Abstract
Objective
There is widespread agreement about the key role of hemoglobin for oxygen transport. Both observational and interventional studies have examined the relationship between hemoglobin levels and maximal oxygen uptake () in humans. However, there exists considerable variability in the scientific literature regarding the potential relationship between hemoglobin and . Thus, we aimed to provide a comprehensive analysis of the diverse literature and examine the relationship between hemoglobin levels (hemoglobin concentration and mass) and (absolute and relative ) among both observational and interventional studies.
Methods
A systematic search was performed on December 6th, 2021. The study procedures and reporting of findings followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Article selection and data abstraction were performed in duplicate by two independent reviewers. Primary outcomes were hemoglobin levels and values (absolute and relative). For observational studies, meta-regression models were performed to examine the relationship between hemoglobin levels and values. For interventional studies, meta-analysis models were performed to determine the change in values (standard paired difference) associated with interventions designed to modify hemoglobin levels or . Meta-regression models were then performed to determine the relationship between a change in hemoglobin levels and the change in values.
Results
Data from 384 studies (226 observational studies and 158 interventional studies) were examined. For observational data, there was a positive association between absolute and hemoglobin levels (hemoglobin concentration, hemoglobin mass, and hematocrit (P<0.001 for all)). Prespecified subgroup analyses demonstrated no apparent sex-related differences among these relationships. For interventional data, there was a positive association between the change of absolute (standard paired difference) and the change in hemoglobin levels (hemoglobin concentration (P<0.0001) and hemoglobin mass (P = 0.006)).
Conclusion
These findings suggest that values are closely associated with hemoglobin levels among both observational and interventional studies. Although our findings suggest a lack of sex differences in these relationships, there were limited studies incorporating females or stratifying results by biological sex.
Introduction
Maximal oxygen uptake () represents the highest rate of oxygen uptake and utilization during large muscle-mass exercise [1]. The concept of was first described by the Nobel prize laureate A.V. Hill in the early 1920’s [2, 3] and is a preeminent physiological variable in the field of human integrative physiology [4–6]. In addition to describing cardiorespiratory fitness among athletes and otherwise healthy humans [7–10], also serves as a powerful prognostic index of both the life span and health span among humans, including those with chronic disease [11, 12]. Although there is debate about which steps along the oxygen transport pathway limit in healthy humans, there is widespread agreement about the key role of oxygen transport via hemoglobin in the blood [13, 14]. In this context, the relationship between hemoglobin levels and in humans has been extensively studied [15–17].
Rationale
Many different approaches have been used to study the relationship between hemoglobin levels and in humans, including both observational studies and interventional studies. When designing a study to estimate the causal effect of an intervention on a physiological outcome (for example, the change in hemoglobin levels on ), interventional studies are generally considered to be the least susceptible to bias [18]. For interventional studies, researchers control the assignment of the treatment or exposure which may limit bias associated with unmeasured confounders. Observational study designs, however, are more susceptible to unmeasured confounding influences, and generally do not involve a researcher-controlled intervention. Thus, interventional and observational study designs are inherently different from an epistemological standpoint [19]. Although a strong relationship between hemoglobin levels and in humans has been demonstrated in both observational studies [16, 20, 21] and interventional studies [22–24], some uncertainty remains. As an example, although it is clear that an increase in hemoglobin levels is associated with an increase in (a process referred to as ‘blood doping’ in sport [24]), uncertainty remains about the potential relationship between hemoglobin levels and among other interventions (e.g., dietary supplementation). In this framework, there are ambiguous areas associated with biological diversity and heterogenous study designs, and an unbiased synthesis of information may provide key insights from the scientific corpus [25]. Thus, the present work was performed to provide a comprehensive systematic review and meta-analysis on the relationship between hemoglobin levels and in humans.
Objectives
This systematic review and meta-analysis aimed to evaluate the relationship between hemoglobin levels and by pooling data from observational studies and interventional studies. Moreover, as a secondary objective, prespecified analyses aimed to determine whether this relationship is influenced by population characteristics (i.e., biological sex) or intervention characteristics (subsequently defined in methods section) which may modify hemoglobin levels or .
Methods
Registration and protocol
This systematic review and meta-analysis followed the recommendations of and reported findings according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [26]. The study protocol has been registered in the International Prospective Register of Systematic Reviews, ‘PROSPERO’, (CRD42022329010). All changes to the study protocol are reported in the Methods section. In accordance with the Code of Federal Regulations, 45 CFR 46.102, this study was exempt from obtaining institutional review board approval from Mayo Clinic and from obtaining informed patient consent because it represents secondary use of publicly available data sets.
Information sources and search strategy
On December 6, 2021, MEDLINE was searched for eligible articles by three authors (S.A.K., C.C.W., J.W.S.). Keywords used in the search included: [(oxygen consumption) OR (oxygen uptake) OR (VO2) OR (VO2max)] AND [(hemoglobin) OR (hematocrit) OR (hemodilution) OR (transfusion) OR (venesection) OR (anemia) OR (polycythemia) OR (erythrocythemia) OR (erythropoietin) OR (blood loss) OR (blood doping)] AND (exercise). Only published material was identified and exported to a web-based systematic review management software (Covidence, Melbourne, Australia). The search was not limited by study design or publication period. To be eligible for inclusion, full-text translations must have been available in English. References of included articles were examined for potential inclusion.
Eligibility criteria
Eligible participants included healthy adults (age 18 years or older) and adults with disordered erythropoiesis (anemia, polycythemia, iron deficiency with anemia, iron deficiency without anemia, or thalassemia) without secondary pathologies.
Eligible articles met prespecified inclusion criteria. Eligible articles reported hemoglobin levels (hemoglobin concentration or hemoglobin mass) and achieved during exercise. Eligible tests typified a standard protocol and included a graded or incremental exercise test to task failure with continuous pulmonary gas exchange measurements. Task failure criteria included a combination of at least two of the following criteria: 1) “classical” plateau in ; 2) elevated respiratory exchange ratio (RER, greater than 1.0, 1.1, or 1.15); 3) near maximal, self-reported rating of perceived effort (e.g., Borg Scale6-20 ≥ 17); and 4) maximum heart rate greater than 90% of age-predicted maximum heart rate. In this framework, studies that assessed or estimated were not eligible for inclusion.
Eligible exercise modalities which may be associated with an accurate representation of included running, cycling, swimming, rowing, inclined walking, and exercise using a large muscle-mass ergometer (ski, kayak, or row ergometer). Eligible tests were performed in standard environmental conditions. Thus, ineligible tests included tests performed using non-normoxic inspirates, an altitude greater than approximately sea level, or substantial deviation from room temperature (~20°C).
Eligible hemoglobin concentrations were determined using blood samples obtained via venipuncture or arterial catheterization. Measurements of hemoglobin concentration are associated with both good stability and validity [27, 28], and thus, are often considered the gold-standard measurement of circulating hemoglobin. Notably, however, there is heterogeneity in methodological assessments and associated biological variability due to factors such as the source of blood sample, body positioning during blood collection, circadian variation, and measurement device [29]. Eligible hemoglobin mass values were determined using either a carbon monoxide rebreathing protocol [30] or dye dilution with a fluorescent or radioactive tracer [31]. Similar to measurements of hemoglobin concentration; however, these techniques are subject to considerable heterogeneity in methodological assessment. Additionally, confounding physiological factors such as changes in circulating volumes and incomplete distribution/mixing of the tracer may negatively influence the reliability of these techniques [32].
Selection process and data collection process
Both the selection process and data abstraction process were performed in duplicate and independently by two reviewers from a cohort of seven potential reviewers (K.L.W., E.K.G., O.H.M, S.A.K., R.J.R, S.M.H, and J.W.S.). Conflicts regarding article selection and data abstraction were independently verified by a third reviewer. Disagreements were discussed until consensus. Further information on the article selection process is presented in Fig 1.
Fig 1. PRISMA flow diagram.
Flow diagram displaying the selection of articles through different phases of the systematic review, and categorization of included articles.
Data items
Data abstraction was performed using a standardized data abstraction form. Abstracted data included participant characteristics (sample size, sex, age, height, weight, body mass index, absolute , and relative ) and hematological characteristics (hemoglobin levels (both hemoglobin concentration and hemoglobin mass), and hematocrit) as available.
If an article reported multiple measurements of for the same condition associated with a single measurement of hemoglobin level, the greatest value of was used for analyses. If an article reported hematological data from both arterial and venous blood samples, data associated with the arterial blood sample(s) was used for analyses.
If data associated with primary outcomes (hemoglobin levels and values) were not numerically reported in the text, authors were contacted via email to obtain numerical data. If the data could not be provided but were presented in figures, WebPlotDigitizer [33] was used to extract means and standard deviations. If data were reported with standard error (SE), the following equation was used to convert data to standard deviation (SD): .
Article categorization
Included articles were categorized as observational or interventional according to study design [18]. Observational articles included studies which reported the primary outcomes (hemoglobin levels and values) without respect to a defined event or intervention. Interventional articles included allocation to a clearly defined event or intervention and reported the primary outcomes (hemoglobin levels and values) before and after the clearly defined event or intervention. Interventional articles were further categorized into five mutually exclusive subgroups according to the intervention: 1) blood transfusion or donation, 2) erythropoiesis stimulating agents, 3) dietary supplementation, 4) environmental hypoxia, or 5) aerobic (de)training.
Study risk of bias assessment and reporting bias assessment
Risk of bias assessment was conducted using the Risk Of Bias In Non-Randomized Studies—of Interventions (ROBINS-I) [34] for interventional studies and the Risk Of Bias In Non-randomized Studies–of Exposures (ROBINS-E) [35] for observational studies. Two reviewers from a cohort of three potential reviewers (K.L.W., E.K.G, J.W.S.) applied the risk of bias assessment independently, and data were independently verified by a third reviewer. Disagreements were discussed until consensus. Egger’s [regression] test was used to assess potential publication bias in meta-analyses.
Effect measures
Applied meta-regressions used absolute and relative as the effect measure. Meta-analysis models used the standard paired difference as the effect measure to quantify changes in following a performed intervention. The tau-squared (τ2) statistic was used to estimate between-study variance and the Higgins I-squared statistic (I2) was used to estimate relative heterogeneity within meta-analyses [36].
Synthesis methods
For the primary outcomes (hemoglobin levels and values), we performed meta-analysis and meta-regression using random-effects models to account for variability between studies [37]. Analyses were performed separately for observational studies and interventional studies. Outcome data were pooled from each study to provide one single data point. A minimum of two studies were required to perform meta-analyses [38]. Additional prespecified, exploratory analyses were performed stratified by biological sex by pooling data for male and female subgroups within each study as available. Analyses were performed using Comprehensive Meta-Analysis software (CMA 3.3, Biostat, Englewood, New Jersey, USA). Figures were created using SigmaPlot software (SigmaPlot 12.5, Systat Software Inc, Chicago, Illinois, USA). Results are reported with 95% confidence intervals (CIs), statistical significance was set at α = 0.05, and all tests were two-tailed.
Observational studies
For analyses of observational studies, an amalgam was created using all observational articles and data associated with interventional articles that were measured before the defined event or intervention (so called “baseline data”). The primary analysis investigated the relationship between hemoglobin levels and values. Because hemoglobin level was described by two metrics (hemoglobin concentration and hemoglobin mass) and was described by two metrics (absolute and relative ), the primary analysis was associated with four separate comparisons. Meta-regression models were performed to determine heterogeneity of values associated with hemoglobin levels. Exploratory meta-regression models were then performed with subgroups stratified by biological sex. Results of meta-regression models are presented as (slope coefficient; 95% Confidence Interval (CI); R2; τ2; P-value; n (number of studies or subgroups)).
Interventional studies
For analyses of interventional studies, standard paired difference was used to quantify the change of absolute and relative (separately) in response to the defined, researcher-controlled event or intervention. Meta-analyses were performed for subgroups of interventional studies as defined in the ‘Article Categorization’ section herein. Results of meta-analyses are presented as (pooled standard paired difference; 95% CI; Z-value; τ2; P-value; n (number of studies)).
Meta-regression models were performed to assess the relationship between study-level covariates (hemoglobin levels) and the effect size of the change in values (standard paired difference). Similar to analyses of observational studies, the primary meta-regression models were associated with four separate comparisons because both hemoglobin levels and values are described by two separate variables.
Results
Study selection and study characteristics
The process of study selection and article categorization is represented in the PRISMA flow diagram (Fig 1). A total of 3,625 articles were identified in the initial literature search, and duplicates were removed (n = 1). After screening titles and abstracts, 2,550 articles were deemed ineligible for inclusion. Of the remaining 1,074 full text articles, 381 articles were included. The references of the included 381 articles were then carefully inspected, and an additional 3 articles were deemed eligible for inclusion. Thus, 384 articles were included. Of the 384 included articles, 226 articles were categorized as observational [15, 20, 39–262], and 158 articles were categorized as interventional. The 158 interventional articles were further categorized into five mutually exclusive subgroups according to the intervention: 1) blood transfusion or donation (n = 29) [263–291], 2) erythropoiesis stimulating agents (n = 19) [292–310], 3) dietary supplementation (n = 25) [311–335], 4) environmental hypoxia (n = 40) [336–375], or 5) aerobic (de)training (n = 45) [376–420].
Ten of the included articles reported data for participants with disordered erythropoiesis (n = 6 beta-thalassemia, n = 2 iron deficiency anemia, n = 2 iron deficiency non-anemia). Within these ten articles, no measurements of hemoglobin mass were reported.
Risk of bias in studies
The results of the risk of bias assessment for observational articles and interventional articles are presented in S1 and S2 Tables, respectively. Among observational articles, 83% (188 of 226 articles) had a low risk of bias, 12% (28 of 226 articles) had a moderate risk of bias, and 4% (10 of 226 articles) had a high risk of bias. The moderate and high risk of bias were primarily associated with selection of participants and selection of reported results, primarily owing to the inclusion of participants with disordered erythropoiesis. Among interventional articles, 86% (136 of 158 articles) had a low risk of bias, 13% (20 of 158 articles) had a moderate risk of bias, and 1% (2 of 158 articles) had a high risk of bias. The moderate and high risk of bias were primarily associated with missing data and selection of reported results.
Egger’s regression tests demonstrated no significant publication bias among any interventional subgroups, including: 1) blood transfusion or donation (intercept, -0.805; P = 0.558; n = 29 studies), 2) erythropoiesis stimulating agents (intercept, 1.573; P = 0.136; n = 19 studies), 3) dietary supplementation (intercept, -1.070; P = 0.394; n = 25 studies), 4) environmental hypoxia (intercept, 0.290; P = 0.768; n = 40 studies), and 5) aerobic (de)training (intercept, 0.307; P = 0.741; n = 45 studies).
Results of syntheses
Observational analyses
For observational analyses, data were amalgamated from 226 observational articles and “baseline data” from 158 interventional articles. Observational data included 5,990 male participants and 3,310 female participants. Of the 384 included articles, 228 articles reported only data associated with male participants, 52 articles reported only data associated with female participants, 78 articles reported pooled data for males and females, and 26 articles reported data stratified by biological sex.
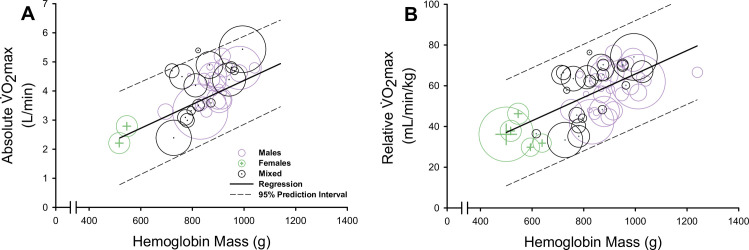
Meta-regression models demonstrated a strong, positive association between study-level data of values and hemoglobin levels (hemoglobin mass (Fig 2) and hemoglobin concentration (S1 Fig)), see Table 1. For data obtained from participants with disordered erythropoiesis, meta-regression models demonstrated a positive association between study-level data of hemoglobin concentration and absolute (slope coefficient, 0.255; 95% CI, 0.018 to 0.491; R2 = 0.42; τ2 = 0.28; P = 0.035; n = 6 studies). When excluding participants with disordered erythropoiesis, a positive association between hemoglobin concentration and absolute remained (slope coefficient, 0.402; 95% CI, 0.273 to 0.532; R2 = 0.28; τ2 = 0.79; P<0.0001; n = 193 studies).
Fig 2. The association between hemoglobin mass and maximal oxygen uptake ().
Bubble plots and meta-regressions displaying the positive association between hemoglobin mass and both absolute (A) and relative (B). Data for males are represented as purple bubbles, data for females are represented as green bubbles with plus sign symbols, and data for studies presenting males and females pooled (mixed) are represented as black bubbles with middle dot symbols. Each bubble represents a group from a single study and the size of bubbles represents the number of participants within the group. The solid line indicates the meta-regression line, and the dashed lines indicate the 95% prediction interval associated with the meta-regression.
Table 1. Hemoglobin levels as covariates of absolute and relative maximal oxygen uptake () derived from observational analyses.
| Absolute (L·min-1) | |||||
|---|---|---|---|---|---|
| Coefficient [95% CI] | P value | R 2 | τ 2 | n | |
| Hemoglobin mass | |||||
| All data | 0.005 [0.003, 0.007] | <0.0001 | 0.49 | 0.34 | 42 |
| Males | 0.006 [0.004, 0.009] | <0.0001 | 0.66 | 0.41 | 23 |
| Females | 0.011 [0.008, 0.015] | <0.0001 | 0.92 | 0.31 | 8 |
| Hemoglobin concentration | |||||
| All data | 0.404 [0.298, 0.510] | <0.0001 | 0.29 | 0.77 | 199 |
| Males | 0.148 [0.022, 0.274] | 0.022 | 0.15 | 0.51 | 141 |
| Females | 0.226 [0.060, 0.393] | 0.008 | 0.22 | 0.31 | 41 |
| Relative (mL·min -1 ·kg -1 ) | |||||
| Hemoglobin mass | |||||
| All data | 0.058 [0.039, 0.077] | <0.0001 | 0.55 | 130.27 | 58 |
| Males | 0.054 [0.024, 0.084] | <0.0001 | 0.19 | 67.75 | 37 |
| Females | 0.137 [0.016, 0.258] | 0.027 | 0.34 | 28.44 | 8 |
| Hemoglobin concentration | |||||
| All data | 4.649 [2.650, 6.648] | <0.0001 | 0.10 | 121.87 | 284 |
| Males | 0.882 [-1.120, 2.884] | 0.388 | 0.02 | 83.77 | 175 |
| Females | 5.999 [3.249, 8.728] | <0.0001 | 0.45 | 67.59 | 63 |
Data depict results of independent meta-regressions in determination of hemoglobin levels (hemoglobin mass and hemoglobin concentration) as covariates of absolute and relative . Data were pooled from each study to provide one single data point for ‘All data’ derived coefficients (n denotes the number of studies). Additional independent analyses were performed for subgroups of data reporting males only and females (n denotes the number of subgroups). Abbreviations: CI, confidence interval.
Meta-regression models also demonstrated a positive association between study-level data of hematocrit and both absolute (slope coefficient, 0.146; 95% CI, 0.091 to 0.200; R2 = 0.19; τ2 = 1.14; P<0.0001; n = 136 studies) and relative (slope coefficient, 1.449; 95% CI, 0.965 to 1.932; R2 = 0.20; τ2 = 105.69; P<0.0001; n = 195 studies; S2 Fig). Exploratory analyses stratifying meta-regression models by biological sex found no sex differences in the relationship between hemoglobin mass and values (Table 1).
Interventional analyses
For analyses of interventional articles, data from 158 articles were included. From pooled interventional data, the meta-analysis model demonstrated a significant increase in absolute following the performed interventions (pooled standard paired difference, 0.183; 95% CI, 0.056 to 0.310; Z = 2.829; τ2 = 0.24; I2 = 11%; P = 0.005; n = 106 studies). The meta-regression model demonstrated a positive association between the change in hemoglobin concentration and the effect size of the change in absolute (standard paired difference), (slope coefficient, 0.271; 95% CI, 0.158 to 0.385; R2 = 0.25; τ2 = 0.19; P<0.0001; n = 100 studies). An additional meta-regression model demonstrated a positive association between the change in hemoglobin mass and the effect size of the change in absolute (standard paired difference), (slope coefficient, 0.008; 95% CI, 0.002 to 0.014; R2 = 0.04; τ2 = 0.31; P = 0.006; n = 24 studies). The 158 interventional articles were further categorized into five mutually exclusive subgroups according to the intervention. Thus, prespecified subanalyses were performed to examine the relationship between changes in hemoglobin levels and changes in values within the interventional subgroups, independently.
Blood transfusion or donation
Analyses included 29 articles associated with blood transfusion or donation. Of the 29 articles, 7 articles examined blood transfusion, 17 articles examined blood donation, and 5 articles examined both blood transfusion and donation. Overall, data were reported for a total of 310 male participants and 84 female participants. Only one article reported data for females only, limiting potential analyses stratified by sex.
