Abstract
Dietary fiber (DF) is an essential, albeit under-consumed, component in the North American diet. DF is thought to have anti-inflammatory disease-modifying effects via DF-related gut microbiota degradation products called short chain fatty acids. Thus far studies have shown the greatest associations between DF intake and risk reduction in obesity, improved weight loss outcomes, and risk reduction of cardiovascular disease (CVD). There is weak evidence associating DF intake and inflammatory bowel disease (IBD) risk, IBD remission, reduced risk of Crohn’s disease (CD flares, and no evidence showing any benefit towards ulcerative colitis (UC) specifically. Evidence on DF intake and the risk reduction of colorectal cancer (CRC) has been equivocal. Studies were limited by a lack of randomization or in controlling fiber types and sources. Based on the current beneficial associations of DF on obesity management and CVD, counseling patients to increase DF intake may be a cost-effective measure to decrease the burden of chronic disease.
Introduction
Dietary fiber (DF) is an essential part of a healthy human diet. It is associated with diversification of gut microbiota and is thought to play a role in various pathological conditions.1 The average American DF intake ranges between 11.8g–19.8g, while the daily intake recommendation is 25–35grams. The gut microbiome plays an important role in degrading dietary fiber into short-chain fatty acids (SCFAs) which have several anti-inflammatory effects associated with modifying various chronic diseases. Many studies have shown an association between increased DF intake and reduced risk of cardiovascular disease and obesity. There is also some weak evidence indicating that DF intake is associated with reduced risk of development of inflammatory bowel disease and the risk of Crohn’s disease flares. This review aims to discuss the current understanding of DF and the role it may play in obesity, cardio-vascular disease (CVD), colorectal cancer (CRC), and inflammatory bowel disease (IBD).
Classification
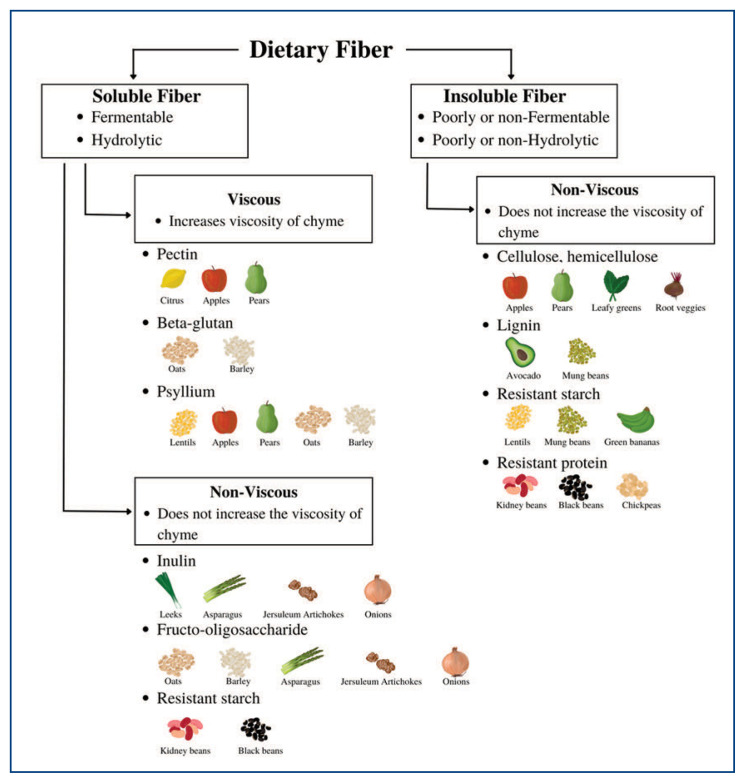
There are numerous definitions of DF based on factors such as the size of the carbohydrate polymer, solubility, and the location of fermentation in the gut. According to the Codex Alimentarius Commission, which is a collection of internationally adopted food standards, DF includes carbohydrate polymers with ten or more monomeric units that are neither digested nor absorbed by the endogenous enzymes of the small intestine of the human gastrointestinal system.2 The definition of the United States Food and Drug Administration (FDA) differs slightly and also includes carbohydrates of three to nine units.3 DF can also be classified based on factors such as their primary food source, chemical structure, water solubility, viscosity, and fermentability. Commonly, based on its water solubility, DF is classified as insoluble fiber (IF) or soluble fiber (SF; Figure 1).4 The solubility of fiber influences its physiological role as described further below.
