Abstract
Background
The degree of inflammation and immune status is widely recognized to be associated with intrahepatic cholangiocarcinoma (ICC) and is closely linked to poor postoperative survival. The purpose of this study was to evaluate whether the systemic immune-inflammatory index (SII) and the albumin bilirubin (ALBI) grade together exhibit better predictive strength compared to SII and ALBI separately in patients with ICC undergoing curative surgical resection.
Methods
A retrospective analysis was performed on a cohort of 374 patients with histologically confirmed ICC who underwent curative surgical resection from January 2016 to January 2020 at three medical centers. The cohort was divided into a training set comprising 258 patients and a validation set consisting of 116 patients. Subsequently, the prognostic predictive abilities of three indicators, namely SII, ALBI, and SII+ALBI grade, were evaluated. Independent risk factors were identified through univariate and multivariate analyses. The identified independent risk factors were then utilized to construct a nomogram prediction model, and the predictive strength of the nomogram prediction model was assessed through Receiver Operating Characteristic (ROC) survival curves and calibration curves.
Results
Univariate analysis of the training set, consisting of 258 eligible patients with ICC, revealed that SII, ALBI, and SII+ALBI grade were significant prognostic factors for overall survival (OS) and recurrence-free survival (RFS) (p < 0.05). Multivariate analysis revealed the independent significance of SII+ALBI grade as a risk factor for postoperative OS and RFS (p < 0.05). Furthermore, we conducted an analysis of the correlation between SII, ALBI, SII+ALBI grade, and clinical features, indicating that SII+ALBI grade exhibited stronger associations with clinical and pathological characteristics compared to SII and ALBI. We constructed a predictive model for postoperative survival in ICC based on SII+ALBI grade, as determined by the results of multivariate analysis. Evaluation of the model’s predictive strength was performed through ROC survival curves and calibration curves in the training set and validation set, revealing favorable predictive performance.
Conclusion
The SII+ALBI grade, a novel classification based on inflammatory and immune status, serves as a reliable prognostic indicator for postoperative OS and RFS in patients with ICC.
Keywords: intrahepatic cholangiocarcinoma, SII, ALBI, SII+ALBI grade, nomogram, prognosis
1. Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most prevalent primary liver cancer distinguished by its aggressive nature, accounting for approximately 15–20% of all biliary malignancies (1, 2). The worldwide incidence of ICC has been consistently rising at a yearly rate of 15% over the past few decades (1). Curative surgical resection currently stands as the gold-standard treatment for ICC. However, only about 20%–40% of patients who get curative surgical resection survive 5 years or more (3, 4). Therefore, the identification of novel prognostic indicators for distinguishing ICC patients who would benefit from curative surgical resection is crucial for developing personalized treatment strategies.
Increasing evidence suggests that in addition to common factors such as lymph node metastasis, tumor size, and vascular invasion, nutritional status and inflammatory levels play a significant predictive role in the prognosis of curative surgical resection for tumors (5, 6). Among them, the Systemic Immune-Inflammation Index (SII) is a fresh quantitative indicator used to assess individual immune status and inflammation levels (7, 8). It is calculated based on parameters such as platelet, neutrophil, and lymphocyte counts. SII is frequently used to assess patients’ preoperative nutritional status and precisely evaluate their individual surgical risks (8). Additionally, the Albumin–bilirubin (ALBI) grade is a composite indicator that comprehensively evaluates patients’ liver function and reserves. Its introduction was first compared to Child-Pugh classification in hepatocellular carcinoma (HCC) patients in 2015, demonstrating superior predictive capability for survival following liver resection and postoperative liver failure (9). A growing body of literature indicates a close association between SII, ALBI, and the prediction of prognosis and survival in patients with HCC, ICC, and other malignancies (9–13). However, whether the combined application of SII and ALBI can improve the prognostic prediction in patients with ICC remains inconclusive. This research seeks to identify the combined application of SII and ALBI in predicting postoperative survival after curative resection for ICC and attempt to construct a survival prognostic model based on SII and ALBI.
2. Materials and methods
2.1. Patient selection
This study included all patients who received curative surgical resection for ICC between January 2016 and January 2020 at People’s Hospital of Zhengzhou University, Cancer Hospital of Zhengzhou University, and The First Affiliated Hospital of Zhengzhou University. Following were the inclusion criteria: 1) Patients whose pathological confirmation with ICC followed a curative surgical resection; 2) Patients aged 18 years or older; 3) No prior anticancer treatment before surgery; 4) No concurrent occurrence of other malignant tumors. Following were the exclusion criteria: 1) Perioperative mortality; 2) Patients with hematological disorders and autoimmune diseases; 3) Incomplete clinical or laboratory data; 4) Patients requiring a second surgery for tumor recurrence; 5) Incomplete follow-up information. 258 patients from People’s Hospital of Zhengzhou University and Cancer Hospital of Zhengzhou University were chosen as the training set, while a total of 116 patients from The First Affiliated Hospital of Zhengzhou University were chosen as the validation set. The 8th edition of the American Joint Committee on Cancer (AJCC) staging method was used to evaluate all patients who were included, and all patients were monitored until January 2023.
This study received ethical approval from the Institutional Review Boards of Zhengzhou University People’s Hospital (Ref No. 2023-012), Zhengzhou University Cancer Hospital (Ref No. 2023-203), and Zhengzhou University First Affiliated Hospital (2021-KY-1137-002). Written informed consent was obtained from all patients prior to their participation in the study.
2.2. Clinical variables
Patient clinical and pathological data included age, gender, HBV infection, obstructive jaundice, tumor differentiation, tumor number, tumor size, perineural invasion, microvascular invasion, and the AJCC 8th TNM Stage. Laboratory test results were collected from one week before surgery, including carbohydrate antigen 19-9 (CA19-9), carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP), alanine transaminase (ALT), aspartate transaminase (AST), albumin, bilirubin, white blood cell count (WBC), lymphocyte count (LY), neutrophil count (NEUT), platelet count (PLT), hemoglobin (HGB), prothrombin time (PT), international normalized ratio (INR) and activated partial thromboplastin time (APTT). Additionally, the calculation methods for the two immune-inflammatory markers, ALBI and SII, were as follows: ALBI = log10bilirubin (mol/L) * 0.66 - albumin (g/L) * 0.085, SII = platelet count * neutrophil count/lymphocyte count. Subsequently, Subsequently, the X-tile software (Yale University, New Haven, CT, USA) was employed to compute the optimal cutoff values for overall survival (OS) and recurrence-free survival (RFS) with respect to SII and ALBI. Based on the results, ALBI ≥ -2.50 was defined as the high ALBI group, and ALBI < -2.50 as the low ALBI group. Similarly, SII ≥ 470 was defined as the high SII group, and SII < 470 as the low SII group. In the subsequent analysis, the combination of low SII and low ALBI was defined as SII+ALBI Grade A, the combination of high SII and high ALBI was defined as SII+ALBI Grade C, and the remaining combinations were defined as SII+ALBI Grade B.