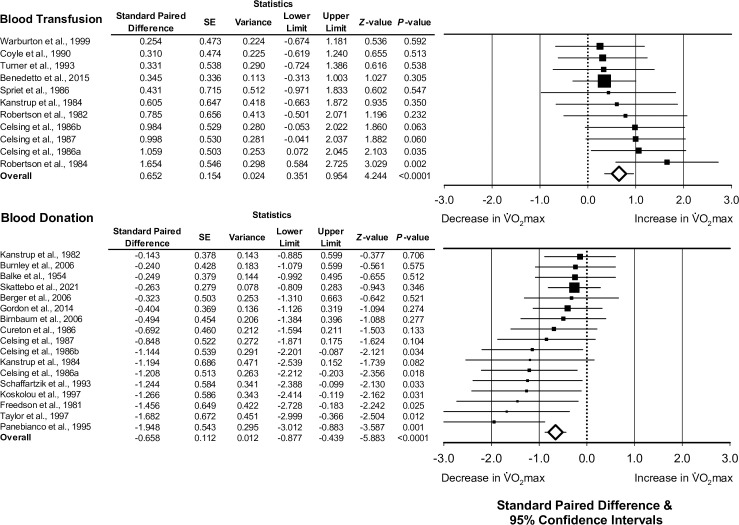
For interventional articles associated with blood transfusion, the median amount of blood transfused was 500 mL (range, 400 to 900 mL). Data for the meta-analysis of blood transfusion studies were associated with an increase in absolute (pooled standard paired difference, 0.652; 95% CI, 0.351 to 0.954; Z = 4.244; τ2 = 0.02; I2 = 2%; P<0.0001; n = 11 studies; Fig 3). When removing one study examining blood transfusion among a clinical population (participants with beta-thalassemia), a significant increase in absolute following transfusion remained (pooled standard paired difference, 0.734; 95% CI, 0.395 to 1.073; Z = 4.245; τ2 = 0.03; I2 = 3%; P<0.0001; n = 10 studies).
Fig 3. The association between blood donation or transfusion and change in maximal oxygen uptake (absolute ).
Forest plots depicting the effect size of the change of absolute (standard paired difference) following blood transfusion or donation. Different size symbols indicate relative weights used in meta-analyses and are proportional to study size. Abbreviations: SE, standard error.
For articles associated with blood donation, the median amount of blood drawn from the donor was 500 mL (range, 300 to 1350 mL). Data for the meta-analysis of blood donation studies were associated with a decrease in absolute (pooled standard paired difference, -0.658; 95% CI, -0.877 to -0.439; Z = -5.883; τ2 = 0.07; I2 = 39%; P<0.0001; n = 17 studies; Fig 3).
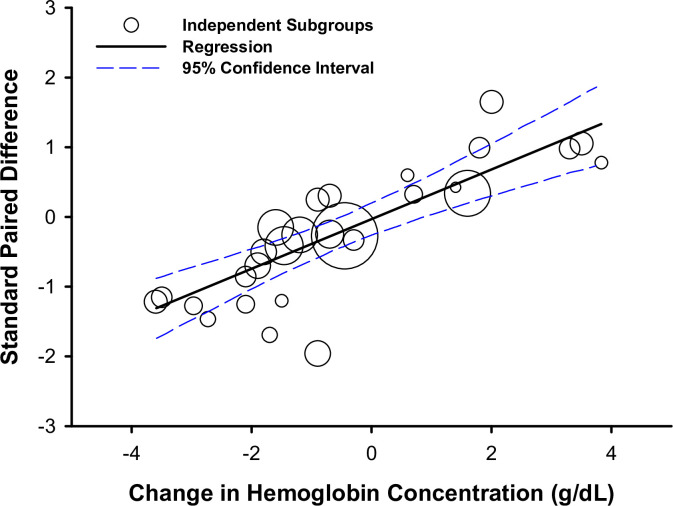
As described above, the 29 articles included in this section were associated with 22 datasets examining blood transfusion and 12 datasets examining blood donation (34 subgroups). The overall meta-regression model, including both blood transfusion and blood donation, demonstrated a positive association between the change in hemoglobin concentration and the effect size of the change in absolute (standard paired difference), (slope coefficient, 0.357; 95% CI, 0.260 to 0.454; R2 = 0.99; τ2<0.01; P<0.0001; n = 28 subgroups; Fig 4A).
Fig 4. The association between change in hemoglobin concentration and change in absolute maximal oxygen uptake () following blood donation or transfusion.
Meta-regression and bubble plot depicting the relationship between the change in hemoglobin concentration and the effect size of the change in absolute (standard paired difference) among independent subgroups following blood transfusion or donation. Different size symbols indicate relative weights used in the meta-regression and are proportional to study sample size.
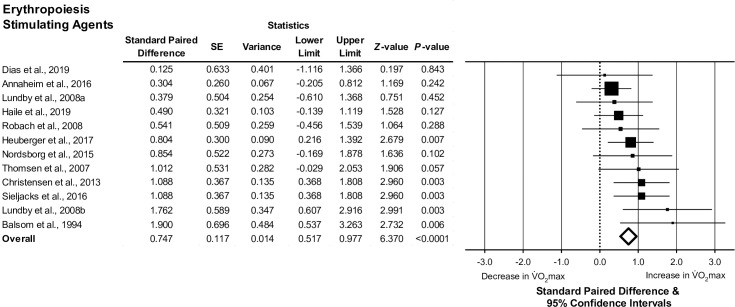
Erythropoiesis stimulating agents
Analyses included 19 articles associated with erythropoiesis stimulating agents, 18 articles examined erythropoietin supplementation and one article examined xenon inhalation. Of the 19 articles, data were reported for a total of 230 male participants and 17 female participants. No articles reported data for females only, limiting potential analyses stratified by sex. The meta-analysis model demonstrated an increase in absolute following administration of erythropoieses stimulating agents (pooled standard paired difference, 0.740; 95% CI, 0.510 to 0.969; Z = 6.315; τ2 = 0.03; I2 = 1%; P<0.0001; n = 12 studies; Fig 5). For studies examining the effects of erythropoietin alone, there was a significant increase in absolute following treatment (pooled standard paired difference, 0.789; 95% CI, 0.526 to 1.052; Z = 5.873; τ2 = 0.03; I2 = 1%; P<0.0001; n = 11 studies). Following treatment with erythropoiesis stimulating agents, the relationships between the change in hemoglobin concentration and changes in values were not statistically significant (absolute , P = 0.150; relative , P = 0.050).
Fig 5. The association between erythropoiesis stimulating agents and change in maximal oxygen uptake (absolute ).
Forest plot depicting the effect size of the change in absolute (standard paired difference) following administration of erythropoiesis stimulating agents. Different size symbols indicate relative weights used in meta-analyses and are proportional to study size. Abbreviations: SD, standard error.
Dietary supplementation
Analyses included 25 articles associated with dietary supplementation—15 articles examined iron supplementation, 4 articles examined plant-based supplementation (echinacea (n = 2), beetroot (n = 1), and higenamine (n = 1)), 3 articles examined dietary restrictions (ketogenic diet (n = 1), caloric deficit (n = 1), and time restricted feeding (n = 1)), and 3 articles examined other dietary supplements (sodium phosphate (n = 1), vitamin C (n = 1), and beta-methylbutyrate (n = 1)). Of the 25 articles, data were reported for a total of 190 male participants and 305 female participants. Six articles reported data for males only, 13 articles reported data for females only, 4 articles reported pooled data for males and females, and 2 articles reported data stratified by biological sex. Due to an insufficient number of studies, stratified analyses were only performed to separately examine the effects of iron and echinacea supplementation.
There was a broad spectrum of heterogenous substances used for dietary supplementation, as described above. Thus, the change in hemoglobin concentration associated with dietary supplementation was heterogenous and ranged from a decrease of 1.2 g/dL to an increase of 1.3 g/dL (median, increase of 0.1 g/dL). The meta-analysis model demonstrated an increase in absolute following dietary supplementation (pooled standard paired difference, 0.274; 95% CI, 0.084 to 0.464; Z = 2.827; τ2 = 0.89; I2 = 69%; P = 0.005; n = 16 studies). Additionally, there was an increase in absolute following iron supplementation (pooled standard paired difference, 0.341; 95% CI, 0.050 to 0.631; Z = 2.301; τ2 = 1.37; I2 = 31%; P = 0.021; n = 9 studies). Among the two studies examining the effects of echinacea supplementation, data regarding absolute were not reported. There was no significant change in relative following echinacea supplementation (pooled standard paired difference, 0.330; 95% CI, -0.118 to 0.778; Z = 1.444; τ2 = 0.29; I2 = 74%; P = 0.149; n = 2 studies).
Meta-regressional analysis found no significant relationship between the effect size of the change in absolute (standard paired difference) and the change in hemoglobin concentration (P = 0.200).
Environmental hypoxia
Analyses included 40 articles associated with environmental hypoxia, 29 articles examined high-altitude acclimatization or sojourn and 11 articles examined chronic normobaric hypoxia. Of the 40 articles, data were reported for a total of 454 male participants and 80 female participants. Only one article reported data for females only, limiting potential analyses stratified by sex. Of the 40 included articles, 13 articles reported a change in hemoglobin mass. The median change in hemoglobin mass was 20 g increase from baseline, and there was a broad range across studies (16 g decrease to 55 g increase).
The meta-analysis model demonstrated an increase in absolute associated with environmental hypoxia (pooled standard difference, 0.203; 95% CI, 0.027 to 0.379; Z = 2.263; τ2 = 0.20; I2 = 5%; P = 0.024; n = 24 studies). Of the articles examining high-altitude sojourn or acclimatization, there was no significant change in absolute (pooled standard difference, 0.004; 95% CI, -0.217 to 0.224; Z = 0.031; τ2 = 0.13; I2 = 4%; P = 0.975; n = 16 studies). For articles examining the effects of chronic normobaric hypoxia, there was a significant increase in absolute (pooled standard difference, 0.550; 95% CI, 0.259 to 0.841; Z = 3.702; τ2 = 0.01; I2 = 2%; P<0.0001; n = 8 studies). Meta-regressional analysis demonstrated no significant relationship between the effect size of the change in (standard paired difference) and the change in hemoglobin concentration (P = 0.854) or the change in hemoglobin mass (P = 0.531) after environmental hypoxia.
Aerobic (de)training
Analyses included 45 articles associated with aerobic (de)training, 36 articles examined aerobic training and 9 articles examined aerobic detraining. Of the 45 articles, data were reported for a total of 495 male participants and 167 female participants, and 9 articles reported data for females alone. The median duration of aerobic training was 5.5 weeks (range, 1 to 22 weeks) and the median duration of detraining was 6 weeks (range, 1 to 52 weeks).
The meta-analysis model demonstrated an increase in absolute following aerobic training (pooled standard paired difference, 0.544; 95% CI, 0.378 to 0.709; Z = 6.452; τ2 = 0.24; I2 = 41%; P<0.0001; n = 23 studies). A separate meta-analysis model demonstrated a decrease in absolute following aerobic detraining (pooled standard paired difference, -0.800; 95% CI, -1.088 to -0.511; Z = -5.431; τ2 = 0.04; I2 = 2%; P<0.0001; n = 7 studies). Pooling data from both aerobic training and detraining, there was no relationship between the change in hemoglobin concentration and the effect size of the change in absolute (P = 0.255).
Discussion
This systematic review and meta-analysis found that maximal oxygen uptake () was positively associated with hemoglobin levels (both hemoglobin mass and hemoglobin concentration). This strong association between and hemoglobin was observed in both observational studies and interventional studies. Given that oxygen transport to the muscle depends, in part, on hemoglobin levels, the strong relationship between and hemoglobin is not surprising [16, 22, 263]. Notably, this relationship between and hemoglobin was observed across a broad range of studies and was not different between males and females. These findings suggest that although complex regulation is associated with oxygen transport to muscle (including several convective and diffusive stages), there is a robust relationship between and hemoglobin due to the central role of hemoglobin in oxygen transport.
Observational analyses
Altogether, observational analyses highlight the importance of hemoglobin levels in determination of . Analyses demonstrate a positive association between hemoglobin levels (hemoglobin mass and hemoglobin concentration) and . In addition, there was also a positive relationship between hematocrit and . However, we must acknowledge that these analyses are generally constrained to a range of ‘healthy’ hematocrit values from 30% to 55%. At hematocrit values greater than 55%, there may be a plateau, or decrease in due to increased blood viscosity and added resistance to skeletal muscle and pulmonary perfusion [421, 422]. This limitation may also be apparent when examining hemoglobin mass and hemoglobin concentration in relation to . Under conditions of large values of hemoglobin mass or concentration, it is possible that other physiological factors (i.e., oxygen diffusion capacity, cardiac output, and mitochondrial capacity) would limit .
There are well-known sex differences in physiological determinants of [423–425]—on average, males have more muscular strength/power, greater cardiac output, greater lung volume, and greater hemoglobin values (both hemoglobin concentration and hemoglobin mass), and thus greater compared to females [426, 427]. Therefore, the question remains of whether the observed relationship between hemoglobin levels and is driven by differences in hemoglobin levels, rather than simply by sex differences. When performing meta-regressional analyses stratified for biological sex, there were no sex-related differences in the association between hemoglobin levels and . These results suggest that the relationship between hemoglobin levels and VO2max, particularly the derived regression coefficients, are similar between sexes.
Interventional analyses
We sought to examine the relationship between hemoglobin levels and values following researcher-controlled interventions designed to modify hemoglobin levels or . When pooling all interventional data, our analyses demonstrated the change in absolute is positively associated with changes in hemoglobin levels, consistent with previous findings and general physiological principles [15, 22, 263]. Among studies with a direct physiological manipulation of hemoglobin levels (interventional blood transfusion or donation), a 1 g·dL-1 change in hemoglobin concentration corresponded to a ~5% change in absolute . Notably, alterations in both blood volume and plasma volume among the blood donors and recipients likely contribute to the observed change in VO2max.
Interventional studies that did not involve a direct physiological manipulation of hemoglobin levels had more divergent findings. Overall, there was no significant relationship between the change in hemoglobin concentration and subsequent percent change in absolute within studies examining erythropoieses stimulating agents, dietary supplementation, environmental hypoxia, or aerobic (de)training. These findings may be due, in part, to potential alterations in blood and plasma volumes that may occur during these interventions [93, 263, 402]. For instance, high-altitude acclimatization is associated with considerable interindividual variability in plasma volume reduction (and blood volume reduction) [428], which often leads to a greater hemoglobin concentration without significant changes in hemoglobin mass. However, a reduction in blood volume limits maximum cardiac output, blunting improvements in [429, 430]. In a similar instance, aerobic training often improves , despite a reduction in hemoglobin concentration due to increased blood volume [431, 432]. Therefore, the association between changes in hemoglobin levels and changes in values is often contingent on alterations of other circulatory parameters such as blood volume and cardiac output during exercise.
Limitations
This systematic review and meta-analysis has several limitations. First, our primary literature search was conducted using one database. Second, we limited the focus of our primary analyses to a single relationship between hemoglobin and , and thus did not consider the potential confounding effects of other relevant physiological factors, such as blood volume, plasma volume, maximum cardiac output, and muscle oxygen diffusivity. Alterations among these variables likely influence the relationship between hemoglobin and . In this context, our analyses were simplistic by design. Additionally, we did not include patient-level data, and this limited the scope of our analytical models. Our methodological approach enabled the inclusion of a heterogenous collection of studies that comprised diverse participant characteristics and study designs. In this framework, including a large diversity of literature may be associated with broad generalizability of these findings, but this approach also limited mechanistic insights that may be gleaned from these analyses. For example, the analyses of dietary supplementation were heterogeneous, potentially owing to between-study differences in the type of dietary supplementation, dose of dietary supplement, duration of intervention, and other putative confounding factors. Our approach did not include these analyses primarily because data were largely unavailable.
Third, the measurement of hemoglobin may be variable. There are several metrics and methods used to quantify hemoglobin which may lead to variability between studies, and the measurement of hemoglobin is associated with biological diversity due to confounding physiological factors, such as changes in circulatory volume, circadian variations, and body positioning during blood collection [29]. In this context, we believe the high level of concordance among study outcomes despite the heterogeneity associated with hemoglobin measurement offers compelling evidence for the relationship between hemoglobin levels and .
Conclusion
This systematic review and meta-analysis found a strong relationship between and hemoglobin levels across a heterogenous pool of studies. Thus, these findings suggest that there is a robust relationship between and hemoglobin levels. These findings offer a comprehensive synthesis of the heterogeneous scientific corpus describing the relationship between hemoglobin and . These broad findings and simplified approach offer a foundation to chart future directions and testable hypotheses in this field.
Supporting information
(PDF)
Bubble plot and meta-regression displaying the positive association between hemoglobin concentration and both absolute (A) and relative (B). Data for males are represented as purple bubbles, data for females are represented as green bubbles with plus sign symbols, and data for studies presenting males and females pooled (mixed) are represented as black bubbles with middle dot symbols. Each bubble represents a group from a single study and the size of bubbles represents the number of participants within the group. The solid line indicates the meta-regression line, and the dashed lines indicate the 95% prediction interval associated with the meta-regression.
(DOCX)
Bubble plot and meta-regression displaying the positive association between hematocrit and both absolute (A) and relative (B). Data for males are represented as purple bubbles, data for females are represented as green bubbles with plus sign symbols, and data for studies presenting males and females pooled (mixed) are represented as black bubbles with middle dot symbols. Each bubble represents a group from a single study and the size of bubbles represents the number of participants within each group. The solid line indicates the meta-regression line, and the dashed lines indicate the 95% prediction interval associated with the meta-regression.
(DOCX)
(DOCX)
(DOCX)
(XLSX)
Acknowledgments
We thank the research teams and investigators for their rigorous efforts that contributed to each of the individual studies that made this review possible. Additionally, we thank members of the Human and Integrative Physiology and Clinical Pharmacology Laboratory at Mayo Clinic for intellectual discussions and feedback on earlier versions of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files. Additionally, the data underlying the results presented in the study are publicly available from the references listed herein.