Figure 1.
Classifications of DF types and their sources. Fibers can be classified further based on the ability to change the viscosity of chyme: the partially digested food materials and digestive secretions during digestion. Note that “resistant” refers to products that can resist breakdown during digestion.
Main Food Sources of Dietary Fiber
Edible fiber includes fiber naturally found in food, as well as those synthetically or enzymatically produced. Most edible DF is found in natural sources. Both SF and IF can be found in legumes, vegetables, nuts/seeds, fruits, and synthetically in cereals (Figure 1).5 Insoluble fiber can bypass digestion in the small intestine and are fermented by specialized microorganisms. They are usually found in cereals, legumes, ripe fruits, and green bananas (Figure 1).5
Current Fiber Recommendations and Intake
The FDA recommends 28g of dietary fiber per day for a 2000 kcal/day diet.6 The American Dietetic Association recommends 14g/1,000 kcal or 25g for adult women and 38g for adult men.7 These recommendations are based on studies showing associations with the protective effects of DF against cardiovascular disease.7 Recommendations vary in children and the elderly based on age. Infants below the age of six months who are exclusively breastfed do not require additional DF intake. Adequate data is not available for infants from the age of 7–12 months. After the age of two years, adequate DF intake is equal to or greater than their age, plus 5g/day. In the elderly, lower values of 14g/1000 kcal are recommended.7
The average daily DF intake in the US is lower than recommended. According to the National Health and Nutrition Examination Survey’s most recent 2017–2018 report on DF intake by Americans, the fiber intake in males ranges from 12.4–19.8g on average and lower in females from 11.8–16g.8 This reported intake is several grams lower than the recommended daily intake. In comparison, the European Food Safety Authorization reports an average daily DF intake of 16–29g for an adult.9 This is a notable increase in the average consumption of fiber relative to the US.
Normal Digestive Physiology and Pathology
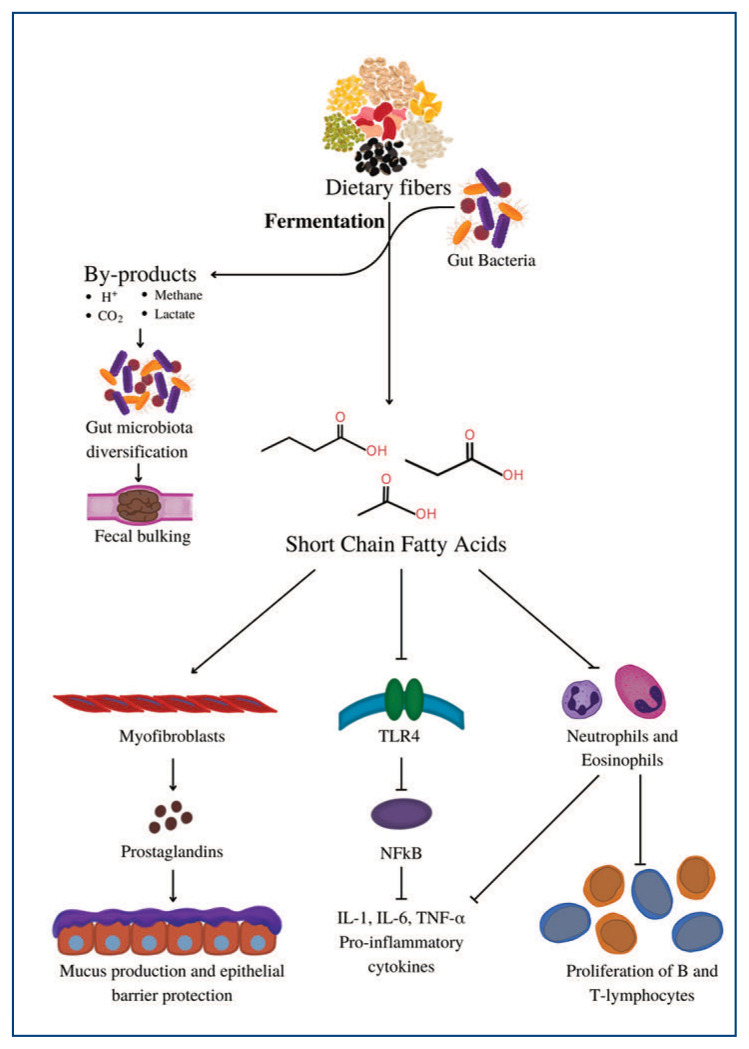
The digestive physiology of DF is dependent on the fiber type, location within the small and large intestines, and specific gut microbiota. DF, while thought to be resistant to absorption, does indeed undergo some chemical and structural changes in the gastrointestinal system, such as hydrolysis. Solubility has been shown to have an association with hydrolysis, with increasing resistance to hydrolysis seen with decreasing water solubility.1 IF is resistant to hydrolysis in the small intestine due to its hydrophobic and crystalline structure formed by hydrogen bonds. This results in a bulking effect, as it can bypass the small intestine with minimal breakdown.1 DF that is resistant to breakdown in the small intestine undergoes fermentation by the gut microbiome of the large intestine. The by-products produced through fermentation provide energy that is required by gut microbes to survive and grow.5 This plays a vital role in the diversification of the gut microbiota and further aids in fecal bulking.1 Some studies indicate that the effect of DF on the diversification of gut microbiota may play a role in disease development and progression.10,11
