Abstract
Alcohol Use Disorder (AUD) can induce long lasting alterations to executive function. This includes altered action control, which can manifest as dysfunctional goal-directed control. Cortical and striatal circuits mediate goal-directed control over behavior, and prior research has found chronic alcohol disrupts these circuits. In particular, prior in vivo and ex vivo work have identified alterations to function and activity of dorsal medial striatum (DMS), which is necessary for goal-directed control. However, unknown is whether these alterations manifest as altered activity of select DMS populations during behavior. Here we examine effects of prior chronic alcohol exposure on calcium activity modulation during action-related behaviors via fiber photometry of genetically-identified DMS populations including the direct and indirect output pathways, and fast-spiking interneurons. We find that prior chronic alcohol exposure leads to increased calcium modulation of the direct pathway during action related behavior. In contrast, prior chronic alcohol exposure led to decreased calcium activity modulation of the indirect pathway and the fast-spiking interneuron population around action-related events. Together, our findings suggest an imbalance in striatal activity during action control. This disruption may contribute to the altered goal-directed control previously reported.
Keywords: Dorsal medial striatum, Alcohol, Decision-making, Direct pathway, Indirect pathway
Introduction
Alterations to executive function as well as continued and dysregulated alcohol use are hallmarks of Alcohol Use Disorder (AUD) and are thought to result in part from effects of chronic alcohol on cortical and striatal brain circuits. For example, goal-directed action control is controlled by the dorsal medial region of the striatum (DMS) which has long been shown to be altered by chronic alcohol (for review see: [1,34,35]). Prior investigations have suggested activity within DMS is differently changed following chronic alcohol and that such changes are responsible for a loss of behavioral control [7,15,36,37,44,45,50]. However, whether and how chronic alcohol alters activity in various DMS neuron populations during action control is not clear.
The DMS is a heterogeneous structure, largely composed of two GABAergic output pathways often referred to as the direct and indirect pathways. The direct pathway contains GABAergic spiny neurons (SPNs) that can be differentiated through their monosynaptic connection with the Substantia Nigra Pars Reticulata as well as their expression of the dopamine type-1 receptor (D1-SPNs). In contrast, the GABAergic SPNs of the indirect pathway make a monosynaptic connection with the Globus Pallidus, and express the dopamine type-2 receptor (D2- SPNs) [17]. Modulating these output pathways are various interneuron populations [48], including fast-spiking interneurons that are often identified and targeted by their expression of Parvalbumin (PV interneurons). These interneurons provide a strong inhibitory clamp of SPN activity [9,16,29,47]. The striatum as a whole is driven by glutamatergic inputs largely from cortex and thalamus. In particular, the DMS receives input from associative cortex and thalamus, and is thus poised to integrate and modulate information important for goal-directed control [27]. Indeed, numerous findings across species show that intact DMS is critical for goal-directed behavior (for review see: [2,3,52]).
Chronic alcohol has been reported to disrupt goal-directed control over both general reward-related behavior as well as a loss of goal-directed control over alcohol self-administration [6,8,18,33,44,45]. This has been associated with specific changes in corticostriatal transmission [37,45], as well as with general changes to transmission within DMS. Generally, chronic alcohol has been associated with reduced GABAergic transmission in DMS [11,13,51], and increased glutamatergic transmission [11,12], although recent findings have suggested this may depend on the specific synapses interrogated (e.g. [7,12,44,45]). Pertinent to the present investigation, a prior examination of chronic alcohol drinking reported potentiated glutamatergic transmission onto the direct pathway, and potentiated GABAergic transmission onto the indirect pathway [7]. Mimicking or countering these changes in transmission in vivo increased or decreased alcohol drinking, respectively [7]. Furthermore, both acute and chronic alcohol has been shown to affect transmission from and onto PV interneurons [41–43] and striatal PV interneurons support compulsive alcohol consumption [42]. As it is hypothesized that alcohol self-administration recruits striatal function that eventually becomes less dependent on DMS [8,19], it suggests chronic alcohol may lead to an imbalance between DMS activity that results in dysfunctional goal-directed control.
Here we examine action-related calcium activity of DMS direct and indirect pathways as well as local PV interneurons following chronic alcohol exposure. We employed a reinforcement schedule that biases goal-directed action control in mice [20,21,25]. Prior alcohol exposure has been found to disrupt goal-directed control under this schedule [44–46]. While we used bulk calcium fiber photometry from genetically or projection-defined populations as a proxy for activity, it should be noted that within striatum, bulk calcium activity is not linearly related to the number of action potentials [31]. However, bulk calcium measurements do provide insight into dendritic as well as somatic calcium activity that likely reflects incoming transmission and is related to plasticity processes. In line with prior hypotheses, we find that prior chronic alcohol induced long-lasting increases in the calcium activity of the direct pathway during decision-making, but reduced calcium activity of the indirect pathway and PV fast-spiking interneurons. Our findings support a hypothesis of DMS population activity imbalance resulting in reduced contribution of DMS to behavioral control.
Methods and materials
Subjects
In the present studies, C57Bl/6 J mice (Jackson Labs), Pvalbtm1(cre)Arbr (PV Cre+) (Jackson Labs) [26], B6.FVB(Cg)-Tg(Drd1-cre) EY266Gsat/Mmucd (D1-Cre+) (MMMRC Repository), and B6.FVB (Cg)-Tg(Adora2a-cre)KG139Gsat/Mmucd (A2a-Cre) [14] (MMMRC Repository) mice were used for experiments. Adult (>8 weeks) male and female mice were housed in groups of 2–4, with mouse chow (Labdiet 5015) and water ad libitum, and were kept on a 14 hr light/10 hr dark cycle. While both males and female mice were included, experiments were not powered to identify sex difference, influence of circulating hormones in males or females, or effect of genotype on the calcium modulation observed. All experiments were conducted during the light phase of the light cycle. All experiments were approved by the University of California San Diego Institutional Animal Care and Use Committee and were carried out in accordance with the National Institutes of Health (NIH) “Principles of Laboratory Care”. Investigators were not blind to the experimental groups. The Animal Care and Use Committee of the University of California, San Diego approved all experiments and experiments were conducted according to the National Institutes of Health (NIH) “Principles of Laboratory Care” guidelines.
