Abstract
Since its inception in 1985, robotic surgery has evolved into a mainstream surgical approach that has become virtually synonymous with minimally invasive surgery (MIS) and adopted across several specialties offering decreased patient morbidity and improved post-operative outcomes. This article discusses the current role of robotics in MIS and its varied applications, prevalence in the community and the future of the field.
Background
Advancements in surgery tend to occur within one of several broad categories – cost reduction, improved patient satisfaction, and improvement in clinical outcomes. Historically, progress was achieved by individuals altering surgical technique or implementing lessons accrued from their own post-operative experiences into intra-operative decision making. While this granular improvement is vital and continues in modern surgery, paradigm shifts in the foundation of surgical principles and instrumentation have accelerated the evolution of modern surgical medicine.
Numerous significant shifts in surgery have occurred in the past few decades including the introduction of endoscopic equipment for not only diagnostic but therapeutic management of disease. Since the dissemination of laparoscopy, surgeons have continued to explore less invasive approaches now in the form of robotic assisted surgery.
It is the natural progression for the evolution of surgery to push the degree of invasiveness towards the extreme of the spectrum over time in order to improve patient satisfaction and clinical outcomes. The typical hindrance of such innovation is related to cost.
However, with modern advancements in optics, mechanics, energy, computing power, and more, this progression and adaptation into everyday practice is occurring at an ever-increasing speed. Robotic surgery continues to be at the forefront of MIS innovation given its ability to minimize human error and increase surgical precision and operative standardization. But, it is important to define what constitutes robotic surgery. Currently, “robotic surgery” is often used synonymously with the most widely recognizable robot, the DaVinci surgical system. This platform by Intuitive consists of three components that allow the surgeon to indirectly control robotic arms that grasp, transect, coagulate, staple, clip and suture. While this master-slave or passive apparatus that allows for telesurgery remains the most popular globally, other surgical robots can be classified into two other categories: supervisory-controlled, and shared control.1
Supervisory-controlled systems allow pre-operative planning with robotic execution under close supervision, such as with aquablation of the prostate in urology. Shared-control systems allow the surgeon and robot to function simultaneously, such as with spine and arthroplasty robots in neurosurgery and orthopedics.1 This article will highlight the current role of robotic systems in minimally invasive surgery.
Robotic-Assisted Laparoscopic Surgery
DaVinci Laparoscopy
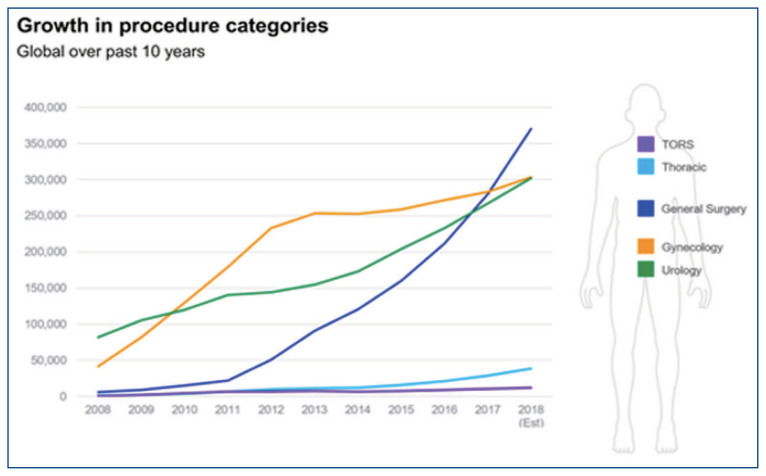
The DaVinci robotic surgical platform (Intuitive surgical Inc., Sunnyvale, CA, USA) was first made commercially available in 2001. It has subsequently undergone three iterations and its most modern format is the DaVinci Xi. Since its inception, this platform has been widely adopted and now used in over 10 million cases over two decades, in five specialties and grown to dominate the robotic assisted laparoscopic (RAL) market (Image 1).2 At least 85% of prostatectomies are performed robotically and the number of hysterectomies performed robotically has increased by 300% over the last decade.3,4 In comparison to open surgery, use of the DaVinci robot has shown significant improvement in clinical outcomes often demonstrating less blood loss, a shorter length of stay and less opioid consumption as supported by numerous multi-specialty reports in the literature.5,6,7,8
Image 1.
Trends in Number of Robotic Cases by Sub-Specialty
These well-described clinical benefits and rapid adoption by a growing number of surgeons ensures that, for the foreseeable future, the DaVinci robot will continue to be a mainstay in the minimally invasive surgical management of oncologic and reconstructive pathologies in cardiothoracic surgery, general surgery, urology and gynecology. In fact, multiple medical specialties now incorporate training modules and robotic skills courses into their curriculum and require residents to have robotic platform specific training in order to graduate.
