Abstract
Charged ions and ion channels play a critical role in regulating the electrical activities of excitable cells. This review discusses the principles of ion channel regulation in the time domain, as well as the diseases that can arise from channel dysfunction and disturbances in ionic balance. Ion channel signaling is a dynamic process that is essential for various physiological functions, including pain sensation, motor control, and the body’s response to stress, such as fight-or-flight response.
Introduction
Ion channels are membrane proteins that create subnanometer-diameter pores that allow specific ions to permeate the cell membrane.1 More than 300 types of ion channel genes have been identified in the human genome, and dysfunctions in these channels, known as channelopathies, are implicated in a wide range of diseases. They are also an important drug target, with numerous therapeutics designed to modulate their activity. Channel regulation is mediated by a wide range of physiological stimuli, including transmembrane voltage, temperature, neurotransmitters, and mechanical force. Recent research has revealed that membrane lipids and lipid-bilayer structures also play a key role in modulating channel function and electrical excitability, allowing for fine-tuning of channel activity during complex physiological responses.
The Roles of Ion Channels: from Nerve Impulses to Cystic Fibrosis
Ionic fluxes from voltage-activated sodium (Na+) and potassium (K+) channels establish the rise and fall of action potentials, commonly referred to as “nerve impulses” or “spikes” (Figure 1). These impulses are crucial to nearly all physiological processes. They underlie the transmission of information between neurons, allowing us to perceive the world around us, and to think. Furthermore, ion channels are essential to the function of other excitable cells, such as muscle cells. In muscle cells, action potentials are responsible for the contraction of muscles, allowing us to move and exert force.
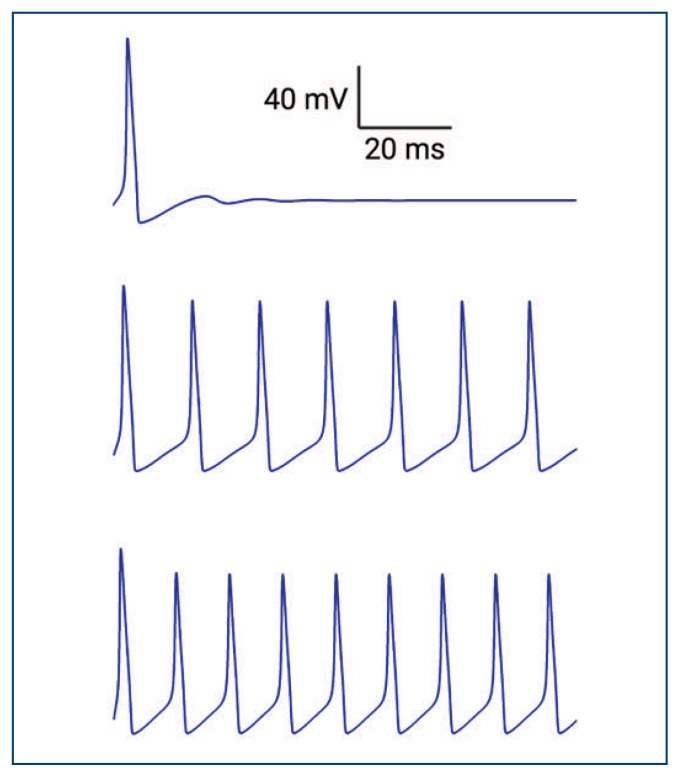
Figure 1.
Simulated action potentials generated using the Hodgkin-Huxley model. Different amounts of current were applied to stimulate trains of action potentials with varying frequencies. The current stimulus ranged from low to high (5 pA, 10 pA, and 20 pA from top to bottom), and the parameters used were based on action potential of the squid giant axon and obtained from the Handbook of Physiology - the Nervous System, Chapter 4, authored by Bertil Hille (1977).
Apart from the channels that shape nerve impulses, there are other channels that are well-known to medical doctors. These channels include the renal outer medullary K+ channel (ROMK) that secretes potassium in the collecting duct system of the kidney, the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channels that controls epithelial mucus transport in the lung, the calcium-activated K+ channels that are important for lacrimal or salivary secretion, and the voltage-activated calcium (Ca2+) channels that regulate the release of hormones from endocrine cells. Mutations in these channels can result in serious diseases such as cystic fibrosis.