Surgical procedures and histological verification
All mice were anesthetized with isoflurane (1–2%) and were given stereotaxically guided injections into the DMS (coordinates from Bregma in mm: anterior [A], +0.5; medial [M], ±1.5; ventral [V]: −3.25) via 500 nl volume Hamilton syringes (Reno, NV). To localize GCaMP6s expression to direct pathway neurons we took two approaches. In one, C57Bl/6 J mice were injected with 300nl AAV-Ef1a-Cre-WPRE in the SNr (coordinates from Bregma in mm: anterior [A], −3.4; medial [M], ±1.5; ventral [V]: −4.3) and injected with 300 nL AAV-CAG-Flex-GCaMP6s in the DMS (N = 2 M 1F Air, 2 M 2F CIE). In the second, D1 Cre+ mice were injected with 300 nL AAV-CAG-Flex-GCaMP6s in the DMS (N = 3 M Air, 5 M CIE). To localize to parvalbumin positive and the indirect pathway, PV-Cre+, and A2a-Cre+ mice received unilateral injections of 300 nL AAV-CAG-Flex-GCaMP6s (pAAV.CAG.Flex.GCaMP6s.WPRE.SV40 targeted to DMS, respectively. Post injection, an optic fiber was implanted immediately above the injection site (V: −3.15). After completion of behavioral procedures, mice were deeply anesthetized, brains extracted and placed into 4% Paraformaldehyde for 24–48 hrs and then transferred to 1X PBS. A subset of D1+ Cre and A2a-Cre+ mice were perfused with Calcium Chloride to allow for imaging of GCaMP6s expression in the absence of immune-histochemical procedures. Spread of GCaMP6s expression was not assessed in PV Cre+ mice due to the sparsity of PV Cre+ neurons in DMS. Optic fiber placement was qualified by examining tracts in 100-μm-thick brain slices under a macro fluorescence microscope (Olympus MVX10). Only mice with 1) signal detection and 2) fiber placement within DMS were included for analyses.
Chronic intermittent ethanol exposure and repeated withdrawal procedure
At least one week post surgical procedures, mice were exposed to ethanol vapor or air and repeated withdrawal procedures [4,5,23,33,44]. All mice underwent four rounds, with each round consisting of 16 hr of vapor exposure followed by an 8 hr withdrawal, repeated for four consecutive days followed by 3 days of no vapor exposure. 190 proof ethanol (Koptec, Pennsylvania) was volatilized by bubbling air through a flask containing 95% ethanol at a rate of 2–3 L/min. The resulting ethanol vapor was combined with a separate air stream to give a total flow rate of approximately 10 L/min, which was delivered to the mice in their home cages in Plexiglas chambers (Plas Labs Inc). Blood ethanol concentrations (BEC) were collected at the end of each round from sentinel mice (mean BEC across the four rounds of vapor = 36.7 ± 3.7 mM). No pyrazole or loading ethanol injections were given prior to placement in vapor chambers [44].
Behavioral training procedures
To avoid effects of acute withdrawal [24], food restriction began three days after the last day of vapor procedures, to reduce body weight to ~85–90% of baseline, with this restriction maintained throughout training and testing. Behavioral training procedures began five days post last vapor procedure. Mice were placed in sound attenuating operant boxes (Med-Associates) and were trained to press a single lever (left or right) for a food reinforcer (Purified Food Pellet, 20 mg, BioServ #F0071) delivered in a food receptacle. Under a Random Time (RT) schedule, mice were first trained to make a head entry to retrieve the outcome, which was delivered on average every 60 s (RT60) in the absence of levers. Next, mice were trained on a continuous reinforcement schedule (CRF) in which each lever press produced a single outcome. The maximum number of outcomes they could potentially earn across three daily CRF sessions were 5, 15, and 30 outcomes, respectively. Following CRF training, mice were then trained under a random ratio (RR) schedule of reinforcement that biases goal-directed control in mice [25,45]. Mice received two days of training in RR10 (on average the 10th lever press produces the outcome), followed by 4–5 days under RR20. A subset of mice (A2a Cre+ n = 6, D1 Cre+ n = 5, PV Cre+ n = 10) stayed at RR10 training for the entire duration of RR training due to overall low levels of lever pressing. Sessions ended after 30 outcomes were earned or after 60 min had elapsed.
Fiber photometry procedures and analyses
Bifurcated optic fibers (400 uM, Thorlabs) (to allow simultaneous recording of two mice) were attached for the last 4–6 days of instrumental training, beginning on the first or second day of random ratio training. A blue LED (470 nm, Thorlabs) was used for excitation of dorsal striatum. Regions of interest for each optic fiber were selected by Bonsai software [32] and fluorescence emissions were focused through a 10 × objective (Olympus) onto a CMOS camera (FLIR Systems). Fluorescence intensity and analog signals for lever press, head entries, and outcome delivery were acquired simultaneously, thresholded, and timestamped for later analyses using Bonsai software. After each session, Bonsai software saved photometry signals and behavioral timestamps within comma-separated value files (.csv) that were then imported into Matlab (Mathworks Inc., Natick, MA) for subsequent analysis using custom scripts. Raw fluorescence intensity signals underwent running median (5th order) and low pass (high cutoff frequency of 1 Hz) filtering to reduce noise and electrical artifacts. To correct for photobleaching in which a signal captured from fluorophores degrades by continuous light exposure during the session, we high pass filtered the signal with a low cutoff frequency of 0.001 Hz. We then performed a quality check on the filtered fluorescence intensity signal for low expression and fiber decoupling. Briefly, sessions that did not exceed a 15 second moving window calculation of the signal’s 97.5 percentile by a minimum 1% fluorescence change [39] or did not pass a visual inspection for within-session fiber-ferrule decoupling artifacts were excluded from further analyses.
Statistical analyses
All analyses were two-tailed and statistical significance was defined as an α of p < 0.05. Statistical analysis was performed using GraphPad Prism 8.3.0 (GraphPad Software). Behavioral training data, including lever presses, response rate, and rewards earned were analyzed using two-way repeated measures ANOVAs with Treatment Group (Air vs. CIE) and Day as factors.