However, the original DaVinci patents have now expired and significant robotic system development and competition is expected, particularly in regards to novel technology, reduced cost and size reduction.9 Current DaVinci platforms do not utilize haptic feedback, the experience of touch by applying resistance to the user, or biometric integration such as eye tracking cameras or head tracking robotic arms that can better replicate a traditional open surgical environment.10 Additionally, the sizable physical footprint of the DaVinci robot tower, arms and console has proven restrictive in its implementation as well as the significant cost of the system, as the Xi still costs approximately $1.5 million per system.11 With the expected increase in number of robotic laparoscopic cases over the next decade, numerous companies have entered and expanded the market (Table 1).
Table 1.
Current and Popular Robotic Surgical Systems Worldwide (MP – multiport, N/a – not applicable)60
| Robot | Uses | Port | Arms | Haptic Feedback | Eye Tracking | US FDA Approval |
|---|---|---|---|---|---|---|
| DaVinci Xi | MIS | MP | 4 | No | No | Yes |
| DaVinci SP | MIS NOTES LESS |
SP | 1 | No | No | Yes |
| Senhance | MIS | MP | 4 | Yes | Yes | Yes |
| Revo-i | MIS | MP | 4 | Yes | No | Pending (Korea) |
| SPORT | MIS NOTES LESS |
SP | 1 | No | No | Pre-Clinical |
| Versius | MIS | MP | 4 | No | No | Pending (Europe) |
| MiroSurge | MIS | MP | 3 | Yes | No | Pre-Clinical |
| SurgiBot | LESS | SP | 1 | N/a | N/a | Pending (China) |
| NeoGuide | NOTES | N/a | 1 | No | N/a | Yes |
| Invendoscopy | NOTES | N/a | 1 | No | N/a | Yes |
| Flex Robotic | NOTES | N/a | 1 | No | N/a | Yes |
| Monarch | NOTES | N/a | 1 | No | N/a | Yes |
| Ion | NOTES | N/a | 1 | No | N/a | Yes |
| PROCEPT | NOTES | N/a | 1 | N/a | N/a | Yes |
| Roboflex | NOTES | N/a | 1 | No | N/a | Yes |
Laparo-endoscopic Single-site Surgery (LESS)
The SinglePort (SP) system, created in 2018, is Intuitive’s venture into the LESS space. Through a 2.5cm incision, a solitary port releases 1 camera and 3 robotic arms which are controlled from the console by the surgeon similar to the Xi. This approach allows for better cosmetic outcomes – a single incision, quicker patient recovery, and similar optics and dexterity of the instruments. Clinical adoption has continued to increase. Between 2020 and 2021, there has been a 56% increase in the number of SP robotic systems nationwide.
Few companies thus far have made a meaningful impact in the LESS space. However, the STRAS system, version 2 (iCUBE, Strasbourg, France) is a flexible endoscopic system capable of single port intraluminal surgery. Although still in preclinical development, its main advantage over current LESS platforms is its significantly smaller size and the option for table mounting arms. The Single Port Orifice Robotic Technology (SPORT) Surgical System (Titan Medical Inc., Toronto, Canada) also uses a 2.5cm incision to deliver two articulating instruments and a camera for LESS. It is pending FDA approval but has demonstrated success in a single port partial nephrectomy in animal models.11
Non-Laparoscopic Robotic Surgical Platforms
Natural Orifice Translumenal Endoscopic Surgery (NOTES)
Robotic NOTES is an exciting area of research and advancement in MIS. The NeoGuide Endoscopy System (Intuitive Surgical Inc., Sunnyvale, CA) and the Invendoscopy E210 System (Ambu, ballerup, Denmark) are flexible self-propelling colonoscopes that have had FDA approval since 2006 and 2016, respectively.12,13 The lightweight systems are easier to manipulate and apply less force to the colon wall to reduce the colonic looping phenomena that is responsible for the majority of post-operative pain. Similarly, the Flex Robotic system (Medrobotics Corp., Raynham, MA, USA) is a robotic platform intended to increase accessibility to deep organs. Its current indications include transoral procedures and recent feasibility studies demonstrate a role for rectal cancer resection.14 This platform has had FDA approval since 2015.
Although these systems are segregated into their own unique approaches, their enhanced visualization has spurred ongoing research to assess the potential to combine NOTES, LESS and other laparoscopic robotic approaches for more diverse and complex surgical applications.