2.3. Statistical analysis
The Kolmogorov-Smirnov test was used in research to determine if continuous variables were normally distributed. Mean and standard deviation (SD) were used to represent normally distributed data, whereas interquartile range (IQR) was used to represent non-normally distributed variables. For group comparisons, the Mann-Whitney rank sum test and the student t-test were used. The baseline features of categorical variables were compared using the chi-square test and Fisher’s exact test. Cox proportional hazards regression analysis was used for the univariate analysis. Cox backward stepwise regression models were employed for the multivariate analysis. GraphPad Prism (version 8.0) was used to create Kaplan-Meier survival curves for OS and RFS based on the grouping of ALBI, SII, and ALBI+SII. Additionally, ROC survival curves were drawn, and the three groups’ areas under the curve (AUC) were contrasted. Statistical significance was defined as p<0.05.
2.4. Follow-up
For the included patients, follow-up began after the surgical procedure. Within the first year postoperatively, monthly follow-up visits were conducted, followed by follow-up visits every three months for the next two years. The last follow-up was performed on January 2023. Overall survival was determined as the interval between the date of curative surgical resection and the last examination or the date of death from any cause. Recurrence-free survival was determined as the interval between the date of curative surgical resection and the most recent follow-up, the occurrence of tumor recurrence or advancement in any way, or the patient’s death for any reason.
2.5. Development and assessment of nomogram
Based on the results of the Cox backward stepwise regression model, predictive models for OS and RFS were constructed using nomogram models. The accuracy of the models was assessed by plotting ROC survival curves and calibration curves for the training and validation sets based on the models. The construction and evaluation of the models were performed using R software (version 4.2.1).
3. Result
A total of 374 patients (172 male and 202 female) who underwent curative surgical resection for pathologically confirmed ICC from January 2016 to January 2020 were included in this study. The median age of the patients was 59 years, ranging from 28 to 80 years. The median follow-up time was 12 months (1-91 months). The 1-year, 2-year, and 3-year OS rates were 52.1%, 23.3%, and 10.9%, respectively. The 1-year, 2-year, and 3-year RFS rates were 29.2%, 15.5%, and 5.3%, respectively. As can be seen in Table 1 , the baseline data and clinicopathological traits of the training set (n=258) and validation set (n=116) were examined for their association. The two cohorts’ distributions were balanced (p>0.05).
Table 1.
Comparison of clinicopathological characteristics in training and validation sets.
| Variables | All patients (n=374) |
Training set (n=258) |
Validation set (n=116) |
p value |
|---|---|---|---|---|
| Sex | 0.884 | |||
| Male | 172(46.0%) | 118(45.7%) | 54(46.6%) | |
| Female | 202(54.0%) | 140(54.3%) | 62(53.4%) | |
| Age (years) | 0.076 | |||
| ≤65 | 250(66.8%) | 165(64.0%) | 85(73.3%) | |
| >65 | 124(33.2%) | 93(36.0%) | 31(26.7%) | |
| Obstructive jaundice | 0.965 | |||
| No | 310(82.9%) | 214(82.9%) | 96(82.8%) | |
| Yes | 64(17.1%)) | 44(17.1%) | 20(17.2%) | |
| HBV infection | 0.467 | |||
| No | 239(63.9%) | 168(65.1%) | 71(61.2%) | |
| Yes | 135(36.1%) | 90(34.9%) | 45(38.8%) | |
| AFP (ng/ml) | 0.085 | |||
| <20 | 309(82.6%) | 219(84.9%) | 90(77.6%) | |
| ≥20 | 65(17.4%) | 39(15.1%) | 26(22.4%) | |
| CEA (ng/ml) | 0.491 | |||
| <5 | 242(64.7%) | 164(63.6%) | 78(67.2%) | |
| ≥5 | 132(35.3%) | 94(36.4%) | 38(32.8%) | |
| CA19-9 (U/ml) | 0.387 | |||
| <37 | 149(39.8%) | 99(38.4%) | 50(43.1%) | |
| ≥37 | 225(60.2%) | 159(61.6%) | 66(56.9%) | |
| Child–Pugh Grade | 0.639 | |||
| Grade A | 334(89.3%) | 233(90.3%) | 101(87.1%) | |
| Grade B | 40(10.7%) | 25(9.7%) | 15(12.9%) | |
| Tumor number | 0.995 | |||
| = 1 | 303(81.0%) | 209(81.0%) | 94(81.0%) | |