For each trace of fiber photometry data, peri‑event changes in fluorescence intensity were calculated via z-score normalization of each corresponding behavioral epoch (i.e. −2 s to 5 s around the lever press at time 0) to a preceding baseline period (ie: for lever press from −5 to −2 prior to lever press). For behavioral epochs, we included analyses centered around each lever press, the first lever press of a bout of lever pressing [45], as well as the first head entry made following reward delivery. The latter includes a reward-related period that may include perception, consumption, and evaluation. We then analyzed this data two ways. First, z-scored fluorescence traces were combined across all mice within a group to preserve the variance seen within a subject. Comparisons between Air and CIE mice were made running permutation tests (1000 shuffles) that required at least 5 consecutive samples to be different from one another [39]. Second, we analyzed the data per animal in a single session to account for the potential differences between subjects. Comparisons between Air and CIE mice were made using permutation testing [28]. Population Ca2+ activity traces were then smoothed with MATLAB’s Savitzky–Golay smoothdata method using a 20 sample sliding window for visual display purposes only.
Results
Chronic ethanol effects on direct pathway calcium activity during actions
Post surgical and vapor procedures (Fig. 1A–C), Air (final n = 6, 5 M 1F) and CIE (final n = 9, 7 M 2F) mice expressing GCaMP6s in the direct pathway similarly acquired lever-press related behaviors. Two-way ANOVAs performed on lever press (Fig. 1D), lever press rate (Fig. 1E), and rewards earned (Fig. 1F) all showed a main effect of Day (Fs’ > 6.23, ps’ < 0.01), but no main effect of Treatment Group nor interaction. Thus, as previously reported, prior chronic alcohol exposure did not lead to gross alterations in acquisition of lever-press related behaviors.
Fig. 1.
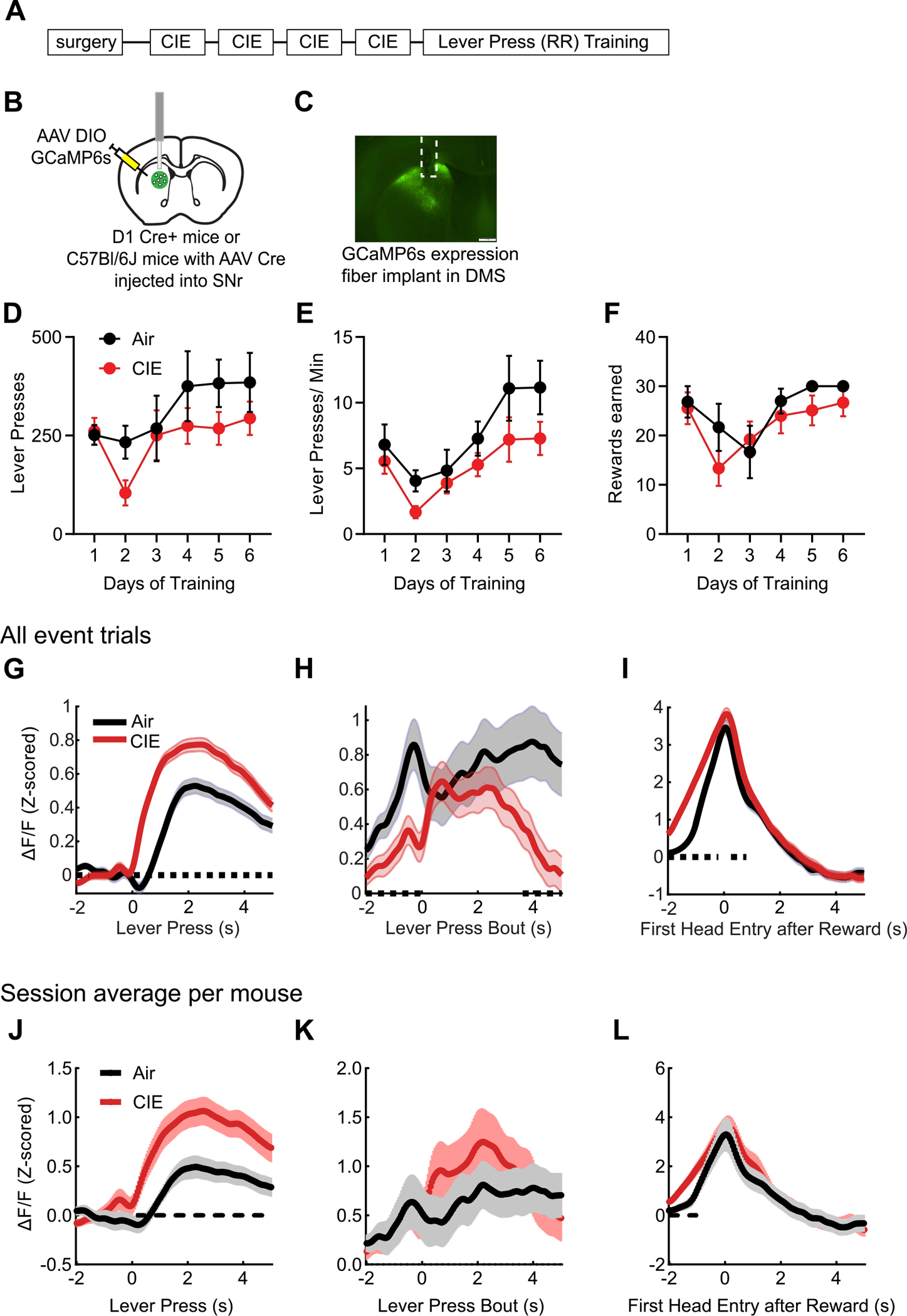
Chronic alcohol increases calcium activity of the direct pathway. A. Schematic of experimental timeline. B. Surgical targeting of GCaMP6s to the direct pathway and implantation of optic fiber, and C. example of optic fiber placement within DMS in animal perfused with 10 mM CaCl2 to show GCaMP6s expression. Lever presses (D), Lever press rate (E), and rewards earned (F) during training. For D-F, plotted are means and error bars representing ± SEM. Average (SEM) calcium activity traces by trial from Air and CIE mice in relation to lever pressing (G), onset of lever press bouts (H), and first head entry following reward delivery (I). Average (SEM) calcium activity traces by per mouse session averages during lever presses (J), onset of lever press bouts (K), and first head entry following reward delivery (L). Differences between Treatment groups was evaluated using permutation testing, with dashed line indicating p < 0.05.