DF undergoes anaerobic fermentation by the gut microbiome to be degraded into products known as short-chain fatty acids (SCFAs) including butyrate, propionic acid, and acetic acid. SCFAs have several effects including anti-inflammatory actions through the inhibition of cytokines, activation of dendritic cells that are involved in anti-viral mechanisms, epithelial barrier protection, boosting IgA secretions, and mucus production.10, 11 The microbes of the gut play a crucial role in the metabolism of DF, which in turn likely contribute to their associated protective role in chronic inflammatory conditions (Figure 2).10, 11 We further describe the role of dietary fiber in chronic disease below.
Figure 2.
DF fermentation byproducts, protons (H+), carbon dioxide (CO2), methane, and lactate, aid in the diversification of gut microbiota resulting in increased fecal bulking. SCFAs act on myofibroblasts to produce prostaglandins that produce mucus membranes and provide epithelial barrier protection. Inhibition of toll-like receptor 4 (TLR4) downregulates nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) to attenuate the production of proinflammatory cytokines such as interleukin-1(IL-1), IL-6, and tumor necrosis factor-alpha (TNF-α). Inhibition of the activation of neutrophils and eosinophils prevents further cytokine activation and proliferation of B and T cells.
Obesity
Obesity has become a pandemic worldwide due to the increasing availability of calorie-dense food and simple carbohydrates. There is a growing body of research showing the association between DF and obesity. Existing literature suggests a protective effect of high DF intake on ideal body weight through the generation of SCFAs.12 Some hypothesized mechanisms to explain the obesity modulating effects of SCFA include that they provide extra sources of energy, suppress insulin-mediated fat accumulation via G-protein coupled receptor-43 expressed in the intestinal epithelium, immune cells, and adipocytes, regulate fasting-induced adipose factor, and regulate leptin secretion via free fatty acid receptor 3.13, 14, 15
In a 12-week, single-center, double-blind, placebo-controlled trial, 53 adults who had overweight or obesity were randomly assigned to a pea fiber (PF) or a control diet.16 Pea fiber intake significantly altered the types of fecal SCFAs and bile acid (BA) levels. The PF group was found to have higher acetate levels and reduced isovalerate, cholate, deoxycholate, and total BA content. Although the exact mechanism of action and role of these metabolite changes are unclear, these SCFAs and BAs may be involved in obesity by acting on signaling pathways, gut epithelium barrier function, and inhibition of proinflammatory cytokine production.16 The correlation of PF consumption, and DF in general, with alterations in the gut microbe metabolites may be a possible mechanism involved in the obesity modulating effects of DF.