| >1 | 71(19.0%) | 49(19.0%) | 22(19.0%) | |
| Tumor size | 0.366 | |||
| <5.0cm | 158(42.2%) | 105(40.7%) | 53(45.7%) | |
| ≥5.0cm | 216(57.8%) | 153(59.3%) | 63(54.3%) | |
| Tumor differentiation | 0.627 | |||
| Well | 36(9.6%) | 27(10.5%) | 9(7.8%) | |
| Moderate | 280(74.9%) | 193(74.8%) | 87(75.0%) | |
| Poor | 58(15.4%) | 38(14.7%) | 20(17.2%) | |
| Perineural invasion | 0.109 | |||
| No | 194(51.9%) | 141(54.7%) | 53(45.7%) | |
| Yes | 180(48.1%) | 117(45.3%) | 63(54.3%) | |
| Microvascular invasion | 0.727 | |||
| No | 205(54.8%) | 143(55.4%) | 62(53.4%) | |
| Yes | 169(45.2%) | 115(44.6%) | 54(46.6%) | |
| AJCC 8th edition T stage | 0.053 | |||
| T1a/T1b | 171(45.7%) | 117(45.3%) | 54(46.6%) | |
| T2 | 147(39.3%) | 95(36.8%) | 52(44.8%) | |
| T3/T4 | 56(15.0%) | 46(17.8%) | 10(8.6%) | |
| AJCC 8th edition N stage | 0.252 | |||
| N0 | 279(74.6%) | 188(72.9%) | 91(78.4%) | |
| N1 | 95(25.4%) | 70(27.1%) | 25(21.6%) | |
| AJCC 8th edition M stage | 0.311 | |||
| M0 | 368(98.4%) | 255(98.8%) | 113(97.4%) | |
| M1 | 6(1.6%) | 3(1.2%) | 3(2.6%) | |
| ALT (ng/ml) | 53(45-61) | 50(44-59) | 60(44-75) | 0.259 |
| AST (ng/ml) | 48(42-53) | 45(38-52) | 53(41-65) | 0.222 |
| Albumin (ng/ml) | 40.54(39.90-41.18) | 40.80(40.05-41.54) | 39.98(38.75-41.20) | 0.245 |
| Bilirubin (ng/ml) | 28.16(22.35-33.97) | 26.88(20.08-33.68) | 31.00(19.79-42.20) | 0.520 |
| PT (s) | 12.30(12.17-12.44) | 12.27(12.11-12.44) | 12.37(12.13-12.62) | 0.509 |
| INR | 1.21(1.00-1.41) | 1.17(0.95-1.39) | 1.28(0.83-1.74) | 0.611 |
| APTT (s) | 32.00(31.36-32.65) | 31.92(31.10-32.74) | 32.18(31.16-33.20) | 0.718 |
| WBC (109/L) | 6.88(6.59-7.16) | 6.76(6.44-7.09) | 7.13(6.55-7.70) | 0.248 |
| HGB (g/L) | 131(129-133) | 130(128-133) | 131(128-135) | 0.710 |
| NEUT (109/L) | 5.19(4.65-5.72) | 4.94(4.38-5.51) | 5.73(4.54-6.92) | 0.183 |
| LY (109/L) | 1.66(1.43-1.88) | 1.71(1.39-2.03) | 1.53(1.41-1.65) | 0.470 |
| PLT (109/L) | 215(207-223) | 215(205-225) | 215(200-231) | 0.946 |
| SII | 706(741-946) | 796(685-907) | 950(728-1172) | 0.172 |
| ALBI | -2.67(-2.74 - -2.60) | -2.70(-2.77 – -2.62) | -2.60(-2.73 – -2.48) | 0.207 |
3.1. Survival analysis for OS and RFS
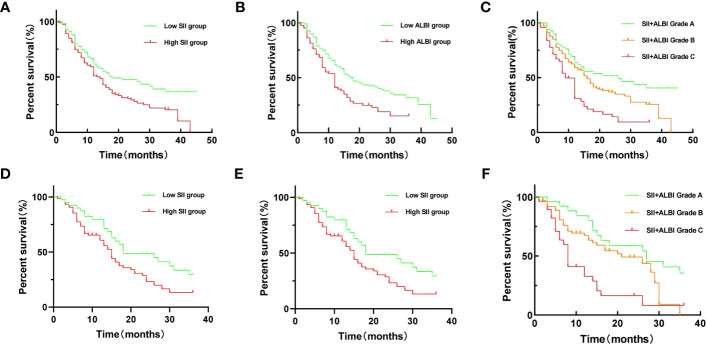
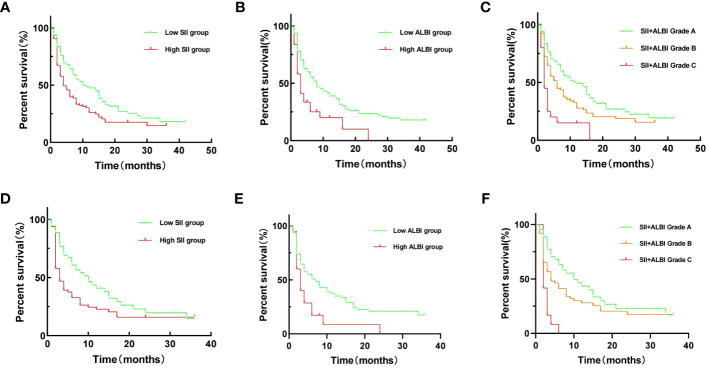
Through univariate survival analysis of the included variables, we found that SII [OS: hazard ratio (HR) = 1.574, 95% CI = 1.126-2.201, p = 0.008; RFS: HR = 1.590, 95% CI = 1.181-2.140, p = 0.002], ALBI [OS: HR = 1.692, 95% CI = 1.220-2.346, p = 0.002; RFS: HR = 1.980, 95% CI = 1.291-3.038, p = 0.002], and SII+ALBI grade [OS: HR = 2.717, 95% CI = 1.701-4.341, p < 0.001; RFS: HR = 3.078, 95% CI = 1.822-5.198, p < 0.001] were prognostic factors for OS and RFS in patients with ICC after surgical resection ( Figures 1 , 2 ). Additionally, the results of the multivariate survival analysis also indicated that SII+ALBI grade [OS: HR = 2.230, 95% CI = 1.371-3.628, p = 0.001; RFS: HR = 2.355, 95% CI = 1.359-4.082, p = 0.001] was an independent risk factor for OS and RFS in postoperative ICC patients ( Figures 1 , 2 ). The detailed results of the univariate and multivariate analyses are presented in Table 2 .
Figure 1.
Kaplan–Meier overall survival (OS) curves of patients with intrahepatic cholangiocarcinoma (ICC) after radical resection according to different prognostic factors in the training set and validation set. (A–C) Kaplan–Meier OS curves according to the Systemic Immune-Inflammation Index (SII)(A), albumin–bilirubin (ALBI) (B), and SII+ALBI grade (C) of training set. (D–F) Kaplan–Meier OS curves according to SII (A), ALBI (B), and SII+ALBI grade (C) of validation set.
Figure 2.
Kaplan–Meier recurrence-free survival (RFS) curves of patients with intrahepatic cholangiocarcinoma (ICC) after radical resection according to different prognostic factors in the training set and validation set. (A–C) Kaplan–Meier OS curves according to the Systemic Immune-Inflammation Index (SII) (A), albumin–bilirubin (ALBI) (B), and SII+ALBI grade (C) of training set. (D–F) Kaplan–Meier OS curves according to SII (A), ALBI (B), and SII+ALBI grade (C) of validation set.
Table 2.