Bronchoscopy Platforms
Bronchoscopy and transthoracic needle aspiration are the two main approaches for diagnostic biopsy of peripheral lung lesions. However, the diagnostic yield of bronchoscopy ranges from 67–84% compared to 92% in needle aspiration due to “getting lost” in the peripheral airways.15,16 The addition of robotic guidance systems, Monarch platform by Aurius health in 2018 and Ion Endoluminal System by Intuitive Surgical in 2019, has the potential to increase yields close to 95% and increase the ability to localize and precisely puncture peripheral nodules.17,18 These systems increase structural support with a locking outer sheath but have an inner flexible controllable bronchoscope with electromagnetic navigation guidance and continuous visualization. This allows for 4-way adjustable angulation to reach farther than conventional bronchoscopy, prevent accidental displacement during sampling, and visualize and tamponade bleeding.19 As this technology expands, there is the potential for adding ablative therapies for the treatment of oligometastatic or inoperable peripheral lung tumors.20
Ureteroscopy Platforms
Ergonomic deficiencies during stone manipulation, laser disintegration, surgeon fatigue, exposure to radiation during fluoroscopy and the need for assistance while performing ureteroscopy has led to the development of robotic ureteroscopes. The first clinical application of robotic ureteroscopy (URS) was introduced in 2008 by Desai and colleagues with the Sensei-Magellan system.21 Since that time the Avicenna Roboflex system, introduced in 2013, has been the only system that remains in clinical use.22 The surgeon sits at the console controlling a flexible arm that can rotate, advance, retract and deflect as well as manipulate irrigation, lasers and stone baskets. Treatment times, safety profiles, and three-month stone free rates were similar to conventional URS.23 As these systems develop, the ability to have tactile feedback from the tip of the ureteroscope, 3D positioning and memory to find stones based off of pre-operative imaging, and adaptive intelligent control of the laser settings as the stone is being fragmented could allow for further utility and offset the higher cost of using a robotic system.22
Aquablation
Aquablation (AA) is the newest robotic platform being used in the field of urology. It is a surgeon-planned, ultrasound-guided, robotically executed technique to resect prostate tissue athermally using a high velocity water jet.24 It is approved for use in men with benign prostatic hyperplasia with 30–150g prostates with or without a median lobe.
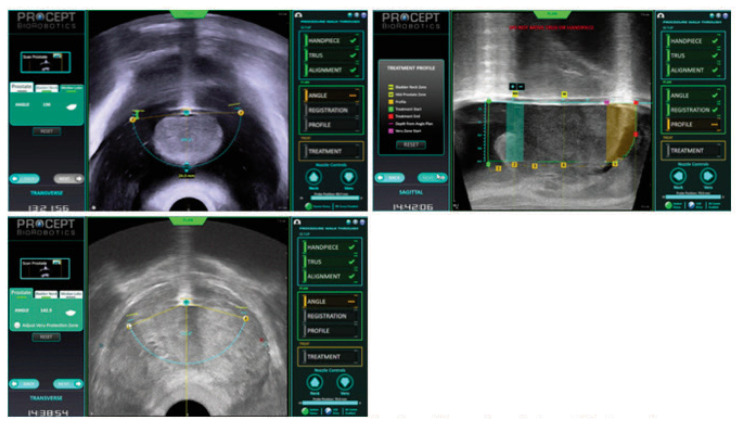
The AA system, developed by Procept, uses a transrectal ultrasound to visualize the prostate. The proprietary software is then used to map the prostate dimensions and the anatomic areas of the median lobe, transitional zone and peri-verumontanum tissue (Image 2, 3). A robotically controlled transurethral waterjet is then programmed by the surgeon to resect a specific amount of prostate tissue in a precise location, up to 0.25mm at a time. The robotic system is then activated by the surgeon and the pre-determined tissue is removed within minutes.
Image 2.
The Procept aquabeam handpiece
Image 3.
Prostatic mapping of the median lobe, mid-gland, and dynamic points – sphincter and bladder neck
This has quickly gained popularity in the urologic community given its short learning curve, use of common urologic skills (transrectal ultrasound and rigid cystoscopy), short operative times, wider range of patient inclusion and encouraging clinical outcomes. There is a 6% incidence of restarting medications or requiring surgical retreatments compared to 12.3% with the gold standard TURP, preservation of sexual function and a 50% reduction in retrograde ejaculation compared to alternative surgical approaches for BPH.25,26 Due to these advantages, the number of AA systems has increased by 52% over the last 9 months.
Robotic Arm-assisted Arthroplasty
The success of knee and hip arthroplasty relies on surgeons’ technical ability to achieve optimal position and alignment of the prosthesis.27 Computer-assistance with navigation allows for pre-planning and real-time intra-operative feedback while the robotic arm stabilizes and optimizes positioning of the prosthesis and surgical instruments. ROBODOC (Integrated surgical systems, Davis, CA, USA), introduced in 1992, was an active system independently performing the osteotomy and only allowed for the surgeon to start and stop the procedure.