Understanding the molecular and structural mechanisms of ion channels has led to the development of treatments for many channel-related diseases. Cystic fibrosis, a life-threatening disease caused by congenital CFTR mutations, is one such condition affecting more than 80,000 people globally. It is characterized by the buildup of sticky mucus, lung infection, and lung damage. With gene therapies targeting CFTR, this disease is now mostly treatable.
Ion Channels Process Different Sensory Signals
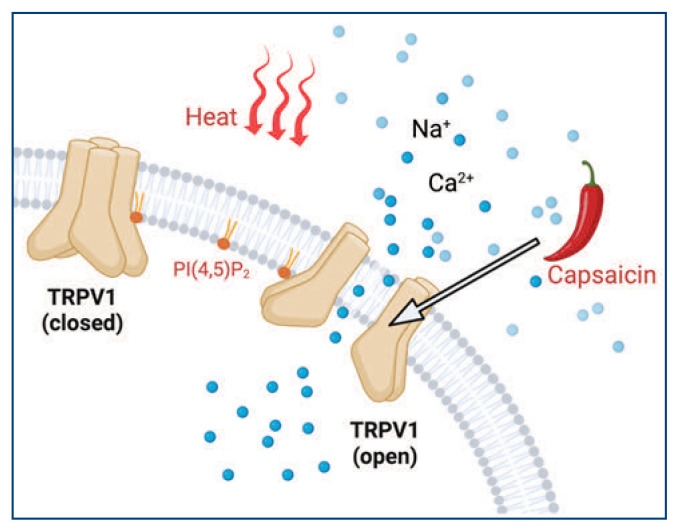
Ion channels are crucial for processing sensory information, including pH, osmolarity, pressure, and temperature. Most ion channels have evolved the ability to respond to multiple external stimuli. One example is the transient receptor potential (TRP) channels, whose name is derived from a mutant strain of the fruit fly Drosophila that loses sustained light response in their photoreceptors. TRP channels can be activated by temperature, voltage, and lipids, as well as capsaicin, menthol, and cannabidiol (CBD). Particularly, capsaicin is a compound found in chili peppers and is known to activate the TRPV1 channel. It is used as a topical analgesic for the treatment of neuropathic pain (Figure 2). In addition, nabiximols (Sativex) is an oromucosal spray that contains CBD and activates the TRPV1 channel. It is approved for the treatment of multiple sclerosis-related spasticity and neuropathic pain.
Figure 2.
Schematic cartoon showing the multimodal activation of TRPV1 channels by both heat and capsaicin binding. Membrane lipid PI(4,5)P2 binds to TRPV1 channels directly.
Ion channels achieve their multimodal property through different protein domain evolution or modification. It has been proposed that TRP channels lost some voltage-sensing amino acids and gained hydrophobic/hydrophilic ones elsewhere, which conferred temperature sensitivity on TRP channels.2 The exact molecular mechanism of the TRP thermo-sensing remains debatable. In general, channels are most often found to be dually activated by voltage and ligand. This multimodal feature of ion channels greatly diversifies the mechanism whereby they could be intricately and dynamically modulated to control cellular excitability. Furthermore, because of this feature and the measurable channel activity at ultrahigh temporal resolution (through on-line patch-clamp electrophysiology), ion channels have become one of the favored macromolecules by protein biochemists to study protein allostery, ligand-binding mechanisms and protein thermodynamics.