Univariate and multivariate analyses of the prognosis for intrahepatic cholangiocarcinoma (ICC) after radical resection in the training set.
| Variables | OS | RFS | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| HR (95%CI) | p value | HR (95%CI) | p value | HR (95%CI) | p value | HR (95%CI) | p value | |
| Sex Female vs. Male |
0.918(0.672-1.256) | 0.594 | 1.040(0.773-1.400) | 0.794 | ||||
| Age (years) >65 vs. ≤65 |
0.947(0.683-1.314) | 0.744 | 0.806(0.590-1.101) | 0.176 | ||||
| Obstructive jaundice Yes vs. no |
0.923(0.533-1.600) | 0.776 | 0.928(0.575-1.499) | 0.761 | ||||
| HBV infection Yes vs. no |
0.722(0.515-1.012) | 0.059 | 0.861(0.629-1.179) | 0.351 | ||||
| AFP (ng/ml) ≥20 vs. <20 |
0.856(0.549-1.333) | 0.491 | 1.051(0.702-1.575) | 0.808 | ||||
| CEA (ng/ml) ≥5 vs. <5 |
1.958(1.427-2.685) | <0.001 | 1.417(1.044-1.923) | 0.025 | ||||
| CA19-9 (U/ml) ≥37 vs. <37 |
2.067(1.464-2.920) | <0.001 | 1.520(1.037-2.228) | 0.032 | 1.779(1.297-2.440) | <0.001 | 1.764(1.273-2.445) | 0.001 |
| ALT (ng/ml) | 1.001(0.999-1.003) | 0.292 | 1.001(0.999-1.002) | 0.404 | ||||
| AST (ng/ml) | 1.003(1.000-1.005) | 0.032 | 1.002(0.999-1.004) | 0.127 | ||||
| Albumin(ng/ml) | 0.959(0.933-0.986) | 0.003 | 0.966(0.941-0.992) | 0.010 | ||||
| Bilirubin(ng/ml) | 1.003(1.001-1.005) | 0.055 | 1.004(1.001-1.006) | 0.002 | ||||
| PT(s) | 1.057(0.948-1.180) | 0.318 | 1.028(0.927-1.139) | 0.606 | ||||
| INR | 0.938(0.821-1.071) | 0.342 | 0.959(0.873-1.054) | 0.385 | ||||
| APTT(s) | 0.997(0.973-1.021) | 0.782 | 0.991(0.970-1.013) | 0.442 | ||||
| Child–Pugh Grade Grade A vs. Grade B |
2.146(1.216-3.789) | 0.008 | ||||||
| SII High group vs. low group |
1.574(1.126-2.201) | 0.008 | 1.590(1.181-2.140) | 0.002 | ||||
| ALBI High group vs. low group |
1.692(1.220-2.346) | 0.002 | 1.980(1.291-3.038) | 0.002 | ||||
| SII+ALBI Grade Grade B vs.Grade A Grade C vs.Grade A |
1.519(1.013-2.278) 2.717(1.701-4.341) |
0.037 <0.001 |
1.347(1.013-2.053) 2.230((1.371-3.628) |
0.037 0.001 |
1.493(1.091-2.042) 3.078(1.822-5.198) |
0.012 <0.001 |
1.225(1.004-1.696) 2.355(1.359-4.082) |
0.032 0.002 |
| Tumor number 1 vs. >1 |
1.426(0.978-2.079) | 0.065 | 1.853(1.306-2.628) | 0.001 | 1.614(1.114-2.339) | 0.011 | ||
| Tumor size (cm) >5.0 vs. ≤5.0 |
1.402(1.013-1.939) | 0.041 | 1.293(0.954-1.751) | 0.097 | ||||
| Tumor differentiation Moderate vs. well Poor vs. well |
2.193(1.142-4.209) 3.258(1.578-6.729) |
0.018 0.001 |
1.685(1.105-3.314) 2.654(1.244-5.662) |
0.028 0.012 |
1.646(1.063-2.812) 2.333(1.251-4.350) |
0.029 0.008 |
1.334(1.073-2.302) 2.068(1.089-3.925) |
0.035 0.026 |
| Perineural invasion Yes vs. no |
1.691(1.232-2.322) | 0.001 | 1.246(0.927-1.676) | 0.145 | ||||
| Microvascular invasion Yes vs. no |
1.993(1.451-2.737) | <0.001 | 1.548(1.112-2.156) | 0.010 | 1.473(1.092-1.987) | 0.011 | 1.364(1.004-1.853) | 0.047 |
| AJCC 8th edition T stage T2 vs. T1a/T1bT3/T4 vs. T1a/T1b |
1.342(0.947-1.903) |
0.098 | 1.062(0.689-1.637) | 0.784 | ||||
| 1.514(0.982-2.332) | 0.060 | 1.527(0.985-2.367) | 0.058 | |||||
| AJCC 8th edition N stage N1 vs. N0 |
1.840(1.307-2.592) | <0.001 | 1.452(1.011-2.085) | 0.043 | 1.421(1.027-1.965) | 0.034 | ||
| AJCC 8th edition M stage M1 vs. M0 |
1.620(0.400-6.556) | 0.499 | 0.988(0.244-3.991) | 0.986 | ||||
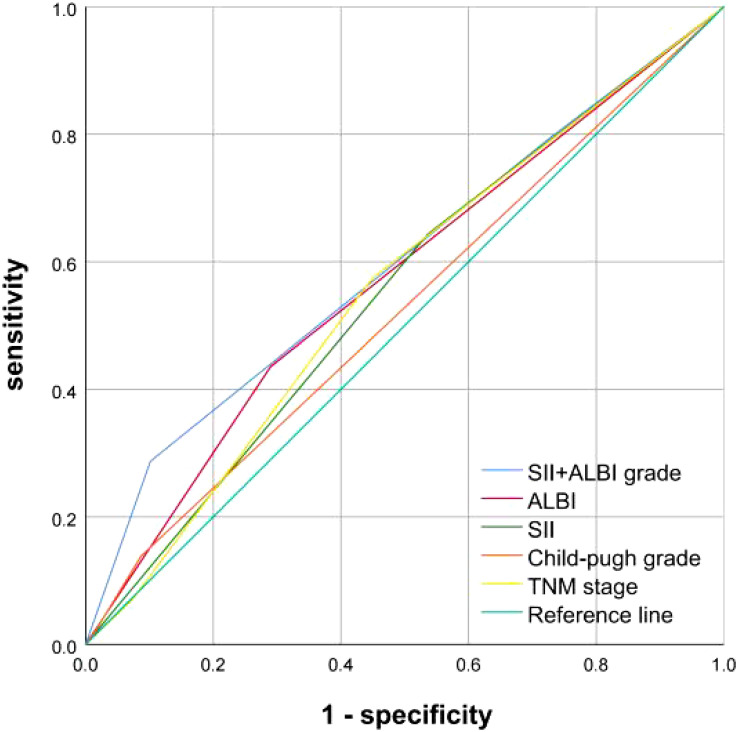
In addition, we plotted the ROC survival curves for SII, ALBI, SII+ALBI grade, Child-pugh Grade and AJCC 8th TNM stage. By comparing the area under the ROC curves, we found that SII+ALBI grade demonstrated a superior survival predictive effect ( Figure 3 ).