Several studies have examined the role of DF on weight management outcomes. A prospective study with a sample size of 120,877 non-obese individuals observed an inverse correlation between long-term weight gain and intake of DF.17 In another study, children between the ages of 7–12 years old with overweight or obesity were randomly assigned to receive either oligofructose-enriched inulin or a maltodextrin placebo once daily for 16 weeks. Oligofructose enriched inulin is a mixture of non-digestible carbohydrates, containing oligosaccharide and inulin, both of which are fermentable plant fructan DF. Reduced fat mass was observed in the inulin group, suggesting that increased intake of DF may have beneficial effects on obesity.18 More recently, the RESOLVE study initiated a two-phase experimental protocol to examine the role of lifestyle modifications on weight loss outcomes. Phase one consisted of a three-week intervention in diet, exercise, and education under a controlled environment with close supervision. This was followed by a second phase wherein participants maintained these lifestyle changes under distant supervision for 12 months. It was found that fiber intake was the only nutritional variable that systematically predicted a significant reduction in health indicators like weight, central fat, and body mass index (BMI).19
A limitation of the RESOLVE study was the second phase of distant supervision, which was not in a controlled setting. The above studies were also limited by varying DF sources and types, limiting generalizability. For example, some studies have examined individual fiber types, such as inulin, 18 others with a pea fiber source,16 and the RESOLVE study examined generalized DF content. 19 With the plethora of the types and sources of DF, it is unclear whether certain DF types or sources have the same obesity modulating effects.
From the above limited studies, DF intake has been associated with reduced obesity and improved weight loss outcomes. Gut microbe-related DF degradation products, such as SCFAs and BAs are thought to be the physiological drivers in these obesity modulating effects. Further studies that include randomization are required to further elucidate whether individual fiber types or sources play an obesity modulating role.
Cardio-Vascular Disease
Coronary artery disease (CAD) is the leading cause of mortality worldwide. Its etiology is a culmination of factors involving lifestyle, environment, and genetics. Diet has been widely regarded as a modifiable risk factor for the prevention of CVD. A high fiber intake may modify risk factors that can contribute to atherosclerotic disease including dyslipidemia, obesity, hypertension, and diabetes.20,21 Current evidence suggests an inverse relationship between CVD and intake of DF. The Pooling Project of Cohort Studies on Diet and Coronary Disease pooled data across 10 prospective cohort studies. This study found a significant inverse relationship between DF intake and the risk of CAD.22 There was a dose-response relationship for every 10g/day increase in DF intake, with a 14% reduction in risk of all coronary events, and a 27% reduction in the risk of coronary mortality.22 This association was independent of age, sex, CVD risk factors such as BMI and hypertension, lifestyle factors such as smoking and physical activity, and other dietary factors such as energy and fat intake.22 Another meta-analysis analyzed the effects of 10g/day increment increases of DF and found a pooled relative risk of 0.91 for CVD.23 A meta-analysis of 19 studies indicated that an increased intake of total fiber, IF, and fiber from cereals, vegetables, and fruits lowered the risk of CVD and coronary heart disease (CHD).24 There was a reduction of risk with each increase of 7g/day in total fiber intake, with relative risks of 0.91 for both CVD and CHD.24
There are several limitations to the studies associating DF to CVD risk. Given that intake of DF was not randomized, causality is difficult to establish. The definition of DF and the definition and measurement of specific fiber sources such as cereal fiber, vegetable fiber, and whole grains were not standardized. For example, Pereira et al. found significant associations between cereal and fruit DF intake in reducing CVD risk, however non-significant findings were associated with vegetable fiber intake.22 Further studies controlling for fiber type and source may be warranted. Many of the studies also failed to control other dietary variables including vitamins, minerals, and beta-carotene. Considering there is some evidence for the contribution of these nutrients in decreasing CVD risk, failing to control for them can potentially impact findings.25 Finally, the influence of socioeconomic factors remains unclear as most studies were in a predominantly Caucasian population in the US and Europe. Given that the food trends and sources of fiber intake are different in different ethnicities and races, the relationship needs to be adjusted for these factors as well.25
From the above studies, DF intake has been associated with a reduction in the risk of CVD. Currently, the consensus on the amount and type of fiber has yet to be reached, but as described above, an increase in 7–10g/day was associated with a reduction in CVD risk. These studies are limited by a lack of randomization, control for DF types and sources, control of various socioeconomic factors, and control for other dietary nutrients that impact CVD. Further studies are required to better elucidate the long-term effects of DF on CVD risk.