Multimodal properties partially explain why the same channel could be therapeutic targets for seemingly different diseases or channelopathies. For example, not only has the TRPV1 channel been a therapeutic target to treat pain, but also to atopic dermatitis, overactive bladder, and cluster headaches. Moreover, the hyperpolarization-activated cyclic nucleotide (HCN)-gated channel, sometimes referred to as “pacemaker channel,” is dually regulated by voltage and ligand. Congenital mutations of HCN channels lead to epilepsy, chronic pain and heart diseases such as cardiac arrhythmia.3 Recent research suggests that HCN2 channels expressed in small nociceptor neurons of the dorsal root ganglion (DRG) can be targeted for treating neuropathic pain.4
Nearly All Second Messengers Regulate Ion Channels
Our body requires signaling molecules, also known as messengers, to function properly. While ion channels respond to more than a thousand forms of first messengers such as extracellular neurotransmitters, odorants, hormones, and pheromones, only a few intracellular second messengers are commonly used in mammalian cells, resulting in a significant convergence in signal transduction. The second messengers utilized in mammalian cells include cyclic adenosine monophosphate (cAMP), cyclic guanosine monophosphate (cGMP), inositol 1,4,5-triphosphate (IP3), phosphoinositides, diacylglycerol (DAG), G-protein βγ subunits, and cationic Ca2+, Mg2+, and Na+ ions.
Medical drugs have been developed to manipulate second-messenger systems. For instance, phosphodiesterase (PDE) inhibitors such as sildenafil (Viagra) can increase the levels of cAMP or cGMP by inhibiting the breakdown of these second messengers by phosphodiesterase enzymes.5 Furthermore, almost all of the second messengers can regulate specific channels through direct binding. For example, cationic Ca2+ and Na+ ions bind to and potentiate Slowpoke family voltage-activated K+ channels, which are widely expressed in the nervous system.1,6 The increase in intracellular concentration of second messengers often requires rapid action. Ca2+ and Na+ can enter cells through cation channels such as voltage-gated Ca2+ channels, Na+ channels, or TRP channels, diffuse quickly, and reach their effectors within microseconds, perfectly meeting the requirements of fast signal transduction.
In contrast, other second messengers generally relay to their effectors in signaling cascades within seconds to minutes, which is slower than the cationic second messengers. In some cases, the level of second messengers decreases in the cell to deactivate downstream channels. For instance, decreasing the level of cGMP turns off cyclic nucleotide-gated (CNG) channels to achieve phototransduction in the retina, while decreasing signaling phosphoinositides (particularly the key lipid phosphatidylinositol 4,5-bisphosphate PI(4,5)P2) upon the activation of Gq/11-type G protein-coupled receptors (GPCRs) closes K+ and Ca2+ channels. From an evolutionary perspective, the “reduction of second messengers” strategy, using PDE or phospholipases (PLC), does not require direct consumption of ATP, making it more energy-efficient.
Mood disorders are often associated with disturbances in the second messenger system, particularly the phosphoinositide-based system. The “inositol depletion hypothesis” has been proposed to provide a mechanistic understanding of mood disorders, especially bipolar disorder and depression. This hypothesis is partly supported by the mood-stabilizing agent lithium, which specifically inhibits inositol recycling. Recent findings have further underscored the medical importance of inositol. For example, supplementing myo-inositols can increase cellular phosphoinositide levels, potentiate various types of K+ channels, and reduce the hyperexcitability of neurons.7 Inositol was not previously considered an essential nutrient as it can be synthesized from glucose. However, recent studies have shown that certain individuals, particularly those with insulin resistance and diabetes, may develop inositol deficiencies. This deficiency may be related to the connection between diabetes and mood disorders, commonly referred to as “diabetes mood swings.” For pancreatic β-cells under insulin resistance, low cellular glucose levels may lead to low myo-inositol and phosphoinositide levels, which can suppress ATP-sensitive potassium (KATP) channels,8 leading to a vicious cycle of high insulin secretion. Inositol may have broad health benefits beyond its currently accepted role in treating polycystic ovary syndrome, a common comorbidity of insulin resistance. Further research is needed to establish the connection between phosphoinositide-based second messengers, ion channels, and mood disorders.