Figure 3.
Comparison of SII, ALBI, SII+ALBI grade, Child-pugh Grade and TNM stage in predicting OS.
3.2. Correlation analysis of SII, ALBI and SII+ALBI with clinical and pathological features
Through chi-square tests, we found that compared to SII and ALBI, SII+ALBI grade exhibited better correlations with age, obstructive jaundice, HBV infection, CA19-9, CEA, Child–Pugh Grade, tumor size, tumor differentiation, perineural invasion (p<0.05, Table 3 ).
Table 3.
Relationship of SII, ALBI and SII+ALBI grade with clinicopathological characteristics of intrahepatic cholangiocarcinoma (ICC) after radical resection in the training set.
| SII | X2 | p-value | ALBI | X2 | p-value | SII+ALBI Grade | X2 | p value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low group | High group | Low group |
High group | Grade A | Grade B | Grade C | |||||||
| Sex | 1.768 | 0.184 | 0.012 | 0.912 | 3.294 | 0.193 | |||||||
| Male | 60 | 80 | 97 | 43 | 44 | 69 | 27 | ||||||
| Female | 41 | 77 | 81 | 37 | 26 | 70 | 22 | ||||||
| Age | 0.911 | 0.340 | 0.002 | 0.964 | 7.010 | 0.030 | |||||||
| ≤65 | 61 | 104 | 114 | 51 | 38 | 99 | 28 | ||||||
| >65 | 40 | 53 | 64 | 29 | 32 | 40 | 21 | ||||||
| Obstructive jaundice | 0.017 | 0.897 | 18.259 | <0.001 | 10.157 | 0.006 | |||||||
| No | 83 | 130 | 159 | 54 | 62 | 118 | 33 | ||||||
| Yes | 18 | 27 | 19 | 26 | 8 | 21 | 16 | ||||||
| HBV infection | 6.834 | 0.009 | 3.805 | 0.051 | 11.015 | 0.004 | |||||||
| No | 56 | 112 | 109 | 59 | 35 | 95 | 38 | ||||||
| Yes | 45 | 45 | 69 | 21 | 35 | 44 | 11 | ||||||
| AFP (ng/ml) | 0.947 | 0.331 | 1.351 | 0.245 | 2.280 | 0.320 | |||||||
| <20 | 83 | 136 | 148 | 71 | 56 | 119 | 44 | ||||||
| ≥20 | 18 | 21 | 30 | 9 | 14 | 20 | 5 | ||||||
| CEA (ng/ml) | 9.779 | 0.002 | 0.269 | 0.604 | 7.978 | 0.019 | |||||||
| <5 | 76 | 88 | 115 | 49 | 54 | 83 | 27 | ||||||
| ≥5 | 25 | 69 | 63 | 31 | 16 | 56 | 22 | ||||||
| CA19-9 (U/ml) | 5.880 | 0.015 | 7.205 | 0.007 | 16.720 | <0.001 | |||||||
| <37 | 48 | 51 | 78 | 21 | 41 | 44 | 14 | ||||||
| ≥37 | 53 | 106 | 100 | 59 | 29 | 95 | 35 | ||||||
| SII | 0.008 | 0.930 | 156.891 | <0.001 | |||||||||
| Low group | 70 | 31 | 70 | 31 | 0 | ||||||||
| High group | 108 | 49 | 0 | 108 | 49 | ||||||||
| ALBI | 0.008 | 0.930 | 145.410 | <0.001 | |||||||||
| Low group | 70 | 108 | 70 | 108 | 0 | ||||||||
| High group | 31 | 49 | 0 | 31 | 49 | ||||||||
| SII+ALBI grade | 156.891 | <0.001 | 145.410 | <0.001 | |||||||||
| Grade A | 70 | 0 | 70 | 0 | |||||||||
| Grade B | 31 | 108 | 108 | 31 | |||||||||
| Grade C | 0 | 49 | 0 | 49 | |||||||||
| Child–Pugh grade | 0.171 | 0.679 | 54.684 | <0.001 | 31.612 | <0.001 | |||||||
| Grade A | 91 | 142 | 177 | 56 | 69 | 130 | 34 | ||||||
| Grade B | 10 | 15 | 1 | 24 | 1 | 9 | 15 | ||||||
| Tumor Number | 2.840 | 0.092 | 0.004 | 0.947 | 2.383 | 0.304 | |||||||
| =1 | 87 | 122 | 144 | 65 | 61 | 109 | 39 | ||||||
| >1 | 14 | 35 | 34 | 15 | 9 | 30 | 10 | ||||||
| Tumor Size(cm) | 19.244 | <0.001 | 2.223 | 0136 | 7.382 | 0.025 | |||||||
| ≤5 | 58 | 47 | 67 | 38 | 38 | 49 | 18 | ||||||
| >5 | 43 | 110 | 111 | 42 | 32 | 90 | 31 | ||||||
| Tumor differentiation | 1.062 | 0.588 | 5.498 | 0.064 | 6.736 | 0.151 | |||||||
| Well | 13 | 14 | 23 | 4 | 10 | 17 | 11 | ||||||
| Moderate | 73 | 120 | 133 | 60 | 49 | 108 | 36 | ||||||
| Poor | 15 | 23 | 22 | 16 | 11 | 14 | 2 | ||||||
| Perineural invasion | 9.145 | 0.002 | 10.043 | 0.002 | 19.220 | <0.001 | |||||||
| No | 67 | 74 | 109 | 32 | 52 | 72 | 17 | ||||||
| Yes | 34 | 83 | 69 | 48 | 18 | 67 | 32 | ||||||
| Microvascular invasion | 0.500 | 0.480 | 1.296 | 0.255 | 1.679 | 0.432 | |||||||
| No | 58 | 84 | 102 | 40 | 42 | 76 | 24 | ||||||
| Yes | 42 | 73 | 75 | 40 | 27 | 63 | 25 | ||||||
| AJCC 8th edition T stage | 7.729 | 0.021 | 3.851 | 0.146 | 3.737 | 0.443 | |||||||
| T1a/T1b | 55 | 62 | 80 | 37 | 38 | 59 | 20 | ||||||
| T2 | 35 | 60 | 61 | 34 | 22 | 52 | 21 | ||||||
| T3/T4 | 11 | 35 | 37 | 9 | 10 | 28 | 8 | ||||||
