Abstract
Achieving a peak aminoglycoside concentration (Cmax)/MIC of ≥10 within 48 h of initiation of therapy for pneumonia caused by gram-negative organisms results in a 90% probability of therapeutic response by day 7. Targeting an MIC of 1 μg/ml, empirical aminoglycoside loading doses of 348 (25th- to 75th-percentile range, 275 to 432) mg were calculated to obtain a Cmax/MIC of 10 in our patient population. Individualized pharmacokinetic monitoring coupled with MIC data should determine subsequent dosing regimens to minimize the potential for toxicity and maximize the probability of clinical response.
Aminoglycosides have been used for treating a wide variety of serious infections for over 30 years. However, few data exist on how best to maximize dosages and scheduling to achieve the best therapeutic outcome for a given patient. As aminoglycosides exhibit concentration-dependent killing, peak concentrations (Cmax) and Cmax/MIC ratios have been postulated to be the best predictors of therapeutic efficacy (5, 6, 8–12, 14). Traditionally, aminoglycosides have been administered in clinician-determined doses (i.e., similar doses for patients of similar weight) or with individualized pharmacokinetic monitoring (IPM) with Cmax targets associated with efficacy (8–10, 12, 14). However, since aminoglycosides show concentration-dependent killing, it appears more appropriate to target the pharmacodynamic parameter Cmax/MIC in an attempt to optimize efficacy (7).
In patients with documented pneumonia caused by gram-negative organisms, data demonstrate that achieving a Cmax/MIC ratio of ≥10 within 48 h of initiation of therapy results in a 90% probability of temperature and leukocyte count normalization by day 7 of therapy (7). Clinician-determined and Cmax target dosing methods may lead to a delay in reaching appropriate Cmax/MIC ratios. The aim of this analysis was to determine an initial aminoglycoside dosing regimen to immediately achieve pharmacodynamic parameters predictive of optimization of therapeutic response in patients with pneumonia caused by gram-negative organisms.
(This work was presented in part at the 98th Annual Meeting of the American Society for Clinical Pharmacology and Therapeutics, San Diego, Calif., 1997.)
This was a retrospective analysis of prospectively collected pharmacokinetic data for 78 consecutively treated adult medical and surgical patients admitted to Bassett Healthcare from 1983 to 1993. All data were collected by the Clinical Pharmacy Service. Patients with pneumonia caused by gram-negative organisms who received gentamicin or tobramycin for ≥72 h were eligible for analysis. Diagnosis of pneumonia was done according to the Centers for Disease Control criteria (2): (i) a new, otherwise unexplained pulmonary infiltrate on a chest radiograph, (ii) growth of a sole pathogenic organism in a purulent sputum culture, and (iii) leukocytosis (>10,000/mm3) and/or fever (≥38°C). Patients with cystic fibrosis or neutropenia were excluded.
Initial aminoglycoside dosing regimens were chosen by the patient’s physician (clinician-determined method) and only altered if the regimen was found to be a significant overdose or underdose by empirical calculations with hospital-specific patient population pharmacokinetic parameters (1). Pharmacokinetic analysis was performed within 72 h of initiation of therapy with the collection of one predose serum concentration, recording of the duration and time of dose infusion, collection of one postdistributional serum concentration at least 30 min after the end of the infusion, and collection of one postdose serum concentration at least one estimated half-life after the first postdose concentration.
Pharmacokinetic data were analyzed by the method of Sawchuk and Zaske (13), fitting the data to a one-compartment, intravenous-infusion model. Aminoglycoside doses were modified to obtain a 1-h Cmax of 7 to 10 μg/ml and a minimum concentration (Cmin) of <2 μg/ml before redosing (Cmax target method). By the Microscan system (Dade, West Sacramento, Calif.) the MIC at which 90% of the isolates are inhibited (MIC90) of both gentamicin and tobramycin for sterile body fluid isolates (nonurine) of Escherichia coli, Serratia marcesens, and Citrobacter, Klebsiella, Enterobacter, and Proteus species at Bassett Healthcare for 1996 was <1 μg/ml. The MIC90 for P. aeruginosa was 5 μg of gentamicin/ml and 1 μg of tobramycin/ml. Using patient-specific pharmacokinetic parameters and the MIC90 of 1 μg of tobramycin/ml, we modeled an empirical aminoglycoside loading dose to achieve a Cmax/MIC of 10 (thus, a Cmax of 10 μg/ml). The following equation was used to determine these doses (13): Cmax = [ko/(kelV)](1 − e−kelt1)e−kelt2, where Cmax is 10 mg/liter at time t2, ko is the aminoglycoside infusion rate in milligrams per hour, kel is the elimination rate constant in hour−1, V is the volume of distribution in liters, t1 is the infusion duration in hours (set at 1 h), and t2 is the length of the distribution phase (set at 1.7 h secondary to the prolonged distribution time for larger aminoglycoside doses) (4).