Fast and Slow G Protein-Dependent Signaling Pathways Regulate Ion Channels
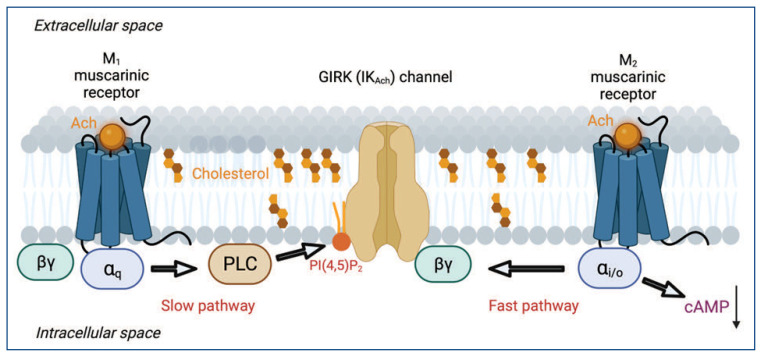
When compared to the immediate effects on ion channels caused by voltage, temperature, and mechanical force, or by ligand binding to ionotropic receptors, GPCR-and G protein-mediated signaling pathways to channels are generally slower. The channel conformational change is typically the only rate-limiting factor for these immediate effects. The G protein-dependent signaling has significant interest to medical researchers since GPCR is the largest family of proteins that are targeted by FDA-approved therapeutic drugs. Among the G protein-dependent pathways, the relatively fast pathway is mediated by the G-protein βγ subunits released by Gi/o-coupled GPCR (i being inhibiting adenylyl cyclases) in a membrane-delimited and pertussis toxin-sensitive manner9,10 (Figure 3). The type of channel activated by βγ subunits is called the G-protein activated inwardly rectifying K+ channel, GIRK10. It only takes one to five seconds from the application of GPCR agonist to observe a peak of GIRK current in live-cell in-vitro systems;7 this process can be as fast as a few hundred milliseconds. GIRK channel is also called IKAch channel because vagal nerve stimulation releases acetylcholine (Ach) to reduce heart rate by activating IKAch. It was thought that this fast diffusion-limited signaling is partially due to the overabundance of Gαi/o subunits relative to other Gα subunits. The GIRK channel is not alone; Gβγ subunits regulate voltage-gated Ca2+ channels and TRPM3 channels via a similar mechanism.11,12
Figure 3.
Fast and slow G protein-dependent signaling pathways for modulating ion channels. Activation of the Gi/o-coupled M2 muscarinic acetylcholine receptors releases the G protein βγ subunits, which activates GIRK channels. On the other hand, activation of the Gq-coupled M1 muscarinic acetylcholine receptors activates PLC, which hydrolyzes membrane lipid PI(4,5)P2, an essential activator of GIRK channels.
Scientists have also discovered a slower G protein-dependent signaling pathway in the sympathetic neurons of the superior cervical ganglion (SCG) that helps our bodies deal with stress. This pathway is mediated by the activation of Gq/11-coupled GPCR and the subsequent recruitment of phospholipase C to hydrolyze PI(4,5)P2 (Figure 3). It is pertussis toxin insensitive and is about five-fold slower than the Gβγ subunit-mediated pathway in vitro.7,13 During this process, IP3 and DAG are generated, both of which have downstream channels as their effectors. One physiological significance of the PLC/PI(4,5)P2 pathway, which has been a popular area of focus for the past two decades, is its role in regulating over 100 types of ion channels. These channels are directly controlled by PI(4,5)P2 as their ligand, and include HCN, TRP, inward rectifiers, and many other voltage-gated channels.13,14 In most cases, when PI(4,5)P2 is hydrolyzed, the channel activities subside.7 Furthermore, regarding the Gs-coupled GPCR pathway (e.g., β-adrenergic receptors, and s being stimulating adenylyl cyclases), cAMP levels are elevated with similar kinetics (~20 to 50 s), and the effect can be more sustainable. Importantly, the modulation of HCN pacemaker channels through cAMP is crucial for the sympathetic regulation of the heartbeat. Collectively, the varied kinetics of G protein-dependent regulation of ion channels ensures that the cellular excitability can be modulated with high spatiotemporal precision.