| AJCC 8th edition N stage | 2.403 | 0.121 | 0.671 | 0.413 | 0.398 | 0.819 | |||||||
| N0 | 79 | 109 | 127 | 61 | 53 | 100 | 35 | ||||||
| N1 | 22 | 48 | 51 | 19 | 17 | 39 | 14 | ||||||
| AJCC 8th edition M stage | 0.043 | 0.836 | 1.804 | 0.179 | 1.244 | 0.537 | |||||||
| M0 | 100 | 155 | 177 | 78 | 70 | 137 | 48 | ||||||
| M1 | 1 | 2 | 1 | 2 | 0 | 2 | 1 | ||||||
3.3. Development and assessment of nomogram
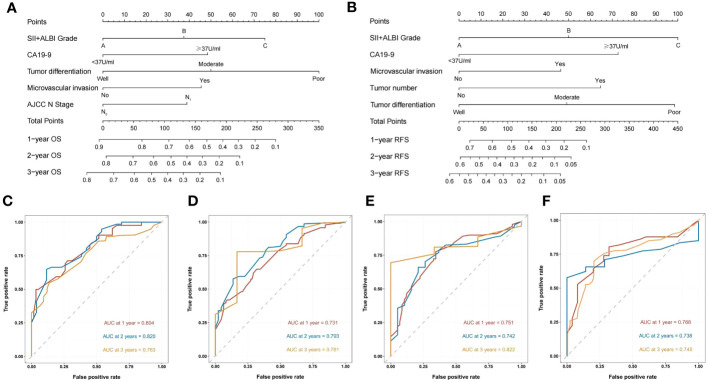
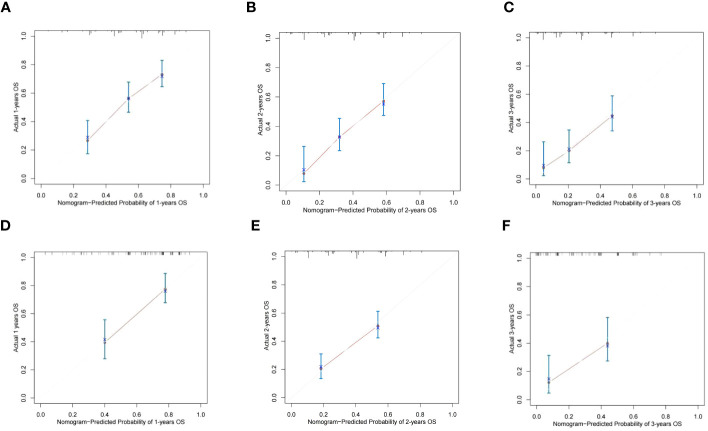
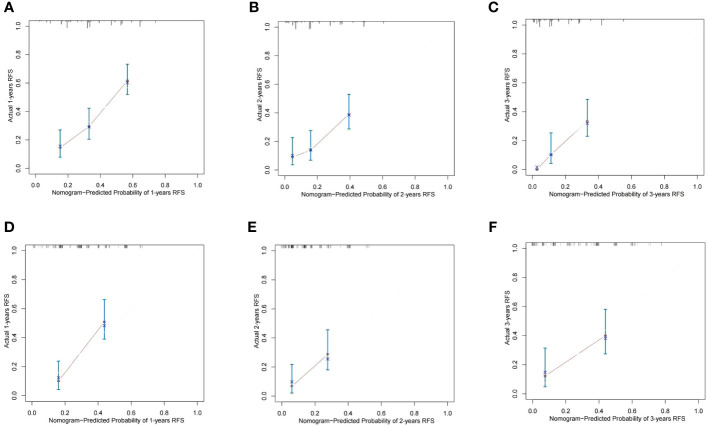
Based on the results of Cox multivariate survival analysis, we established a nomogram prediction model using R software for postoperative OS and RFS in patients with ICC, incorporating various variables including SII+ALBI grade ( Figure 4 ). In addition, we plotted the ROC survival curves for the training and validation sets based on the predictive model. The AUC values for 1–3-year OS in the training set were 0.804, 0.820, and 0.763, respectively, while for the validation set, they were 0.731, 0.793, and 0.781. The AUC values for 1–3-year RFS in the training set were 0.751, 0.742, and 0.822, respectively, and for the validation set they were 0.768, 0.738, and 0.745 ( Figure 4 ). We also plotted the calibration curves of the training and validation sets for 1–3-year survival using both models, and the results consistently demonstrated the excellent predictive ability of the model for postoperative survival in ICC patients ( Figures 5 , 6 ).
Figure 4.
Construction and validation of the nomograms. Nomograms incorporating SII + ALBI Grade and other clinicopathological parameters for OS (A) and RFS (B) prediction in the training cohort. ROC survival curves of the training set for OS (C) and RFS (D) based on the model. ROC survival curves of the validation set for OS (E) and RFS (F) based on the model.
Figure 5.
The calibration curves of the nomograms between predicted and observed 1-, 2-, and 3-year OS of patients in the training set (A–C) and the validation set (D–F). The dashed line of 45° represents the perfect prediction of the nomogram.
Figure 6.
The calibration curves of the nomograms between predicted and observed 1-, 2-, and 3-year RFS in the training set (A–C) and the validation set (D–F). The dashed line of 45° represents the perfect prediction of the nomogram.
4. Discussion
Curative surgical resection represents the gold standard for the treatment of ICC (14). The decision to proceed with surgical resection is often based on the patient’s imaging data and the presence of accompanying symptoms. However, even among patients with similar disease stages and grades, there exists significant heterogeneity in the prognosis and clinical response to curative surgical resection (15). Therefore, the identification of a robust intraoperative and postoperative risk prediction tool holds paramount importance.
As a composite index of platelet, lymphocyte, and monocyte counts, SII provides a direct reflection of the body’s inflammatory status. Increasing evidence suggests that platelets and monocytes can interact with tumor cells through various mechanisms, promoting tumor cell survival and metastasis, enhancing cancer cell invasion, proliferation, and immune evasion, thereby modulating the interplay between the host and tumor (16–19). On the other hand, lymphocytes play a crucial role in cell-mediated immune destruction of cancer cells by activated T cells and other lymphocytes, while tumors can also release cytokines such as IFN-γ and TNF-α to regulate various immune functions in the body (20, 21). Furthermore, numerous studies have confirmed that SII is an independent prognostic factor for postoperative survival in various digestive system malignancies, including HCC, ICC, and gallbladder cancer (8, 22–25). Similarly, in our study, a lower SII was significantly associated with improved postoperative survival and reduced recurrence rates, further validating this observation.