Colorectal Cancer
Colorectal cancer (CRC) is one of the leading causes of cancer deaths in the world. The recent intense screening measures have reduced the incidence, but CRC remains the third leading cause of cancer in men and women.26 Understanding the preventative factors of CRC may help to reduce its disease burden. Some components of DF such as wheat bran and phytic acid (inositol hexaphosphate) were found to have chemo-preventive properties against colon carcinogenesis.27
The evidence on the protective effects of DF intake on CRC has been inconsistent due to evidence being mostly observational. Case-control studies were generally affirmative but most observational cohort studies challenged an association of DF to CRC. 28, 29 In a large prospective study, the NIH-AARP Diet and Health Study, analysis of half a million adults for five years found no association between total DF intake and CRC. However, an inverse association was observed between the intake of fiber from grains and CRC.26 Another study conducted with a 15-year follow-up period showed a significant decrease in the incidence of CRC with the intake of whole wheat.30 A similar association was not seen when looking at the incidence of CRC with overall increased DF intake.30 Current studies investigating the role of DF in the development of CRC have been equivocal, and further studies are required to elucidate whether DF has long-term effects in decreasing the risk for CRC.
Inflammatory Bowel Disease
The incidence of IBD has been increasing over recent decades.31 IBD is comprised of CD and ulcerative colitis (UC). Currently, the mainstay treatments for patients with IBD have been biologics to arrest progression and maintain remission. Modifiable factors such as DF intake have been investigated to prevent the development and progression of IBD. The potential role of DF lies in the production of SCFAs. SCFAs such as butyrate has been shown to regulate cytokines involved in intestinal inflammation such as IL-10, IFNγ, and IL-1β.32, 33 In a study comparing women in the highest quantile of fiber intake and women in the lowest quantile of fiber intake, a 40% decrease in risk of CD development was found.33 The benefit was highest with fiber derived from fruits. However, a significant association was not seen in the risk of UC development. Similarly, another study found DF to be protective against CD.34 Data from a systemic review showed that adults with IBD consume significantly less DF compared to healthy adults, and the presence of an inverse association between DF intake and the risk of CD.31
Beyond the risk of developing IBD, studies have also shown a decreased risk of CD flares with fiber intake.35 A small study found a lower rate of treatment failure for the psyllium plus mesalamine group versus only mesalamine (28% versus 35%).36 This association was not seen with UC.
While these studies show some beneficial association between high fiber intake and IBD, the impact on causality is yet to be understood. Significant associations between DF were only shown with CD, whereas no associations with UC were found. The effects on IBD remission have been varied and require further investigation.
Clinical Strategies to Improve Dietary Fiber Intake
The intake of DF is limited in the average Western diet. In a clinical setting, a comprehensive approach toward disease management includes counseling patients on dietary modification, an important part of preventative medicine (Table 1). Advising patients to increase overall DF intake is an easy, cost-effective, and low-harm measure, and may aid in the holistic management of obesity, CVD, and other chronic diseases. Counselling should include investigating the dietary components of a patient’s average meal. Resources regarding fiber-rich food via print materials, links to websites, along with resources on the beneficial effects of DF can be provided to patients. Dietary Guidelines for Americans have information about food sources that are rich in DF along with recommended portion sizes, and the information is detailed yet simple to understand.37 Simple behavioral measures such as replacing processed food snacks with small servings of fruits, eating vegetables or salads, and reading food labels may be beneficial.
Table 1.
Practical Approach to Counselling on Dietary Fiber Intake
|
Conclusion
Current studies have shown the greatest associations between DF intake and risk reduction in obesity and CVD. There is some weak evidence through observational studies indicating that DF intake is associated with a reduced risk of development of IBD and the risk of CD flares. Associations with CRC were equivocal. See Table 2 for a summary of the studies noted in this review. Based on the current findings associating DF intake with reduced risk for obesity and CAD, counselling patients and providing patients with educational resources to increase DF intake may provide long-term benefits. Studies were limited by a lack of randomization or in controlling fiber types and sources. Further investigation is needed into understanding the role of DF in the development of chronic diseases and their implication for treatment.
Table 2.