Mobilizing Local Ca2+ Is the Goal for Channel Signaling
Adequate calcium intake is essential for the proper functioning of muscles, nerves, and the heart. Because of the 20,000-fold concentration gradient of extracellular (2 mM) versus intracellular (resting 100 nM) Ca2+ levels, Ca2+ is ideally suited to be a sensitive and dynamic signaling messenger. Ca2+ is essential for achieving many crucial physiological functions, for example, the release of neurotransmitter vesicles at presynaptic terminals in the brain and the excitation-contraction coupling for cardiac and skeletal muscle contraction.1 Cells often use local Ca2+ release to achieve fast signaling. In other words, Ca2+ does not need to travel far to produce a signaling effect. Besides voltage-activated Ca2+ channels, nonselective cation channels like TRPV channels, and neuronal NMDA glutamate receptors serve the role of permitting Ca2+ entry into cells.1 In addition, IP3 receptors and ryanodine receptors (also known as the ionotropic receptor of caffeine) mobilize Ca2+ from the intracellular endoplasmic reticulum ER (and sarcoplasmic reticulum, SR) Ca2+ store. The release of Ca2+ from IP3 receptors upon the activation of Gq/11-type GPCRs coincide temporally with the hydrolysis of plasma membrane PI(4,5)P2 and the rise of IP3. Nevertheless, Ca2+ release from the ER could also exhibit prolonged oscillation due to feedback regulation of IP3 receptors by local Ca2+. Furthermore, immune cells, like cytotoxic T cells, do not use voltage-activated Ca2+ channels, but rather use Ca2+ release-activated Ca2+ (CRAC) channels15 identified as Orai channels to allow the influx of extracellular Ca2+. Orai activation is initiated by its direct interaction with the ER-residing protein STIM, which senses the low Ca2+ level in the ER store of calcium.15 Therefore, this process is also called the store-operated Ca2+ entry (SOCE) and happens with a timescale of ~ 50 seconds. Elevated cytosolic free Ca2+ ions are regulated by Ca2+-binding proteins like calmodulin and buffered by calbindin/parvalbumin in neurons. Ca2+ ions can also undergo the mitochondrial Ca2+ uptake quickly (less than 100 s) by mitochondrial calcium uniporter (MCU).1 Other intracellular Ca2+ stores such as endosomes and lysosomes can release local Ca2+ through TRPML and TRPM2 channels. Besides IP3, notable Ca2+-mobilizing second messengers include ADP-ribose (ADPR) and nicotinic acid adenine dinucleotide phosphate (NAADP). ADPR is an agonist of Ca2+-permeable TRPM2 channels whereas the ionotropic receptor of NAADP remains uncertain.
There are many diseases linked to Ca2+-permeable channels and imbalances in Ca2+ homeostasis (Table 1). Alzheimer’s and Parkinson’s disease, along with other neurodegenerative diseases, are believed to be connected to alterations in calcium signaling, even though the pathophysiological mechanisms are not yet well understood. The major element in the Alzheimer’s γ-secretase protein complex presenilin-1 was proposed as an ER Ca2+-leak channel, but it could instead function as a Ca2+-leak enhancer by alternative mechanisms. In general, Ca2+ signaling through glutamate receptors are important for synaptic plasticity, learning and memory. Furthermore, mutations in TRPML (ML being mucolipin) are associated with a type of neurodevelopmental disorder called mucolipidosis, whereas mutations in TRPM (M being melastatin) are associated with hypomagnesemia and melanoma. Moreover, mutations in genes encoding the Orai/STIM CRAC system are linked to autoimmune diseases, myopathy, and muscular hypotonia15.
Table 1.