We next examined calcium activity of direct pathway populations (Fig. 1G–L). While direct pathway SPNs showed modulation around lever-press related behaviors, we found largely increased calcium activity modulation in CIE mice compared to Air mice. Permutation testing revealed significantly greater calcium modulation from baseline modulation around a lever press (Fig. 1G) (Air trial n = 9288, CIE trial n = 8179) (ps’ < 0.05). However, grouping lever presses by bout and examining calcium activity centered around the first lever press in a bout showed a reduced increase in calcium modulation in CIE mice compared to Air mice prior to the onset of a lever press bout (Fig. 1H) (Air trial n = 921, CIE trial n = 882) (ps’ < 0.05). Increased calcium activity modulation was also present during the first head entry following reward delivery, with permutation testing showing a large significant difference in calcium activity recruited between Air and CIE mice during this reward-related epoch (Fig. 1I) (Air trial n = 711, CIE trial n = 727) (ps’ < 0.05). This pattern of findings was also seen when we analyzed activity using per mouse averages, except there were no differences at the onset of a lever-press bout (Fig.J-L). Thus, prior CIE exposure largely led to a long-lasting increase in lever-press-related calcium activity modulation in the direct pathway during decision-making.
Chronic ethanol effects on indirect pathway calcium activity during actions
Air (final n = 4, 1 M 3F) and CIE (final n = 6, 2 M 4F) mice expressing GCaMP6s targeted to the indirect pathway (Fig. 2A–C) also acquired lever press behavior to similar degrees. Once again, there was only a main effect of Day across when examining lever presses (Fig. 2D), lever press rate (Fig. 2E), and rewards earned (Fig. 2F) (Fs’ > 6.61, ps’ < 0.05), with no main effects of Treatment Group or interaction.
Fig. 2.
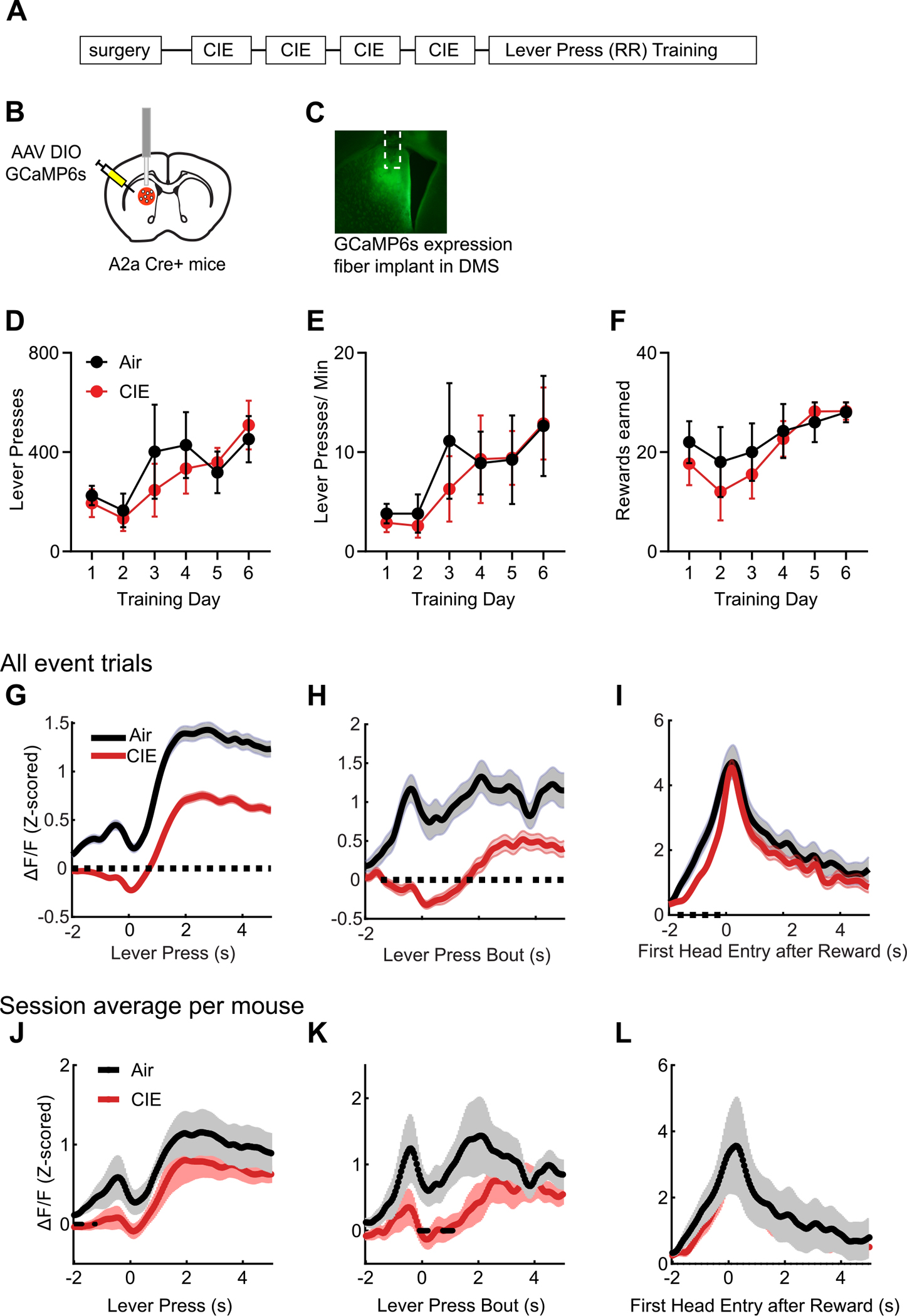
Chronic alcohol decreases calcium activity of the indirect pathway. A. Schematic of experimental timeline. B. Surgical targeting of GCaMP6s to the indirect pathway and implantation of optic fiber. C. Example of optic fiber placement within DMS in animal perfused with 10 mM CaCl2 to show GCaMP6s expression. Lever presses (D), Lever press rate (E), and rewards earned (F) during training. For D-F, plotted are means and error bars representing ± SEM. Average (SEM) calcium activity traces by trial from Air and CIE mice in relation to lever pressing (G), onset of lever press bouts (H), and first head entry following reward delivery (I). Average (SEM) calcium activity traces by per mouse session averages during lever presses (J), onset of lever press bouts (K), and first head entry following reward delivery (L). Differences between Treatment groups was evaluated using permutation testing, with dashed line indicating p < 0.05.
