Abstract
The intracellular accumulation of norfloxacin and pefloxacin in Klebsiella pneumoniae was evaluated. The roles of lipopolysaccharide, capsule, and outer membrane proteins were not important for the intrabacterial accumulation of fluoroquinolones in isogenic strains with known outer membrane alterations. In fluoroquinolone-resistant clinical isolates also expressing GyrA alterations, an active efflux leading to decreased accumulation of the drugs enhanced their resistance to these agents.
Several mechanisms are involved in the resistance to fluoroquinolones (FQ) by gram-negative bacteria. In Escherichia coli, point mutations in the quinolone resistance-determining region (QRDR) of gyrA (21) are the most important cause of resistance. Mutations in gyrB are uncommon in clinical isolates (22), while mutations in parC (19) or (much less frequently) in parE (4) contribute to increased levels of resistance. The gyrA gene of Klebsiella pneumoniae has been cloned and sequenced and is similar (about 85% at the nucleotide level and about 90% at the amino acid level) to its E. coli homolog (5). The role of gyrA mutations in FQ-resistant K. pneumoniae has been demonstrated, these mutations being analogous to those previously observed in E. coli (6, 7). Decreased activity of FQ against E. coli has also been related to a reduced intracellular drug accumulation due to lipopolysaccharide or porin alterations impairing uptake or because of enhanced efflux (10, 11). Cross-resistance between FQ and beta-lactams in K. pneumoniae mutants has been related to outer membrane protein alterations (12, 17). Expression of the ramA locus from K. pneumoniae in E. coli confers resistance to norfloxacin, determines the active efflux of tetracycline and chloramphenicol, and causes almost complete loss of OmpF (8). It has been reported that the high hydrophobicity of FQ is generally associated with decreased accumulation in both E. coli and Pseudomonas aeruginosa (14). The lipopolysaccharide of the outer leaflet of the outer membrane in some bacteria blocks the access of the drug to the microorganism. Other reports indicate that hydrophylic compounds may cross the outer membrane through porins (11).
The objectives of this study were to evaluate the accumulation of two FQ with different hydrophobicities (norfloxacin [NFX] and pefloxacin [PFX]) in K. pneumoniae by studying FQ-susceptible isogenic strains with known outer membrane alterations and FQ-resistant clinical isolates.
(This work was presented in part at the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy, New Orleans, La., 15 to 18 September 1996 [13].)
An environmental isolate, K. pneumoniae C3 (18), and the derived mutants KT717, KT793, and KT5003 (1–3, 9) were used. K. pneumoniae LB2 and LB4 (12) and K. pneumoniae CSUB10S and CSUB10R (kindly provided by J. Liñares [Barcelona, Spain]) are clinical isolates; LB and CSUB isolates are epidemiologically unrelated, as determined by pulse-field gel electrophoresis (data not shown). The outer membrane characteristics of all strains are shown in Table 1. MICs of NFX (Sigma, Madrid, Spain) and PFX (Rhône-Poulenc, Antony, France) were determined by microdilution, according to National Committee for Clinical Laboratory Standards NCCLS guidelines (16). To assess FQ accumulation in K. pneumoniae C3, bacterial cells were incubated with 2, 5, 10, or 50 μg of each FQ per ml at 37°C for different periods of time. For the other strains a concentration of 10 μg/ml was used. The cells were separated from the extracellular solution by centrifugation through a silicon oil barrier (1.029 g/cm3). The entire cell pellet was placed in 2 ml of 0.1 M glycine-HCl buffer (pH 3.0), vortexed, and centrifuged for 5 min at 12,800 × g. The amount of FQ in the supernatant was determined by spectrofluorometry. Bacterially associated FQ was expressed as nanograms of FQ per milligram [dry weight] of cells. The effects of the energy inhibitors carbonyl cyanide m-chlorophenylhydrazone (CCCP) (0.1 mM; Sigma) and 2,4-dinitrophenol (Sigma) were also evaluated. Data are expressed as means ± standard deviations. Differences among groups were compared by analysis of variance, which was used to assess statistical significance at P values of ≤0.05.