Calcium-permeable ion channels and the related channelopathies
| Channel Type | Main Functions | Related Diseases |
|---|---|---|
| Voltage-activated Ca2+ channels | Presynaptic neurotransmitter release, excitation-contraction (EC) coupling in cardiac and skeletal muscles | Hypokalemic periodic paralysis, ataxia, migraine |
| TRP channels | Thermo-sensation, Ca2+ release from intracellular organelles | Chronic pain, episodic pain, mucolipidosis, melanoma, dermatitis, carcinoma, asthma, chronic obstructive pulmonary disease, ischemia |
| Cyclic-nucleotide gated channel | Retinal phototransduction | Retinitis pigmentosa, achromatopsia, macular degeneration |
| Orai (CRAC) channel | T-cell activation, SOCE | Immunodeficiency, myopathy, and muscular hypotonia |
| Mitochondrial calcium uniporter | Ca2+ uptake to mitochondria | Neurodegeneration, ataxia |
| IP3 receptor | ER/SR Ca2+ release | Neurodegeneration, taste perception deficit, heart failure and hypertension |
| Ryanodine receptor | ER/SR Ca2+ release, EC coupling | Malignant hyperthermia, central core disease |
| NMDA receptor | Ca2+ entry in synapse, postsynaptic potential, synaptic plasticity, learning and memory | Alzheimer’s disease, Parkinson’s disease, schizophrenia, epilepsy |
| Piezo channel | Mechano-sensation | Hereditary xerocytosis, lymphedema, osteoarthritis |
| CatSper channel | Ca2+ entry to sperm flagella | Male infertility |
Mechanical Force Activates Ion Channels
A new frontier in drug discovery involves targeting newly characterized channels that detect lipid membrane curvature and deformation caused by changes of cell shape. These channels, known as mechanosensitive channels, produce rapid and often transient currents when subjected to mechanical force applied to the cell membrane. The discovery of human mechanosensitive channels, referred to as “Piezo,” represents an exciting new avenue for investigating the mechanism underlying the dynamic impact of the lipid bilayer on ion channels.16 The opening of Piezo channels is directly coupled to a change in the membrane curvature caused by mechanical force, happening within a few milliseconds after a mechanical stimulus.17 These channels are not only necessary for touch sensation but also act as sensors for red blood cell volume, morphological changes of endothelial cells in blood vessels, pressure in pancreatic ducts, and stretching of the bladder wall. The physiological significance of these findings is profound, and many diseases caused by dysfunctional mechano-transduction are associated with mutations in Piezo genes. Furthermore, hair cells in the inner ear also use mechanical force through the tip-links between hair bundles to gate a type of channel called TMC (transmembrane channel-like protein), whose identity had been a persistent mystery in auditory research.18 The latest research suggests that TMC’s multiple auxiliary subunits, particularly TMIE, lipids, and lipid membrane thickness could all contribute to achieve the opening of TMC channels19. Besides Piezo and TMC, channels like the volume-activated chloride channel, TRPN and the K2P (TREK subtype) channel are other reported mechano-sensors.20
In addition, the dynamic heterogeneity of the lipid-bilayer membrane regulates ion channels. Particularly, membrane nanodomains (often less than 250 nm in size) enriched with sphingomyelin (SM) and cholesterol create membrane compartmentalization to gather molecules for effective signaling.21 These organized nanodomains, previously called “lipid rafts,” could rearrange and coalesce under certain pathophysiological conditions, e.g., inflammation, neuropathy and age-related neurodegeneration. Furthermore, drugs that target cholesterols such as statin could change these domains. In general, many more types of channels could be sensitive to membrane deformation and stretching, considering that transmembrane helices tend to rearrange differently depending on the membrane thickness and curvature. It remains elusive how the lipid disposition of membrane nanodomains as well as the line tension at the boundary of different lipid domains could affect the mechano-sensitivity of ion channels.
Blood Flow and Blood Coagulation Factors Regulate Ion Channels in the Brain
The blood flow in the brain is tightly coupled to neuronal activity, as it supplies oxygen and nutrients to neurons. This coupling, so-called “neurovascular coupling,” involves a variety of ion channels. One fast pathway is that extracellular K+ ions, released from astrocytes in response to neuronal activity, act like first messengers to dilate blood vessels (less than a minute) in the brain and increase cerebral blood flow.22 This effect is achieved by a swift rise in K+ levels, causing the removal of the blockage of inwardly rectifying Kir channels by intracellular magnesium (Mg2+) and polyamines.22 It is worth noting that Mg2+ also blocks NMDA glutamate receptors. Therefore, K+ and Mg2+ act as signaling ions that dynamically modulate cellular excitability in the brain. The potentiation of inwardly rectifying channels by K+, particularly in endothelium cells of blood vessels, provides a positive feedback mechanism to supply more blood to more active brain regions. Interestingly, because of the dependence of Kir channels on PI(4,5)P2, this effect is also sensitive to the Gq/11-coupled GPCR-mediated PI(4,5)P2 dynamics. Furthermore, molecules in the blood-coagulation system, particularly thrombin, regulate voltage-gated ion channels in the brain. Blood coagulation factors released from broken blood vessels (hemorrhage), often caused by stroke and brain trauma, could induce epileptic seizures.23 Similar to the potentiation of cardiac voltage-gated Na+ channels by thrombin24, neuronal Na+ channels are also potentiated by thrombin.23 These results suggest that thrombin, a protein that itself is allosterically activated by Na+ binding,25 can rapidly potentiate Na+ channels and cause epileptic seizures that are associated with intracerebral hemorrhage.23 The exact molecular mechanism underlying the thrombin potentiation of ion channels is uncertain. Essentially, the effect of blood supply to the brain can impact the excitability of neurons through ion-channel mechanisms, leading to significant physiological consequences.