Unlike calcium activity in the direct pathway, calcium activity modulation in the indirect pathway was largely reduced in CIE mice when compared to Air mice. While indirect pathway calcium activity increased prior to lever pressing, permutation testing showed that indirect pathway calcium activity modulation was largely reduced in CIE compared to Air mice around a lever press (Fig. 2G) (Air trial n = 3802, CIE trial n = 4543) as well as at the onset of a lever press bout (Fig. 2H) (Air trial n = 396, CIE trial n = 551), (ps’ < 0.05). While of a lesser magnitude, reward-related indirect pathway calcium activity modulation was overall slightly greater in Air compared to CIE mice (Fig. 2I) (Air trial n = 283, CIE trial n = 347) (ps’ < 0.05). A similar pattern was seen when data was analyzed using session averages per mouse, except no significant difference in modulation were observed during the first head entry after reward (Fig.J-L). Thus, prior chronic alcohol exposure largely decreased calcium activity in the indirect pathway population.
Chronic ethanol effects on striatal interneuron calcium activity during actions
We also examined whether chronic alcohol altered calcium activity modulation in a population of striatal interneurons, the fast-spiking PV interneurons (Fig. 3A–C). Once again, acquisition behavior was similar between Air (final n = 5, 2F 3 M) and CIE (final n = 7, 3F 4 M) exposed mice. Across lever press rate (Fig. 3E) and rewards earned (Fig. 3F), there was a main effect of Day (Fs’ > 2.65, ps’ < 0.05), but no effect of Treatment Group or interaction and no differences in lever presses made (Fig. 3D).
Fig. 3.
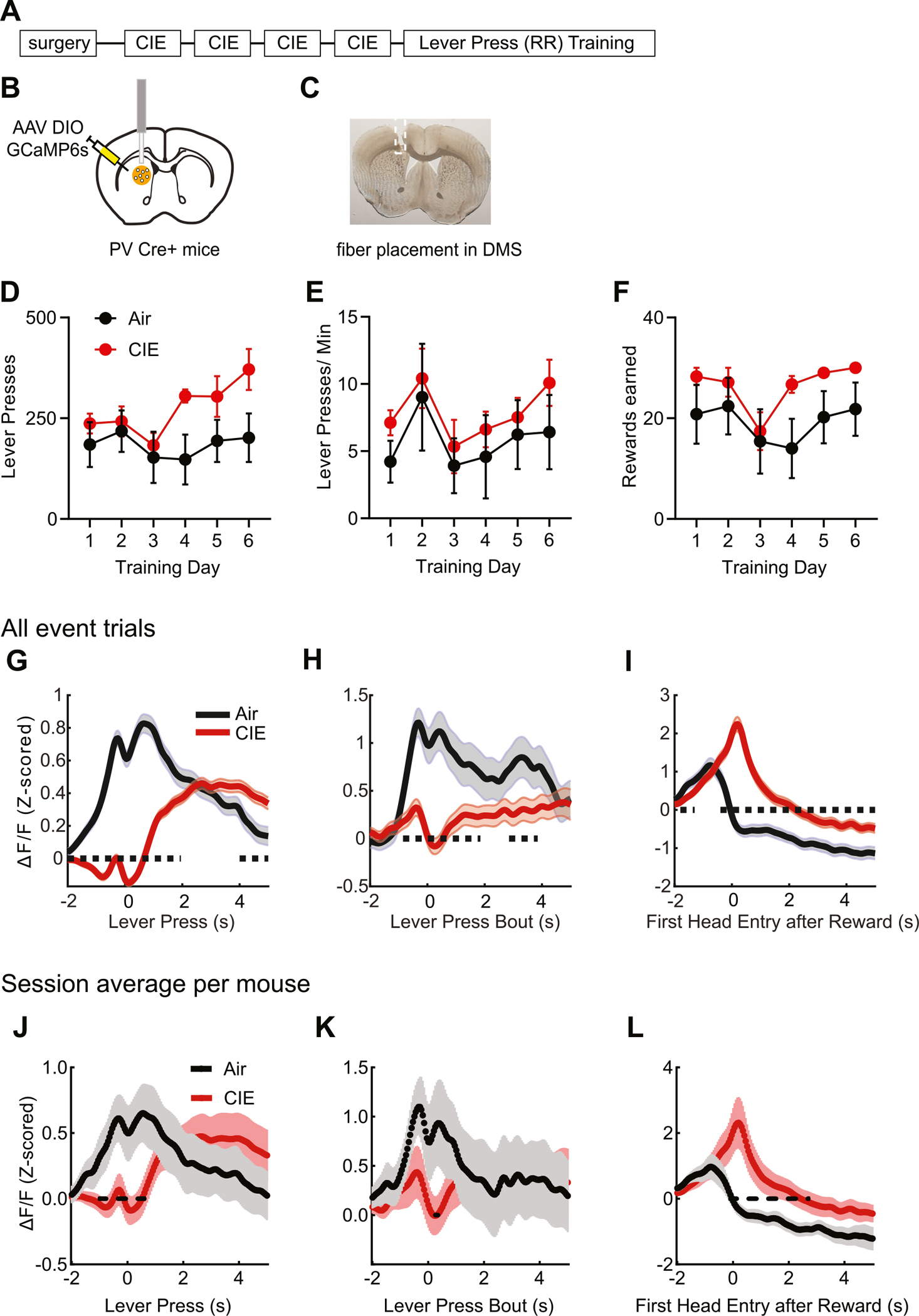
Chronic alcohol decreases calcium activity of local interneurons. A. Schematic of experimental timeline. B. Surgical targeting of GCaMP6s to PV interneurons and implantation of optic fiber. C. Fiber placement in DMS. Lever presses (D), Lever press rate (E), and rewards earned (F) during training. For D-F, plotted are means and error bars representing ± SEM. Average (SEM) calcium activity traces by trial from Air and CIE mice in relation to lever pressing (G), onset of lever press bouts (H), and first head entry following reward delivery (I). Average (SEM) calcium activity traces per mouse session average during lever presses (J), onset of lever press bouts (K), and first head entry following reward delivery (L). Differences between Treatment groups was evaluated using permutation testing, with dashed line indicating p < 0.05.