TABLE 1.
Susceptibilities to and accumulation of NFX in four isogenic and four clinical K. pneumoniae strains
| Strain | Presence in strain ofa:
|
NFX MIC (μg/ml) | Accumulation of NFX (ng/mg [dry weight])b
|
|||||
|---|---|---|---|---|---|---|---|---|
| O-Ag | K-Ag | OmpK34 | OmpK35 | OmpK36 | Control | CCCP | ||
| Isogenic strains | ||||||||
| C3 | O1 | K66 | + | + | + | 0.015 | 366 ± 65 | 346 ± 46 |
| KT717 | O1 | − | + | + | + | 0.03 | 330 ± 67 | ND |
| KT793 | − | − | + | + | + | 0.03 | 299 ± 68 | ND |
| KT5003 | − | − | + | − | − | 0.06 | 314 ± 54 | ND |
| Clinical strains | ||||||||
| LB2 | ND | ND | + | + | − | 2 | 286 ± 24 | 385 ± 65 |
| LB4 | ND | ND | + | − | − | 4 | 116 ± 21 | 263 ± 38 |
| CSUB10S | ND | ND | + | + | − | 4 | 313 ± 6 | ND |
| CSUB10R | ND | ND | + | − | − | 16 | 66 ± 20 | 76 ± 9 |
OmpK34, OmpK35, and OmpK36 are the K. pneumoniae homologs of E. coli OmpA and porins OmpF and OmpC, respectively. O-Ag, O antigen; K-Ag, K antigen; ND, not determined.
Data are means ± standard deviations of five experiments.
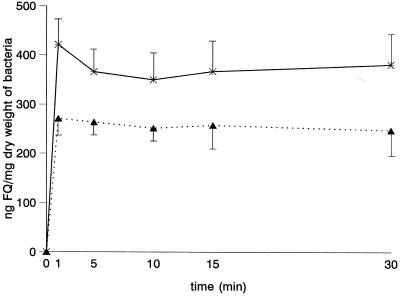
To analyze alterations in the GyrA subunit of DNA gyrase and the ParC subunit of topoisomerase IV, the QRDR of gyrA and the analogous region of parC were sequenced with previously described primers (6). MICs of PFX were identical for K. pneumoniae C3 and the derived mutants. A two-dilution step increase in the MIC of NFX for mutant KT5003 was observed in comparison with the parental strain K. pneumoniae C3. MICs of both quinolones for K. pneumoniae LB4 and CSUB10R were higher than for K. pneumoniae LB2 and CSUB10S, respectively (Table 1). The intracellular accumulation of NFX and PFX in K. pneumoniae C3 was rapid (Fig. 1) and not saturable at extracellular concentrations ranging from 2 to 50 μg/ml. Nonsignificant differences in the accumulation of FQ were observed in isogenic strains KT717, KT793, and KT5003. These results indicate that the roles of cell wall components in the intrabacterial accumulation of FQ with different grades of hydrophobicity are small or nonexistent. The high susceptibilities of the nonclinical strains, K. pneumoniae C3 and the mutants, to FQ are probably related to the absence of specific mechanisms of resistance to FQ. The accumulations of both FQ were significantly lower in the clinical K. pneumoniae isolates LB4 and CSUB10R. Preincubation of strains in the presence of CCCP did not modify the accumulation of NFX in K. pneumoniae C3, but it significantly increased the accumulation of NFX in both K. pneumoniae LB4 and CSUB10R (Table 1). Similar results were obtained with 2,4-dinitrophenol. This suggests that, at least in part, the reduced accumulation observed in both clinical isolates could be caused by an energy-dependent mechanism that pumps FQ out of the bacterial cell. Deguchi et al. have also described an active efflux system for NFX in FQ-resistant K. pneumoniae (7).
FIG. 1.
Time-dependent accumulation of NFX (∗) and PFX (▴) in K. pneumoniae C3.