A semi-active system, such as RIO by MAKO Surgical Corp., is now the most widely used system in modern orthopedics. Using a pre-operative extremity CT scan, a 3D model with pre-programmed boundaries is created that limits the range of movement of the surgical instruments controlled by the robotic arm.28,29 All actions, such as reaming and osteotomy, are performed according to pre-operative planning, but the final manipulation depends on surgeon execution.
Compared to traditional total hip arthroplasty, the MAKO system can reduce varus and valgus deformities of the femoral prosthesis, restore the offset, and more accurately position the acetabular cup prosthesis in the safety zone.30,31,32,33 Although several studies report the benefit for less intra-operative blood loss, low complication rate and shorter hospital stay with robotic systems, the literature doesn’t report a significant difference in short-term clinical efficacy through the Harris hip score or other scoring systems.33,34
The FDA has approved MAKO and NAVIO surgical robots for uni-compartmental/total knee arthroplasty (UKA/TKA) as well. These systems employ visual, tactile and auditory feedback to improve surgical efficiency and achieve a more precise osteotomy alignment.35
Over the last decade, use of robotic systems for UKA has more than doubled in certain regions of the US due to the definite and repeatable evidence of improved femoral and tibial prosthetic positioning accuracy by 3 and 3.4 times, respectively.36,37 Additionally, soft tissue balance improves and the joint dynamics of the knee have better function and greater longevity.35
Patients report superior pain scores at 2 months and functional scores at 3 months when compared with traditional approaches.38 Continued long-term, prospective and randomized-controlled trials are needed to further study the use of MAKO in TKA patients.
Spine Robot Platforms
The Mazor robotic platform (Medtronic Navigation, Louisville, CO, USA; Medtronic Spine, Memphis, TN, USA) was first FDA approved in 2004 and is currently one of the most popular systems in the world for spinal pedicle screw placement. The initial system, SpineAssist, had a patient-mounted track that used pre-operative or intra-operative CT imaging to plan hardware trajectory. The current model, Mazor X Stealth Edition, released in 2019, no longer requires a patient-mounted track, has faster computing speed, the vertebral bodies can be registered individually, and an optic camera allows for self-detection of the robot to avoid intra-operative collisions.
ExcelsiusGPS (Globus Medical, Inc., Audubon, PA, USA) was the first spine robot with a fully integrated navigation platform, real-time instrument tracking and pedicle screw placement without guidewires. Similar to the Mazor X Stealth Edition, intra-operative fluoroscopic imaging can be merged with pre-operative CT scans (Image 3).
These robotic systems aid in maintaining a fixed working angle to reduce inaccuracies and tremors by the surgeon and thus allow for more consistent, safe and improved patient outcomes. While, studies demonstrate a high accuracy rate of robotic assisted pedicle screw placement (91–98%) comparisons with traditional approaches have not shown an overwhelming improvement in accuracy.39,40,41,42 A recent meta-analysis has demonstrated decreased screw revision risk when using robotic-assisted and navigated screw placement over free-hand techniques.43 Additionally, robotic assistance can reduce the risk of proximal facet joint violation, compared to freehand techniques, which can minimize the risk of adjacent segment disease.44,45,46
Limitations to robotic spinal surgery include cost, lack of diverse indications for its use, increased operative times and lack of direct evidence of benefit. In fact, several database studies report increased risk of re-operation, 30 day readmission rate and complication rate with robotic assistance in lumbar spinal fusion.47,48 While the technology continues to improve, more research is needed to increase the implementation of robotics in spine surgery.
The Future
Two on-going areas of intense research include robotic telesurgery and micro-robotics. The new 5G network deployed by telecom companies worldwide offers rapid communication and the potential for telesurgery in order to reduce health care costs and improve patient access to quality care. Studies have determined that a lag time <400ms is imperceptible to the surgeon and this can be achieved using 5G networks. Telesurgery has been performed in China, Germany, Italy and Spain with promising results. Remote nephrectomy was safely performed without complication and conversion with a median distance of 187km between surgeon and patient. The median round trip delay was 26ms and total delay was 200ms.49,50 Over time, the extent and feasibility of telesurgery will continue to be tested. This is promising for use on aircraft carriers, for future space travel and in underserved areas worldwide. As of this year, however, a radical cystectomy was performed remotely from a distance of nearly 3000km with an average total delay lag time of 254ms.51
Investigative micro-robotic prototypes are freely mobile capsule endoscopes that offer a variety of diagnostic, targeted drug delivery and surgical applications.52 One such robot is a millimeter in size and has been, in porcine models, directed with extracorporeal magnets to apply a single functional nitinol clip and stop colonic bleeding following biopsy.53
Research is ongoing around four categories specific to micro-robotics: contained propulsion, miniaturized functionality, accurate telemanipulation and consistent visualization.2
Propulsion can be achieved externally via electromagnetic fields or ultrasonographic energy while internally driven systems are more restricted as they require chemical reactions for motion and a separate navigational source.9,54 Several studies have demonstrated proof-of-concept for cutting, grasping and ablation on the micro-scale.55 There is also early promise with phototaxis of polysterene beads and magnetically directed chrome spheres using MRI for telemanipulation. Finally, studies have demonstrated the ability for live-tracking of ferromagnetically labeled microalgae to model microbots in rats, X-ray angiography tracking of a radio-dense robot in the aorta of a rabbit, and US tracking of a magnetically-labeled robot through muscle tissue in a chicken model.56,57,58,59
The age of microbots is clearly in its infancy. However, as further research is performed to address safety to the patient and operator, cost and accessibility, creation of 3D tracking and the potential role of fluorescence, significant advancements could create another major paradigm shift in minimally invasive surgery.
