Scholar]
- 2.Schievink WI, Maya MM, Jean-Pierre S, et al. A classification system of spontaneous spinal CSF leaks. Neurology 2016; 87(7): 673–679. [published Online First: 20160720]. DOI: 10.1212/wnl.0000000000002986 [DOI] [PubMed] [Google Scholar]
- 3.Amrhein TJ, Kranz PG. Spontaneous intracranial hypotension: imaging in diagnosis and treatment. Radiol Clin North Am 2019; 57(2): 439–451. [published Online First: 20181207]. DOI: 10.1016/j.rcl.2018.10.004 [DOI] [PubMed] [Google Scholar]
- 4.Dobrocky T, Grunder L, Breiding PS, et al. Assessing spinal cerebrospinal fluid leaks in spontaneous intracranial hypotension with a scoring system based on brain magnetic resonance imaging findings. JAMA Neurol 2019; 76(5): 580–587. [published Online First: 2019/02/19]. DOI: 10.1001/jamaneurol.2018.4921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kranz PG, Amrhein TJ, Choudhury KR, et al. Time-dependent changes in dural enhancement associated with spontaneous intracranial hypotension. AJR Am J Roentgenol 2016; 207(6): 1283–1287. [published Online First: 2016/08/25]. DOI: 10.2214/AJR.16.16381 [DOI] [PubMed] [Google Scholar]
- 6.Kim DK, Brinjikji W, Morris PP, et al. Lateral decubitus digital subtraction myelography: tips, tricks, and pitfalls. AJNR Am J Neuroradiol 2020; 41(1): 21–28. [published Online First: 2019/12/21]. DOI: 10.3174/ajnr.A6368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farb RI, Nicholson PJ, Peng PW, et al. Spontaneous intracranial hypotension: a systematic imaging approach for CSF leak localization and management based on MRI and digital subtraction myelography. AJNR Am J Neuroradiol 2019; 40(4): 745–753. [published Online First: 2019/03/30]. DOI: 10.3174/ajnr.A6016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starling A, Hernandez F, Hoxworth JM, et al. Sensitivity of MRI of the spine compared with CT myelography in orthostatic headache with CSF leak. Neurology 2013; 81(20): 1789–1792. [published Online First: 2013/10/11]. DOI: 10.1212/01.wnl.0000435555.13695.22 [DOI] [PubMed] [Google Scholar]
- 9.Kim DK, Carr CM, Benson JC, et al. Diagnostic yield of lateral decubitus digital subtraction myelogram stratified by brain MRI findings. Neurology 2021; 96(9): e1312–e1318. [published Online First: 2021/01/22]. DOI: 10.1212/WNL.0000000000011522 [DOI] [PubMed] [Google Scholar]
- 10.Clark MS, Diehn FE, Verdoorn JT, et al. Prevalence of hyperdense paraspinal vein sign in patients with spontaneous intracranial hypotension without dural CSF leak on standard CT myelography. Diagn Interv Radiol 2018; 24(1): 54–59. [published Online First: 2017/12/09]. DOI: 10.5152/dir.2017.17220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schievink WI, Maya MM, Moser FG, et al. Lateral decubitus digital subtraction myelography to identify spinal CSF-venous fistulas in spontaneous intracranial hypotension. J Neurosurg Spine 2019; 31: 902–905. [published Online First: 2019/09/14]. DOI: 10.3171/2019.6.SPINE19487 [DOI] [PubMed] [Google Scholar]
- 12.Schievink WI, Moser FG, Maya MM, et al. Digital subtraction myelography for the identification of spontaneous spinal CSF-venous fistulas. J Neurosurg Spine 2016; 24(6): 960–964. [published Online First: 2016/02/06]. DOI: 10.3171/2015.10.SPINE15855 [DOI] [PubMed] [Google Scholar]
- 13.Mark I, Madhavan A, Oien M, et al. Temporal characteristics of CSF-venous fistulas on digital subtraction myelography. AJNR Am J Neuroradiol 2023; 44: 492–495. [published Online First: 20230309]. DOI: 10.3174/ajnr.A7809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kranz PG, Gray L, Amrhein TJ. Decubitus CT myelography for detecting subtle CSF leaks in spontaneous intracranial hypotension. AJNR Am J Neuroradiol 2019; 40(4): 754–756. [published Online First: 2019/03/02]. DOI: 10.3174/ajnr.A5995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chazen JL, Robbins MS, Strauss SB, et al. MR myelography for the detection of CSF-venous fistulas. AJNR Am J Neuroradiol 2020; 41(5): 938–940. [published Online First: 2020/05/02]. DOI: 10.3174/ajnr.A6521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mark IT, Morris PP, Brinjikji W, et al. Sacral CSF-venous fistulas and potential imaging techniques. AJNR Am J Neuroradiol 2022; 43(12): 1824–1826. [published Online First: 20221103]. DOI: 10.3174/ajnr.A7699 [DOI] [PubMed] [Google Scholar]
- 17.Mamlouk MD, Ochi RP, Jun P, et al. Decubitus CT myelography for CSF-venous fistulas: a procedural approach. AJNR Am J Neuroradiol 2021; 42(1): 32–36. [published Online First: 2020/10/31]. DOI: 10.3174/ajnr.A6844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinsman KA, Verdoorn JT, Luetmer PH, et al. Renal contrast on CT myelography: diagnostic value in patients with spontaneous intracranial hypotension. AJNR Am J Neuroradiol 2019; 40(2): 376–381. [published Online First: 20190117]. DOI: 10.3174/ajnr.A5934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mark IT, Amans MR, Shah VN, et al. Resisted inspiration: a new technique to aid in the detection of CSF-venous fistulas. AJNR Am J Neuroradiol 2022; 43(10): 1544–1547. [published Online First: 20220922]. DOI: 10.3174/ajnr.A7636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kranz PG, Tanpitukpongse TP, Choudhury KR, et al. How common is normal cerebrospinal fluid pressure in spontaneous intracranial hypotension? Cephalalgia 2016; 36(13): 1209–1217. [published Online First: 20160719]. DOI: 10.1177/0333102415623071 [DOI] [PubMed] [Google Scholar]