Different authors have found discrepant amino acids at position 83 of the GyrA protein in FQ-susceptible K. pneumoniae: Thr (accession no. X16817 [5] and strain C3 [this work]) and Ser (ATCC 13833 [6]). In either case, a change in this position was observed in FQ-resistant strains LB2-LB4 (Tyr) and CSUB10S-CSUB10R (Phe). Both pairs of strains also have changes at position 112 (Val to Ile). These changes should contribute to the observed resistance to FQ, as similar changes have been observed in other gram-negative organisms (6, 20, 21). No changes in the QRDRs of the parC genes of the four clinical isolates studied were observed, in comparison with the sequence for K. pneumoniae C3. New studies are in progress to elucidate the roles of porin deficiency, efflux, and topoisomerase modification in the resistance of K. pneumoniae to FQ.
Deguchi et al. have suggested that the system responsible for active efflux of NFX may contribute to increased resistance to beta-lactams, chloramphenicol, and tetracycline. The relative importance of the efflux systems of K. pneumoniae LB4 and CSUB10R to antimicrobial agents other than FQ is not known. Both strains produce broad- and extended-spectrum beta-lactamases. Loss of porins may explain the increased resistance to beta-lactams in comparison with their respective parental strains LB2 and CSUB10S (12), but this does not discount the importance of the efflux mechanism as a contributor to beta-lactam resistance in K. pneumoniae, as has previously been shown for P. aeruginosa (15). As soon as the genetics of the efflux mechanisms in these two strains are determined, their importance as factors contributing to resistance to antimicrobial agents other than FQ can be defined.
Acknowledgments
This work was supported by grants from Comisión Interministerial de Ciencia y Tecnología (CICYT) of the Spanish government (PB96-0197). S.H.-A. was supported by a predoctoral fellowship from CICYT (FP94-41497233).
REFERENCES
- 1.Albertí S, Marqués G, Camprubí S, Merino S, Tomás J M, Vivanco F, Benedí V J. C1q binding and activation of the complement classical pathway by Klebsiella pneumoniae outer membrane proteins. Infect Immun. 1993;61:852–860. doi: 10.1128/iai.61.3.852-860.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albertí S, Rodríguez-Quiñones F, Schirmer T, Rummel G, Tomás J M, Rosenbusch J P, Benedí V J. A porin from Klebsiella pneumoniae: sequence homology, three-dimensional model, and complement binding. Infect Immun. 1995;63:903–910. doi: 10.1128/iai.63.3.903-910.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benedí V J, Ciurana B, Tomás J M. Isolation and characterization of Klebsiella pneumoniae unencapsulated mutants. J Clin Microbiol. 1989;27:82–87. doi: 10.1128/jcm.27.1.82-87.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breines D M, Ouabdesselam S, Ng E Y, Tankovic J, Shah S, Soussy C J, Hooper D. Quinolone resistance locus nfxD of Escherichia coli is a mutant allele of the parE gene encoding a subunit of topoisomerase IV. Antimicrob Agents Chemother. 1997;41:175–179. doi: 10.1128/aac.41.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimri G P, Das H K. Cloning and sequence analysis of gyrA gene of Klebsiella pneumoniae. Nucleic Acids Res. 1990;18:151–156. doi: 10.1093/nar/18.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deguchi T, Fukuoka A, Yasuda M, Nakano M, Ozeki S, Kanematsu E, Nishino Y, Ishihara S, Ban Y, Kawada Y. Alterations in the GyrA subunit of DNA gyrase and the ParC subunit of topoisomerase IV in quinolone-resistant clinical isolates of Klebsiella pneumoniae. Antimicrob Agents Chemother. 1997;41:699–701. doi: 10.1128/aac.41.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deguchi T, Kawamura T, Yasuda M, Nakano M, Fukuda H, Kato H, Kato N, Okano Y, Kawada Y. In vitro selection of Klebsiella pneumoniae strains with enhanced quinolone resistance during fluoroquinolone treatment of urinary tract infection. Antimicrob Agents Chemother. 1997;41:1609–1611. doi: 10.1128/aac.41.7.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George A M, Hall R M, Stokes H W. Multidrug resistance in Klebsiella pneumoniae: a novel gene, ramA, confers a multidrug resistance phenotype in Escherichia coli. Microbiology. 1995;141:1909–1920. doi: 10.1099/13500872-141-8-1909. [DOI] [PubMed] [Google Scholar]