Funding Statement
This work was supported by the National Heart, Lung, and Blood Institute T-32-HL105355 (K.L.W.), R-35-HL139854 (C.C.W. and M.J.J.), and F-32-HL154320 (J.W.S.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bassett DR, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance: Med Sci Sports Exerc. 2000. Jan;70. [DOI] [PubMed] [Google Scholar]
- 2.Hill AV, Lupton H. Muscular Exercise, Lactic Acid, and the Supply and Utilization of Oxygen. QJM. 1923. Jan 1;os-16(62):135–71. [Google Scholar]
- 3.Hill AV, Long CNH, Lupton H. Muscular exercise, lactic acid and the supply and utilisation of oxygen.—Parts VII–VIII. Proc R Soc Lond Ser B Contain Pap Biol Character. 1924. Dec;97(682):155–76. [Google Scholar]
- 4.Wagner PD. New ideas on limitations to VO2max. Exerc Sport Sci Rev. 2000. Jan 1;28(1):10–4. [PubMed] [Google Scholar]
- 5.Sutton JR. VO2max—new concepts on an old theme. Med Sci Sports Exerc. 1992. Jan 1;24(1):26–9. [PubMed] [Google Scholar]
- 6.Levine BD. ,max: what do we know, and what do we still need to know? J Physiol. 2008. Jan 1;586(Pt 1):25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strasser B, Burtscher M. Survival of the fittest: VO2max, a key predictor of longevity? Front Biosci-Landmark. 2018. Mar 1;23(8):1505–16. doi: 10.2741/4657 [DOI] [PubMed] [Google Scholar]
- 8.Shephard RJ, Allen C, Benade AJ, Davies CT, Di Prampero PE, Hedman R, et al. The maximum oxygen intake. An international reference standard of cardiorespiratory fitness. Bull World Health Organ. 1968;38(5):757–64. [PMC free article] [PubMed] [Google Scholar]
- 9.Wilmore JH, Knuttgen HG. Aerobic Exercise and Endurance. Phys Sportsmed. 2003. May 1;31(5):45–51. [DOI] [PubMed] [Google Scholar]
- 10.Joyner M. Physiological limiting factors and distance running: influence of gender and age on record performances. Exerc Sport Sci Rev. 1993;21:103–33. [PubMed] [Google Scholar]
- 11.Laukkanen JA, Zaccardi F, Khan H, Kurl S, Jae SY, Rauramaa R. Long-term Change in Cardiorespiratory Fitness and All-Cause Mortality: A Population-Based Follow-up Study. Mayo Clin Proc. 2016. Sep 1;91(9):1183–8. doi: 10.1016/j.mayocp.2016.05.014 [DOI] [PubMed] [Google Scholar]
- 12.Booth FW, Roberts CK, Laye MJ. Lack of Exercise Is a Major Cause of Chronic Diseases. In: Comprehensive Physiology [Internet]. John Wiley & Sons, Ltd; 2012. [cited 2022 Sep 3]. p. 1143–211. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/cphy.c110025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mairbäurl H, Weber RE. Oxygen Transport by Hemoglobin. In: Comprehensive Physiology [Internet]. American Cancer Society; 2012. [cited 2021 Sep 13]. p. 1463–89. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/cphy.c080113 [DOI] [PubMed] [Google Scholar]
- 14.Jones JH, Longworth KE, Lindholm A, Conley KE, Karas RH, Kayar SR, et al. Oxygen transport during exercise in large mammals. I. Adaptive variation in oxygen demand. J Appl Physiol. 1989. Aug;67(2):862–70. doi: 10.1152/jappl.1989.67.2.862 [DOI] [PubMed] [Google Scholar]
- 15.Calbet JAL, Rådegran G, Boushel R, Søndergaard H, Saltin B, Wagner PD. Effect of blood haemoglobin concentration on V(O2,max) and cardiovascular function in lowlanders acclimatised to 5260 m. J Physiol. 2002;545(2):715–28. doi: 10.1113/jphysiol.2002.029108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt W, Prommer N. Impact of Alterations in Total Hemoglobin Mass on V˙O2max: Exerc Sport Sci Rev. 2010. Apr;38(2):68–75. [DOI] [PubMed] [Google Scholar]
- 17.Pate RR. Sports Anemia: A Review of the Current Research Literature. Phys Sportsmed. 1983. Feb 1;11(2):115–31. doi: 10.1080/00913847.1983.11708460 [DOI] [PubMed] [Google Scholar]
- 18.de Vocht F, Katikireddi SV, McQuire C, Tilling K, Hickman M, Craig P. Conceptualising natural and quasi experiments in public health. BMC Med Res Methodol. 2021. Feb 11;21:32. doi: 10.1186/s12874-021-01224-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bogan J. Theory and observation in science. 2009. [Google Scholar]
- 20.Hunter GR, Weinsier RL, McCarthy JP, Enette Larson-Meyer D, Newcomer BR. Hemoglobin, muscle oxidative capacity, and VO2max in African-American and Caucasian women. Med Sci Sports Exerc. 2001;33(10):1739–43. doi: 10.1097/00005768-200110000-00019 [DOI] [PubMed] [Google Scholar]
- 21.Woodson RD. Hemoglobin Concentration and Exercise Capacity. Am Rev Respir Dis. 1984. Feb;129(2P2):S72–5. doi: 10.1164/arrd.1984.129.2P2.S72 [DOI] [PubMed] [Google Scholar]
- 22.Calbet JAL, Lundby C, Koskolou M, Boushel R. Importance of hemoglobin concentration to exercise: acute manipulations. Respir Physiol Neurobiol. 2006. Apr 28;151(2–3):132–40. doi: 10.1016/j.resp.2006.01.014 [DOI] [PubMed] [Google Scholar]
- 23.Ekblom BT. Blood boosting and sport. Best Pract Res Clin Endocrinol Metab. 2000. Mar;14(1):89–98. doi: 10.1053/beem.2000.0056 [DOI] [PubMed] [Google Scholar]
- 24.Jones M, Tunstall Pedoe DS. Blood doping—a literature review. Br J Sports Med. 1989. Jun 1;23(2):84–8. doi: 10.1136/bjsm.23.2.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ioannidis JPA. Systematic reviews for basic scientists: a different beast. Physiol Rev. 2023. Jan;103(1):1–5. doi: 10.1152/physrev.00028.2022 [DOI] [PubMed] [Google Scholar]
- 26.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021. Mar 29;n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Billett HH. Hemoglobin and Hematocrit. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations [Internet]. 3rd ed. Boston: Butterworths; 1990. [cited 2021 Mar 7]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK259/ [PubMed] [Google Scholar]
- 28.Whitehead RD Jr, Mei Z, Mapango C, Jefferds MED. Methods and analyzers for hemoglobin measurement in clinical laboratories and field settings. Ann N Y Acad Sci. 2019;1450(1):147–71. doi: 10.1111/nyas.14124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berkow L. Factors affecting hemoglobin measurement. J Clin Monit Comput. 2013. Oct 1;27(5):499–508. doi: 10.1007/s10877-013-9456-3 [DOI] [PubMed] [Google Scholar]
- 30.Siebenmann C, Keiser S, Robach P, Lundby C. CORP: The assessment of total hemoglobin mass by carbon monoxide rebreathing. J Appl Physiol. 2017. Sep;123(3):645–54. doi: 10.1152/japplphysiol.00185.2017 [DOI] [PubMed] [Google Scholar]
- 31.Beutler E, Blume KG, Kaplan JC, Löhr GW, Ramot B, Valentine WN. International Committee for Standardization in Haematology: Recommended Methods for Red-Cell Enzyme Analysis*. Br J Haematol. 1977;35(2):331–40. doi: 10.1111/j.1365-2141.1977.tb00589.x [DOI] [PubMed] [Google Scholar]
- 32.Keiser S, Siebenmann C, Bonne TC, Sørensen H, Robach P, Lundby C. The carbon monoxide re-breathing method can underestimate Hbmass due to incomplete blood mixing. Eur J Appl Physiol. 2013. Sep;113(9):2425–30. doi: 10.1007/s00421-013-2681-0 [DOI] [PubMed] [Google Scholar]
- 33.Rohatgi A. WebPlotDigitizer [Internet]. 2021. Available from: https://automeris.io/WebPlotDigitizer [Google Scholar]
- 34.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016. Oct 12;355:i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan R, Sterne J, Higgins J, Thayer K, Schunemann H, Rooney A, et al. A new instrument to assess Risk of Bias in Non-randomised Studies of Exposures (ROBINS-E): Application to studies of environmental exposure. Cochrane Database of Systematic Reviews. 2017;9(Suppl. 1). [Google Scholar]
- 36.Biggerstaff BJ, Tweedie RL. Incorporating Variability in Estimates of Heterogeneity in the Random Effects Model in Meta-Analysis. Stat Med. 1997;16(7):753–68. doi: [DOI] [PubMed] [Google Scholar]
- 37.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010. Apr;1(2):97–111. doi: 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- 38.Ryan R, Cochrane Consumers and Communication Review Group. Cochrane Consumers and Communication Group: meta-analysis. 2016. Dec; Available from: http://cccrg.cochrane.org/ [Google Scholar]
- 39.Accalai E, Vignati C, Salvioni E, Pezzuto B, Contini M, Cadeddu C, et al. Non-invasive estimation of stroke volume during exercise from oxygen in heart failure patients. Eur J Prev Cardiol. 2020; [DOI] [PubMed] [Google Scholar]
- 40.Agostoni P, Cerino M, Palermo P, Magini A, Bianchi M, Bussotti M, et al. Exercise capacity in patients with beta-thalassaemia intermedia. Br J Haematol. 2005;131(2):278–81. doi: 10.1111/j.1365-2141.2005.05765.x [DOI] [PubMed] [Google Scholar]
- 41.Aguiló A, Tauler P, Pilar Guix M, Villa G, Córdova A, Tur JA, et al. Effect of exercise intensity and training on antioxidants and cholesterol profile in cyclists. J Nutr Biochem. 2003;14(6):319–25. doi: 10.1016/s0955-2863(03)00052-4 [DOI] [PubMed] [Google Scholar]
- 42.Ahlgrim C, Pottgiesser T, Kron J, Duerr H, Baumstark M, Schumacher YO. Relations between haemoglobin mass, cardiac dimensions and aerobic capacity in endurance trained cyclists. J Sports Med Phys Fitness. 2009;49(4):364–71. [PubMed] [Google Scholar]
- 43.Ahmadizad S, Bassami M, Hadian M, Eslami M. Influences of two high intensity interval exercise protocols on the main determinants of blood fluidity in overweight men. Clin Hemorheol Microcirc. 2016;64(4):827–35. doi: 10.3233/CH-168009 [DOI] [PubMed] [Google Scholar]
- 44.Al Asoom LI, Al Makhaita MM, Rafique N, Al Afandi DT, Al Otaibi WM, Alsuwat HS, et al. Effects of (-3.7)α Deletion and Sickle-Cell Trait on Ventilatory and Hemodynamic Responses to Maximum Exercise in Young Saudi Females. J Blood Med. 2020;11:371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Altehoefer C, Schmid A, Büchert M, Ghanem NA, Heinrich L, Langer M. Characterization of hematopoietic bone marrow in male professional cyclists by magnetic resonance imaging of the lumbar spine. J Magn Reson Imaging JMRI. 2002;16(3):284–8. doi: 10.1002/jmri.10157 [DOI] [PubMed] [Google Scholar]
- 46.Anchisi S, Moia C, Ferretti G. Oxygen delivery and oxygen return in humans exercising in acute normobaric hypoxia. Pflugers Arch. 2001;442(3):443–50. doi: 10.1007/s004240100553 [DOI] [PubMed] [Google Scholar]
- 47.Ascha M, Bhattacharyya A, Ramos JA, Tonelli AR. Pulse Oximetry and Arterial Oxygen Saturation during Cardiopulmonary Exercise Testing. Med Sci Sports Exerc. 2018;50(10):1992–7. doi: 10.1249/MSS.0000000000001658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Awad elKarim MA. The effect of schistosomiasis on 24-hour energy expenditure. J Trop Med Hyg. 1986;89(6):303–7. [PubMed] [Google Scholar]
- 49.Balcerek B, Steinach M, Lichti J, Maggioni MA, Becker PN, Labes R, et al. A broad diversity in oxygen affinity to haemoglobin. Sci Rep. 2020. Dec;10(1):16920. doi: 10.1038/s41598-020-73560-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barac-Nieto M, Spurr GB, Maksud MG, Lotero H. Aerobic work capacity in chronically undernourished adult males. J Appl Physiol. 1978;44(2):209–15. doi: 10.1152/jappl.1978.44.2.209 [DOI] [PubMed] [Google Scholar]
- 51.Bárány P, Freyschuss U, Pettersson E, Bergström J. Treatment of anaemia in haemodialysis patients with erythropoietin: long-term effects on exercise capacity. Clin Sci Lond Engl 1979. 1993;84(4):441–7. doi: 10.1042/cs0840441 [DOI] [PubMed] [Google Scholar]
- 52.Baron B, Dekerle J, Robin S, Neviere R, Dupont L, Matran R, et al. Maximal lactate steady state does not correspond to a complete physiological steady state. Int J Sports Med. 2003;24(8):582–7. doi: 10.1055/s-2003-43264 [DOI] [PubMed] [Google Scholar]
- 53.Baron B, Noakes TD, Dekerle J, Moullan F, Robin S, Matran R, et al. Why does exercise terminate at the maximal lactate steady state intensity? Br J Sports Med. 2008;42(10):828–33. doi: 10.1136/bjsm.2007.040444 [DOI] [PubMed] [Google Scholar]
- 54.Bejder J, Bonne TC, Nyberg M, Sjøberg KA, Nordsborg NB. Physiological determinants of elite mountain bike cross-country Olympic performance. J Sports Sci. 2019;37(10):1154–61. doi: 10.1080/02640414.2018.1546546 [DOI] [PubMed] [Google Scholar]
- 55.Bennett-Guerrero E, Lockhart EL, Bandarenko N, Campbell ML, Natoli MJ, Jamnik VK, et al. A randomized controlled pilot study of VO(2) max testing: a potential model for measuring relative in vivo efficacy of different red blood cell products. Transfusion (Paris). 2017;57(3):630–6. doi: 10.1111/trf.13918 [DOI] [PubMed] [Google Scholar]
- 56.Berger N, Cooley D, Graham M, Harrison C, Best R. Physiological Responses and Nutritional Intake during a 7-Day Treadmill Running World Record. Int J Env Res Public Health. 2020;17(16). doi: 10.3390/ijerph17165962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berger N, Cooley D, Graham M, Harrison C, Campbell G, Best R. Consistency Is Key When Setting a New World Record for Running 10 Marathons in 10 Days. Int J Env Res Public Health. 2021;18(22). doi: 10.3390/ijerph182212066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bilé A, Le Gallais D, Mercier B, Martinez P, Ahmaidi S, Préfaut C. Anaerobic exercise components during the force-velocity test in sickle cell trait. Int J Sports Med. 1996;17(4):254–8. doi: 10.1055/s-2007-972842 [DOI] [PubMed] [Google Scholar]
- 59.Bonetti DL, Hopkins WG, Kilding AE. High-intensity kayak performance after adaptation to intermittent hypoxia. Int J Sports Physiol Perform. 2006;1(3):246–60. doi: 10.1123/ijspp.1.3.246 [DOI] [PubMed] [Google Scholar]
- 60.Böning D, Cristancho E, Serrato M, Reyes O, Mora M, Coy L, et al. Hemoglobin mass and peak oxygen uptake in untrained and trained female altitude residents. Int J Sports Med. 2004;25(8):561–8. doi: 10.1055/s-2004-820963 [DOI] [PubMed] [Google Scholar]
- 61.Böning D, Rojas J, Serrato M, Ulloa C, Coy L, Mora M, et al. Hemoglobin mass and peak oxygen uptake in untrained and trained residents of moderate altitude. Int J Sports Med. 2001;22(8):572–8. doi: 10.1055/s-2001-18530 [DOI] [PubMed] [Google Scholar]
- 62.Bonner HW, Tate CA, Buffington CK. Changes in erythrocyte 2,3 diphosphoglycerate in women following short term maximal exercise. Eur J Appl Physiol. 1975;34(4):227–32. doi: 10.1007/BF00999936 [DOI] [PubMed] [Google Scholar]
- 63.Bouten J, Colosio AL, Bourgois G, Lootens L, VAN Eenoo P, Bourgois JG, et al. Acute Apnea Does Not Improve 3-km Cycling Time Trial Performance. Med Sci Sports Exerc. 2020;52(5):1116–25. doi: 10.1249/MSS.0000000000002236 [DOI] [PubMed] [Google Scholar]
- 64.Branch JD 3rd, Pate RR, Bourque SP, Convertino VA, Durstine JL, Ward DS. Effects of exercise mode on hematologic adaptations to endurance training in adult females. Aviat Space Environ Med. 1997;68(9):788–94. [PubMed] [Google Scholar]
- 65.Branch JD 3rd, Pate RR, Bodary PF, Convertino VA. Red cell volume and [erythropoietin] responses during exposure to simulated microgravity. Aviat Space Environ Med. 1998;69(4):347–51. [PubMed] [Google Scholar]
- 66.Buick FJ, Gledhill N, Froese AB, Spriet L, Meyers EC. Effect of induced erythrocythemia on aerobic work capacity. J Appl Physiol. 1980;48(4):636–42. doi: 10.1152/jappl.1980.48.4.636 [DOI] [PubMed] [Google Scholar]
- 67.Burden RJ, Pollock N, Whyte GP, Richards T, Moore B, Busbridge M, et al. Effect of Intravenous Iron on Aerobic Capacity and Iron Metabolism in Elite Athletes. Med Sci Sports Exerc. 2015;47(7):1399–407. doi: 10.1249/MSS.0000000000000568 [DOI] [PubMed] [Google Scholar]
- 68.Burtscher M, Nachbauer W, Wilber R. The upper limit of aerobic power in humans. Eur J Appl Physiol. 2011;111(10):2625–8. doi: 10.1007/s00421-011-1885-4 [DOI] [PubMed] [Google Scholar]
- 69.Calbet JAL, Boushel R, Rådegran G, Søndergaard H, Wagner PD, Saltin B. Determinants of maximal oxygen uptake in severe acute hypoxia. Am J Physiol-Regul Integr Comp Physiol. 2003. Feb 1;284(2):R291–303. doi: 10.1152/ajpregu.00155.2002 [DOI] [PubMed] [Google Scholar]
- 70.Calbet JAL, Boushel R, Rådegran G, Søndergaard H, Wagner PD, Saltin B. Why is VO2max after altitude acclimatization still reduced despite normalization of arterial O2 content? Am J Physiol-Regul Integr Comp Physiol. 2003. Feb 1;284(2):R304–16. doi: 10.1152/ajpregu.00156.2002 [DOI] [PubMed] [Google Scholar]
- 71.Calbet JAL, Rådegran G, Boushel R, Søndergaard H, Saltin B, Wagner PD. Plasma volume expansion does not increase maximal cardiac output or VO2 max in lowlanders acclimatized to altitude. Am J Physiol Heart Circ Physiol. 2004;287(3):H1214–24. doi: 10.1152/ajpheart.00840.2003 [DOI] [PubMed] [Google Scholar]
- 72.Calbet JAL, Losa-Reyna J, Torres-Peralta R, Rasmussen P, Ponce-González JG, Sheel AW, et al. Limitations to oxygen transport and utilization during sprint exercise in humans: evidence for a functional reserve in muscle O2 diffusing capacity. J Physiol. 2015;593(20):4649–64. doi: 10.1113/JP270408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Caldemeyer KS, Smith RR, Harris A, Williams T, Huang Y, Eckert GJ, et al. Hematopoietic bone marrow hyperplasia: correlation of spinal MR findings, hematologic parameters, and bone mineral density in endurance athletes. Radiology. 1996;198(2):503–8. doi: 10.1148/radiology.198.2.8596857 [DOI] [PubMed] [Google Scholar]
- 74.Caldwell JT, Wardlow GC, Branch PA, Ramos M, Black CD, Ade CJ. Effect of exercise-induced muscle damage on vascular function and skeletal muscle microvascular deoxygenation. Physiol Rep. 2016. Nov;4(22):e13032. doi: 10.14814/phy2.13032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carr AJ, Saunders PU, Vallance BS, Garvican-Lewis LA, Gore CJ. Increased Hypoxic Dose After Training at Low Altitude with 9h Per Night at 3000m Normobaric Hypoxia. J Sports Sci Med. 2015;14(4):776–82. [PMC free article] [PubMed] [Google Scholar]
- 76.Chanda M, Nantakomol D, Suksom D, Palasuwan A. Cell-derived microparticles after exercise in individuals with G6PD Viangchan. Clin Hemorheol Microcirc. 2015;60(2):241–51. [DOI] [PubMed] [Google Scholar]
- 77.Ciekot-Sołtysiak M, Kusy K, Podgórski T, Zieliński J. Training-induced annual changes in red blood cell profile in highly-trained endurance and speed-power athletes. J Sports Med Phys Fitness. 2018;58(12):1859–66. doi: 10.23736/S0022-4707.17.07819-7 [DOI] [PubMed] [Google Scholar]