Summary of the Studies Noted in This Review
| Study | Study design | Results |
|---|---|---|
| Obesity | ||
| Kim et al. 12 | Meta-analysis of 21 randomized control trials, with a median intervention duration of 6 weeks. |
|
| Mozaffarian et al.17 | Prospective trial with 120,877 healthy individuals followed over four years |
|
| Nicolucci et al. 18 | Single-center, double-blind, placebo-controlled trial including children 7–12 years old with overweight or obesity |
|
| RESOLVE 19 | Randomized control trial with 87 participants 3-week intensive diet–exercise intervention |
|
| Cardio-vascular disease | ||
| Pereira et al. 22 | Pooled cohort analysis 10 prospective cohort studies with a 6–10 year follow-up |
|
| Kim et al. 25 | Meta-analysis of 15 studies |
|
| Threapleton et al. 24 | Meta-analysis of 22 cohort studies |
|
| Colorectal cancer | ||
| NIH-AARP Diet and Health Study 26 | Prospective trial including 291,988 men and 197,623 women aged 50–71 years old. |
|
| NIH-AARP Diet and Health Study 30 | Same as above |
|
| Inflammatory Bowel Disease | ||
| Ananthakrishnan et al. 33 | Prospective study 170,776 women, followed up over 26 years |
|
| Amre et al. 34 | Case control study of 130 CD patients and 202 controls |
|
| Hou et al. 31 | Systematic review 19 studies, 2609 IBD patients |
|
| Brotherton et al. 35 | Longitudinal internet-based cohort of more than 14,000 participants with IBD |
|
CAD= Coronary Artery Disease; CD= Crohn’s Disease; CRC= Colorectal carcinoma; CVD= Coronary Vascular Disease; DF= Dietary fiber; IBD= Inflammatory Bowel Disease; UC= Ulcerative Colitis
Footnotes
Ifrah Fatima, MD, Internal Medicine Resident, University of Missouri-Kansas City, Kansas City, Missouri (UMKC-KCMO). Imali Gamage, Medical Student, Saba University School of Medicine, Caribbean Netherlands. Reuben Joaquim Ricardo De Almeida, MD, (pictured), Internal Medicine Resident, UMKC-KCMO. Geetha Kamath, MD, is Assistant Professor, Department of Internal Medicine, University Health Weight Management Clinic, UMKC-KCMO. Peminda Cabandugama, MD, is Assistant Professor, Cleveland Clinic, Cleveland, Ohio.
Disclosure
None reported.
References
- 1.Capuano E. The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Critical Reviews in Food Science and Nutrition. 2017;16:3543–3564. doi: 10.1080/10408398.2016.1180501. [DOI] [PubMed] [Google Scholar]
- 2.Jones JM. CODEX-aligned dietary fiber definitions help to bridge the ‘fiber gap.’. Nutrition Journal. 2014. p. 1. [DOI] [PMC free article] [PubMed]
- 3.Questions and Answers on Dietary Fiber | FDA. U.S. Food and Drug Administration; FDA; n.d. Retrieved April 17, 2022 from https://www.fda.gov/food/food-labeling-nutrition/questions-and-answers-dietary-fiber#define_dietary_fiber. [Google Scholar]
- 4.Deehan EC, Duar RM, Armet AM, Perez-Muñoz ME, Jin M, Walter J. Modulation of the Gastrointestinal Microbiome with Nondigestible Fermentable Carbohydrates To Improve Human Health. Microbiology Spectrum. 2017:5. doi: 10.1128/microbiolspec.BAD-0019-2017. [DOI] [PubMed] [Google Scholar]
- 5.Stephen AM, Champ MM-J, Cloran SJ, Fleith M, van Lieshout L, Mejborn H, Burley VJ. Dietary fibre in Europe: current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutrition Research Reviews. 2017;2:149–190. doi: 10.1017/s095442241700004x. [DOI] [PubMed] [Google Scholar]
- 6.Korczak Renee, Slavin Joanne L. Nutrition Reviews. Supplement_1. Oxford University Press (OUP); Jul, 2020. Definitions, Regulations, and New Frontiers for Dietary Fiber and Whole Grains; pp. 6–12. Crossref. [DOI] [PubMed] [Google Scholar]
- 7.Slavin Joanne. Journal of the American Dietetic Association no 10. Elsevier BV; Oct., 2008. Position of the American Dietetic Association: Health Implications of Dietary Fiber; pp. 1716–31. Crossref. [DOI] [PubMed] [Google Scholar]
- 8.US Department of Agriculture, Agricultural Research Service. Nutrient Intakes from Food and Beverages: Mean Amounts Consumed per Individual, by Gender and Age, What We Eat in America, NHANES 2017–2018 2020 [Google Scholar]