Definitive aminoglycoside regimens for achieving a Cmax/MIC of 10 by using the MIC for the organism isolated in each patient (which in all cases was ≤8 μg/ml) were also determined by the following equations (13): τ = ln (Cmax/Cmin)/kel, where τ is the dosing interval in hours and Cmin is 0.5 μg/ml; and MD = [CmaxkelV(1 − e−kelτ)]/[(1 − e−kt1)e−kelt2], where MD is the maintenance dose of aminoglycoside in milligrams. Data are presented as medians and 25th- to 75th-percentile range.
Patient demographics are shown in Table 1. This was an elderly population with some degree of renal impairment. Measured pharmacokinetic parameters are shown in Table 2.
TABLE 1.
Patient demographics
| Characteristic | No. (%) of patients |
|---|---|
| Age (yr) | 69 (58–75)a |
| Sex | |
| Male | 52 (66.7) |
| Female | 26 (33.3) |
| Aminoglycoside | |
| Gentamicin | 38 (48.7) |
| Tobramycin | 40 (51.3) |
| Initial calculated creatinine clearance (ml/min/1.73 m2)b | 67.2 (50.0–85.0)a |
| Concurrent antibiotic therapy | |
| β-Lactam (sensitive) | 56 (71.8) |
| β-Lactam (resistant) | 17 (21.8) |
| None | 5 (6.4) |
| Isolated organisms | |
| Acinetobacter sp. | 1 (1.3) |
| Citrobacter sp. | 5 (6.4) |
| Enterobacter sp. | 9 (11.5) |
| E. coli | 1 (1.3) |
| Klebsiella sp. | 9 (11.5) |
| Proteus sp. | 1 (1.3) |
| P. aeruginosa | 45 (57.7) |
| Serratia sp. | 6 (7.7) |
| Stenotrophomonas sp. | 1 (1.3) |
| MIC of aminoglycoside used (μg/ml) | 1.0 (0.5–4.0)a |
Median (25th to 75th percentile).
Calculated by the Cockcroft-Gault equation (3).
TABLE 2.
Measured pharmacokinetic variables
| Variable | Valuea |
|---|---|
| Initial clinician-determined dose (mg/kg) | 1.6 (1.4–1.9) |
| Initial dosing interval (h) | 12 (12–12) |
| First measured Cmax (μg/ml) | 5.3 (3.9–6.3) |
| First measured Cmin (μg/ml) | 0.6 (0.3–1.1) |
| First measured Cmax/MIC | 3.6 (1.4–6.2) |
| Aminoglycoside clearance (ml/min/1.73 m2) | 71.5 (50.4–91.3) |
| Half-life (h) | 3.5 (2.6–5.0) |
| Volume of distribution (liters) | 20.2 (16.3–27.8) |
| Volume of distribution (liter/kg) | 0.32 (0.27–0.38) |
| Adjusted dose (mg/kg) | 2.4 (1.9–2.8) |
| Adjusted dosing interval (h) | 12 (12–18) |
| Second measured Cmax (μg/ml) | 6.7 (5.2–7.6) |
| Second measured Cmin (μg/ml) | 0.8 (0.5–1.1) |
Data presented as median (25th to 75th percentile).
Assuming an MIC of 1 μg/ml, an empiric median tobramycin loading dose of 348 (275 to 432) mg (or 5.3 [4.3 to 6.7] mg/kg of total body weight) would have achieved a postdistributional Cmax of at least 10 μg/ml and a Cmax/MIC ratio of at least 10 in 50% of our patient population. A 7 mg/kg loading dose of tobramycin would achieve an initial Cmax of at least 10 μg/ml in 90% of our patients. Utilizing patient-specific pharmacokinetic parameters and the isolated-bacterium-specific MIC, a definitive aminoglycoside dose of 460 (280 to 1,160) mg, or 7.0 (4.2 to 17.0) mg/kg, given every 8, 12, 18, 24, 36, 48, and 60 h in 14, 25, 26, 19, 10, 3, and 3% of our patients, respectively, would have achieved a Cmax of 10 (5 to 40) μg/ml, a Cmax/MIC of 10, and a Cmin of 0.5 μg/ml in all patients.