- 78.Connes P, Sara F, Hardy-Dessources MD, Marlin L, Etienne F, Larifla L, et al. Effects of short supramaximal exercise on hemorheology in sickle cell trait carriers. Eur J Appl Physiol. 2006;97(2):143–50. doi: 10.1007/s00421-006-0155-3 [DOI] [PubMed] [Google Scholar]
- 79.Córdova A, Mielgo-Ayuso J, Fernandez-Lazaro CI, Caballero-García A, Roche E, Fernández-Lázaro D. Effect of Iron Supplementation on the Modulation of Iron Metabolism, Muscle Damage Biomarkers and Cortisol in Professional Cyclists. Nutrients. 2019;11(3):500. doi: 10.3390/nu11030500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cordova Martinez A, Escanero JF. Iron, transferrin, and haptoglobin levels after a single bout of exercise in men. Physiol Behav. 1992;51(4):719–22. doi: 10.1016/0031-9384(92)90107-d [DOI] [PubMed] [Google Scholar]
- 81.Costill DL, Branam L, Eddy D, Fink W. Alterations in red cell volume following exercise and dehydration. J Appl Physiol. 1974;37(6):912–6. doi: 10.1152/jappl.1974.37.6.912 [DOI] [PubMed] [Google Scholar]
- 82.Cox RH, Thomas TR, Hinton PS, Donahue OM. Effects of acute 60 and 80% VO2max bouts of aerobic exercise on state anxiety of women of different age groups across time. Res Q Exerc Sport. 2004;75(2):165–75. doi: 10.1080/02701367.2004.10609148 [DOI] [PubMed] [Google Scholar]
- 83.Crouter SE, DellaValle DM, Haas JD. Relationship between physical activity, physical performance, and iron status in adult women. Appl Physiol Nutr Metab Physiol Appl Nutr Metab. 2012;37(4):697–705. doi: 10.1139/h2012-044 [DOI] [PubMed] [Google Scholar]
- 84.Davies B, Shapiro CM, Daggett A, Gatt JA, Jakeman P. Physiological changes and sleep responses during and following a world record continuous walking record. Br J Sports Med. 1984;18(3):173–80. doi: 10.1136/bjsm.18.3.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davies CT, Chukweumeka AC, Van Haaren JP. Iron-deficiency anaemia: its effect on maximum aerobic power and responses to exercise in African males aged 17–40 years. Clin Sci. 1973;44(6):555–62. doi: 10.1042/cs0440555 [DOI] [PubMed] [Google Scholar]
- 86.Davies CT, Van Haaren JP. Effect of treatment on physiological responses to exercise in East African industrial workers with iron deficiency anaemia. Br J Ind Med. 1973;30(4):335–40. doi: 10.1136/oem.30.4.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Bisschop C, Martinot JB, Leurquin-Sterk G, Faoro V, Guénard H, Naeije R. Improvement in lung diffusion by endothelin A receptor blockade at high altitude. J Appl Physiol Bethesda Md 1985. 2012;112(1):20–5. doi: 10.1152/japplphysiol.00670.2011 [DOI] [PubMed] [Google Scholar]
- 88.Deitrick RW. Intravascular haemolysis in the recreational runner. Br J Sports Med. 1991;25(4):183–7. doi: 10.1136/bjsm.25.4.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dellavalle DM, Haas JD. Iron status is associated with endurance performance and training in female rowers. Med Sci Sports Exerc. 2012;44(8):1552–9. doi: 10.1249/MSS.0b013e3182517ceb [DOI] [PubMed] [Google Scholar]
- 90.Dempsey JA, Thomson JM, Forster HV, Cerny FC, Chosy LW. HbO2 dissociation in man during prolonged work in chronic hypoxia. J Appl Physiol. 1975;38(6):1022–9. doi: 10.1152/jappl.1975.38.6.1022 [DOI] [PubMed] [Google Scholar]
- 91.Dengel DR, Weyand PG, Black DM, Cureton KJ. Effect of varying levels of hypohydration on responses during submaximal cycling. Med Sci Sports Exerc. 1992;24(10):1096–101. [PubMed] [Google Scholar]
- 92.Deuster PA, Dolev E, Kyle SB, Anderson RA, Schoomaker EB. Magnesium homeostasis during high-intensity anaerobic exercise in men. J Appl Physiol Bethesda Md 1985. 1987;62(2):545–50. doi: 10.1152/jappl.1987.62.2.545 [DOI] [PubMed] [Google Scholar]
- 93.Diaz-Canestro C, Siebenmann C, Montero D. Blood Oxygen Carrying Capacity Determines Cardiorespiratory Fitness in Middle-Age and Older Women and Men. Med Sci Sports Exerc. 2021;53(11):2274–82. doi: 10.1249/MSS.0000000000002720 [DOI] [PubMed] [Google Scholar]
- 94.Dominelli PB, Wiggins CC, Baker SE, Shepherd JRA, Roberts SK, Roy TK, et al. Influence of high affinity haemoglobin on the response to normoxic and hypoxic exercise. J Physiol. 2020. Apr;598(8):1475–90. doi: 10.1113/JP279161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Drinkwater BL, Horvath SM, Wells CL. Aerobic power of females, ages 10 to 68. J Gerontol. 1975;30(4):385–94. doi: 10.1093/geronj/30.4.385 [DOI] [PubMed] [Google Scholar]
- 96.Drinkwater BL, Kramár PO, Bedi JF, Folinsbee LJ. Women at altitude: cardiovascular responses to hypoxia. Aviat Space Environ Med. 1982;53(5):472–7. [PubMed] [Google Scholar]
- 97.Duda K, Zoladz JA, Majerczak J, Kolodziejski L, Konturek SJ. The effect of exercise performed before and 24 hours after blood withdrawal on serum erythropoietin and growth hormone concentrations in humans. Int J Sports Med. 2003;24(5):326–31. doi: 10.1055/s-2003-40709 [DOI] [PubMed] [Google Scholar]
- 98.El-Sayed MS, Rattu AJ. Changes in lipid profile variables in response to submaximal and maximal exercise in trained cyclists. Eur J Appl Physiol. 1996;73(1–2):88–92. doi: 10.1007/BF00262814 [DOI] [PubMed] [Google Scholar]
- 99.Eriksson BO, Engström I, Karlberg P, Saltin B, Thorén C. A physiological analysis of former girl swimmers. Acta Paediatr Scand Suppl. 1971;217:68–72. doi: 10.1111/j.1651-2227.1971.tb05697.x [DOI] [PubMed] [Google Scholar]
- 100.Ertl AC, Bernauer EM, Hom CA. Plasma volume shifts with immersion at rest and two exercise intensities. Med Sci Sports Exerc. 1991;23(4):450–7. [PubMed] [Google Scholar]
- 101.Falk B, Burstein R, Ashkenazi I, Spilberg O, Alter J, Zylber-Katz E, et al. The effect of caffeine ingestion on physical performance after prolonged exercise. Eur J Appl Physiol. 1989;59(3):168–73. doi: 10.1007/BF02386182 [DOI] [PubMed] [Google Scholar]
- 102.Freund H, Lonsdorfer J, Oyono-Enguéllé S, Lonsdorfer A, Dah C, Bogui P. Lactate exchange and removal abilities in sickle cell trait carriers during and after incremental exercise. Int J Sports Med. 1995;16(7):428–34. doi: 10.1055/s-2007-973032 [DOI] [PubMed] [Google Scholar]
- 103.Garvican LA, Martin DT, Clark SA, Schmidt WF, Gore CJ. Variability of erythropoietin response to sleeping at simulated altitude: a cycling case study. Int J Sports Physiol Perform. 2007;2(3):327–31. doi: 10.1123/ijspp.2.3.327 [DOI] [PubMed] [Google Scholar]
- 104.Gass EM, Gass GC. Thermoregulatory responses to repeated warm water immersion in subjects who are paraplegic. Spinal Cord. 2001;39(3):149–55. doi: 10.1038/sj.sc.3101117 [DOI] [PubMed] [Google Scholar]
- 105.Gass GC, Camp EM, Watson J, Eager D, Wicks L, Ng A. Prolonged exercise in highly trained female endurance runners. Int J Sports Med. 1983;4(4):241–6. doi: 10.1055/s-2008-1026042 [DOI] [PubMed] [Google Scholar]
- 106.Gass GC, McLellan TM, Gass EM. Effects of prolonged exercise at a similar percentage of maximal oxygen consumption in trained and untrained subjects. Eur J Appl Physiol. 1991;63(6):430–5. [DOI] [PubMed] [Google Scholar]
- 107.Gatterer H, Menz V, Burtscher M. Acute Moderate Hypoxia Reduces One-Legged Cycling Performance Despite Compensatory Increase in Peak Cardiac Output: A Pilot Study. Int J Env Res Public Health. 2021;18(7). doi: 10.3390/ijerph18073732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gimenez M, Florentz M. Serum enzyme variations in men during an exhaustive “square-wave” endurance exercise test. Eur J Appl Physiol. 1984;52(2):219–24. doi: 10.1007/BF00433396 [DOI] [PubMed] [Google Scholar]
- 109.Gimenez M, Mohan-Kumar T, Humbert JC, De Talance N, Buisine J. Leukocyte, lymphocyte and platelet response to dynamic exercise. Duration or intensity effect? Eur J Appl Physiol. 1986;55(5):465–70. [DOI] [PubMed] [Google Scholar]
- 110.Gimenez M, Mohan-Kumar T, Humbert JC, de Talance N, Teboul M, Ponz JL, et al. Haematological and hormonal responses to dynamic exercise in patients with chronic airway obstruction. Eur J Clin Invest. 1987;17(1):75–80. doi: 10.1111/j.1365-2362.1987.tb01229.x [DOI] [PubMed] [Google Scholar]
- 111.Goodrich JA, Ryan BJ, Byrnes WC. The Influence of Oxygen Saturation on the Relationship Between Hemoglobin Mass and VO (2) max. Sports Med Int Open. 2018;2(4):E98–104. doi: 10.1055/a-0655-7207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gordon D, Marshall K, Connell A, Barnes RJ. Influence of blood donation on oxygen uptake kinetics during moderate and heavy intensity cycle exercise. Int J Sports Med. 2010;31(5):298–303. doi: 10.1055/s-0030-1248321 [DOI] [PubMed] [Google Scholar]
- 113.Gore CJ, Hahn AG, Scroop GC, Watson DB, Norton KI, Wood RJ, et al. Increased arterial desaturation in trained cyclists during maximal exercise at 580 m altitude. J Appl Physiol. 1996;80(6):2204–10. doi: 10.1152/jappl.1996.80.6.2204 [DOI] [PubMed] [Google Scholar]
- 114.Govus AD, Peeling P, Abbiss CR, Lawler NG, Swinkels DW, Laarakkers CM, et al. Live high, train low—influence on resting and post-exercise hepcidin levels. Scand J Med Sci Sports. 2017;27(7):704–13. doi: 10.1111/sms.12685 [DOI] [PubMed] [Google Scholar]
- 115.Gray AB, Telford RD, Weidemann MJ. The effect of intense interval exercise on iron status parameters in trained men. Med Sci Sports Exerc. 1993;25(7):778–82. doi: 10.1249/00005768-199307000-00004 [DOI] [PubMed] [Google Scholar]
- 116.Green HJ, Hughson RL, Thomson JA, Sharratt MT. Supramaximal exercise after training-induced hypervolemia. I. Gas exchange and acid-base balance. J Appl Physiol Bethesda Md 1985. 1987;62(5):1944–53. doi: 10.1152/jappl.1987.62.5.1944 [DOI] [PubMed] [Google Scholar]
- 117.Grover RF, Reeves JT, Grover EB, Leathers JE. Muscular exercise in young men native to 3,100 m altitude. J Appl Physiol. 1967;22(3):555–64. doi: 10.1152/jappl.1967.22.3.555 [DOI] [PubMed] [Google Scholar]
- 118.Gurney T, Brouner J, Spendiff O. Twenty-one days of spirulina supplementation lowers heart rate during submaximal cycling and augments power output during repeated sprints in trained cyclists. Appl Physiol Nutr Metab. 2021;1–9. doi: 10.1139/apnm-2021-0344 [DOI] [PubMed] [Google Scholar]
- 119.Halson SL, Lancaster GI, Jeukendrup AE, Gleeson M. Immunological responses to overreaching in cyclists. Med Sci Sports Exerc. 2003;35(5):854–61. doi: 10.1249/01.MSS.0000064964.80040.E9 [DOI] [PubMed] [Google Scholar]
- 120.Hausswirth C, Bigard AX, Berthelot M, Thomaïdis M, Guezennec CY. Variability in energy cost of running at the end of a triathlon and a marathon. Int J Sports Med. 1996;17(8):572–9. doi: 10.1055/s-2007-972897 [DOI] [PubMed] [Google Scholar]
- 121.Heaps CL, González-Alonso J, Coyle EF. Hypohydration causes cardiovascular drift without reducing blood volume. Int J Sports Med. 1994;15(2):74–9. doi: 10.1055/s-2007-1021023 [DOI] [PubMed] [Google Scholar]
- 122.Heinicke K, Wolfarth B, Winchenbach P, Biermann B, Schmid A, Huber G, et al. Blood volume and hemoglobin mass in elite athletes of different disciplines. Int J Sports Med. 2001;22(7):504–12. doi: 10.1055/s-2001-17613 [DOI] [PubMed] [Google Scholar]
- 123.Heyman E, Daussin F, Wieczorek V, Caiazzo R, Matran R, Berthon P, et al. Muscle Oxygen Supply and Use in Type 1 Diabetes, From Ambient Air to the Mitochondrial Respiratory Chain: Is There a Limiting Step? Diabetes Care. 2020;43(1):209–18. doi: 10.2337/dc19-1125 [DOI] [PubMed] [Google Scholar]
- 124.Hinghofer-Szalkay HG, Mekonen W, Rössler A, Schwaberger G, Lamprecht M, Hofmann P. Post-exercise decrease of plasma hyaluronan: increased clearance or diminished production? Physiol Res. 2002;51(2):139–44. [PubMed] [Google Scholar]
- 125.Holmberg HC, Calbet JAL. Insufficient ventilation as a cause of impaired pulmonary gas exchange during submaximal exercise. Respir Physiol Neurobiol. 2007;157(2–3):348–59. doi: 10.1016/j.resp.2006.12.013 [DOI] [PubMed] [Google Scholar]
- 126.Horvath SM, Agnew JW, Wagner JA, Bedi JF. Maximal aerobic capacity at several ambient concentrations of carbon monoxide at several altitudes. Res Rep Health Eff Inst. 1988;(21):1–21. [PubMed] [Google Scholar]
- 127.Hsieh SS, Freedson PS, Mroz MC, Stewart PM. Exercise intensity and erythrocyte 2,3-diphosphoglycerate concentration. Med Sci Sports Exerc. 1986;18(1):82–6. [PubMed] [Google Scholar]
- 128.Hue O, Voltaire B, Galy O, Costes O, Callis A, Hertogh C, et al. Effects of 8 days acclimation on biological and performance response in a tropical climate. J Sports Med Phys Fitness. 2004;44(1):30–7. [PubMed] [Google Scholar]
- 129.Hutchinson PL, Cureton KJ, Outz H, Wilson G. Relationship of cardiac size to maximal oxygen uptake and body size in men and women. Int J Sports Med. 1991;12(4):369–73. doi: 10.1055/s-2007-1024696 [DOI] [PubMed] [Google Scholar]
- 130.Hütler M, Woweries S, Leithäuser R, Böning D, Beneke R. Exercise-induced changes in blood levels of alpha-tocopherol. Eur J Appl Physiol. 2001;85(1–2):151–6. doi: 10.1007/s004210100427 [DOI] [PubMed] [Google Scholar]
- 131.Jacobs RA, Rasmussen P, Siebenmann C, Díaz V, Gassmann M, Pesta D, et al. Determinants of time trial performance and maximal incremental exercise in highly trained endurance athletes. J Appl Physiol Bethesda Md 1985. 2011;111(5):1422–30. doi: 10.1152/japplphysiol.00625.2011 [DOI] [PubMed] [Google Scholar]
- 132.Jimenez C, Melin B, Koulmann N, Allevard AM, Launay JC, Savourey G. Plasma volume changes during and after acute variations of body hydration level in humans. Eur J Appl Physiol. 1999;80(1):1–8. doi: 10.1007/s004210050550 [DOI] [PubMed] [Google Scholar]
- 133.Johnson D, Roberts J, Gordon D. Effect of an acute blood donation on oxygen uptake kinetics in moderate and heavy domains over a period of 96 hours. Transfusion (Paris). 2020;60(12):2896–906. [DOI] [PubMed] [Google Scholar]
- 134.Kargotich S, Keast D, Goodman C, Crawford GP, Morton AR. The influence of blood volume changes on leucocyte and lymphocyte subpopulations in elite swimmers following interval training of varying intensities. Int J Sports Med. 1997;18(5):373–80. doi: 10.1055/s-2007-972649 [DOI] [PubMed] [Google Scholar]
- 135.Kiyamu M, Rivera-Chira M, Brutsaert TD. Aerobic capacity of Peruvian Quechua: a test of the developmental adaptation hypothesis. Am J Phys Anthropol. 2015;156(3):363–73. doi: 10.1002/ajpa.22655 [DOI] [PubMed] [Google Scholar]
- 136.Koch G, Röcker L. Plasma volume and intravascular protein masses in trained boys and fit young men. J Appl Physiol. 1977;43(6):1085–8. doi: 10.1152/jappl.1977.43.6.1085 [DOI] [PubMed] [Google Scholar]
- 137.Koehler K, Braun H, de Marees M, Geyer H, Thevis M, Mester J, et al. Glycerol administration before endurance exercise: metabolism, urinary glycerol excretion and effects on doping-relevant blood parameters. Drug Test Anal. 2014;6(3):202–9. doi: 10.1002/dta.1446 [DOI] [PubMed] [Google Scholar]
- 138.Koivisto-Mørk AE, Svendsen IS, Skattebo Ø, Hallén J, Paulsen G. Impact of baseline serum ferritin and supplemental iron on altitude-induced hemoglobin mass response in elite athletes. Scand J Med Sci Sports. 2021;31(9):1764–73. doi: 10.1111/sms.13982 [DOI] [PubMed] [Google Scholar]
- 139.Koponen AS, Peltonen JE, Päivinen MK, Aho JM, Hägglund HJ, Uusitalo AL, et al. Low total haemoglobin mass, blood volume and aerobic capacity in men with type 1 diabetes. Eur J Appl Physiol. 2013;113(5):1181–8. doi: 10.1007/s00421-012-2532-4 [DOI] [PubMed] [Google Scholar]
- 140.Lawrenson L, Hoff J, Richardson RS. Aging attenuates vascular and metabolic plasticity but does not limit improvement in muscle VO(2) max. Am J Physiol Heart Circ Physiol. 2004;286(4):H1565–72. doi: 10.1152/ajpheart.01070.2003 [DOI] [PubMed] [Google Scholar]
- 141.Lebrun CM, McKenzie DC, Prior JC, Taunton JE. Effects of menstrual cycle phase on athletic performance. Med Sci Sports Exerc. 1995;27(3):437–44. [PubMed] [Google Scholar]
- 142.Lespagnol E, Tagougui S, Fernandez BO, Zerimech F, Matran R, Maboudou P, et al. Circulating biomarkers of nitric oxide bioactivity and impaired muscle vasoreactivity to exercise in adults with uncomplicated type 1 diabetes. Diabetologia. 2021;64(2):325–38. doi: 10.1007/s00125-020-05329-8 [DOI] [PubMed] [Google Scholar]
- 143.Levine L, Rose MS, Francesconi P, Neufer PD, Sawka MN. Fluid replacement during sustained activity in the heat: nutrient solution vs. water. Aviat Space Environ Med. 1991;62(6):559–64. [PubMed] [Google Scholar]
- 144.Lindinger MI, Spriet LL, Hultman E, Putman T, McKelvie RS, Lands LC, et al. Plasma volume and ion regulation during exercise after low- and high-carbohydrate diets. Am J Physiol. 1994;266(6 Pt 2):R1896–906. doi: 10.1152/ajpregu.1994.266.6.R1896 [DOI] [PubMed] [Google Scholar]
- 145.Logan-Sprenger HM, Heigenhauser GJF, Jones GL, Spriet LL. The effect of dehydration on muscle metabolism and time trial performance during prolonged cycling in males. Physiol Rep. 2015;3(8):e12483. doi: 10.14814/phy2.12483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lucía A, Hoyos J, Santalla A, Pérez M, Chicharro JL. Curvilinear VO(2):power output relationship in a ramp test in professional cyclists: possible association with blood hemoglobin concentration. Jpn J Physiol. 2002;52(1):95–103. [DOI] [PubMed] [Google Scholar]
- 147.Lukaski HC. Low dietary zinc decreases erythrocyte carbonic anhydrase activities and impairs cardiorespiratory function in men during exercise. Am J Clin Nutr. 2005;81(5):1045–51. doi: 10.1093/ajcn/81.5.1045 [DOI] [PubMed] [Google Scholar]
- 148.Lundby C, Calbet JAL, van Hall G, Saltin B, Sander M. Pulmonary gas exchange at maximal exercise in Danish lowlanders during 8 wk of acclimatization to 4,100 m and in high-altitude Aymara natives. Am J Physiol Regul Integr Comp Physiol. 2004;287(5):R1202–8. doi: 10.1152/ajpregu.00725.2003 [DOI] [PubMed] [Google Scholar]
- 149.Lundgren KM, Aspvik NP, Langlo KAR, Braaten T, Wisløff U, Stensvold D, et al. Blood Volume, Hemoglobin Mass, and Peak Oxygen Uptake in Older Adults: The Generation 100 Study. Front Sports Act Living. 2021;3:638139. doi: 10.3389/fspor.2021.638139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lyle RM, Weaver CM, Sedlock DA, Rajaram S, Martin B, Melby CL. Iron status in exercising women: the effect of oral iron therapy vs increased consumption of muscle foods. Am J Clin Nutr. 1992;56(6):1049–55. doi: 10.1093/ajcn/56.6.1049 [DOI] [PubMed] [Google Scholar]