Conclusion
Robotic technology has created a unique paradigm shift in the surgical care of the patient beginning several decades ago. With new systems and applications routinely coming to market, it continues to be an exciting avenue for new research and development as well as improving the patient care experience. As advancements in computer processing, optics, mechanics and haptic feedback continue to rapidly progress, robotic surgery has cemented its role in modern surgical medicine and its near universal adoption across surgical and medical specialties offers promise to continue to improve medical care around the world.
Footnotes
Patrick Probst, MD, is a Urologist with Kansas City Urology Care in North Kansas City, Missouri.
Disclosure
None reported.
References
- 1.Alluri RK, Avrumova F, Sivaganesan A, Vaishnav AS, Lebl DR, Qureshi SA. Overview of Robotic Technology in Spine Surgery. HSS J. 2021 Oct 17;3:308–316. doi: 10.1177/15563316211026647. Epub 2021 Jul 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khandalavala K, Shimon T, Flores L, Armijo PR, Oleynikov D. Emerging surgical robotic technology: a progression toward microbots. Ann Laparosc Endosc Surg. 5:3. 202. [Google Scholar]
- 3.Golla V, Williams SB. Cost-effectiveness of Robotic-Assisted Prostatectomy in the UK—Are We Doing Enough? JAMA Netw Open. 2022;5(4):e225747. doi: 10.1001/jamanetworkopen.2022.5747. [DOI] [PubMed] [Google Scholar]
- 4.Carbonnel M, Moawad GN, Tarazi MM, Revaux A, Kennel T, Favre-Inhofer A, Ayoubi JM. Robotic Hysterectomy for Benign Indications: What Have We Learned from a Decade? JSLS. 2021 Jan–Mar;25(1):e2020.00091. doi: 10.4293/JSLS.2020.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lantz A, Bock D, Akre O, Angenete E, Bjartell A, Carlsson S, Modig KK, Nyberg M, Kollberg KS, Steineck G, Stranne J, Wiklund P, Haglind E. Functional and Oncological Outcomes After Open Versus Robot-assisted Laparoscopic Radical Prostatectomy for Localised Prostate Cancer: 8-Year Follow-up. Eur Urol. 2021 doi: 10.1016/j.eururo.2021.07.025. [DOI] [PubMed] [Google Scholar]
- 6.Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, Buda A, Yan X, Shuzhong Y, Chetty N, Isla D, Tamura M, Zhu T, Robledo KP, Gebski V, Asher R, Behan V, Nicklin JL, Coleman RL, Obermair A. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N Engl J Med. 2018 Nov 15;379(20):1895–1904. doi: 10.1056/NEJMoa1806395. [DOI] [PubMed] [Google Scholar]
- 7.Shearman AD, Sephton BM, Wilson J, Nathwani DK. Robotic-assisted unicompartmental knee arthroplasty is associated with earlier discharge from physiotherapy and reduced length-of-stay compared to conventional navigated techniques. Arch Orthop Trauma Surg. 2021 Dec;141(12):2147–2153. doi: 10.1007/s00402-021-04207-1. [DOI] [PubMed] [Google Scholar]
- 8.Witt RG, Hirata Y, Prakash LR, Newhook TE, Maxwell JE, Kim MP, Tran Cao HS, Lee JE, Vauthey JN, Katz MHG, Tzeng CD, Ikoma N. Comparative analysis of opioid use between robotic and open pancreatoduodenectomy. J Hepatobiliary Pancreat Sci. 2022 Jul 7; doi: 10.1002/jhbp.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceylan H, Yasa IC, Kilic U, et al. Translational prospects of untethered medical microrobots. Progress in Biomedical Engineering. 2019;1:012002. [Google Scholar]
- 10.Cadeddu JA. Re: Early Experience with the Senhance®-Laparoscopic/Robotic Platform in the US. J Urol. 2019 10109701JU0000576800809701c. [Google Scholar]
- 11.Rassweiler JJ, Autorino R, Klein J, et al. Future of robotic surgery in urology. BJU Int. 2017;120:822–41. doi: 10.1111/bju.13851. [DOI] [PubMed] [Google Scholar]
- 12.Eickhoff A, van Dam J, Jakobs R, et al. Computer-assisted colonoscopy (the NeoGuide Endoscopy System): results of the first human clinical trial (“PACE study”) Am J Gastroenterol. 2007;102:261–6. doi: 10.1111/j.1572-0241.2006.01002.x. [DOI] [PubMed] [Google Scholar]