Conclusion
Since Hodgkin and Huxley’s discovery of the separate Na+ and K+ conductance in nerve action potentials in the 1950s, the study of ion channels has advanced significantly. As of 2023, multiple high-resolution protein structures exist for nearly all channel subfamilies in various states. Ion channels are precisely organized and play a crucial role in dynamic signaling, which is often unique to the animal kingdom. Despite these advancements, significant questions remain regarding the signaling complexes formed by ion channels in native membranes, their conformational dynamics during physiological adaptation, allostery of the multimodal protein regulation, and how they interact with other molecules such as membrane lipids.
Acknowledgments
The author thanks Prof. Bertil Hille (University of Washington, Seattle) for comments on the manuscript and teaching the action potential simulation using Igor Pro (Wavemetrics), and Prof. Joel Eissenberg and Natalie L. Macchi (Saint Louis University) for editing the manuscript.
Footnotes
Gucan Dai, PhD (pictured), is in the Edward A. Doisy Department of Biochemistry & Molecular Biology, Saint Louis University School of Medicine, St. Louis, Missouri.
Disclosure
Figures were created using BioRender.
References
- 1.Hille B. Ion Channels of Excitable Membranes. 3rd edn. Sinauer Associates; 2001. [Google Scholar]
- 2.Chowdhury S, Jarecki BW, Chanda B. A molecular framework for temperature-dependent gating of ion channels. Cell. 2014;158:1148–1158. doi: 10.1016/j.cell.2014.07.026. https://doi.org:10.1016/j.cell.2014.07.026 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol. 2003;65:453–480. doi: 10.1146/annurev.physiol.65.092101.142734. https://doi.org:10.1146/annurev.physiol.65.092101.142734 . [DOI] [PubMed] [Google Scholar]
- 4.Emery EC, Young GT, Berrocoso EM, Chen L, McNaughton PA. HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science. 2011;333:1462–1466. doi: 10.1126/science.1206243. https://doi.org:10.1126/science.1206243 . [DOI] [PubMed] [Google Scholar]
- 5.Bobin P, et al. Cyclic nucleotide phosphodiesterases in heart and vessels: A therapeutic perspective. Arch Cardiovasc Dis. 2016;109:431–443. doi: 10.1016/j.acvd.2016.02.004. https://doi.org:10.1016/j.acvd.2016.02.004 . [DOI] [PubMed] [Google Scholar]
- 6.Butler A, Tsunoda S, McCobb DP, Wei A, Salkoff L. mSlo, a complex mouse gene encoding “maxi” calcium-activated potassium channels. Science. 1993;261:221–224. doi: 10.1126/science.7687074. https://doi.org:10.1126/science.7687074 . [DOI] [PubMed] [Google Scholar]
- 7.Dai G, Yu H, Kruse M, Traynor-Kaplan A, Hille B. Osmoregulatory inositol transporter SMIT1 modulates electrical activity by adjusting PI(4,5)P2 levels. Proc Natl Acad Sci U S A. 2016;113:E3290–3299. doi: 10.1073/pnas.1606348113. https://doi.org:10.1073/pnas.1606348113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashcroft FM. ATP-sensitive potassium channelopathies: focus on insulin secretion. J Clin Invest. 2005;115:2047–2058. doi: 10.1172/JCI25495. https://doi.org:10.1172/JCI25495 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfaffinger PJ, Martin JM, Hunter DD, Nathanson NM, Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985;317:536–538. doi: 10.1038/317536a0. https://doi.org:10.1038/317536a0 . [DOI] [PubMed] [Google Scholar]
- 10.Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The βγ subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987;325:321–326. doi: 10.1038/325321a0. https://doi.org:10.1038/325321a0 . [DOI] [PubMed] [Google Scholar]
- 11.Herlitze S, et al. Modulation of Ca2+ channels by G-protein βγ subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. https://doi.org:10.1038/380258a0 . [DOI] [PubMed] [Google Scholar]