- 151.Lyons TP, Riedesel ML, Meuli LE, Chick TW. Effects of glycerol-induced hyperhydration prior to exercise in the heat on sweating and core temperature. Med Sci Sports Exerc. 1990;22(4):477–83. [PubMed] [Google Scholar]
- 152.Mackenzie RWA, Watt PW, Maxwell NS. Acute normobaric hypoxia stimulates erythropoietin release. High Alt Med Biol. 2008;9(1):28–37. doi: 10.1089/ham.2007.1043 [DOI] [PubMed] [Google Scholar]
- 153.Magazanik A, Weinstein Y, Dlin RA, Derin M, Schwartzman S, Allalouf D. Iron deficiency caused by 7 weeks of intensive physical exercise. Eur J Appl Physiol. 1988;57(2):198–202. doi: 10.1007/BF00640663 [DOI] [PubMed] [Google Scholar]
- 154.Mairbäurl H, Humpeler E, Schwaberger G, Pessenhofer H. Training-dependent changes of red cell density and erythrocytic oxygen transport. J Appl Physiol. 1983;55(5):1403–7. doi: 10.1152/jappl.1983.55.5.1403 [DOI] [PubMed] [Google Scholar]
- 155.Mairbäurl H, Schobersberger W, Hasibeder W, Schwaberger G, Gaesser G, Tanaka KR. Regulation of red cell 2,3-DPG and Hb-O2-affinity during acute exercise. Eur J Appl Physiol. 1986;55(2):174–80. doi: 10.1007/BF00715001 [DOI] [PubMed] [Google Scholar]
- 156.Maksud MG, Baron A. Physiological responses to exercise in chronic cigarette and marijuana users. Eur J Appl Physiol. 1980;43(2):127–34. doi: 10.1007/BF00422443 [DOI] [PubMed] [Google Scholar]
- 157.Marconi C, Marzorati M, Grassi B, Basnyat B, Colombini A, Kayser B, et al. Second generation Tibetan lowlanders acclimatize to high altitude more quickly than Caucasians. J Physiol. 2004;556(Pt 2):661–71. doi: 10.1113/jphysiol.2003.059188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Marlin L, Connes P, Antoine-Jonville S, Tripette J, Montout-Hedreville M, Sanouiller A, et al. Cardiorespiratory responses during three repeated incremental exercise tests in sickle cell trait carriers. Eur J Appl Physiol. 2008;102(2):181–7. doi: 10.1007/s00421-007-0570-0 [DOI] [PubMed] [Google Scholar]
- 159.Marrades RM, Roca J, Campistol JM, Diaz O, Barberá JA, Torregrosa JV, et al. Effects of erythropoietin on muscle O2 transport during exercise in patients with chronic renal failure. J Clin Invest. 1996;97(9):2092–100. doi: 10.1172/JCI118646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Martin TD, Green MS, Whitehead MT, Scheett TP, Webster MJ, Hudson GM. Six weeks of oral Echinacea purpurea supplementation does not enhance the production of serum erythropoietin or erythropoietic status in recreationally active males with above-average aerobic fitness. Appl Physiol Nutr Metab Physiol Appl Nutr Metab. 2019;44(7):791–5. doi: 10.1139/apnm-2018-0783 [DOI] [PubMed] [Google Scholar]
- 161.McInnis MD, Newhouse IJ, von Duvillard SP, Thayer R. The effect of exercise intensity on hematuria in healthy male runners. Eur J Appl Physiol. 1998;79(1):99–105. doi: 10.1007/s004210050480 [DOI] [PubMed] [Google Scholar]
- 162.McKelvie RS, Jones NL, Heigenhauser GJ. Effect of progressive incremental exercise and beta-adrenergic blockade on erythrocyte ion concentrations. Can J Physiol Pharmacol. 1997;75(1):19–25. doi: 10.1139/cjpp-75-1-19 [DOI] [PubMed] [Google Scholar]
- 163.McKenzie DC, Goodman LS, Nath C, Davidson B, Matheson GO, Parkhouse WS, et al. Cardiovascular adaptations in Andean natives after 6 wk of exposure to sea level. J Appl Physiol Bethesda Md 1985. 1991;70(6):2650–5. doi: 10.1152/jappl.1991.70.6.2650 [DOI] [PubMed] [Google Scholar]
- 164.McMurray RG. Plasma volume changes during submaximal swimming. Eur J Appl Physiol. 1983;51(3):347–56. doi: 10.1007/BF00429071 [DOI] [PubMed] [Google Scholar]
- 165.Medbø JI, Sejersted OM. Acid-base and electrolyte balance after exhausting exercise in endurance-trained and sprint-trained subjects. Acta Physiol Scand. 1985;125(1):97–109. doi: 10.1111/j.1748-1716.1985.tb07696.x [DOI] [PubMed] [Google Scholar]
- 166.Metin G, Atukeren P, Alturfan AA, Gulyasar T, Kaya M, Gumustas MK. Lipid peroxidation, erythrocyte superoxide-dismutase activity and trace metals in young male footballers. Yonsei Med J. 2003;44(6):979–86. doi: 10.3349/ymj.2003.44.6.979 [DOI] [PubMed] [Google Scholar]
- 167.Miller BF, Lindinger MI, Fattor JA, Jacobs KA, Leblanc PJ, Duong M, et al. Hematological and acid-base changes in men during prolonged exercise with and without sodium-lactate infusion. J Appl Physiol Bethesda Md 1985. 2005;98(3):856–65. doi: 10.1152/japplphysiol.00753.2004 [DOI] [PubMed] [Google Scholar]
- 168.Montero D, Lundby C. Refuting the myth of non-response to exercise training: “non-responders” do respond to higher dose of training. J Physiol. 2017;595(11):3377–87. doi: 10.1113/JP273480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mora-Rodriguez R, Aguado-Jimenez R, Del Coso J, Estevez E. A standard blood bank donation alters the thermal and cardiovascular responses during subsequent exercise. Transfusion (Paris). 2012;52(11):2339–47. doi: 10.1111/j.1537-2995.2012.03613.x [DOI] [PubMed] [Google Scholar]
- 170.Moraga FA, Osorio J, Jiménez D, Calderón-Jofré R, Moraga D. Aerobic Capacity, Lactate Concentration, and Work Assessment During Maximum Exercise at Sea Level and High Altitude in Miners Exposed to Chronic Intermittent Hypobaric Hypoxia (3,800 m). Front Physiol. 2019;10:1149–1149. doi: 10.3389/fphys.2019.01149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Moretti D, Mettler S, Zeder C, Lundby C, Geurts-Moetspot A, Monnard A, et al. An intensified training schedule in recreational male runners is associated with increases in erythropoiesis and inflammation and a net reduction in plasma hepcidin. Am J Clin Nutr. 2018;108(6):1324–33. doi: 10.1093/ajcn/nqy247 [DOI] [PubMed] [Google Scholar]
- 172.Morgan AL, Sinning WE, Weldy DL. Age effects on body fluid distribution during exercise in the heat. Aviat Space Environ Med. 2002;73(8):750–7. [PubMed] [Google Scholar]
- 173.Mounier R, Pialoux V, Mischler I, Coudert J, Fellmann N. Effect of hypervolemia on heart rate during 4 days of prolonged exercises. Int J Sports Med. 2003;24(7):523–9. doi: 10.1055/s-2003-42019 [DOI] [PubMed] [Google Scholar]
- 174.Muza SR, Sawka MN, Young AJ, Dennis RC, Gonzalez RR, Martin JW, et al. Elite special forces: physiological description and ergogenic influence of blood reinfusion. Aviat Space Environ Med. 1987;58(10):1001–4. [PubMed] [Google Scholar]
- 175.Nanas S, Vasileiadis I, Dimopoulos S, Sakellariou D, Kapsimalakou S, Papazachou O, et al. New insights into the exercise intolerance of beta-thalassemia major patients. Scand J Med Sci Sports. 2009;19(1):96–102. doi: 10.1111/j.1600-0838.2008.00778.x [DOI] [PubMed] [Google Scholar]
- 176.Novosadová J. The changes in hematocrit, hemoglobin, plasma volume and proteins during and after different types of exercise. Eur J Appl Physiol. 1977;36(3):223–30. [DOI] [PubMed] [Google Scholar]
- 177.Nuss R, Loehr JP, Daberkow E, Graham L, Lane PA. Cardiopulmonary function in men with sickle cell trait who reside at moderately high altitude. J Lab Clin Med. 1993;122(4):382–7. [PubMed] [Google Scholar]
- 178.O’Toole ML, Paolone AM, Ramsey RE, Irion G. The effects of heat acclimation on plasma volume and plasma protein of females. Int J Sports Med. 1983;4(1):40–4. doi: 10.1055/s-2008-1026014 [DOI] [PubMed] [Google Scholar]
- 179.Oöpik V, Timpmann S, Kadak K, Medijainen L, Karelson K. The Effects of Sodium Citrate Ingestion on Metabolism and 1500-m Racing Time in Trained Female Runners. J Sports Sci Med. 2008;7(1):125–31. [PMC free article] [PubMed] [Google Scholar]
- 180.Otsuka J, Okamoto Y, Fujii N, Enoki Y, Maejima D, Nishiyasu T, et al. Effects of Isomaltulose Ingestion on Thermoregulatory Responses during Exercise in a Hot Environment. Int J Env Res Public Health. 2021;18(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Oyono-Enguéllé S, Le Gallais D, Lonsdorfer A, Dah C, Freund H, Bogui P, et al. Cardiorespiratory and metabolic responses to exercise in HbSC sickle cell patients. Med Sci Sports Exerc. 2000;32(4):725–31. doi: 10.1097/00005768-200004000-00002 [DOI] [PubMed] [Google Scholar]
- 182.Painter P, Krasnoff JB, Kuskowski M, Frassetto L, Johansen KL. Effects of modality change and transplant on peak oxygen uptake in patients with kidney failure. Am J Kidney Dis Off J Natl Kidney Found. 2011;57(1):113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pastene J, Germain M, Allevard AM, Gharib C, Lacour JR. Water balance during and after marathon running. Eur J Appl Physiol. 1996;73(1–2):49–55. doi: 10.1007/BF00262808 [DOI] [PubMed] [Google Scholar]
- 184.Pedlar CR, Whyte GP, Burden R, Moore B, Horgan G, Pollock N. A case study of an iron-deficient female Olympic 1500-m runner. Int J Sports Physiol Perform. 2013;8(6):695–8. doi: 10.1123/ijspp.8.6.695 [DOI] [PubMed] [Google Scholar]
- 185.Peeling P, Dawson B, Goodman C, Landers G, Wiegerinck ET, Swinkels DW, et al. Cumulative effects of consecutive running sessions on hemolysis, inflammation and hepcidin activity. Eur J Appl Physiol. 2009;106(1):51–9. doi: 10.1007/s00421-009-0988-7 [DOI] [PubMed] [Google Scholar]
- 186.Peltonen JE, Hägglund H, Koskela-Koivisto T, Koponen AS, Aho JM, Rissanen APE, et al. Alveolar gas exchange, oxygen delivery and tissue deoxygenation in men and women during incremental exercise. Respir Physiol Neurobiol. 2013. Aug;188(2):102–12. doi: 10.1016/j.resp.2013.05.014 [DOI] [PubMed] [Google Scholar]
- 187.Peters HP, Wiersma WC, Akkermans LM, Bol E, Kraaijenhagen RJ, Mosterd WL, et al. Gastrointestinal mucosal integrity after prolonged exercise with fluid supplementation. Med Sci Sports Exerc. 2000;32(1):134–42. doi: 10.1097/00005768-200001000-00020 [DOI] [PubMed] [Google Scholar]
- 188.Petersen AC, Leikis MJ, McMahon LP, Kent AB, McKenna MJ. Effects of endurance training on extrarenal potassium regulation and exercise performance in patients on haemodialysis. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc—Eur Ren Assoc. 2009;24(9):2882–8. doi: 10.1093/ndt/gfp157 [DOI] [PubMed] [Google Scholar]
- 189.Petkus DL, Murray-Kolb LE, Scott SP, Southmayd EA, De Souza MJ. Iron status at opposite ends of the menstrual function spectrum. J Trace Elem Med Biol Organ Soc Miner Trace Elem GMS. 2019;51:169–75. doi: 10.1016/j.jtemb.2018.10.016 [DOI] [PubMed] [Google Scholar]
- 190.Piehl Aulin K, Svedenhag J, Wide L, Berglund B, Saltin B. Short-term intermittent normobaric hypoxia—haematological, physiological and mental effects. Scand J Med Sci Sports. 1998;8(3):132–7. doi: 10.1111/j.1600-0838.1998.tb00182.x [DOI] [PubMed] [Google Scholar]
- 191.Pivarnik JM, Montain SJ, Graves JE, Pollock ML. Alterations in plasma volume, electrolytes and protein during incremental exercise at different pedal speeds. Eur J Appl Physiol. 1988;57(1):103–9. doi: 10.1007/BF00691247 [DOI] [PubMed] [Google Scholar]
- 192.Pivarnik JM, Goetting MP, Senay LC Jr. The effects of body position and exercise on plasma volume dynamics. Eur J Appl Physiol. 1986;55(4):450–6. doi: 10.1007/BF00422750 [DOI] [PubMed] [Google Scholar]
- 193.Pivarnik JM, Leeds EM, Wilkerson JE. Effects of endurance exercise on metabolic water production and plasma volume. J Appl Physiol. 1984;56(3):613–8. doi: 10.1152/jappl.1984.56.3.613 [DOI] [PubMed] [Google Scholar]
- 194.Ponce-González JG, Olmedillas H, Calleja-González J, Guerra B, Sanchis-Moysi J. Physical fitness, adiposity and testosterone concentrations are associated to playing position in professional basketballers. Nutr Hosp. 2015;31(6):2624–32. doi: 10.3305/nh.2015.31.6.8977 [DOI] [PubMed] [Google Scholar]
- 195.Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, et al. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol Bethesda Md 1985. 1998;85(1):68–75. doi: 10.1152/jappl.1998.85.1.68 [DOI] [PubMed] [Google Scholar]
- 196.Prommer N, Heinicke K, Viola T, Cajigal J, Behn C, Schmidt WFJ. Long-term intermittent hypoxia increases O2-transport capacity but not VO2max. High Alt Med Biol. 2007;8(3):225–35. doi: 10.1089/ham.2007.8309 [DOI] [PubMed] [Google Scholar]
- 197.Prommer N, Thoma S, Quecke L, Gutekunst T, Völzke C, Wachsmuth N, et al. Total hemoglobin mass and blood volume of elite Kenyan runners. Med Sci Sports Exerc. 2010;42(4):791–7. doi: 10.1249/MSS.0b013e3181badd67 [DOI] [PubMed] [Google Scholar]
- 198.Ready AE. Physiological characteristics of male and female middle distance runners. Can J Appl Sport Sci J Can Sci Appl Au Sport. 1984;9(2):70–7. [PubMed] [Google Scholar]
- 199.Regensteiner JG, Sippel J, McFarling ET, Wolfel EE, Hiatt WR. Effects of non-insulin-dependent diabetes on oxygen consumption during treadmill exercise. Med Sci Sports Exerc. 1995;27(6):875–81. [PubMed] [Google Scholar]
- 200.Richalet JP, Bittel J, Herry JP, Savourey G, Le Trong JL, Auvert JF, et al. Use of a hypobaric chamber for pre-acclimatization before climbing Mount Everest. Int J Sports Med. 1992;13 Suppl 1:S216–20. doi: 10.1055/s-2007-1024644 [DOI] [PubMed] [Google Scholar]
- 201.Rietjens GJWM, Kuipers H, Hartgens F, Keizer HA. Red blood cell profile of elite olympic distance triathletes. A three-year follow-up. Int J Sports Med. 2002;23(6):391–6. doi: 10.1055/s-2002-33736 [DOI] [PubMed] [Google Scholar]
- 202.Robergs RA, Quintana R, Parker DL, Frankel CC. Multiple variables explain the variability in the decrement in VO2max during acute hypobaric hypoxia. Med Sci Sports Exerc. 1998;30(6):869–79. doi: 10.1097/00005768-199806000-00015 [DOI] [PubMed] [Google Scholar]
- 203.Robertson EY, Saunders PU, Pyne DB, Aughey RJ, Anson JM, Gore CJ. Reproducibility of performance changes to simulated live high/train low altitude. Med Sci Sports Exerc. 2010;42(2):394–401. doi: 10.1249/MSS.0b013e3181b34b57 [DOI] [PubMed] [Google Scholar]
- 204.Robinson TA, Hawley JA, Palmer GS, Wilson GR, Gray DA, Noakes TD, et al. Water ingestion does not improve 1-h cycling performance in moderate ambient temperatures. Eur J Appl Physiol. 1995;71(2–3):153–60. [DOI] [PubMed] [Google Scholar]
- 205.Robinson Y, Cristancho E, Böning D. Erythrocyte aspartate aminotransferase activity as a possible indirect marker for stimulated erythropoiesis in male and female athletes. Lab Hematol Off Publ Int Soc Lab Hematol. 2007;13(2):49–55. doi: 10.1532/LH96.07005 [DOI] [PubMed] [Google Scholar]
- 206.Rotstein A, Falk B, Einbinder M, Zigel L. Changes in plasma volume following intense intermittent exercise in neutral and hot environmental conditions. J Sports Med Phys Fitness. 1998;38(1):24–9. [PubMed] [Google Scholar]
- 207.Roy JLP, Hunter GR, Fernandez JR, McCarthy JP, Larson-Meyer DE, Blaudeau TE, et al. Cardiovascular factors explain genetic background differences in VO2max. Am J Hum Biol Off J Hum Biol Counc. 2006;18(4):454–60. doi: 10.1002/ajhb.20509 [DOI] [PubMed] [Google Scholar]
- 208.Sara F, Hardy-Dessources MD, Voltaire B, Etienne-Julan M, Hue O. Lactic response in sickle cell trait carriers in comparison with subjects with normal hemoglobin. Clin J Sport Med Off J Can Acad Sport Med. 2003;13(2):96–101. doi: 10.1097/00042752-200303000-00006 [DOI] [PubMed] [Google Scholar]
- 209.Sara F, Hardy-Dessources MD, Marlin L, Connes P, Hue O. Lactate distribution in the blood compartments of sickle cell trait carriers during incremental exercise and recovery. Int J Sports Med. 2006;27(6):436–43. doi: 10.1055/s-2005-865844 [DOI] [PubMed] [Google Scholar]
- 210.Sara F, Connes P, Hue O, Montout-Hedreville M, Etienne-Julan M, Hardy-Dessources MD. Faster lactate transport across red blood cell membrane in sickle cell trait carriers. J Appl Physiol Bethesda Md 1985. 2006;100(2):427–32. doi: 10.1152/japplphysiol.00771.2005 [DOI] [PubMed] [Google Scholar]
- 211.Saunders PU, Telford RD, Pyne DB, Cunningham RB, Gore CJ, Hahn AG, et al. Improved running economy in elite runners after 20 days of simulated moderate-altitude exposure. J Appl Physiol Bethesda Md 1985. 2004;96(3):931–7. doi: 10.1152/japplphysiol.00725.2003 [DOI] [PubMed] [Google Scholar]
- 212.Sawka MN, Francesconi RP, Pimental NA, Pandolf KB. Hydration and vascular fluid shifts during exercise in the heat. J Appl Physiol. 1984;56(1):91–6. doi: 10.1152/jappl.1984.56.1.91 [DOI] [PubMed] [Google Scholar]
- 213.Schierbauer J, Hoffmeister T, Treff G, Wachsmuth NB, Schmidt WFJ. Effect of Exercise-Induced Reductions in Blood Volume on Cardiac Output and Oxygen Transport Capacity. Front Physiol. 2021;12:679232. doi: 10.3389/fphys.2021.679232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Schleh MW, Dumke CL. Comparison of Sports Drink Versus Oral Rehydration Solution During Exercise in the Heat. Wilderness Environ Med. 2018;29(2):185–93. doi: 10.1016/j.wem.2018.01.005 [DOI] [PubMed] [Google Scholar]
- 215.Schmidt W, Eckardt KU, Hilgendorf A, Strauch S, Bauer C. Effects of maximal and submaximal exercise under normoxic and hypoxic conditions on serum erythropoietin level. Int J Sports Med. 1991;12(5):457–61. doi: 10.1055/s-2007-1024713 [DOI] [PubMed] [Google Scholar]
- 216.Schmidt W, Heinicke K, Rojas J, Manuel Gomez J, Serrato M, Mora M, et al. Blood volume and hemoglobin mass in endurance athletes from moderate altitude. Med Sci Sports Exerc. 2002;34(12):1934–40. doi: 10.1097/00005768-200212000-00012 [DOI] [PubMed] [Google Scholar]
- 217.Schmidt WFJ, Hoffmeister T, Haupt S, Schwenke D, Wachsmuth NB, Byrnes WC. Chronic Exposure to Low Dose Carbon Monoxide Alters Hemoglobin Mass and VO2max. Med Sci Sports Exerc. 2020; doi: 10.1249/MSS.0000000000002330 [DOI] [PubMed] [Google Scholar]
- 218.Schobersberger W, Tschann M, Hasibeder W, Steidl M, Herold M, Nachbauer W, et al. Consequences of 6 weeks of strength training on red cell O2 transport and iron status. Eur J Appl Physiol. 1990;60(3):163–8. doi: 10.1007/BF00839152 [DOI] [PubMed] [Google Scholar]
- 219.Schommer K, Wiesegart N, Menold E, Haas U, Lahr K, Buhl H, et al. Training in normobaric hypoxia and its effects on acute mountain sickness after rapid ascent to 4559 m. High Alt Med Biol. 2010;11(1):19–25. doi: 10.1089/ham.2009.1019 [DOI] [PubMed] [Google Scholar]