- 13.Groth S, Rex DK, Rösch T, et al. High cecal intubation rates with a new computer-assisted colonoscope: a feasibility study. Am J Gastroenterol. 2011;106:1075–80. doi: 10.1038/ajg.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmichael H, D’Andrea AP, Skancke M, et al. Feasibility of transanal total mesorectal excision (taTME) using the Medrobotics Flex® System. Surg Endosc. 2020;34:485–91. doi: 10.1007/s00464-019-07019-y. [DOI] [PubMed] [Google Scholar]
- 15.Folch EE, Pritchett MA, Nead MA, et al. Electromagnetic Navigation Bronchoscopy for Peripheral Pulmonary Lesions: One-Year Results of the Prospective, Multicenter NAVIGATE Study. J Thorac Oncol. 2019;14:445–58. doi: 10.1016/j.jtho.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 16.DiBardino DM, Yarmus LB, Semaan RW. Transthoracic needle biopsy of the lung. J Thorac Dis. 2015;7:S304–16. doi: 10.3978/j.issn.2072-1439.2015.12.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen A, Silvestri GA, Gildea TR, et al. Robotic Bronchoscopy for Peripheral Pulmonary Lesions: A Multicenter Pilot and Feasibility Study (BENEFIT) Chest New Orleans. doi: 10.1016/j.chest.2020.08.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yarmus LB, Mallow C, Pastis N, et al. First-in-Human Use of a Hybrid Real-Time Ultrasound-Guided Fine-Needle Acquisition System for Peripheral Pulmonary Lesions: A Multicenter Pilot Study. Respiration. 2019;98:527–33. doi: 10.1159/000504025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen AC, Gillespie CT. Robotic Endoscopic Airway Challenge: REACH Assessment. Ann Thorac Surg. 2018;106:293–7. doi: 10.1016/j.athoracsur.2018.01.051. [DOI] [PubMed] [Google Scholar]
- 20.Agrawal A, Hogarth DK, Murgu S. Robotic bronchoscopy for pulmonary lesions: a review of existing technologies and clinical data. J Thorac Dis. 2020 Jun;12(6):3279–3286. doi: 10.21037/jtd.2020.03.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aron M, Desai MM. Flexible robotics. Urol Clin North Am. 2009;36:157–62. viii. doi: 10.1016/j.ucl.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Rassweiler-Seyfried MC, Herrmann J, Klein J, Michel MS, Rassweiler J, Grüne B. Robot-assisted flexible ureterorenoscopy: state of the art in 2022. Mini-invasive Surg. 2022;6:41. [Google Scholar]
- 23.Geavlete P, et al. Robotic flexible ureteroscopy versus classic flexible ureteroscopy in renal stones: The initial Romanian experience. Chir. 2016;111:326–9. [PubMed] [Google Scholar]
- 24.Probst P, Desai M. Expectations Facing Reality: Complication Management after Aquablation Treatment for Lower Urinary Tract Symptoms. Eur Urol Focus. 2022 May 16; doi: 10.1016/j.euf.2022.04.013. S2405-4569(22)00109-2. [DOI] [PubMed] [Google Scholar]
- 25.Gilling Peter. WATER: A Double-Blind, Randomized, Controlled Trial of Aquablation vs Transurethral Resection of the Prostate in Benign Prostatic Hyperplasia. J Urol. 2018;199:1252–1261. doi: 10.1016/j.juro.2017.12.065. [DOI] [PubMed] [Google Scholar]
- 26.Desai Mihir. Aquablation for benign prostatic hyperplasia in large prostate (80–150mL): 6-month results from the WATER II trial. BJU Int. 2019;8(1):e1498. doi: 10.1111/bju.14703. [DOI] [PubMed] [Google Scholar]
- 27.Mason JB, Fehring TK, Estok R, et al. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22:1097–1106. doi: 10.1016/j.arth.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Bargar WL. Robots in orthopaedic surgery: past, present, and future. Clin Orthop Relat Res. 2007;463:31–36. [PubMed] [Google Scholar]
- 29.van der List JP, Chawla H, Joskowicz L, et al. Current state of computer navigation and robotics in unicompartmental and total knee arthroplasty: a systematic review with meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2016;24:3482–3495. doi: 10.1007/s00167-016-4305-9. [DOI] [PubMed] [Google Scholar]