- 9.Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre. EFSA Journal. 2010. p. 3. [DOI]
- 10.Chénard T, Prévost K, Dubé J, Massé E. Immune System Modulations by Products of the Gut. Microbiota Vaccines. 2020;3:461. doi: 10.3390/vaccines8030461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shenoy S. Gut microbiome, Vitamin D, ACE2 interactions are critical factors in immune-senescence and inflammaging: key for vaccine response and severity of COVID-19 infection. Inflammation Research. 2021;1:13–26. doi: 10.1007/s00011-021-01510-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SJ, de Souza RJ, Choo VL, Ha V, Cozma AI, Chiavaroli L, Mirrahimi A, Blanco Mejia S, Di Buono M, Bernstein AM, Leiter LA, Kris-Etherton PM, Vuksan V, Beyene J, Kendall CW, Jenkins DJ, Sievenpiper JL. Effects of dietary pulse consumption on body weight: a systematic review and meta-analysis of randomized controlled trials. The American Journal of Clinical Nutrition. 2016a;5:1213–1223. doi: 10.3945/ajcn.115.124677. [DOI] [PubMed] [Google Scholar]
- 13.Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, Takahashi T, Miyauchi S, Shioi G, Inoue H, Tsujimoto G. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nature Communications. 2013;1 doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary Gut Microbial Metabolites, Short-chain Fatty Acids, and Host Metabolic Regulation. Nutrients. 2015;4:2839–2849. doi: 10.3390/nu7042839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaibi MS, Stocker CJ, O’Dowd J, Davies A, Bellahcene M, Cawthorne MA, Brown AJH, Smith DM, Arch JRS. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Letters. 2010;11:2381–2386. doi: 10.1016/j.febslet.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 16.Mayengbam S, Lambert JE, Parnell JA, Tunnicliffe JM, Nicolucci AC, Han J, Sturzenegger T, Shearer J, Mickiewicz B, Vogel HJ, Madsen KL, Reimer RA. Impact of dietary fiber supplementation on modulating microbiota–host–metabolic axes in obesity. The Journal of Nutritional Biochemistry. 2019:228–236. doi: 10.1016/j.jnutbio.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in Diet and Lifestyle and Long-Term Weight Gain in Women and Men. New England Journal of Medicine. 2011;25:2392–2404. doi: 10.1056/nejmoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicolucci AC, Hume MP, Martínez I, Mayengbam S, Walter J, Reimer RA. Prebiotics Reduce Body Fat and Alter Intestinal Microbiota in Children Who Are Overweight or with Obesity. Gastroenterology. 2017;3:711–722. doi: 10.1053/j.gastro.2017.05.055. [DOI] [PubMed] [Google Scholar]
- 19.Tremblay A, Clinchamps M, Pereira B, Courteix D, Lesourd B, Chapier R, Obert P, Vinet A, Walther G, Chaplais E, Bagheri R, Baker JS, Thivel D, Drapeau V, Dutheil F. Dietary Fibres and the Management of Obesity and Metabolic Syndrome: The RESOLVE Study. Nutrients. 2020;10:2911. doi: 10.3390/nu12102911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lairon D, Arnault N, Bertrais S, Planells R, Clero E, Hercberg S, Boutron-Ruault M-C. Dietary fiber intake and risk factors for cardiovascular disease in French adults. The American Journal of Clinical Nutrition. 2005;6:1185–1194. doi: 10.1093/ajcn/82.6.1185. [DOI] [PubMed] [Google Scholar]
- 21.Soliman GA. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients. 2019;5:1155. doi: 10.3390/nu11051155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pereira MA, O’Reilly E, Augustsson K, Fraser GE, Goldbourt U, Heitmann BL, Hallmans G, Knekt P, Liu S, Pietinen P, Spiegelman D, Stevens J, Virtamo J, Willett WC, Ascherio A. Dietary Fiber and Risk of Coronary Heart Disease Archives of Internal Medicine. 2004;4:370. doi: 10.1001/archinte.164.4.370. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y, Je Y. Dietary fibre intake and mortality from cardiovascular disease and all cancers: A meta-analysis of prospective cohort studies. Archives of Cardiovascular Diseases. 2016;1:39–54. doi: 10.1016/j.acvd.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Threapleton DE, Greenwood DC, Evans CEL, Cleghorn CL, Nykjaer C, Woodhead C, Cade JE, Gale CP, Burley VJ. Dietary fibre intake and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2013 dec 19;2:f6879–f6879. doi: 10.1136/bmj.f6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satija A, Hu FB. Cardiovascular Benefits of Dietary Fiber Current Atherosclerosis Reports. 2012;6:505–514. doi: 10.1007/s11883-012-0275-7. [DOI] [PubMed] [Google Scholar]