Calcium activity of PV Cre+ interneurons showed reduced modulation in CIE compared to AIR mice during lever pressing (Fig. 3G) (Air trial n = 3446, CIE trial n = 7024), and at the onset of lever press bouts (Fig. 3H) (Air trial n = 426, CIE trial n = 700). However, compared to Air mice, CIE mice showed increased calcium activity modulation of PV Cre+ interneurons during reward-related epochs (Fig. 3I) (Air trial n = 356, CIE trial n = 616) (ps’ <0.05). Analyses on session averages per mouse also found the same pattern of calcium modulation (Fig. J-L). This suggests that prior chronic alcohol exposure led to reduced action-related recruitment of calcium activity in PV interneurons, but increased calcium modulation during reward-related epochs in DMS during action control.
Discussion
Here we show that prior chronic alcohol exposure leads to a long-lasting imbalance in the activity of DMS populations during action-related behaviors. This imbalance was reflected in increased modulation of calcium activity in direct pathway SPNs, while largely decreased calcium activity modulation was observed in indirect pathway SPNs as well as in PV DMS interneurons. Such changes are in line with prior findings suggesting that chronic alcohol results in potentiated recruitment of direct pathway SPNs and increased inhibition of indirect pathway neurons [7]. Here, we show for the first time that such chronic-alcohol induced changes are reflected in the endogenous calcium activity of DMS pathways during action-related behaviors. These findings provide evidence that chronic alcohol induces an imbalance in DMS activity, and such an imbalance may lead to less functional control by DMS over behavior.
While intact DMS function has been widely implicated as necessary for goal-directed control ([2,22,52]), the proper balance of activity between direct and indirect pathways has recently been more appreciated [30]. Indeed, both direct and indirect pathways are engaged during lever pressing [10] and can support optogenetic-intracranial self--stimulation [49]. Effective control of behavior by DMS is thought to engage cortical and thalamic recruitment of balanced activity of direct and indirect pathways [30]. Recent examples show that chronic alcohol-induced changes in strength of select cortical inputs can lead to loss of goal-directed control [44,45]. Interestingly, this was a reduction of orbitofrontal cortex transmission onto the direct, but not indirect, pathway in DMS [44,45]. Thus, one interpretation has been reduced cortical drive (albeit from one area) into DMS leads to a loss of DMS recruitment and goal-directed control.
However, other works examining other prefrontal cortex projections have shown potentiated inputs onto D1 SPNs of the direct pathway in DMS [37], as well as decreased thalamic input to striatum [38]. In the present findings, we saw increased calcium activity modulation in D1 SPNs, but decreased modulation in D2 SPNs and a local interneuron population. Intriguingly, we also saw reduced modulation of PV interneurons in the DMS. Recent work ablated this population in the dorsal lateral striatum and found reduced organized ethanol consumption with ethanol consumption showing increased sensitivity to the addition of quinine [42]. As interneurons can provide a strong clamp on SPN firing and are recruited in part through cortical input [16,40], our data suggest less modulation of PV interneurons may reflect less engagement of this population to shape SPN activity patterns during actions. These findings along with prior works showing alcohol-induced changes to SPNs themselves suggests that at minimum, dysfunctional or a loss of DMS control over behavior could result from a gain or loss in strength of specific cortical or thalamic inputs conveying relevant information, and/or through functional changes in the DMS neurons themselves. Further work examining how chronic alcohol disrupts the ability of converging inputs to recruit appropriate DMS activity is clearly warranted.
Care should be taken in interpreting the present results as bulk population calcium in striatal SPNs is likely not reflective of action potential firing [31]. Given the extent of SPN arborization, the bulk signals collected in the present data likely reflect a combination of threshold and subthreshold calcium activity in the soma, dendritic arbors, and axon collaterals within striatum. We did not examine sensitivity to outcome devaluation in the present studies and therefore cannot say whether the current subjects were more reliant on habitual or goal-directed control, although prior works have shown chronic alcohol-exposure disrupts goal-directed control for non-alcohol reward [6,8,44,46]. However, our data do suggest that chronic alcohol resulted in altered calcium modulation in identified SPN populations during action performance. As prior findings showed DMS inactivation was ineffective at altering behavioral control following chronic alcohol [8], our data suggest that alteration to activity of DMS populations may underlie deficits in using DMS for behavioral control. We further hypothesize that this imbalance in recruitment likely contributes to the reduced contribution of DMS to goal-directed control following chronic alcohol.