- 30.Amirouche F, Solitro GF, Chandrasekaran S, et al. Validating a modified circle theorem method for the measurement of acetabular cup anteversion on plain radiography with intra-operative data from robotic assisted total hip arthroplasty. J Arthroplasty. 2016;31:323–329. doi: 10.1016/j.arth.2015.07.042. [DOI] [PubMed] [Google Scholar]
- 31.Domb BG, Redmond JM, Louis SS, et al. Accuracy of component positioning in 1980 total hip arthroplasties: a comparative analysis by surgical technique and mode of guidance. J Arthroplasty. 2015;30:2208–2218. doi: 10.1016/j.arth.2015.06.059. [DOI] [PubMed] [Google Scholar]
- 32.Lim SJ, Ko KR, Park CW, et al. Robot-assisted primary cementless total hip arthroplasty with a short femoral stem: a prospective randomized short-term outcome study. Comput Aided Surg. 2015;20:41–46. doi: 10.3109/10929088.2015.1076044. [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Xiong J, Wang P, et al. Robotic-assisted compared with conventional total hip arthroplasty: systematic review and meta-analysis. Postgrad Med. 2018;94:335–341. doi: 10.1136/postgradmedj-2017-135352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siebel T, Käfer W. Clinical outcome following robotic assisted versus conventional total hip arthroplasty: a controlled and prospective study of seventy-one patients. Z Orthop Ihre Grenzgeb. 2005;143:391–398. doi: 10.1055/s-2005-836776. [DOI] [PubMed] [Google Scholar]
- 35.Chen X, Deng S, Sun ML, He R. Robotic arm-assisted arthroplasty: The latest developments. Chin J Traumatol. 2022 May;25(3):125–131. doi: 10.1016/j.cjtee.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boylan M, Suchman K, Vigdorchik J, et al. Technology-assisted hip and knee arthroplasties: an analysis of utilization trends. J Arthroplasty. 2017;33:1019–1023. doi: 10.1016/j.arth.2017.11.033. [DOI] [PubMed] [Google Scholar]
- 37.Citak M, Suero EM, Citak M, et al. Unicompartmental knee arthroplasty: is robotic technology more accurate than conventional technique? Knee. 2013;20:268–271. doi: 10.1016/j.knee.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Blyth MJG, Anthony I, Rowe P, et al. Robotic arm assisted versus conventional unicompartmental knee arthroplasty: exploratory secondary analysis of a randomised controlled trial. Bone Joint Res. 2017;6:631–639. doi: 10.1302/2046-3758.611.BJR-2017-0060.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devito DP, Kaplan L, Dietl R, et al. Clinical acceptance and accuracy assessment of spinal implants guided with SpineAssist surgical robot: retrospective study. Spine (Phila Pa 1976) 2010;35:2109–2115. doi: 10.1097/BRS.0b013e3181d323ab. [DOI] [PubMed] [Google Scholar]
- 40.Kantelhardt SR, Martinez R, Baerwinkel S, Burger R, Giese A, Rohde V. Perioperative course and accuracy of screw positioning in conventional, open robotic-guided and percutaneous robotic-guided, pedicle screw placement. Eur Spine J. 2011;20:860–868. doi: 10.1007/s00586-011-1729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keric N, Doenitz C, Haj A, et al. Evaluation of robot-guided minimally invasive implantation of 2067 pedicle screws. Neurosurg Focus. 2017;42:E11. doi: 10.3171/2017.2.FOCUS16552. [DOI] [PubMed] [Google Scholar]
- 42.Ringel F, Stuer C, Reinke A, et al. Accuracy of robot-assisted placement of lumbar and sacral pedicle screws: a prospective randomized comparison to conventional freehand screw implantation. Spine (Phila Pa 1976) 2012;37(8):E496–E501. doi: 10.1097/BRS.0b013e31824b7767. [DOI] [PubMed] [Google Scholar]
- 43.Staartjes VE, Klukowska AM, Schroder ML. Pedicle screw revision in robot-guided, navigated, and freehand thoracolumbar instrumentation: a systematic review and meta-analysis. World Neurosurg. 2018;116:433–443. doi: 10.1016/j.wneu.2018.05.159. [DOI] [PubMed] [Google Scholar]
- 44.Hyun SJ, Kim KJ, Jahng TA, Kim HJ. Minimally invasive robotic versus open fluoroscopic-guided spinal instrumented fusions: a randomized controlled trial. Spine (Phila Pa 1976) 2017;42(6):353–358. doi: 10.1097/BRS.0000000000001778. [DOI] [PubMed] [Google Scholar]