- 9.Hernández-Allés S, Albertí, Rubires X, Merino S, Tomás J M, Benedí V J. Isolation of FC3-11, a bacteriophage specific for the Klebsiella pneumoniae porin OmpK36, and its use for the isolation of porin-deficient mutants. Can J Microbiol. 1995;41:399–406. [Google Scholar]
- 10.Hirai K, Aoyama H, Irikura T, Iyobe S, Mitsuhashi S. Differences in susceptibility to quinolones of outer membrane mutants of Salmonella typhimurium and Escherichia coli. Antimicrob Agents Chemother. 1986;29:535–538. doi: 10.1128/aac.29.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hooper D C, Wolfson J S, Souza S, Tung C, McHugh G L, Swartz M N. Genetic and biochemical characterization of norfloxacin resistance in Escherichia coli. Antimicrob Agents Chemother. 1986;29:639–644. doi: 10.1128/aac.29.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martínez-Martínez L, Hernández-Allés S, Albertí S, Tomás J M, Benedí V J, Jacoby G A. In vivo selection of porin-deficient mutants of Klebsiella pneumoniae with increased resistance to cefoxitin and expanded-spectrum cephalosporins. Antimicrob Agents Chemother. 1996;40:342–348. doi: 10.1128/aac.40.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martínez-Martínez L, García I, Ballesta S, Benedí V J, Tomás J M, Pascual A. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Energy-dependent accumulation of fluoroquinolones in quinolone-resistant Klebsiella pneumoniae, abstr. C38; p. 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCaffrey C, Botasso A, Pace J, Georgopapadakou N H. Quinolone accumulation in Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus. Antimicrob Agents Chemother. 1992;36:1601–1605. doi: 10.1128/aac.36.8.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakae T. Multiantibiotic resistance caused by active drug extrusion in Pseudomonas aeruginosa and other gram-negative bacteria. Microbiol SEM. 1997;13:273–284. [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Methods for dilution susceptibility tests for bacteria that grow aerobically. 4th ed. Approved standard M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 17.Sanders C C, Sanders W E, Goering R V, Vermer V. Selection of multiple antibiotic resistance by quinolones, β-lactams, and aminoglycosides with special reference to cross-resistance between unrelated drug classes. Antimicrob Agents Chemother. 1984;26:787–801. doi: 10.1128/aac.26.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomás J M, Benedí V J, Ciurana B, Jofré J. Role of capsule and O antigen in resistance of Klebsiella pneumoniae to serum bactericidal activity. Infect Immun. 1986;54:85–89. doi: 10.1128/iai.54.1.85-89.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vila J, Ruiz J, Goñi P, Jiménez De Anta M T. Detection of mutations in parC in quinolone-resistant clinical isolates of Escherichia coli. Antimicrob Agents Chemother. 1996;40:491–493. doi: 10.1128/aac.40.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vila J, Ruiz J, Marco F, Barcelo A, Goñi P, Giralt E, Jiménez De Anta M T. Association between double mutation in gyrA gene of ciprofloxacin-resistant clinical isolates of Escherichia coli and MICs. Antimicrob Agents Chemother. 1994;38:2477–2479. doi: 10.1128/aac.38.10.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshida H, Bogaki M, Nakamura M, Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob Agents Chemother. 1990;34:1271–1272. doi: 10.1128/aac.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida H, Bogaki M, Nakamura M, Yamanaka L, Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrB gene of Escherichia coli. Antimicrob Agents Chemother. 1991;35:1647–1650. doi: 10.1128/aac.35.8.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]