Albumin-bilirubin, calculated based on serum albumin and bilirubin levels, provides an intuitive reflection of a patient’s immune status and liver function; ALBI was initially proposed by Johnson et al. in 2014 as an alternative to the Child-Pugh classification for assessing liver function in HCC patients, overcoming its limitations (26). Increasing evidence suggests that ALBI is a reliable indicator of liver functional reserve. A multicenter cohort study demonstrated that the predictive performance of the Barcelona Clinic Liver Cancer (BCLC) staging system based on ALBI score is comparable to or even superior to that based on the Child-Pugh classification (27). Subsequently, the predictive ability of ALBI for the prognosis of HCC and ICC patients has been validated in multiple independent cohorts, including those from Japan, China, and other countries (28–30). Consistent with the findings of these studies, in our research, the low ALBI group exhibited significantly higher OS and RFS rates compared to the high ALBI group.
In our study, we took into consideration the patients’ inflammatory status, immune capacity, and liver function, by combining SII and ALBI, which were categorized into three grades: A, B, and C. Through the construction of Kaplan-Meier survival curves and ROC survival curves, we found that the SII+ALBI grade had better predictive ability and discrimination when compared separately to SII and ALBI. Therefore, we included the SII+ALBI grade as an independent grade index in our model and confirmed that the nomogram predictive model incorporating SII+ALBI grade for OS and RFS demonstrated good predictive performance. Additionally, we analyzed the correlation between SII+ALBI grade and clinical and pathological characteristics. Surprisingly, for indicators such as microvascular invasion and 8th edition AJCC N stage, which showed no significant correlation with individual SII or ALBI, the SII+ALBI classification still exhibited a correlation. Therefore, we believe that the SII+ALBI classification can better reflect the patients’ clinical and pathological characteristics to a certain extent.
After reviewing relevant research, we found that our study is the first to combine SII and ALBI and construct a prognostic survival model based on SII+ALBI grade. In our model, SII+ALBI grade carries a significant weight, which is closely related to representing the immune-inflammatory status and liver function. Additionally, we plotted ROC survival curves and calibration curves for the training and validation sets based on the predictive model. The results demonstrated excellent predictive ability of the model for postoperative survival in patients with ICC.
In addition, our study has the following limitations. Firstly, although it is a multicenter retrospective study, the sample size involved in the study is relatively small, with a total of 374 cases. Secondly, due to the retrospective nature of this study, selection bias is unavoidable, and we only included patients who underwent surgical resection without receiving other treatments prior to surgery. Thirdly, despite our efforts to minimize the impact of confounding factors on the study results, individual differences in various laboratory parameters cannot be completely eliminated. Therefore, further large-scale prospective multicenter studies are still needed to validate our findings.
5. Conclusion
In conclusion, this multicenter study included a sample of 374 patients with ICC who underwent surgical resection in three tertiary hospitals. Based on univariate, multivariate, and clinical significance analyses, multiple relevant indicators incorporating the SII+ALBI grade were incorporated to construct a nomogram predictive model for OS and RFS. The model demonstrated excellent accuracy in survival prediction. To our knowledge, this is the first clinical prediction model for ICC that includes the SII+ALBI grade. We believe that this model can provide better guidance for the management of ICC and has the potential for broad application.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study received ethical approval from the Institutional Review Boards of Zhengzhou University People’s Hospital (Ref No. 2023-012), Zhengzhou University Cancer Hospital (Ref No. 2023-203), and Zhengzhou University First Affiliated Hospital (2021-KY-1137-002). Written informed consent was obtained from all patients prior to their participation in the study. The studies were conducted in accordance with the local legislation and institutional requirements.
Author contributions
HZ is the first author. HZ, QL and HY conceived and designed the study. HZ, GH, ZY, KC and BM collected and offered the data. HZ, GH and ZY performed follow-up and statistical analysis. HZ wrote the manuscript. HY critically revised the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank AiMi Academic Services (www.aimieditor.com) for English language editing and review services.
Funding Statement
This work was financially supported by Henan Province key research and development and promotion special projects (222102310709).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol (2014) 60(6):1268–89. doi: 10.1016/j.jhep.2014.01.021 [DOI] [PubMed] [Google Scholar]
- 2. Rahnemai-Azar AA, Weisbrod A, Dillhoff M, Schmidt C, Pawlik TM. Intrahepatic cholangiocarcinoma: molecular markers for diagnosis and prognosis. Surg Oncol (2017) 26(2):125–37. doi: 10.1016/j.suronc.2016.12.009 [DOI] [PubMed] [Google Scholar]
- 3. Jutric Z, Johnston WC, Hoen HM, Newell PH, Cassera MA, Hammill CW, et al. Impact of lymph node status in patients with intrahepatic cholangiocarcinoma treated by major hepatectomy: A review of the national cancer database. HPB: Off J Int Hepato Pancreato Biliary Assoc (2016) 18(1):79–87. doi: 10.1016/j.hpb.2015.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. Capecitabine compared with observation in resected biliary tract cancer (Bilcap): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol (2019) 20(5):663–73. doi: 10.1016/s1470-2045(18)30915-x [DOI] [PubMed] [Google Scholar]
- 5. Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg (2014) 149(6):565–74. doi: 10.1001/jamasurg.2013.5137 [DOI] [PubMed] [Google Scholar]
- 6. Philip M, Rowley DA, Schreiber H. Inflammation as a tumor promoter in cancer induction. Semin Cancer Biol (2004) 14(6):433–9. doi: 10.1016/j.semcancer.2004.06.006 [DOI] [PubMed] [Google Scholar]