- 220.Schumacher YO, Schmid A, König D, Berg A. Effects of exercise on soluble transferrin receptor and other variables of the iron status. Br J Sports Med. 2002;36(3):195–9. doi: 10.1136/bjsm.36.3.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Schumacher YO, Pottgiesser T, Ahlgrim C, Ruthardt S, Dickhuth HH, Roecker K. Haemoglobin mass in cyclists during stage racing. Int J Sports Med. 2008;29(5):372–8. doi: 10.1055/s-2007-965335 [DOI] [PubMed] [Google Scholar]
- 222.Schumacher YO, Ruthardt S, Schmidt M, Ahlgrim C, Roecker K, Pottgiesser T. Total haemoglobin mass but not cardiac volume adapts to long-term endurance exercise in highly trained spinal cord injured athletes. Eur J Appl Physiol. 2009;105(5):779–85. doi: 10.1007/s00421-008-0963-8 [DOI] [PubMed] [Google Scholar]
- 223.Shizukuda Y, Smith KP, Tripodi DJ, Arena R, Yau YY, Bolan CD, et al. Changes in exercise capacity in subjects with cardiac asymptomatic hereditary hemochromatosis during a follow-up after 5 yrs. Am J Phys Med Rehabil. 2012;91(5):418–24. doi: 10.1097/PHM.0b013e3182465f5f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Smith MS. Physiological profile of senior and junior England international amateur boxers. J Sports Sci Med. 2006;5(CSSI):74–89. [PMC free article] [PubMed] [Google Scholar]
- 225.Sohn EY, Kato R, Noetzli LJ, Gera A, Coates T, Harmatz P, et al. Exercise performance in thalassemia major: correlation with cardiac iron burden. Am J Hematol. 2013;88(3):193–7. doi: 10.1002/ajh.23370 [DOI] [PubMed] [Google Scholar]
- 226.Sperlich B, Zinner C, Pfister R, Holmberg HC, Michels G. Repeated apnea-induced contraction of the spleen in cyclists does not enhance performance in a subsequent time-trial. Eur J Appl Physiol. 2015;115(1):205–12. doi: 10.1007/s00421-014-3003-x [DOI] [PubMed] [Google Scholar]
- 227.Spodaryk K. The relationship between iron status and perceived exertion in trained and untrained women. J Physiol Pharmacol Off J Pol Physiol Soc. 1993;44(4):415–23. [PubMed] [Google Scholar]
- 228.Staessen J, Fagard R, Hespel P, Lijnen P, Vanhees L, Amery A. Plasma renin system during exercise in normal men. J Appl Physiol Bethesda Md 1985. 1987;63(1):188–94. doi: 10.1152/jappl.1987.63.1.188 [DOI] [PubMed] [Google Scholar]
- 229.Steiner T, Wehrlin JP. Does hemoglobin mass increase from age 16 to 21 and 28 in elite endurance athletes? Med Sci Sports Exerc. 2011;43(9):1735–43. doi: 10.1249/MSS.0b013e3182118760 [DOI] [PubMed] [Google Scholar]
- 230.Steiner T, Maier T, Wehrlin JP. Effect of Endurance Training on Hemoglobin Mass and V˙O2max in Male Adolescent Athletes. Med Sci Sports Exerc. 2019;51(5):912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Stephenson LA, Kolka MA. Plasma volume during heat stress and exercise in women. Eur J Appl Physiol. 1988;57(4):373–81. doi: 10.1007/BF00417979 [DOI] [PubMed] [Google Scholar]
- 232.Stephenson LA, Kolka MA, Francesconi R, Gonzalez RR. Circadian variations in plasma renin activity, catecholamines and aldosterone during exercise in women. Eur J Appl Physiol. 1989;58(7):756–64. doi: 10.1007/BF00637388 [DOI] [PubMed] [Google Scholar]
- 233.Stevenson ET, Davy KP, Seals DR. Maximal aerobic capacity and total blood volume in highly trained middle-aged and older female endurance athletes. J Appl Physiol Bethesda Md 1985. 1994;77(4):1691–6. doi: 10.1152/jappl.1994.77.4.1691 [DOI] [PubMed] [Google Scholar]
- 234.Stewart GM, Chase S, Cross TJ, Wheatley-Guy CM, Joyner M, Curry T, et al. Effects of an allosteric hemoglobin affinity modulator on arterial blood gases and cardiopulmonary responses during normoxic and hypoxic low-intensity exercise. J Appl Physiol. 2020. Jun 1;128(6):1467–76. doi: 10.1152/japplphysiol.00185.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Stewart GM, Cross TJ, Joyner MJ, Chase SC, Curry T, Lehrer-Graiwer J, et al. Impact of Pharmacologically Left Shifting the Oxygen–Hemoglobin Dissociation Curve on Arterial Blood Gases and Pulmonary Gas Exchange During Maximal Exercise in Hypoxia. High Alt Med Biol [Internet]. 2021. Jun 21 [cited 2021 Jul 13]; Available from: https://www.liebertpub.com/doi/10.1089/ham.2020.0159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Stringer WW, Hansen JE, Wasserman K. Cardiac output estimated noninvasively from oxygen uptake during exercise. J Appl Physiol Bethesda Md 1985. 1997;82(3):908–12. doi: 10.1152/jappl.1997.82.3.908 [DOI] [PubMed] [Google Scholar]
- 237.Svendsen IS, Killer SC, Carter JM, Randell RK, Jeukendrup AE, Gleeson M. Impact of intensified training and carbohydrate supplementation on immunity and markers of overreaching in highly trained cyclists. Eur J Appl Physiol. 2016;116(5):867–77. doi: 10.1007/s00421-016-3340-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Tagougui S, Leclair E, Fontaine P, Matran R, Marais G, Aucouturier J, et al. Muscle oxygen supply impairment during exercise in poorly controlled type 1 diabetes. Med Sci Sports Exerc. 2015;47(2):231–9. doi: 10.1249/MSS.0000000000000424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Telford RD, Sly GJ, Hahn AG, Cunningham RB, Bryant C, Smith JA. Footstrike is the major cause of hemolysis during running. J Appl Physiol Bethesda Md 1985. 2003;94(1):38–42. doi: 10.1152/japplphysiol.00631.2001 [DOI] [PubMed] [Google Scholar]
- 240.Tomkiewicz-Pajak L, Plazak W, Kolcz J, Pajak J, Kopec G, Dluzniewska N, et al. Iron deficiency and hematological changes in adult patients after Fontan operation. J Cardiol. 2014;64(5):384–9. doi: 10.1016/j.jjcc.2014.02.021 [DOI] [PubMed] [Google Scholar]
- 241.Treff G, Schmidt W, Wachsmuth N, Völzke C, Steinacker JM. Total haemoglobin mass, maximal and submaximal power in elite rowers. Int J Sports Med. 2014;35(7):571–4. doi: 10.1055/s-0033-1358476 [DOI] [PubMed] [Google Scholar]
- 242.Trikas A, Tentolouris K, Katsimaklis G, Antoniou J, Stefanadis C, Toutouzas P. Exercise capacity in patients with beta-thalassemia major: relation to left ventricular and atrial size and function. Am Heart J. 1998;136(6):988–90. doi: 10.1016/s0002-8703(98)70154-1 [DOI] [PubMed] [Google Scholar]
- 243.Upton SJ, Hagan RD, Lease B, Rosentswieg J, Gettman LR, Duncan JJ. Comparative physiological profiles among young and middle-aged female distance runners. Med Sci Sports Exerc. 1984;16(1):67–71. [PubMed] [Google Scholar]
- 244.van der Zwaard S, van der Laarse WJ, Weide G, Bloemers FW, Hofmijster MJ, Levels K, et al. Critical determinants of combined sprint and endurance performance: an integrative analysis from muscle fiber to the human body. FASEB J Off Publ Fed Am Soc Exp Biol. 2018;32(4):2110–23. doi: 10.1096/fj.201700827R [DOI] [PubMed] [Google Scholar]
- 245.Vasileiadis I, Roditis P, Dimopoulos S, Ladis V, Pangalis G, Aessopos A, et al. Impaired oxygen kinetics in beta-thalassaemia major patients. Acta Physiol Oxf Engl. 2009;196(3):357–63. doi: 10.1111/j.1748-1716.2008.01937.x [DOI] [PubMed] [Google Scholar]
- 246.Vistisen B, Nybo L, Xu X, Høy CE, Kiens B. Minor amounts of plasma medium-chain fatty acids and no improved time trial performance after consuming lipids. J Appl Physiol Bethesda Md 1985. 2003;95(6):2434–43. doi: 10.1152/japplphysiol.00118.2003 [DOI] [PubMed] [Google Scholar]
- 247.Vogt S, Altehoefer C, Bueltermann D, Pottgiesser T, Prettin S, Schmid A, et al. Magnetic resonance imaging of the lumbar spine and blood volume in professional cyclists. Eur J Appl Physiol. 2008;102(4):411–6. doi: 10.1007/s00421-007-0599-0 [DOI] [PubMed] [Google Scholar]
- 248.Walker AJ, McFadden BA, Sanders DJ, Bozzini BN, Conway SP, Arent SM. Early Season Hormonal and Biochemical Changes in Division I Field Hockey Players: Is Fitness Protective? J Strength Cond Res. 2020;34(4):975–81. doi: 10.1519/JSC.0000000000003492 [DOI] [PubMed] [Google Scholar]
- 249.Warrington G, Ryan C, Murray F, Duffy P, Kirwan JP. Physiological and metabolic characteristics of elite tug of war athletes. Br J Sports Med. 2001;35(6):396–401. doi: 10.1136/bjsm.35.6.396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wasserman K, Stringer WW, Casaburi R, Zhang YY. Mechanism of the exercise hyperkalemia: an alternate hypothesis. J Appl Physiol Bethesda Md 1985. 1997;83(2):631–43. doi: 10.1152/jappl.1997.83.2.631 [DOI] [PubMed] [Google Scholar]
- 251.Wenger CB, Latzka WA. Effects of pyridostigmine bromide on physiological responses to heat, exercise, and hypohydration. Aviat Space Environ Med. 1992;63(1):37–45. [PubMed] [Google Scholar]
- 252.Wetter TJ, St Croix CM, Pegelow DF, Sonetti DA, Dempsey JA. Effects of exhaustive endurance exercise on pulmonary gas exchange and airway function in women. J Appl Physiol Bethesda Md 1985. 2001;91(2):847–58. doi: 10.1152/jappl.2001.91.2.847 [DOI] [PubMed] [Google Scholar]
- 253.Wiebe CG, Gledhill N, Warburton DE, Jamnik VK, Ferguson S. Exercise cardiac function in endurance-trained males versus females. Clin J Sport Med Off J Can Acad Sport Med. 1998;8(4):272–9. doi: 10.1097/00042752-199810000-00004 [DOI] [PubMed] [Google Scholar]
- 254.Wilkerson JE, Batterton DL, Horvath SM. Exercise-induced changes in blood ammonia levels in humans. Eur J Appl Physiol. 1977;37(4):255–63. doi: 10.1007/BF00430955 [DOI] [PubMed] [Google Scholar]
- 255.Wilkerson JE, Horvath SM, Gutin B, Molnar S, Diaz FJ. Plasma electrolyte content and concentration during treadmill exercise in humans. J Appl Physiol. 1982;53(6):1529–39. doi: 10.1152/jappl.1982.53.6.1529 [DOI] [PubMed] [Google Scholar]
- 256.Witte KKA, Desilva R, Chattopadhyay S, Ghosh J, Cleland JGF, Clark AL. Are hematinic deficiencies the cause of anemia in chronic heart failure? Am Heart J. 2004;147(5):924–30. doi: 10.1016/j.ahj.2003.11.007 [DOI] [PubMed] [Google Scholar]
- 257.Wolfel EE, Groves BM, Brooks GA, Butterfield GE, Mazzeo RS, Moore LG, et al. Oxygen transport during steady-state submaximal exercise in chronic hypoxia. J Appl Physiol Bethesda Md 1985. 1991;70(3):1129–36. doi: 10.1152/jappl.1991.70.3.1129 [DOI] [PubMed] [Google Scholar]
- 258.Wu EY, Ramanathan M, Hsia CC. Role of hematocrit in the recruitment of pulmonary diffusing capacity: comparison of human and dog. J Appl Physiol Bethesda Md 1985. 1996;80(3):1014–20. doi: 10.1152/jappl.1996.80.3.1014 [DOI] [PubMed] [Google Scholar]
- 259.Yaspelkis BB 3rd, Ivy JL. Effect of carbohydrate supplements and water on exercise metabolism in the heat. J Appl Physiol Bethesda Md 1985. 1991;71(2):680–7. doi: 10.1152/jappl.1991.71.2.680 [DOI] [PubMed] [Google Scholar]
- 260.Zavorsky GS, Lands LC, Schneider W, Carli F. Comparison of fingertip to arterial blood samples at rest and during exercise. Clin J Sport Med Off J Can Acad Sport Med. 2005;15(4):263–70. doi: 10.1097/01.jsm.0000171287.99174.b7 [DOI] [PubMed] [Google Scholar]
- 261.Zheng ZQ, Geng ZH, Liu JX, Guo ST. Compressed food with added functional oligopeptides improves performance during military endurance training. Asia Pac J Clin Nutr. 2017;26(6):1066–75. doi: 10.6133/apjcn.122016.06 [DOI] [PubMed] [Google Scholar]
- 262.Zhu YI, Haas JD. Iron depletion without anemia and physical performance in young women. Am J Clin Nutr. 1997;66(2):334–41. doi: 10.1093/ajcn/66.2.334 [DOI] [PubMed] [Google Scholar]
- 263.Kanstrup IL, Ekblom B. Blood volume and hemoglobin concentration as determinants of maximal aerobic power. Med Sci Sports Exerc. 1984. Jun 1;16(3):256–62. [PubMed] [Google Scholar]
- 264.Balke B, Grillo GP, Konecci EB, Luft UC. Work capacity after blood donation. J Appl Physiol. 1954. Nov;7(3):231–8. doi: 10.1152/jappl.1954.7.3.231 [DOI] [PubMed] [Google Scholar]
- 265.Benedetto D, Rao CM, Cefalù C, Aguglia DO, Cattadori G, D’Ascola DG, et al. Effects of blood transfusion on exercise capacity in thalassemia major patients. PloS One. 2015;10(5):e0127553–e0127553. doi: 10.1371/journal.pone.0127553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Berger NJA, Campbell IT, Wilkerson DP, Jones AM. Influence of acute plasma volume expansion on VO2 kinetics, VO2 peak, and performance during high-intensity cycle exercise. J Appl Physiol Bethesda Md 1985. 2006;101(3):707–14. doi: 10.1152/japplphysiol.00154.2006 [DOI] [PubMed] [Google Scholar]
- 267.Birnbaum L, Dahl T, Boone T. Effect of blood donation on maximal oxygen consumption. J Sports Med Phys Fitness. 2006;46(4):535–9. [PubMed] [Google Scholar]
- 268.Burnley M, Roberts CL, Thatcher R, Doust JH, Jones AM. Influence of blood donation on O2 uptake on-kinetics, peak O2 uptake and time to exhaustion during severe-intensity cycle exercise in humans. Exp Physiol. 2006;91(3):499–509. doi: 10.1113/expphysiol.2005.032805 [DOI] [PubMed] [Google Scholar]
- 269.Celsing F, Ekblom B. Anemia causes a relative decrease in blood lactate concentration during exercise. Eur J Appl Physiol. 1986;55(1):74–8. doi: 10.1007/BF00422897 [DOI] [PubMed] [Google Scholar]
- 270.Celsing F, Nyström J, Pihlstedt P, Werner B, Ekblom B. Effect of long-term anemia and retransfusion on central circulation during exercise. J Appl Physiol Bethesda Md 1985. 1986;61(4):1358–62. doi: 10.1152/jappl.1986.61.4.1358 [DOI] [PubMed] [Google Scholar]
- 271.Celsing F, Svedenhag J, Pihlstedt P, Ekblom B. Effects of anaemia and stepwise-induced polycythaemia on maximal aerobic power in individuals with high and low haemoglobin concentrations. Acta Physiol Scand. 1987;129(1):47–54. doi: 10.1111/j.1748-1716.1987.tb08038.x [DOI] [PubMed] [Google Scholar]
- 272.Coyle EF, Hopper MK, Coggan AR. Maximal oxygen uptake relative to plasma volume expansion. Int J Sports Med. 1990;11(2):116–9. doi: 10.1055/s-2007-1024774 [DOI] [PubMed] [Google Scholar]
- 273.Cureton K, Bishop P, Hutchinson P, Newland H, Vickery S, Zwiren L. Sex difference in maximal oxygen uptake. Effect of equating haemoglobin concentration. Eur J Appl Physiol. 1986;54(6):656–60. doi: 10.1007/BF00943356 [DOI] [PubMed] [Google Scholar]
- 274.Eliassen HS, Hervig T, Backlund S, Sivertsen J, Iversen VV, Kristoffersen M, et al. Immediate effects of blood donation on physical and cognitive performance-A randomized controlled double-blinded trial. J Trauma Acute Care Surg. 2018;84(6S Suppl 1):S125–31. doi: 10.1097/TA.0000000000001917 [DOI] [PubMed] [Google Scholar]
- 275.Freedson PS. The influence of hemoglobin concentration on exercise cardiac output. Int J Sports Med. 1981;2(2):81–6. doi: 10.1055/s-2008-1034587 [DOI] [PubMed] [Google Scholar]
- 276.Gordon D, Wood M, Porter A, Vetrivel V, Gernigon M, Caddy O, et al. Influence of blood donation on the incidence of plateau at VO2max. Eur J Appl Physiol. 2014;114(1):21–7. doi: 10.1007/s00421-013-2743-3 [DOI] [PubMed] [Google Scholar]
- 277.Hill DW, Vingren JL, Burdette SD. Effect of plasma donation and blood donation on aerobic and anaerobic responses in exhaustive, severe-intensity exercise. Appl Physiol Nutr Metab Physiol Appl Nutr Metab. 2013;38(5):551–7. doi: 10.1139/apnm-2012-0361 [DOI] [PubMed] [Google Scholar]
- 278.Kanstrup IL, Ekblom B. Acute hypervolemia, cardiac performance, and aerobic power during exercise. J Appl Physiol. 1982;52(5):1186–91. doi: 10.1152/jappl.1982.52.5.1186 [DOI] [PubMed] [Google Scholar]
- 279.Koskolou MD, Roach RC, Calbet JA, Rådegran G, Saltin B. Cardiovascular responses to dynamic exercise with acute anemia in humans. Am J Physiol. 1997;273(4):H1787–93. doi: 10.1152/ajpheart.1997.273.4.H1787 [DOI] [PubMed] [Google Scholar]
- 280.Meurrens J, Steiner T, Ponette J, Janssen HA, Ramaekers M, Wehrlin JP, et al. Effect of Repeated Whole Blood Donations on Aerobic Capacity and Hemoglobin Mass in Moderately Trained Male Subjects: A Randomized Controlled Trial. Sports Med—Open. 2016;2(1):43–43. doi: 10.1186/s40798-016-0067-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Panebianco RA, Stachenfeld N, Coplan NL, Gleim GW. Effects of blood donation on exercise performance in competitive cyclists. Am Heart J. 1995;130(4):838–40. doi: 10.1016/0002-8703(95)90085-3 [DOI] [PubMed] [Google Scholar]
- 282.Robertson RJ, Gilcher R, Metz KF, Skrinar GS, Allison TG, Bahnson HT, et al. Effect of induced erythrocythemia on hypoxia tolerance during physical exercise. J Appl Physiol. 1982;53(2):490–5. doi: 10.1152/jappl.1982.53.2.490 [DOI] [PubMed] [Google Scholar]
- 283.Robertson RJ, Gilcher R, Metz KF, Caspersen CJ, Allison TG, Abbott RA, et al. Hemoglobin concentration and aerobic work capacity in women following induced erythrocythemia. J Appl Physiol. 1984;57(2):568–75. doi: 10.1152/jappl.1984.57.2.568 [DOI] [PubMed] [Google Scholar]
- 284.Schaffartzik W, Barton ED, Poole DC, Tsukimoto K, Hogan MC, Bebout DE, et al. Effect of reduced hemoglobin concentration on leg oxygen uptake during maximal exercise in humans. J Appl Physiol Bethesda Md 1985. 1993;75(2):491–490. doi: 10.1152/jappl.1993.75.2.491 [DOI] [PubMed] [Google Scholar]
- 285.Skattebo Ø, Johansen ES, Capelli C, Hallén J. Effects of 150- and 450-mL Acute Blood Losses on Maximal Oxygen Uptake and Exercise Capacity. Med Sci Sports Exerc. 2021;53(8):1729–38. doi: 10.1249/MSS.0000000000002618 [DOI] [PubMed] [Google Scholar]
- 286.Spriet LL, Gledhill N, Froese AB, Wilkes DL. Effect of graded erythrocythemia on cardiovascular and metabolic responses to exercise. J Appl Physiol Bethesda Md 1985. 1986;61(5):1942–8. doi: 10.1152/jappl.1986.61.5.1942 [DOI] [PubMed] [Google Scholar]
- 287.Taylor NA, Patterson MJ, Cotter JD, Macfarlane DJ. Effects of artificially-induced anaemia on sudomotor and cutaneous blood flow responses to heat stress. Eur J Appl Physiol. 1997;76(4):380–6. doi: 10.1007/s004210050265 [DOI] [PubMed] [Google Scholar]
- 288.Turner DL, Hoppeler H, Noti C, Gurtner HP, Gerber H, Schena F, et al. Limitations to VO2max in humans after blood retransfusion. Respir Physiol. 1993;92(3):329–41. doi: 10.1016/0034-5687(93)90017-5 [DOI] [PubMed] [Google Scholar]
- 289.Warburton DE, Gledhill N, Jamnik VK, Krip B, Card N. Induced hypervolemia, cardiac function, VO2max, and performance of elite cyclists. Med Sci Sports Exerc. 1999;31(6):800–8. doi: 10.1097/00005768-199906000-00007 [DOI] [PubMed] [Google Scholar]
- 290.Woodson RD, Wills RE, Lenfant C. Effect of acute and established anemia on O2 transport at rest, submaximal and maximal work. J Appl Physiol. 1978;44(1):36–43. doi: 10.1152/jappl.1978.44.1.36 [DOI] [PubMed] [Google Scholar]