- 26.Schatzkin A, Mouw T, Park Y, Subar AF, Kipnis V, Hollenbeck A, Leitzmann MF, Thompson FE. Dietary fiber and whole-grain consumption in relation to colorectal cancer in the NIH-AARP Diet and Health Study. The American Journal of Clinical Nutrition. 2007;5:1353–1360. doi: 10.1093/ajcn/85.5.1353. [DOI] [PubMed] [Google Scholar]
- 27.Reddy BS. Prevention of colon carcinogenesis by components of dietary fiber. Anticancer research. 1999;19(5A):3681–3683. [PubMed] [Google Scholar]
- 28.Howe GR, Benito E, Castelleto R, Cornée J, Estève J, Gallagher RP, Iscovich JM, Deng-ao J, Kaaks R, Kune GA, Kune S, L’Abbé KA, Lee HP, Lee M, Miller AB, Peters RK, Potter JD, Riboli E, Slattery ML, Shu Z. Dietary Intake of Fiber and Decreased Risk of Cancers of the Colon and Rectum: Evidence From the Combined Analysis of 13 Case-Control Studies. JNCI: Journal of the National Cancer Institute. 1992;24:1887–1896. doi: 10.1093/jnci/84.24.1887. [DOI] [PubMed] [Google Scholar]
- 29.Bazyk AE, Olson JE, Sellers TA, Kushi LH, Anderson KE, Lazovich D, Folsom AR, Bostick RM. Diet and risk of colon cancer in a large prospective study of older women: an analysis stratified on family history (Iowa, United States) Cancer Causes and Control. 1998;4:357–367. doi: 10.1023/a:1008886715597. [DOI] [PubMed] [Google Scholar]
- 30.Hullings AG, Sinha R, Liao LM, Freedman ND, Graubard BI, Loftfield E. Whole grain and dietary fiber intake and risk of colorectal cancer in the NIH-AARP Diet and Health Study cohort. The American Journal of Clinical Nutrition. 2020;3:603–612. doi: 10.1093/ajcn/nqaa161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hou JK, Abraham B, El-Serag H. Dietary Intake and Risk of Developing Inflammatory Bowel Disease: A Systematic Review of the Literature. American Journal of Gastroenterology. 2011;4:563–573. doi: 10.1038/ajg.2011.44. [DOI] [PubMed] [Google Scholar]
- 32.Asarat M, Apostolopoulos V, Vasiljevic T, Donkor O. Short-Chain Fatty Acids Regulate Cytokines and Th17/Treg Cells in Human Peripheral Blood Mononuclear Cellsin vitro. Immunological Investigations. 2016;3:205–222. doi: 10.3109/08820139.2015.1122613. [DOI] [PubMed] [Google Scholar]
- 33.Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Korzenik JR, Fuchs CS, Willett WC, Richter JM, Chan AT. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology. 2013;145(5):970–977. doi: 10.1053/j.gastro.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amre DK, D’Souza S, Morgan K, Seidman G, Lambrette P, Grimard G, Israel D, Mack D, Ghadirian P, Deslandres C, Chotard V, Budai B, Law L, Levy E, Seidman EG. Imbalances in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for Crohn’s disease in children. The American journal of gastroenterology. 2007;102(9):2016–2025. doi: 10.1111/j.1572-0241.2007.01411.x. [DOI] [PubMed] [Google Scholar]
- 35.Brotherton CS, Martin CA, Long MD, Kappelman MD, Sandler RS. Avoidance of Fiber Is Associated With Greater Risk of Crohn’s Disease Flare in a 6-Month Period. Clinical Gastroenterology and Hepatology. 2016;8:1130–1136. doi: 10.1016/j.cgh.2015.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Copaci I, et al. Digestive and Liver Disease. Elsevier BV; May, 2000. Maintenance of Remission of Ulcerative Colitis (UC): Mesalamine, Dietary Fiber, S Boulardi; p. A24. Crossref. [DOI] [Google Scholar]
- 37.Food Sources of Dietary Fiber | Dietary Guidelines for Americans. Home | Dietary Guidelines for Americans. [Accessed 4 Aug. 2022.]. https://www.dietaryguidelines.gov/resources/2020-2025-dietary-guidelines-online-materials/food-sources-select-nutrients/food-0 .