- 7. Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res (2014) 20(23):6212–22. doi: 10.1158/1078-0432.Ccr-14-0442 [DOI] [PubMed] [Google Scholar]
- 8. Tsilimigras DI, Moris D, Mehta R, Paredes AZ, Sahara K, Guglielmi A, et al. The systemic immune-inflammation index predicts prognosis in intrahepatic cholangiocarcinoma: an international multi-institutional analysis. HPB: Off J Int Hepato Pancreato Biliary Assoc (2020) 22(12):1667–74. doi: 10.1016/j.hpb.2020.03.011 [DOI] [PubMed] [Google Scholar]
- 9. Wang YY, Zhong JH, Su ZY, Huang JF, Lu SD, Xiang BD, et al. Albumin-bilirubin versus child-pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg (2016) 103(6):725–34. doi: 10.1002/bjs.10095 [DOI] [PubMed] [Google Scholar]
- 10. Wang D, Hu X, Xiao L, Long G, Yao L, Wang Z, et al. Prognostic nutritional index and systemic immune-inflammation index predict the prognosis of patients with hcc. J gastrointestinal Surg (2021) 25(2):421–7. doi: 10.1007/s11605-019-04492-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li H, Wang JJ, Zhang M, Ren B, Li JX, Xu L, et al. Prognostic significance of systemic immune-inflammation index in patients with intrahepatic cholangiocarcinoma undergoing hepatic resection. World J gastrointestinal Oncol (2020) 12(4):467–82. doi: 10.4251/wjgo.v12.i4.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang J, Bao Y, Chen W, Duan Y, Sun D. Nomogram based on systemic immune inflammation index and prognostic nutrition index predicts recurrence of hepatocellular carcinoma after surgery. Front Oncol (2020) 10:551668. doi: 10.3389/fonc.2020.551668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaneko S, Kurosaki M, Tsuchiya K, Yasui Y, Inada K, Kirino S, et al. Prognosis of intrahepatic cholangiocarcinoma stratified by albumin-bilirubin grade. Hepatol Res (2021) 51(8):902–8. doi: 10.1111/hepr.13673 [DOI] [PubMed] [Google Scholar]
- 14. Mansour JC, Aloia TA, Crane CH, Heimbach JK, Nagino M, Vauthey JN. Hilar cholangiocarcinoma: expert consensus statement. HPB: Off J Int Hepato Pancreato Biliary Assoc (2015) 17(8):691–9. doi: 10.1111/hpb.12450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Q, Chen C, Zhang J, Wu H, Qiu Y, Song T, et al. Prediction efficacy of prognostic nutritional index and albumin-bilirubin grade in patients with intrahepatic cholangiocarcinoma after radical resection: A multi-institutional analysis of 535 patients. Front Oncol (2021) 11:769696. doi: 10.3389/fonc.2021.769696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell (2011) 20(5):576–90. doi: 10.1016/j.ccr.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2y2 receptor. Cancer Cell (2013) 24(1):130–7. doi: 10.1016/j.ccr.2013.05.008 [DOI] [PubMed] [Google Scholar]
- 18. Halazun KJ, Hardy MA, Rana AA, Woodland DC4, Luyten EJ, Mahadev S, et al. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg (2009) 250(1):141–51. doi: 10.1097/SLA.0b013e3181a77e59 [DOI] [PubMed] [Google Scholar]
- 19. Dan J, Zhang Y, Peng Z, Huang J, Gao H, Xu L, et al. Postoperative neutrophil-to-lymphocyte ratio change predicts survival of patients with small hepatocellular carcinoma undergoing radiofrequency ablation. PloS One (2013) 8(3):e58184. doi: 10.1371/journal.pone.0058184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guo XJ, Lu JC, Zeng HY, Zhou R, Sun QM, Yang GH, et al. Ctla-4 synergizes with pd1/pd-L1 in the inhibitory tumor microenvironment of intrahepatic cholangiocarcinoma. Front Immunol (2021) 12:705378. doi: 10.3389/fimmu.2021.705378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lasek W, Janyst M, Wolny R, Zapała Ł, Bocian K, Drela N. Immunomodulatory effects of inosine pranobex on cytokine production by human lymphocytes. Acta Pharm (Zagreb Croatia) (2015) 65(2):171–80. doi: 10.1515/acph-2015-0015 [DOI] [PubMed] [Google Scholar]
- 22. Xu Y, Han H, Cao W, Fu H, Liu Y, Yan L, et al. Establishment and validation of a predictive model of recurrence in primary hepatocellular carcinoma after resection. J gastrointestinal Oncol (2023) 14(1):278–86. doi: 10.21037/jgo-22-1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Safcak D, Drazilova S, Gazda J, Andrasina I, Adamcova-Selcanova S, Balazova L, et al. Inflammatory indexes as prognostic factors of survival in geriatric patients with hepatocellular carcinoma: A case control study of eight slovak centers. J Clin Med (2022) 11(14). doi: 10.3390/jcm11144183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li J, Cao D, Huang Y, Xiong Q, Tan D, Liu L, et al. The prognostic and clinicopathological significance of systemic immune-inflammation index in bladder cancer. Front Immunol (2022) 13:865643. doi: 10.3389/fimmu.2022.865643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang S, Du J, Zhong X, Tan P, Xu H, Zhang J, et al. The prognostic value of the systemic immune-inflammation index for patients with bladder cancer after radical cystectomy. Front Immunol (2022) 13:1072433. doi: 10.3389/fimmu.2022.1072433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the Albi grade. J Clin Oncol (2015) 33(6):550–8. doi: 10.1200/jco.2014.57.9151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chan AW, Kumada T, Toyoda H, Tada T, Chong CC, Mo FK, et al. Integration of albumin-bilirubin (Albi) score into barcelona clinic liver cancer (Bclc) system for hepatocellular carcinoma. J Gastroenterol Hepatol (2016) 31(7):1300–6. doi: 10.1111/jgh.13291 [DOI] [PubMed] [Google Scholar]
- 28. Toyoda H, Lai PB, O’Beirne J, Chong CC, Berhane S, Reeves H, et al. Long-term impact of liver function on curative therapy for hepatocellular carcinoma: application of the Albi grade. Br J Cancer (2016) 114(7):744–50. doi: 10.1038/bjc.2016.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hiraoka A, Kumada T, Kudo M, Hirooka M, Tsuji K, Itobayashi E, et al. Albumin-bilirubin (Albi) grade as part of the evidence-based clinical practice guideline for hcc of the Japan society of hepatology: A comparison with the liver damage and child-pugh classifications. Liver Cancer (2017) 6(3):204–15. doi: 10.1159/000452846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma XL, Zhou JY, Gao XH, Tian L, Wu J, Zhang CY, et al. Application of the albumin-bilirubin grade for predicting prognosis after curative resection of patients with early-stage hepatocellular carcinoma. Clinica chimica acta; Int J Clin Chem (2016) 462:15–22. doi: 10.1016/j.cca.2016.08.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.