Acknowledgements
This work was funded by the National Institutes of Health AA026077 (C.M.G.) and AA026776 (R.R.) as well as the National Science Foundation Graduate Research Fellowship DGE-2038238 (E.T.B). All authors contributed to data collection and formal analyses. R.R and C.M.G contributed to the conceptualization. E.T.B and C.M.G. contributed to writing the original draft, and all authors contributed to review and editing of the manuscript.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- [1].Abrahao KP, Salinas AG, Lovinger DM, Alcohol and the brain: neuronal molecular targets, synapses, and circuits, Neuron 96 (6) (2017) 1223–1238, 10.1016/j.neuron.2017.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Balleine BW, The meaning of behavior: discriminating reflex and volition in the brain, Neuron 104 (1) (2019) 47–62, 10.1016/j.neuron.2019.09.024. [DOI] [PubMed] [Google Scholar]
- [3].Balleine BW, P O’Doherty J, Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action, Neuropsychopharmacology 35 (1) (2010) 48–69, 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Becker HC, Positive relationship between the number of prior ethanol withdrawal episodes and the severity of subsequent withdrawal seizures, Psychopharmacology (Berl.) 116 (1) (1994) 26–32, 10.1007/BF02244867. [DOI] [PubMed] [Google Scholar]
- [5].Becker HC, Lopez MF, Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice, Alcoholism, Clinic. Experim. Res 28 (12) (2004) 1829–1838, 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- [6].Cazares C, Schreiner DC, Gremel CM, Different effects of alcohol exposure on action and outcome-related orbitofrontal cortex activity, eNeuro 8 (2) (2021), 10.1523/ENEURO.0052-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cheng Y, Huang CCY, Ma T, Wei X, Wang X, Lu J, Wang J, Distinct synaptic strengthening of the striatal direct and indirect pathways drives alcohol consumption, Biol. Psychiatry 81 (11) (2017) 918–929, 10.1016/j.biopsych.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Corbit LH, Nie H, Janak PH, Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum, Biol. Psychiatry 72 (5) (2012) 389–395, 10.1016/j.biopsych.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cowan RL, Wilson CJ, Emson PC, Heizmann CW, Parvalbumin-containing gabaergic interneurons in the rat neostriatum, J. Comp. Neurol. 302 (2) (1990) 197–205, 10.1002/cne.903020202. [DOI] [PubMed] [Google Scholar]
- [10].Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM, Concurrent activation of striatal direct and indirect pathways during action initiation, Nature 494 (7436) (2013) 238–242, 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].C Cuzon Carlson V, GABA and glutamate synaptic coadaptations to chronic ethanol in the striatum, in: Grant KA, Lovinger DM (Eds.), The Neuropharmacology of Alcohol, Springer International Publishing, 2018, pp. 79–112, 10.1007/164_2018_98. [DOI] [PubMed] [Google Scholar]
- [12].Cuzon Carlson VC, Grant KA, M Lovinger D, Synaptic adaptations to chronic ethanol intake in male rhesus monkey dorsal striatum depend on age of drinking onset, Neuropharmacology 131 (2018) 128–142, 10.1016/J.NEUROPHARM.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cuzon Carlson VC, Seabold GK, Helms CM, Garg N, Odagiri M, Rau AR, Daunais J, Alvarez VA, Lovinger DM, A Grant K, Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates, Neuropsychopharmacology 36 (12) (2011) 2513–2528, 10.1038/npp.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Durieux PF, Bearzatto B, Guiducci S, Buch T, Waisman A, Zoli M, Schiffmann SN, de Kerchove d’Exaerde A, D2R striatopallidal neurons inhibit both locomotor and drug reward processes, Nat. Neurosci. 12 (4) (2009), 10.1038/nn.2286. [DOI] [PubMed] [Google Scholar]
- [15].Fanelli RR, Klein JT, Reese RM, Robinson DL, Dorsomedial and dorsolateral striatum exhibit distinct phasic neuronal activity during alcohol self-administration in rats, Eur. J. Neurosci. 38 (4) (2013) 2637–2648, 10.1111/ejn.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fino E, Vandecasteele M, Perez S, Saudou F, Venance L, Region-specific and state-dependent action of striatal GABAergic interneurons, Nat. Commun. 9 (1) (2018), 10.1038/s41467-018-05847-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gerfen CR, Bolam JP, Chapter 1—The Neuroanatomical Organization of the Basal Ganglia, in: H. Steiner, Tseng KY (Eds.), Handbook of Behavioral Neuroscience Vol. 20, Elsevier, 2010, pp. 3–28, 10.1016/B978-0-12-374767-9.00001-9. [DOI] [Google Scholar]
- [18].Gillan CM, Kosinski M, Whelan R, Phelps EA, Daw ND, Enander J, Schreiber LRN, Gillan C, Fineberg NA, Sahakian BJ, Robbins TW, Harrison NA, Wood J, Daw ND, Dayan P, Grant JE, Bullmore ET, Characterizing a psychiatric symptom dimension related to deficits in goal-directed control, Elife 5 (2016) 301–320, 10.7554/eLife.11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Giuliano C, Belin D, Everitt BJ, Compulsive Alcohol Seeking Results from a Failure to Disengage Dorsolateral Striatal Control over Behavior, J. Neurosci. 39 (9) (2019) 1744–1754, 10.1523/JNEUROSCI.2615-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gremel C, Chancey J, Atwood B, Luo G, Neve R, Ramakrishnan C, Deisseroth K, Lovinger D, Costa R, Endocannabinoid modulation of orbitostriatal circuits gates habit formation, Neuron (2016) 1–13, 10.1016/j.neuron.2016.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gremel CM, Costa RM, Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions, Nat. Commun. 4 (2013) 2264, 10.1038/ncomms3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gremel CM, Lovinger DM, Associative and sensorimotor cortico-basal ganglia circuit roles in effects of abused drugs, Genes, Brain Behav. 16 (1) (2017) 71–85, 10.1111/gbb.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Griffin WC, Lopez MF, Becker HC, Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice, Alcohol. Clin. Exp. Res. 33 (11) (2009) 1893–1900, 10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Heilig M, Egli M, Crabbe JC, Becker HC, Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict. Biol 15 (2) (2010) 169–184, 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hilário MRF, Clouse E, Yin HH, Costa RM, Endocannabinoid signaling is critical for habit formation, Front. Integr. Neurosci 1 (6) (2007), 10.3389/neuro.07.006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, Arber S, A developmental switch in the response of DRG neurons to ETS transcription factor signaling, PLoS Biol. 3 (5) (2005) e159, 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hunnicutt BJ, Jongbloets BC, Birdsong WT, Gertz KJ, Zhong H, Mao T, Averbeck BB, Lehman J, Jacobson M, Haber SN, Balleine BW, Liljeholm M, Ostlund SB, Bastian M, Heymann S, Jacomy M, Belin D, Jonkman S, Dickinson A, Dong HW, A comprehensive excitatory input map of the striatum reveals novel functional organization, Elife 5 (2016) 9497–9505, 10.7554/eLife.19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jean-Richard-dit-Bressel P, Clifford CWG, McNally GP, Analyzing event-related transients: confidence intervals, permutation tests, and consecutive thresholds, Front. Mol. Neurosci 13 (2020), 10.3389/fnmol.2020.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC, Striatal interneurones: chemical, physiological and morphological characterization, Trends Neurosci. 18 (12) (1995) 527–535, 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- [30].Klaus A, da Silva JA, Costa RM, What, If, and When to Move: Basal Ganglia Circuits and Self-Paced Action Initiation, Annu. Rev. Neurosci. 42 (1) (2019) 459–483, 10.1146/annurev-neuro-072116-031033. [DOI] [PubMed] [Google Scholar]