- 291.Ziegler AK, Grand J, Stangerup I, Nielsen HJ, Dela F, Magnussen K, et al. Time course for the recovery of physical performance, blood hemoglobin, and ferritin content after blood donation. Transfusion (Paris). 2015;55(4):898–905. doi: 10.1111/trf.12926 [DOI] [PubMed] [Google Scholar]
- 292.Annaheim S, Jacob M, Krafft A, Breymann C, Rehm M, Boutellier U. RhEPO improves time to exhaustion by non-hematopoietic factors in humans. Eur J Appl Physiol. 2016;116(3):623–33. doi: 10.1007/s00421-015-3322-6 [DOI] [PubMed] [Google Scholar]
- 293.Audran M, Gareau R, Matecki S, Durand F, Chenard C, Sicart MT, et al. Effects of erythropoietin administration in training athletes and possible indirect detection in doping control. Med Sci Sports Exerc. 1999;31(5):639–45. doi: 10.1097/00005768-199905000-00003 [DOI] [PubMed] [Google Scholar]
- 294.Balsom PD, Ekblom B, Sjödin B. Enhanced oxygen availability during high intensity intermittent exercise decreases anaerobic metabolite concentrations in blood. Acta Physiol Scand. 1994;150(4):455–6. doi: 10.1111/j.1748-1716.1994.tb09711.x [DOI] [PubMed] [Google Scholar]
- 295.Birkeland KI, Stray-Gundersen J, Hemmersbach P, Hallen J, Haug E, Bahr R. Effect of rhEPO administration on serum levels of sTfR and cycling performance. Med Sci Sports Exerc. 2000;32(7):1238–43. doi: 10.1097/00005768-200007000-00009 [DOI] [PubMed] [Google Scholar]
- 296.Caillaud C, Connes P, Ben Saad H, Mercier J. Erythropoietin enhances whole body lipid oxidation during prolonged exercise in humans. J Physiol Biochem. 2015;71(1):9–16. doi: 10.1007/s13105-014-0374-8 [DOI] [PubMed] [Google Scholar]
- 297.Christensen B, Nellemann B, Larsen MS, Thams L, Sieljacks P, Vestergaard PF, et al. Whole body metabolic effects of prolonged endurance training in combination with erythropoietin treatment in humans: a randomized placebo controlled trial. Am J Physiol Endocrinol Metab. 2013;305(7):E879–89. doi: 10.1152/ajpendo.00269.2013 [DOI] [PubMed] [Google Scholar]
- 298.Connes P, Perrey S, Varray A, Préfaut C, Caillaud C. Faster oxygen uptake kinetics at the onset of submaximal cycling exercise following 4 weeks recombinant human erythropoietin (r-HuEPO) treatment. Pflugers Arch. 2003;447(2):231–8. doi: 10.1007/s00424-003-1174-0 [DOI] [PubMed] [Google Scholar]
- 299.Dias KA, Lawley JS, Gatterer H, Howden EJ, Sarma S, Cornwell WK 3rd, et al. Effect of acute and chronic xenon inhalation on erythropoietin, hematological parameters, and athletic performance. J Appl Physiol Bethesda Md 1985. 2019;127(6):1503–10. doi: 10.1152/japplphysiol.00289.2019 [DOI] [PubMed] [Google Scholar]
- 300.Durussel J, Daskalaki E, Anderson M, Chatterji T, Wondimu DH, Padmanabhan N, et al. Haemoglobin mass and running time trial performance after recombinant human erythropoietin administration in trained men. PloS One. 2013;8(2):e56151–e56151. doi: 10.1371/journal.pone.0056151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Guadalupe-Grau A, Plenge U, Helbo S, Kristensen M, Andersen PR, Fago A, et al. Effects of an 8-weeks erythropoietin treatment on mitochondrial and whole body fat oxidation capacity during exercise in healthy males. J Sports Sci. 2015;33(6):570–8. doi: 10.1080/02640414.2014.951872 [DOI] [PubMed] [Google Scholar]
- 302.Haider T, Diaz V, Albert J, Alvarez-Sanchez M, Thiersch M, Maggiorini M, et al. A Single 60.000 IU Dose of Erythropoietin Does Not Improve Short-Term Aerobic Exercise Performance in Healthy Subjects: A Randomized, Double-Blind, Placebo-Controlled Crossover Trial. Front Physiol. 2020;11:537389. doi: 10.3389/fphys.2020.537389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Haile DW, Durussel J, Mekonen W, Ongaro N, Anjila E, Mooses M, et al. Effects of EPO on Blood Parameters and Running Performance in Kenyan Athletes. Med Sci Sports Exerc. 2019;51(2):299–307. doi: 10.1249/MSS.0000000000001777 [DOI] [PubMed] [Google Scholar]
- 304.Heuberger JAAC Rotmans JI, Gal P Stuurman FE, van ‘t Westende J, Post TE, et al. Effects of erythropoietin on cycling performance of well trained cyclists: a double-blind, randomised, placebo-controlled trial. Lancet Haematol. 2017;4(8):e374–86. doi: 10.1016/S2352-3026(17)30105-9 [DOI] [PubMed] [Google Scholar]
- 305.Lundby C, Achman-Andersen NJ, Thomsen JJ, Norgaard AM, Robach P. Testing for recombinant human erythropoietin in urine: problems associated with current anti-doping testing. J Appl Physiol Bethesda Md 1985. 2008;105(2):417–9. [DOI] [PubMed] [Google Scholar]
- 306.Lundby C, Robach P, Boushel R, Thomsen JJ, Rasmussen P, Koskolou M, et al. Does recombinant human Epo increase exercise capacity by means other than augmenting oxygen transport? J Appl Physiol Bethesda Md 1985. 2008;105(2):581–7. doi: 10.1152/japplphysiol.90484.2008 [DOI] [PubMed] [Google Scholar]
- 307.Nordsborg NB, Robach P, Boushel R, Calbet JAL, Lundby C. Erythropoietin does not reduce plasma lactate, H+, and K+ during intense exercise. Scand J Med Sci Sports. 2015;25(6):e566–75. [DOI] [PubMed] [Google Scholar]
- 308.Robach P, Calbet JAL, Thomsen JJ, Boushel R, Mollard P, Rasmussen P, et al. The ergogenic effect of recombinant human erythropoietin on VO2max depends on the severity of arterial hypoxemia. PloS One. 2008;3(8):e2996–e2996. doi: 10.1371/journal.pone.0002996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Sieljacks P, Thams L, Nellemann B, Larsen MS, Vissing K, Christensen B. Comparative Effects of Aerobic Training and Erythropoietin on Oxygen Uptake in Untrained Humans. J Strength Cond Res. 2016;30(8):2307–17. doi: 10.1519/JSC.0000000000001314 [DOI] [PubMed] [Google Scholar]
- 310.Thomsen JJ, Rentsch RL, Robach P, Calbet JAL, Boushel R, Rasmussen P, et al. Prolonged administration of recombinant human erythropoietin increases submaximal performance more than maximal aerobic capacity. Eur J Appl Physiol. 2007;101(4):481–6. doi: 10.1007/s00421-007-0522-8 [DOI] [PubMed] [Google Scholar]
- 311.Baumann CW, Bond KL, Rupp JC, Ingalls CP, Doyle JA. Echinacea purpurea supplementation does not enhance VO2max in distance runners. J Strength Cond Res. 2014;28(5):1367–72. doi: 10.1097/JSC.0000000000000206 [DOI] [PubMed] [Google Scholar]
- 312.Brownlie T 4th, Utermohlen V, Hinton PS, Giordano C, Haas JD. Marginal iron deficiency without anemia impairs aerobic adaptation among previously untrained women. Am J Clin Nutr. 2002;75(4):734–42. doi: 10.1093/ajcn/75.4.734 [DOI] [PubMed] [Google Scholar]
- 313.Cheng HY, Frise MC, Curtis MK, Bart NK, Petousi N, Talbot NP, et al. Intravenous iron delivers a sustained (8-week) lowering of pulmonary artery pressure during exercise in healthy older humans. Physiol Rep. 2019;7(13):e14164. doi: 10.14814/phy2.14164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Durkalec-Michalski K, Nowaczyk PM, Główka N, Ziobrowska A, Podgórski T. Is a Four-Week Ketogenic Diet an Effective Nutritional Strategy in CrossFit-Trained Female and Male Athletes? Nutrients. 2021;13(3). doi: 10.3390/nu13030864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Garvican LA, Saunders PU, Cardoso T, Macdougall IC, Lobigs LM, Fazakerley R, et al. Intravenous iron supplementation in distance runners with low or suboptimal ferritin. Med Sci Sports Exerc. 2014;46(2):376–85. doi: 10.1249/MSS.0b013e3182a53594 [DOI] [PubMed] [Google Scholar]
- 316.Hinton PS, Giordano C, Brownlie T, Haas JD. Iron supplementation improves endurance after training in iron-depleted, nonanemic women. J Appl Physiol Bethesda Md 1985. 2000;88(3):1103–11. doi: 10.1152/jappl.2000.88.3.1103 [DOI] [PubMed] [Google Scholar]
- 317.Hinton PS, Sinclair LM. Iron supplementation maintains ventilatory threshold and improves energetic efficiency in iron-deficient nonanemic athletes. Eur J Clin Nutr. 2007;61(1):30–9. doi: 10.1038/sj.ejcn.1602479 [DOI] [PubMed] [Google Scholar]
- 318.Klingshirn LA, Pate RR, Bourque SP, Davis JM, Sargent RG. Effect of iron supplementation on endurance capacity in iron-depleted female runners. Med Sci Sports Exerc. 1992;24(7):819–24. [PubMed] [Google Scholar]
- 319.Kreider RB, Miller GW, Williams MH, Somma CT, Nasser TA. Effects of phosphate loading on oxygen uptake, ventilatory anaerobic threshold, and run performance. Med Sci Sports Exerc. 1990;22(2):250–6. [PubMed] [Google Scholar]
- 320.LaManca JJ, Haymes EM. Effects of iron repletion on VO2max, endurance, and blood lactate in women. Med Sci Sports Exerc. 1993;25(12):1386–92. [PubMed] [Google Scholar]
- 321.Lukaski HC, Hall CB, Siders WA. Altered metabolic response of iron-deficient women during graded, maximal exercise. Eur J Appl Physiol. 1991;63(2):140–5. doi: 10.1007/BF00235184 [DOI] [PubMed] [Google Scholar]
- 322.Magazanik A, Weinstein Y, Abarbanel J, Lewinski U, Shapiro Y, Inbar O, et al. Effect of an iron supplement on body iron status and aerobic capacity of young training women. Eur J Appl Physiol. 1991;62(5):317–23. doi: 10.1007/BF00634966 [DOI] [PubMed] [Google Scholar]
- 323.Marley A, Grant MC, Babraj J. Weekly Vitamin D(3) supplementation improves aerobic performance in combat sport athletes. Eur J Sport Sci. 2021;21(3):379–87. doi: 10.1080/17461391.2020.1744736 [DOI] [PubMed] [Google Scholar]
- 324.McDonagh STJ, Vanhatalo A, Fulford J, Wylie LJ, Bailey SJ, Jones AM. Dietary nitrate supplementation attenuates the reduction in exercise tolerance following blood donation. Am J Physiol Heart Circ Physiol. 2016;311(6):H1520–9. doi: 10.1152/ajpheart.00451.2016 [DOI] [PubMed] [Google Scholar]
- 325.McMurray RG, Ben-Ezra V, Forsythe WA, Smith AT. Responses of endurance-trained subjects to caloric deficits induced by diet or exercise. Med Sci Sports Exerc. 1985;17(5):574–9. [PubMed] [Google Scholar]
- 326.Moro T, Tinsley G, Longo G, Grigoletto D, Bianco A, Ferraris C, et al. Time-restricted eating effects on performance, immune function, and body composition in elite cyclists: a randomized controlled trial. J Int Soc Sports Nutr. 2020;17(1):65. doi: 10.1186/s12970-020-00396-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Newhouse IJ, Clement DB, Taunton JE, McKenzie DC. The effects of prelatent/latent iron deficiency on physical work capacity. Med Sci Sports Exerc. 1989;21(3):263–8. [PubMed] [Google Scholar]
- 328.Peeling P, Blee T, Goodman C, Dawson B, Claydon G, Beilby J, et al. Effect of iron injections on aerobic-exercise performance of iron-depleted female athletes. Int J Sport Nutr Exerc Metab. 2007;17(3):221–31. doi: 10.1123/ijsnem.17.3.221 [DOI] [PubMed] [Google Scholar]
- 329.Pompano LM, Haas JD. Efficacy of iron supplementation may be misinterpreted using conventional measures of iron status in iron-depleted, nonanemic women undergoing aerobic exercise training. Am J Clin Nutr. 2017;106(6):1529–38. doi: 10.3945/ajcn.117.152777 [DOI] [PubMed] [Google Scholar]
- 330.Rasic JS, Ivanovic ND, Andjelkovic MS, Nedeljkovic IP, Nikolic IR, Stojanovic SD, et al. Influence of Higenamine on Exercise Performance of Recreational Female Athletes: A Randomized Double-Blinded Placebo-Controlled Trial. Front Psychol. 2021;12:633110. doi: 10.3389/fpsyg.2021.633110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Schoene RB, Escourrou P, Robertson HT, Nilson KL, Parsons JR, Smith NJ. Iron repletion decreases maximal exercise lactate concentrations in female athletes with minimal iron-deficiency anemia. J Lab Clin Med. 1983;102(2):306–12. [PubMed] [Google Scholar]
- 332.Stevenson JL, Krishnan S, Inigo MM, Stamatikos AD, Gonzales JU, Cooper JA. Echinacea-Based Dietary Supplement Does Not Increase Maximal Aerobic Capacity in Endurance-Trained Men and Women. J Diet Suppl. 2016;13(3):324–38. doi: 10.3109/19390211.2015.1036189 [DOI] [PubMed] [Google Scholar]
- 333.Taniguchi M, Imamura H, Shirota T, Okamatsu H, Fujii Y, Toba M, et al. Improvement in iron deficiency anemia through therapy with ferric ammonium citrate and vitamin C and the effects of aerobic exercise. J Nutr Sci Vitaminol (Tokyo). 1991;37(2):161–71. doi: 10.3177/jnsv.37.161 [DOI] [PubMed] [Google Scholar]
- 334.Vukovich MD, Dreifort GD. Effect of beta-hydroxy beta-methylbutyrate on the onset of blood lactate accumulation and V(O)(2) peak in endurance-trained cyclists. J Strength Cond Res. 2001;15(4):491–7. [PubMed] [Google Scholar]
- 335.Wachsmuth NB, Aigner T, Völzke C, Zapf J, Schmidt WF. Monitoring recovery from iron deficiency using total hemoglobin mass. Med Sci Sports Exerc. 2015;47(2):419–27. doi: 10.1249/MSS.0000000000000420 [DOI] [PubMed] [Google Scholar]
- 336.Basset FA, Joanisse DR, Boivin F, St-Onge J, Billaut F, Doré J, et al. Effects of short-term normobaric hypoxia on haematology, muscle phenotypes and physical performance in highly trained athletes. Exp Physiol. 2006;91(2):391–402. doi: 10.1113/expphysiol.2005.031682 [DOI] [PubMed] [Google Scholar]
- 337.Bender PR, Groves BM, McCullough RE, McCullough RG, Huang SY, Hamilton AJ, et al. Oxygen transport to exercising leg in chronic hypoxia. J Appl Physiol Bethesda Md 1985. 1988;65(6):2592–7. doi: 10.1152/jappl.1988.65.6.2592 [DOI] [PubMed] [Google Scholar]
- 338.Bonne TC, Lundby C, Jørgensen S, Johansen L, Mrgan M, Bech SR, et al. “Live High-Train High” increases hemoglobin mass in Olympic swimmers. Eur J Appl Physiol. 2014;114(7):1439–49. doi: 10.1007/s00421-014-2863-4 [DOI] [PubMed] [Google Scholar]
- 339.Boutellier U, Dériaz O, di Prampero PE, Cerretelli P. Aerobic performance at altitude: effects of acclimatization and hematocrit with reference to training. Int J Sports Med. 1990;11 Suppl 1:S21–6. doi: 10.1055/s-2007-1024849 [DOI] [PubMed] [Google Scholar]
- 340.Brugniaux JV, Schmitt L, Robach P, Nicolet G, Fouillot JP, Moutereau S, et al. Eighteen days of “living high, training low” stimulate erythropoiesis and enhance aerobic performance in elite middle-distance runners. J Appl Physiol Bethesda Md 1985. 2006;100(1):203–11. [DOI] [PubMed] [Google Scholar]
- 341.Chapman RF, Stray-Gundersen J, Levine BD. Individual variation in response to altitude training. J Appl Physiol Bethesda Md 1985. 1998;85(4):1448–56. doi: 10.1152/jappl.1998.85.4.1448 [DOI] [PubMed] [Google Scholar]
- 342.Chen CY, Hou CW, Bernard JR, Chen CC, Hung TC, Cheng LL, et al. Rhodiola crenulata- and Cordyceps sinensis-based supplement boosts aerobic exercise performance after short-term high altitude training. High Alt Med Biol. 2014;15(3):371–9. doi: 10.1089/ham.2013.1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Clark SA, Aughey RJ, Gore CJ, Hahn AG, Townsend NE, Kinsman TA, et al. Effects of live high, train low hypoxic exposure on lactate metabolism in trained humans. J Appl Physiol Bethesda Md 1985. 2004;96(2):517–25. doi: 10.1152/japplphysiol.00799.2003 [DOI] [PubMed] [Google Scholar]
- 344.Czuba M, Waskiewicz Z, Zajac A, Poprzecki S, Cholewa J, Roczniok R. The effects of intermittent hypoxic training on aerobic capacity and endurance performance in cyclists. J Sports Sci Med. 2011;10(1):175–83. [PMC free article] [PubMed] [Google Scholar]
- 345.Czuba M, Maszczyk A, Gerasimuk D, Roczniok R, Fidos-Czuba O, Zając A, et al. The effects of hypobaric hypoxia on erythropoiesis, maximal oxygen uptake and energy cost of exercise under normoxia in elite biathletes. J Sports Sci Med. 2014;13(4):912–20. [PMC free article] [PubMed] [Google Scholar]
- 346.Dehnert C, Hütler M, Liu Y, Menold E, Netzer C, Schick R, et al. Erythropoiesis and performance after two weeks of living high and training low in well trained triathletes. Int J Sports Med. 2002;23(8):561–6. doi: 10.1055/s-2002-35533 [DOI] [PubMed] [Google Scholar]
- 347.Garver MJ, Scheadler CM, Smith LM, Taylor SJ, Harbach CM. Simulated Altitude via Re-Breathing Creates Arterial Hypoxemia but Fails to Improve Elements of Running Performance. Int J Exerc Sci. 2018;11(6):187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Grassi B, Marzorati M, Kayser B, Bordini M, Colombini A, Conti M, et al. Peak blood lactate and blood lactate vs. workload during acclimatization to 5,050 m and in deacclimatization. J Appl Physiol Bethesda Md 1985. 1996;80(2):685–92. [DOI] [PubMed] [Google Scholar]
- 349.Green HJ. Altitude acclimatization, training and performance. J Sci Med Sport. 2000. Sep;3(3):299–312. doi: 10.1016/s1440-2440(00)80039-0 [DOI] [PubMed] [Google Scholar]
- 350.Hahn AG, Gore CJ, Martin DT, Ashenden MJ, Roberts AD, Logan PA. An evaluation of the concept of living at moderate altitude and training at sea level. Comp Biochem Physiol A Mol Integr Physiol. 2001;128(4):777–89. doi: 10.1016/s1095-6433(01)00283-5 [DOI] [PubMed] [Google Scholar]
- 351.Ingjer F, Myhre K. Physiological effects of altitude training on elite male cross-country skiers. J Sports Sci. 1992;10(1):37–47. doi: 10.1080/02640419208729905 [DOI] [PubMed] [Google Scholar]
- 352.Koivisto AE, Paulsen G, Paur I, Garthe I, Tønnessen E, Raastad T, et al. Antioxidant-rich foods and response to altitude training: A randomized controlled trial in elite endurance athletes. Scand J Med Sci Sports. 2018;28(9):1982–95. doi: 10.1111/sms.13212 [DOI] [PubMed] [Google Scholar]
- 353.Lundby C, Nielsen TK, Dela F, Damsgaard R. The influence of intermittent altitude exposure to 4100 m on exercise capacity and blood variables. Scand J Med Sci Sports. 2005;15(3):182–7. doi: 10.1111/j.1600-0838.2004.405.x [DOI] [PubMed] [Google Scholar]
- 354.Morton JP, Cable NT. Effects of intermittent hypoxic training on aerobic and anaerobic performance. Ergonomics. 2005;48(11–14):1535–46. doi: 10.1080/00140130500100959 [DOI] [PubMed] [Google Scholar]
- 355.Mounier R, Pialoux V, Cayre A, Schmitt L, Richalet JP, Robach P, et al. Leukocyte’s Hif-1 expression and training-induced erythropoietic response in swimmers. Med Sci Sports Exerc. 2006;38(8):1410–7. doi: 10.1249/01.mss.0000228955.98215.a1 [DOI] [PubMed] [Google Scholar]
- 356.Nakamoto FP, Ivamoto RK, Andrade MDS, de Lira CAB, Silva BM, da Silva AC. Effect of Intermittent Hypoxic Training Followed by Intermittent Hypoxic Exposure on Aerobic Capacity of Long Distance Runners. J Strength Cond Res. 2016;30(6):1708–20. doi: 10.1519/JSC.0000000000001258 [DOI] [PubMed] [Google Scholar]