Figure 1 compares the median first Cmax/MIC ratio that would be achieved in these patients for a range of aminoglycoside MICs for three different dosing regimens: an empirical 7-mg/kg loading dose, the traditional Cmax target method, and the clinician-determined dosing method. The traditional Cmax target method and the clinician-determined method would only achieve the Cmax/MIC target ratio of 10 for MICs of ≤0.5 μg/ml, while a 7-mg/kg loading dose would achieve Cmax/MIC targets for MICs of ≤1 μg/ml in 90% of the subjects and would reach a median Cmax/MIC of 6.6 (5.2 to 8.1) for an MIC of 2 μg/ml and 3.3 (2.6 to 4.1) for an MIC of 4 μg/ml. Using a large loading dose followed by IPM, and MIC data once available, should ensure the continued achievement of Cmax/MIC targets.
FIG. 1.
Median initial Cmax/MICs for three aminoglycoside dosing regimens for a range of typical MICs for gram-negative bacteria for 72 patients with pneumonia caused by gram-negative organisms.
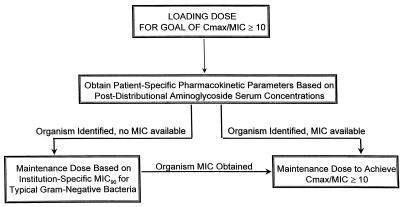
Figure 2 represents a proposed dosing algorithm to maximize the probability of achieving the target Cmax/MIC of 10 in patients with pneumonia caused by gram-negative organisms. This Cmax/MIC target was chosen to achieve the maximum probability of response, considering the MIC90s of our institution’s gram-negative organisms and the upper limit of aminoglycoside dose tolerability. However, other Cmax/MIC targets may be more appropriate. The probability of temperature and leukocyte count resolution by day 7 of therapy has been calculated for the following Cmax/MIC ratios: 4, 65%; 5, 71%; 6, 76%; 7, 81%; 8, 84%; 9, 88%; and 10, 90% (7). For example, in our patient population, more resistant organisms with aminoglycoside MICs of 2 and 4 μg/ml would require median loading doses of 10.6 (8.6 to 13.4) and 21.3 (17.2 to 26.9) mg/kg, respectively, to achieve the Cmax/MIC target of 10. As the safety of these doses has not been established, targeting a Cmax/MIC of 5 in this situation may be a more reasonable approach, even though this Cmax/MIC does not give a ≥90% probability of a temperature or leukocyte response. In addition, the combination of aminoglycosides with an additive or synergistic time-dependent killing agent may be essential. As each institution has different patient population profiles and different bacterial organism sensitivities, early pharmacokinetic optimization is important.
FIG. 2.
Dosing algorithm for aminoglycoside therapy in nosocomial pneumonia caused by gram-negative organisms.
Achieving a postdistributional aminoglycoside Cmax/MIC of 10 may decrease the time to therapeutic response in our patient population (7). Using an MIC90 of 1 μg/ml, with an aminoglycoside volume of distribution of 20.2 (16.3 to 27.8) liters (0.32 [0.27 to 0.38] liter/kg) and a moderate degree of renal impairment, administering an aminoglycoside loading dose of 7 mg/kg with IPM with the first dose will rapidly achieve this goal. As all patients in this analysis had their aminoglycoside dosing intervals adjusted to achieve a Cmin of 0.5 μg/ml (65% were dosed every 8 to 18 h), these data should not be extrapolated to empirical single-daily-dose regimens. The aggressive Cmax/MIC target of 10 may not be practical for all institutions, as aminoglycoside doses for organisms for which the MIC90s are greater than 1 μg/ml may be beyond a clinician’s acceptable range. However, these data stress the need for rapid pharmacokinetic optimization of individual aminoglycoside dosing.