- 45.Li HM, Zhang RJ, Shen CL. Accuracy of pedicle screw placement and clinical outcomes of robot-assisted technique versus conventional freehand technique in spine surgery from nine randomized controlled trials: a meta-analysis. Spine (Phila Pa 1976) 2020;45:E111–E119. doi: 10.1097/BRS.0000000000003193. [DOI] [PubMed] [Google Scholar]
- 46.Zhou LP, Zhang RJ, Li HM, Shen CL. Comparison of cranial facet joint violation rate and four other clinical indexes between robot-assisted and freehand pedicle screw placement in spine surgery: a meta-analysis. Spine (Phila Pa 1976) 2020;45(22):E1532–E1540. doi: 10.1097/BRS.0000000000003632. [DOI] [PubMed] [Google Scholar]
- 47.Lieber AM, Kirchner GJ, Kerbel YE, Khalsa AS. Robotic-assisted pedicle screw placement fails to reduce overall postoperative complications in fusion surgery. Spine J. 2019;19(2):212–217. doi: 10.1016/j.spinee.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 48.Yang DS, Li NY, Kleinhenz DT, Patel S, Daniels AH. Risk of postoperative complications and revision surgery following robot-assisted posterior lumbar spinal fusion. Spine (Phila Pa 1976) 2020;45(24):E1692–E1698. doi: 10.1097/BRS.0000000000003701. [DOI] [PubMed] [Google Scholar]
- 49.Legeza P, Britz GW, Shah A, Sconzert K, Sungur JM, Chinnadurai P, Sinha K, Lumsden AB. Impact of network performance on remote robotic-assisted endovascular interventions in porcine model. J Robot Surg. 2022 Feb;16(1):29–35. doi: 10.1007/s11701-021-01196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J, Yang X, Chu G, et al. Application of Improved Robot-assisted Laparoscopic Telesurgery with 5G Technology in Urology. Eur Urol. 2022 Jul 8; doi: 10.1016/j.eururo.2022.06.018. S0302-2838(22)02469-1. [DOI] [PubMed] [Google Scholar]
- 51.Yang X, Wang Y, Jiao W, et al. Application of 5G technology to conduct tele-surgical robot-assisted laparoscopic radical cystectomy. Int J Med Robot. 2022 Aug;18(4):e2412. doi: 10.1002/rcs.2412. [DOI] [PubMed] [Google Scholar]
- 52.Ciuti G, Caliò R, Camboni D, et al. Frontiers of robotic endoscopic capsules: a review. J Microbio Robot. 2016;11:1–18. doi: 10.1007/s12213-016-0087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valdastri P, Quaglia C, Susilo E, et al. Wireless therapeutic endoscopic capsule: in vivo experiment. Endoscopy. 2008;40:979–82. doi: 10.1055/s-0028-1103424. [DOI] [PubMed] [Google Scholar]
- 54.Chautems C, Zeydan B, Charreyron S, et al. Magnetically powered microrobots: a medical revolution underway? Eur J Cardiothorac Surg. 2017;51:405–7. doi: 10.1093/ejcts/ezw432. [DOI] [PubMed] [Google Scholar]
- 55.Kirson ED, Yaari Y. A novel technique for micro-dissection of neuronal processes. J Neurosci Methods. 2000;98:119–22. doi: 10.1016/s0165-0270(00)00194-1. [DOI] [PubMed] [Google Scholar]
- 56.Martel S. Swimming microorganisms acting as nanorobots versus artificial nanorobotic agents: A perspective view from an historical retrospective on the future of medical nanorobotics in the largest known three-dimensional biomicrofluidic networks. Biomicrofluidics. 2016;10:021301. doi: 10.1063/1.4945734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belharet K, Folio D, Ferreira A. MRI-based microrobotic system for the propulsion and navigation of ferromagnetic microcapsules. Minim Invasive Ther Allied Technol. 2010;19:157–69. doi: 10.3109/13645706.2010.481402. [DOI] [PubMed] [Google Scholar]
- 58.Park S, Cha K, Park J. Development of Biomedical Microrobot for Intravascular Therapy. Int J Adv Robot Syst. 2010;7:1. [Google Scholar]
- 59.Hu W, Lum GZ, Mastrangeli M, et al. Small-scale soft-bodied robot with multimodal locomotion. Nature. 2018;554:81–5. doi: 10.1038/nature25443. [DOI] [PubMed] [Google Scholar]
- 60.Almujalhem A, Rha KH. Surgical robotic systems: What we have now? A urological perspective. BJUI Compass. 2020;1:152–159. doi: 10.1002/bco2.31. [DOI] [PMC free article] [PubMed] [Google Scholar]