- [31].Legaria AA, Matikainen-Ankney BA, Yang B, Ahanonu B, Licholai JA, Parker JG, Kravitz AV, Fiber photometry in striatum reflects primarily nonsomatic changes in calcium, Nat. Neurosci. 25 (9) (2022), 10.1038/s41593-022-01152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lopes G, Bonacchi N, Frazão J, Neto JP, Atallah BV, Soares S, Moreira L, Matias S, Itskov PM, Correia PA, Medina RE, Calcaterra L, Dreosti E, Paton JJ, Kampff AR, Bonsai: an event-based framework for processing and controlling data streams, Front. Neuroinform 9 (2015), 10.3389/fninf.2015.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lopez MF, Becker HC, Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice, Psychopharmacology (Berl.) 181 (4) (2005) 688–696, 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- [34].Lovinger DM, Alvarez VA, Alcohol and basal ganglia circuitry: animal models, Neuropharmacology (2017), 10.1016/j.neuropharm.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lovinger DM, Gremel CM, A Circuit-Based Information Approach to Substance Abuse Research, Trends Neurosci. 44 (2) (2021) 122–135, 10.1016/j.tins.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lu J, Cheng Y, Wang X, Woodson K, Kemper C, Disney E, Wang J, Alcohol intake enhances glutamatergic transmission from D2 receptor-expressing afferents onto D1 receptor-expressing medium spiny neurons in the dorsomedial striatum, Neuropsychopharmacology 44 (6) (2019) 1123–1131, 10.1038/s41386-019-0332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ma T, Cheng Y, Roltsch Hellard E, Wang X, Lu J, Gao X, Huang CCY, Wei XY, Ji JY, Wang J, Bidirectional and long-lasting control of alcohol-seeking behavior by corticostriatal LTP and LTD, Nat. Neurosci. 21 (3) (2018) 373–383, 10.1038/s41593-018-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ma T, Huang Z, Xie X, Cheng Y, Zhuang X, Childs MJ, Gangal H, Wang X, Smith LN, Smith RJ, Zhou Y, Wang J, Chronic alcohol drinking persistently suppresses thalamostriatal excitation of cholinergic neurons to impair cognitive flexibility, J. Clin. Invest. 132 (4) (2022), e154969, 10.1172/JCI154969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Markowitz JE, Gillis WF, Beron CC, Neufeld SQ, Robertson K, Bhagat ND, Peterson RE, Peterson E, Hyun M, Linderman SW, Sabatini BL, Datta SR, The striatum organizes 3D behavior via moment-to-moment action selection, Cell 174 (1) (2018) 44–58, 10.1016/j.cell.2018.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].McKeon PN, Bunce GW, Patton MH, Chen R, Mathur BN, Cortical control of striatal fast-spiking interneuron synchrony, J. Physiol. (Lond.) 600 (9) (2022) 2189–2202, 10.1113/JP282850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Patton MH, Roberts BM, Lovinger DM, Mathur BN, Ethanol disinhibits dorsolateral striatal medium spiny neurons through activation of a presynaptic delta opioid receptor, Neuropsychopharmacology 41 (7) (2016) 1831–1840, 10.1038/npp.2015.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Patton MS, Heckman M, Kim C, Mu C, Mathur BN, Compulsive alcohol consumption is regulated by dorsal striatum fast-spiking interneurons, Neuropsychopharmacology 46 (2) (2021) 351–359, 10.1038/s41386-020-0766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Patton MS, Sheats SH, Siclair AN, Mathur BN, Alcohol potentiates multiple GABAergic inputs to dorsal striatum fast-spiking interneurons, Neuropharmacology 232 (2023), 109527, 10.1016/j.neuropharm.2023.109527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Renteria R, Baltz ET, Gremel CM, Chronic alcohol exposure disrupts top-down control over basal ganglia action selection to produce habits, Nat. Commun. 9 (1) (2018) 211, 10.1038/s41467-017-02615-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Renteria R, Cazares C, Baltz ET, Schreiner DC, Yalcinbas EA, Steinkellner T, Hnasko TS, Gremel CM, Mechanism for differential recruitment of orbitostriatal transmission during actions and outcomes following chronic alcohol exposure, Elife 10 (2021) e67065, 10.7554/eLife.67065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Renteria R, Cazares C, Gremel CM, Habitual ethanol seeking and licking microstructure of enhanced ethanol self-administration in ethanol dependent mice, Alcohol. Clin. Exp. Res. 44 (4) (2020) 880–891, 10.1111/acer.14302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tepper JM, Bolam JP, Functional diversity and specificity of neostriatal interneurons, Curr. Opin. Neurobiol. 14 (6) (2004) 685–692, 10.1016/j.conb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- [48].Tepper JM, Koós T, Chapter 8 —GABAergic Interneurons of the Striatum, in: Steiner H, Tseng KY (Eds.), Handbook of Behavioral Neuroscience Vol. 24, Elsevier, 2016, pp. 157–178, 10.1016/B978-0-12-802206-1.00008-8. [DOI] [Google Scholar]
- [49].Vicente AM, Galvão-Ferreira P, Tecuapetla F, Costa RM, Direct and indirect dorsolateral striatum pathways reinforce different action strategies, Curr. Biol. 26 (7) (2016) R267–R269, 10.1016/j.cub.2016.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wang J, Cheng Y, Wang X, Roltsch Hellard E, Ma T, Gil H, Ben Hamida S, Ron D, Alcohol elicits functional and structural plasticity selectively in dopamine D1 receptor-expressing neurons of the dorsomedial striatum, J. Neurosci. 35 (33) (2015) 11634–11643, 10.1523/JNEUROSCI.0003-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wilcox MV, Cuzon Carlson VC, Sherazee N, Sprow GM, Bock R, Thiele TE, Lovinger DM, Alvarez VA, Repeated binge-like ethanol drinking alters ethanol drinking patterns and depresses striatal GABAergic transmission, Neuropsychopharmacology 39 (3) (2014) 579–594, 10.1038/npp.2013.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yin HH, Knowlton BJ, The role of the basal ganglia in habit formation, Nat. Rev. Neurosci. 7 (6) (2006) 464–476, 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