By reducing the time to therapeutic response, overall courses of aminoglycoside therapy may be shorter; with a potential reduction in the incidence of nephrotoxicity. Furthermore, to reduce the risk of aminoglycoside toxicity, a reduction in dose (and thus overall exposure) can be achieved by utilizing the aminoglycoside with the lowest MIC for the isolated bacterium. Our institution’s microbiology data for 1996 illustrate the importance of rapid organism identification and accurate MIC data to optimize aminoglycoside regimens. This analysis has demonstrated that two commonly used methods for determining the dosages of aminoglycosides (clinician determined and traditional Cmax targets) fall short of achieving optimal pharmacodynamic targets.
As this is a retrospective analysis, it will be necessary to conduct a prospective clinical trial to determine if an aggressive aminoglycoside regimen with subsequent adjustment based on an organism-specific MIC to achieve a Cmax/MIC of 10 and a Cmin of <2 μg/ml in patients with pneumonia caused by gram-negative organisms can reduce the time to therapeutic response and potentially reduce the length of stay in the hospital without increasing toxicity.
Acknowledgments
This work was supported by a grant from Abbott Laboratories Diagnostics Division, Inc.
REFERENCES
- 1.Bertino J S, Jr, Booker L A, Franck P, Rybicki B. Gentamicin pharmacokinetics in patients with malignancies. Antimicrob Agents Chemother. 1991;35:1501–1503. doi: 10.1128/aac.35.7.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control Public Health Service. National nosocomial infection surveillance study. Hospital Infections Program. Washington, D.C: U.S. Department of Health and Human Services; 1994. [Google Scholar]
- 3.Cockcroft D W, Gault M H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 4.Demczar D J, Nafziger A N, Bertino J S., Jr Pharmacokinetics of gentamicin at traditional versus high doses: implications for once-daily aminoglycoside dosing. Antimicrob Agents Chemother. 1997;41:1115–1119. doi: 10.1128/aac.41.5.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deziel-Evans L M, Murphy J E, Job M L. Correlation of pharmacokinetic indices with therapeutic outcome in patients receiving aminoglycosides. Clin Pharm. 1986;5:319–324. [PubMed] [Google Scholar]
- 6.Jackson, G. G., and L. J. Riff. 1971. Pseudomonas bacteremia: pharmacologic and other bases for failure of treatment with gentamicin. J. Infect. Dis. 124(Suppl.):S185–S191. [DOI] [PubMed]
- 7.Kashuba A D M, Nafziger A N, Drusano G L, Bertino J S., Jr . Program and Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Early optimization of aminoglycoside pharmacokinetic goals reduces time to therapeutic response in gram-negative pneumonia, abstr. A100; p. 20. [Google Scholar]
- 8.Klastersky J, Daneau D, Swings G, Weerts D. Antibacterial activity in serum and urine as a therapeutic guide in bacterial infections. J Infect Dis. 1974;129:187–193. doi: 10.1093/infdis/129.2.187. [DOI] [PubMed] [Google Scholar]
- 9.Moore R D, Smith C R, Lietman P S. Association of aminoglycoside plasma levels with therapeutic outcome in gram-negative pneumonia. Am J Med. 1984;77:657–662. doi: 10.1016/0002-9343(84)90358-9. [DOI] [PubMed] [Google Scholar]
- 10.Moore R D, Smith C R, Lietman P S. The association of aminoglycoside plasma levels with mortality in patients with gram-negative bacteremia. J Infect Dis. 1984;149:443–448. doi: 10.1093/infdis/149.3.443. [DOI] [PubMed] [Google Scholar]
- 11.Moore R D, Lietman P S, Smith C R. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987;155:93–99. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- 12.Noone P, Parsons T M C, Pattison J R, Slack R C B, Garfield-Davies D, Hughes K. Experience in monitoring gentamicin therapy during treatment of serious gram-negative sepsis. Br Med J. 1974;1:477–481. doi: 10.1136/bmj.1.5906.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawchuk R J, Zaske D E. Pharmacokinetics of dosing regimens which utilize multiple intravenous infusions: gentamicin in burn patients. J Pharmacokinet Biopharm. 1976;4:183–195. doi: 10.1007/BF01086153. [DOI] [PubMed] [Google Scholar]
- 14.Zaske D E, Bootman J L, Solem L B, Strate R G. Increased burn patient survival with individualized dosages of gentamicin. Surgery. 1982;91:142–149. [PubMed] [Google Scholar]