- 12.Badheka D, et al. Inhibition of Transient Receptor Potential Melastatin 3 ion channels by G-protein βγ subunits. Elife. 2017;6 doi: 10.7554/eLife.26147. https://doi.org:10.7554/eLife.26147 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hille B, Dickson EJ, Kruse M, Vivas O, Suh BC. Phosphoinositides regulate ion channels. Biochim Biophys Acta. 18512015:844–856. doi: 10.1016/j.bbalip.2014.09.010. https://doi.org:10.1016/j.bbalip.2014.09.010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilgemann DW, Ball R. Regulation of cardiac Na+, Ca2+ exchange and KATP potassium channels by PIP2. Science. 1996;273:956–959. doi: 10.1126/science.273.5277.956. https://doi.org:10.1126/science.273.5277.956 . [DOI] [PubMed] [Google Scholar]
- 15.Lewis RS. Store-Operated Calcium Channels: From Function to Structure and Back Again. Cold Spring Harb Perspect Biol. 2020;12 doi: 10.1101/cshperspect.a035055. https://doi.org:10.1101/cshperspect.a035055 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coste B, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483:176–181. doi: 10.1038/nature10812. https://doi.org:10.1038/nature10812 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin YC, et al. Force-induced conformational changes in PIEZO1. Nature. 2019;573:230–234. doi: 10.1038/s41586-019-1499-2. https://doi.org:10.1038/s41586-019-1499-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan B, et al. TMC1 Forms the Pore of Mechanosensory Transduction Channels in Vertebrate Inner Ear Hair Cells. Neuron. 2018;99:736–753e736. doi: 10.1016/j.neuron.2018.07.033. https://doi.org:10.1016/j.neuron.2018.07.033 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong H, et al. Structures of the TMC-1 complex illuminate mechanosensory transduction. Nature. 610(2022):796–803. doi: 10.1038/s41586-022-05314-8. https://doi.org:10.1038/s41586-022-05314-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin P, Jan LY, Jan YN. Mechanosensitive Ion Channels: Structural Features Relevant to Mechanotransduction Mechanisms. Annu Rev Neurosci. 43(2020):207–229. doi: 10.1146/annurev-neuro-070918-050509. https://doi.org:10.1146/annurevneuro-070918-050509 . [DOI] [PubMed] [Google Scholar]
- 21.Hilgemann DW, et al. Lipid signaling to membrane proteins: From second messengers to membrane domains and adapter-free endocytosis. J Gen Physiol. 2018;150:211–224. doi: 10.1085/jgp.201711875. https://doi.org:10.1085/jgp.201711875 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longden TA, Hill-Eubanks DC, Nelson MT. Ion channel networks in the control of cerebral blood flow. J Cereb Blood Flow Metab. 2016;36:492–512. doi: 10.1177/0271678X15616138. https://doi.org:10.1177/0271678X15616138 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isaeva E, Hernan A, Isaev D, Holmes GL. Thrombin facilitates seizures through activation of persistent sodium current. Ann Neurol. 2012;72:192–198. doi: 10.1002/ana.23587. https://doi.org:10.1002/ana.23587 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinet C, Le Grand B, John GW, Coulombe A. Thrombin facilitation of voltage-gated sodium channel activation in human cardiomyocytes: implications for ischemic sodium loading. Circulation. 2002;106:2098–2103. doi: 10.1161/01.cir.0000034510.64828.96. https://doi.org:10.1161/01.cir.0000034510.64828.96 . [DOI] [PubMed] [Google Scholar]
- 25.Cera Di, Thrombin E : a paradigm for enzymes allosterically activated by monovalent cations. C R Biol. 2004;327:1065–1076. doi: 10.1016/j.crvi.2004.07.011. https://doi.org:10.1016/j.crvi.2004.07.011 . [DOI] [PubMed] [Google Scholar]