- 357.Okazaki K, Stray-Gundersen J, Chapman RF, Levine BD. Iron insufficiency diminishes the erythropoietic response to moderate altitude exposure. J Appl Physiol Bethesda Md 1985. 2019;127(6):1569–78. doi: 10.1152/japplphysiol.00115.2018 [DOI] [PubMed] [Google Scholar]
- 358.Park HY, Park W, Lim K. Living High-Training Low for 21 Days Enhances Exercise Economy, Hemodynamic Function, and Exercise Performance of Competitive Runners. J Sports Sci Med. 2019;18(3):427–37. [PMC free article] [PubMed] [Google Scholar]
- 359.Ramos-Campo DJ, Martínez-Sánchez F, Esteban-García P, Rubio-Arias JA, Clemente-Suarez VJ, Jiménez-Díaz JF. The effects of intermittent hypoxia training on hematological and aerobic performance in triathletes. Acta Physiol Hung. 2015;102(4):409–18. doi: 10.1556/036.102.2015.4.8 [DOI] [PubMed] [Google Scholar]
- 360.Ramos-Campo DJ, Martínez-Guardado I, Olcina G, Marín-Pagán C, Martínez-Noguera FJ, Carlos-Vivas J, et al. Effect of high-intensity resistance circuit-based training in hypoxia on aerobic performance and repeat sprint ability. Scand J Med Sci Sports. 2018;28(10):2135–43. doi: 10.1111/sms.13223 [DOI] [PubMed] [Google Scholar]
- 361.Robach P, Schmitt L, Brugniaux JV, Nicolet G, Duvallet A, Fouillot JP, et al. Living high-training low: effect on erythropoiesis and maximal aerobic performance in elite Nordic skiers. Eur J Appl Physiol. 2006;97(6):695–705. doi: 10.1007/s00421-006-0240-7 [DOI] [PubMed] [Google Scholar]
- 362.Robach P, Siebenmann C, Jacobs RA, Rasmussen P, Nordsborg N, Pesta D, et al. The role of haemoglobin mass on VO(2)max following normobaric “live high-train low” in endurance-trained athletes. Br J Sports Med. 2012;46(11):822–7. doi: 10.1136/bjsports-2012-091078 [DOI] [PubMed] [Google Scholar]
- 363.Robach P, Bonne T, Flück D, Bürgi S, Toigo M, Jacobs RA, et al. Hypoxic training: effect on mitochondrial function and aerobic performance in hypoxia. Med Sci Sports Exerc. 2014;46(10):1936–45. doi: 10.1249/MSS.0000000000000321 [DOI] [PubMed] [Google Scholar]
- 364.Robach P, Hansen J, Pichon A, Meinild Lundby AK, Dandanell S, Slettaløkken Falch G, et al. Hypobaric live high-train low does not improve aerobic performance more than live low-train low in cross-country skiers. Scand J Med Sci Sports. 2018;28(6):1636–52. doi: 10.1111/sms.13075 [DOI] [PubMed] [Google Scholar]
- 365.Robertson EY, Saunders PU, Pyne DB, Gore CJ, Anson JM. Effectiveness of intermittent training in hypoxia combined with live high/train low. Eur J Appl Physiol. 2010;110(2):379–87. doi: 10.1007/s00421-010-1516-5 [DOI] [PubMed] [Google Scholar]
- 366.Roels B, Millet GP, Marcoux CJL, Coste O, Bentley DJ, Candau RB. Effects of hypoxic interval training on cycling performance. Med Sci Sports Exerc. 2005;37(1):138–46. doi: 10.1249/01.mss.0000150077.30672.88 [DOI] [PubMed] [Google Scholar]
- 367.Ryan BJ, Goodrich JA, Schmidt W, Kane LA, Byrnes WC. Ten Days of Intermittent, Low-dose Carbon Monoxide Inhalation does not Significantly Alter Hemoglobin Mass, Aerobic Performance Predictors, or Peak-power Exercise Tolerance. Int J Sports Med. 2016;37(11):884–9. doi: 10.1055/s-0042-108197 [DOI] [PubMed] [Google Scholar]
- 368.Saugy JJ, Schmitt L, Hauser A, Constantin G, Cejuela R, Faiss R, et al. Same Performance Changes after Live High-Train Low in Normobaric vs. Hypobaric Hypoxia. Front Physiol. 2016;7:138–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Saunders PU, Telford RD, Pyne DB, Hahn AG, Gore CJ. Improved running economy and increased hemoglobin mass in elite runners after extended moderate altitude exposure. J Sci Med Sport. 2009;12(1):67–72. doi: 10.1016/j.jsams.2007.08.014 [DOI] [PubMed] [Google Scholar]
- 370.Sotiridis A, Debevec T, McDonnell AC, Ciuha U, Eiken O, Mekjavic IB. Exercise cardiorespiratory and thermoregulatory responses in normoxic, hypoxic and hot environment following 10-day continuous hypoxic exposure. J Appl Physiol Bethesda Md 1985. 2018; doi: 10.1152/japplphysiol.01114.2017 [DOI] [PubMed] [Google Scholar]
- 371.Steinacker JM, Liu Y, Böning D, Halder A, Maassen N, Thomas A, et al. Lung diffusion capacity, oxygen uptake, cardiac output and oxygen transport during exercise before and after an himalayan expedition. Eur J Appl Physiol. 1996;74(1–2):187–93. [DOI] [PubMed] [Google Scholar]
- 372.Stray-Gundersen J, Chapman RF, Levine BD. “Living high-training low” altitude training improves sea level performance in male and female elite runners. J Appl Physiol Bethesda Md 1985. 2001;91(3):1113–20. [DOI] [PubMed] [Google Scholar]
- 373.Svedenhag J, Piehl-Aulin K, Skog C, Saltin B. Increased left ventricular muscle mass after long-term altitude training in athletes. Acta Physiol Scand. 1997;161(1):63–70. doi: 10.1046/j.1365-201X.1997.00204.x [DOI] [PubMed] [Google Scholar]
- 374.Wehrlin JP, Zuest P, Hallén J, Marti B. Live high-train low for 24 days increases hemoglobin mass and red cell volume in elite endurance athletes. J Appl Physiol Bethesda Md 1985. 2006;100(6):1938–45. doi: 10.1152/japplphysiol.01284.2005 [DOI] [PubMed] [Google Scholar]
- 375.Yan B, Ge X, Yu J, Hu Y, Girard O. Hypoxic re-exposure retains hematological but not performance adaptations post-altitude training. Eur J Appl Physiol. 2021;121(4):1049–59. doi: 10.1007/s00421-020-04589-x [DOI] [PubMed] [Google Scholar]
- 376.Ade CJ, Broxterman RM, Moore AD, Barstow TJ. Decreases in maximal oxygen uptake following long-duration spaceflight: Role of convective and diffusive O(2) transport mechanisms. J Appl Physiol Bethesda Md 1985. 2017;122(4):968–75. doi: 10.1152/japplphysiol.00280.2016 [DOI] [PubMed] [Google Scholar]
- 377.Aoyagi Y, McLellan TM, Shephard RJ. Effects of training and acclimation on heat tolerance in exercising men wearing protective clothing. Eur J Appl Physiol. 1994;68(3):234–45. doi: 10.1007/BF00376772 [DOI] [PubMed] [Google Scholar]
- 378.Ben Abderrahman A, Prioux J, Chamari K, Ben Ounis O, Tabka Z, Zouhal H. Running interval training and estimated plasma-volume variation. Int J Sports Physiol Perform. 2013;8(4):358–65. doi: 10.1123/ijspp.8.4.358 [DOI] [PubMed] [Google Scholar]
- 379.Bonne TC, Doucende G, Flück D, Jacobs RA, Nordsborg NB, Robach P, et al. Phlebotomy eliminates the maximal cardiac output response to six weeks of exercise training. Am J Physiol Regul Integr Comp Physiol. 2014;306(10):R752–60. doi: 10.1152/ajpregu.00028.2014 [DOI] [PubMed] [Google Scholar]
- 380.Bourque SP, Pate RR, Branch JD. Twelve weeks of endurance exercise training does not affect iron status measures in women. J Am Diet Assoc. 1997;97(10):1116–21. doi: 10.1016/S0002-8223(97)00272-1 [DOI] [PubMed] [Google Scholar]
- 381.Branch JD 3rd, Pate RR, Bourque SP, Convertino VA, Durstine JL, Ward DS. Exercise training and intensity does not alter vascular volume responses in women. Aviat Space Environ Med. 1999;70(11):1070–6. [PubMed] [Google Scholar]
- 382.Bringard A, Pogliaghi S, Adami A, De Roia G, Lador F, Lucini D, et al. Cardiovascular determinants of maximal oxygen consumption in upright and supine posture at the end of prolonged bed rest in humans. Respir Physiol Neurobiol. 2010;172(1–2):53–62. doi: 10.1016/j.resp.2010.03.018 [DOI] [PubMed] [Google Scholar]
- 383.Cardinale DA, Larsen FJ, Lännerström J, Manselin T, Södergård O, Mijwel S, et al. Influence of Hyperoxic-Supplemented High-Intensity Interval Training on Hemotological and Muscle Mitochondrial Adaptations in Trained Cyclists. Front Physiol. 2019;10:730–730. doi: 10.3389/fphys.2019.00730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Convertino VA, Brock PJ, Keil LC, Bernauer EM, Greenleaf JE. Exercise training-induced hypervolemia: role of plasma albumin, renin, and vasopressin. J Appl Physiol. 1980;48(4):665–9. doi: 10.1152/jappl.1980.48.4.665 [DOI] [PubMed] [Google Scholar]
- 385.Coppola L, Grassia A, Coppola A, Tondi G, Peluso G, Mordente S, et al. Effects of a moderate-intensity aerobic program on blood viscosity, platelet aggregation and fibrinolytic balance in young and middle-aged sedentary subjects. Blood Coagul Fibrinolysis Int J Haemost Thromb. 2004;15(1):31–7. doi: 10.1097/00001721-200401000-00006 [DOI] [PubMed] [Google Scholar]
- 386.Costa P, Simão R, Perez A, Gama M, Lanchtermacher R, Musialowski R, et al. A Randomized Controlled Trial Investigating the Effects of Undulatory, Staggered, and Linear Load Manipulations in Aerobic Training on Oxygen Supply, Muscle Injury, and Metabolism in Male Recreational Runners. Sports Med Open. 2019;5(1):32. doi: 10.1186/s40798-019-0200-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Dressendorfer RH, Wade CE, Claybaugh J, Cucinell SA, Timmis GC. Effects of 7 successive days of unaccustomed prolonged exercise on aerobic performance and tissue damage in fitness joggers. Int J Sports Med. 1991;12(1):55–61. doi: 10.1055/s-2007-1024656 [DOI] [PubMed] [Google Scholar]
- 388.Eastwood A, Bourdon PC, Snowden KR, Gore CJ. Detraining decreases Hb(mass) of triathletes. Int J Sports Med. 2012;33(4):253–7. doi: 10.1055/s-0031-1291184 [DOI] [PubMed] [Google Scholar]
- 389.Eastwood A, Bourdon PC, Norton KI, Lewis NR, Snowden KR, Gore CJ. No change in hemoglobin mass after 40 days of physical activity in previously untrained adults. Scand J Med Sci Sports. 2012;22(6):722–8. doi: 10.1111/j.1600-0838.2011.01310.x [DOI] [PubMed] [Google Scholar]
- 390.Farzad B, Gharakhanlou R, Agha-Alinejad H, Curby DG, Bayati M, Bahraminejad M, et al. Physiological and performance changes from the addition of a sprint interval program to wrestling training. J Strength Cond Res. 2011;25(9):2392–9. doi: 10.1519/JSC.0b013e3181fb4a33 [DOI] [PubMed] [Google Scholar]
- 391.Fernandez FA, Martin-Martin R, García-Camacha I, Juarez D, Fidel P, González-Ravé JM. Medium term effects of physical conditioning on breath-hold diving performance. Respir Physiol Neurobiol. 2019;259:70–4. doi: 10.1016/j.resp.2018.07.013 [DOI] [PubMed] [Google Scholar]
- 392.Ferretti G, Antonutto G, Denis C, Hoppeler H, Minetti AE, Narici MV, et al. The interplay of central and peripheral factors in limiting maximal O2 consumption in man after prolonged bed rest. J Physiol. 1997;501(3):677–86. doi: 10.1111/j.1469-7793.1997.677bm.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Friman G. Effect of clinical bed rest for seven days on physical performance. Acta Med Scand. 1979;205(5):389–93. doi: 10.1111/j.0954-6820.1979.tb06070.x [DOI] [PubMed] [Google Scholar]
- 394.Goodman J, Radomski M, Hart L, Plyley M, Shephard RJ. Maximal aerobic exercise following prolonged sleep deprivation. Int J Sports Med. 1989;10(6):419–23. doi: 10.1055/s-2007-1024936 [DOI] [PubMed] [Google Scholar]
- 395.Green HJ, Coates G, Sutton JR, Jones S. Early adaptations in gas exchange, cardiac function and haematology to prolonged exercise training in man. Eur J Appl Physiol. 1991;63(1):17–23. doi: 10.1007/BF00760795 [DOI] [PubMed] [Google Scholar]
- 396.Jacobs RA, Flück D, Bonne TC, Bürgi S, Christensen PM, Toigo M, et al. Improvements in exercise performance with high-intensity interval training coincide with an increase in skeletal muscle mitochondrial content and function. J Appl Physiol Bethesda Md 1985. 2013;115(6):785–93. doi: 10.1152/japplphysiol.00445.2013 [DOI] [PubMed] [Google Scholar]
- 397.Kilbom A. Physical training with submaximal intensities in women. I. Reaction to exercise and orthostasis. Scand J Clin Lab Invest. 1971;28(2):141–61. doi: 10.3109/00365517109086896 [DOI] [PubMed] [Google Scholar]
- 398.Klimek AT, Lubkowska A, Szyguła Z, Chudecka M, Fraczek B. Influence of the ten sessions of the whole body cryostimulation on aerobic and anaerobic capacity. Int J Occup Med Environ Health. 2010;23(2):181–9. doi: 10.2478/v10001-010-0019-2 [DOI] [PubMed] [Google Scholar]
- 399.Maldonado-Martín S, Cámara J, James DVB, Fernández-López JR, Artetxe-Gezuraga X. Effects of long-term training cessation in young top-level road cyclists. J Sports Sci. 2017;35(14):1396–401. doi: 10.1080/02640414.2016.1215502 [DOI] [PubMed] [Google Scholar]
- 400.Maron MB, Horvath SM. Maximal aerobic power after competitive marathon running. Can J Sport Sci J Can Sci Sport. 1988;13(1):50–5. [PubMed] [Google Scholar]
- 401.Menz V, Strobl J, Faulhaber M, Gatterer H, Burtscher M. Effect of 3-week high-intensity interval training on VO2max, total haemoglobin mass, plasma and blood volume in well-trained athletes. Eur J Appl Physiol. 2015;115(11):2349–56. doi: 10.1007/s00421-015-3211-z [DOI] [PubMed] [Google Scholar]
- 402.Mier CM, Domenick MA, Turner NS, Wilmore JH. Changes in stroke volume and maximal aerobic capacity with increased blood volume in men women. J Appl Physiol Bethesda Md 1985. 1996;80(4):1180–6. doi: 10.1152/jappl.1996.80.4.1180 [DOI] [PubMed] [Google Scholar]
- 403.Montero D, Cathomen A, Jacobs RA, Flück D, de Leur J, Keiser S, et al. Haematological rather than skeletal muscle adaptations contribute to the increase in peak oxygen uptake induced by moderate endurance training. J Physiol. 2015;593(20):4677–88. doi: 10.1113/JP270250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Podgórski T, Kryściak J, Konarski J, Domaszewska K, Durkalec-Michalski K, Strzelczyk R, et al. Iron Metabolism in Field Hockey Players During an Annual Training Cycle. J Hum Kinet. 2015;47:107–14. doi: 10.1515/hukin-2015-0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Puhl JL, Runyan WS. Hematological Variations during Aerobic Training of College Women. Res Q Exerc Sport. 1980. Oct 1;51(3):533–41. doi: 10.1080/02701367.1980.10608076 [DOI] [PubMed] [Google Scholar]
- 406.Putman CT, Jones NL, Heigenhauser GJF. Effects of short-term training on plasma acid-base balance during incremental exercise in man. J Physiol. 2003;550(Pt 2):585–603. doi: 10.1113/jphysiol.2003.039743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Rietjens GJWM, Kuipers H, Adam JJ, Saris WHM, van Breda E, van Hamont D, et al. Physiological, biochemical and psychological markers of strenuous training-induced fatigue. Int J Sports Med. 2005;26(1):16–26. doi: 10.1055/s-2004-817914 [DOI] [PubMed] [Google Scholar]
- 408.Rønnestad BR, Ellefsen S, Nygaard H, Zacharoff EE, Vikmoen O, Hansen J, et al. Effects of 12 weeks of block periodization on performance and performance indices in well-trained cyclists. Scand J Med Sci Sports. 2014;24(2):327–35. doi: 10.1111/sms.12016 [DOI] [PubMed] [Google Scholar]
- 409.Rønnestad BR, Hamarsland H, Hansen J, Holen E, Montero D, Whist JE, et al. Five weeks of heat training increases haemoglobin mass in elite cyclists. Exp Physiol. 2021;106(1):316–27. doi: 10.1113/EP088544 [DOI] [PubMed] [Google Scholar]
- 410.Schmidt W, Maassen N, Trost F, Böning D. Training induced effects on blood volume, erythrocyte turnover and haemoglobin oxygen binding properties. Eur J Appl Physiol. 1988;57(4):490–8. doi: 10.1007/BF00417998 [DOI] [PubMed] [Google Scholar]
- 411.Sheykhlouvand M, Khalili E, Agha-Alinejad H, Gharaat M. Hormonal and Physiological Adaptations to High-Intensity Interval Training in Professional Male Canoe Polo Athletes. J Strength Cond Res. 2016;30(3):859–66. doi: 10.1519/JSC.0000000000001161 [DOI] [PubMed] [Google Scholar]
- 412.Shoemaker JK, Green HJ, Coates J, Ali M, Grant S. Failure of prolonged exercise training to increase red cell mass in humans. Am J Physiol. 1996;270(1 Pt 2):H121–6. doi: 10.1152/ajpheart.1996.270.1.H121 [DOI] [PubMed] [Google Scholar]
- 413.Sitkowski D, Cisoń T, Szygula Z, Surała O, Starczewski M, Sadowska D, et al. Hematological Adaptations to Post-Exercise Sauna Bathing with No Fluid Intake: A Randomized Cross-Over Study. Res Q Exerc Sport. 2021;1–9. doi: 10.1080/02701367.2021.1921684 [DOI] [PubMed] [Google Scholar]
- 414.Skattebo Ø, Bjerring AW, Auensen M, Sarvari SI, Cumming KT, Capelli C, et al. Blood volume expansion does not explain the increase in peak oxygen uptake induced by 10 weeks of endurance training. Eur J Appl Physiol. 2020;120(5):985–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Veicsteinas A, Samaja M, Gussoni M, Cerretelli P. Blood O2 affinity and maximal O2 consumption in elite bicycle racers. J Appl Physiol. 1984;57(1):52–8. doi: 10.1152/jappl.1984.57.1.52 [DOI] [PubMed] [Google Scholar]
- 416.Wang J, Ji Y, Zhou L, Xiang Y, Heinonen I, Zhang P. A New Method to Improve Running Economy and Maximal Aerobic Power in Athletes: Endurance Training With Periodic Carbon Monoxide Inhalation. Front Physiol. 2019;10:701–701. doi: 10.3389/fphys.2019.00701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Wirth JC, Lohman TG, Avallone JP Jr, Shire T, Boileau RA. The effect of physical training on the serum iron levels of college-age women. Med Sci Sports. 1978;10(3):223–6. [PubMed] [Google Scholar]
- 418.Zapico AG, Calderón FJ, Benito PJ, González CB, Parisi A, Pigozzi F, et al. Evolution of physiological and haematological parameters with training load in elite male road cyclists: a longitudinal study. J Sports Med Phys Fitness. 2007;47(2):191–6. [PubMed] [Google Scholar]
- 419.Zorbas YG, Yaroshenko YN, Kuznetsov NK, Andreyev VG, Federenko YF. Bone histomorphometric changes in trained subjects during prolonged restriction of muscular activity and chronic hyperhydration. Panminerva Med. 1997;39(4):265–74. [PubMed] [Google Scholar]
- 420.Zorbas YG, Yaroshenko YY, Kuznetsov NK, Matvedev SL. Daily hyperhydration effect on electrolyte deficiency of endurance-trained subjects during prolonged hypokinesia. Biol Trace Elem Res. 1998;64(1–3):259–73. doi: 10.1007/BF02783342 [DOI] [PubMed] [Google Scholar]
- 421.Hillman S, Withers P, Hedrick M, Kimmel P. The effects of erythrocythemia on blood viscosity, maximal systemic oxygen transport capacity and maximal rates of oxygen consumption in an amphibian. J Comp Physiol [B]. 1985. Feb 1;155:577–81. [DOI] [PubMed] [Google Scholar]
- 422.Birchard GF, Tenney SM. Relationship between blood-oxygen affinity and blood volume. Respir Physiol. 1991. Mar;83(3):365–73. doi: 10.1016/0034-5687(91)90055-n [DOI] [PubMed] [Google Scholar]
- 423.Sanborn CF, Jankowski CM. Physiologic considerations for women in sport. Clin Sports Med. 1994. Apr 1;13(2):315–27. [PubMed] [Google Scholar]
- 424.Murphy WG. The sex difference in haemoglobin levels in adults—Mechanisms, causes, and consequences. Blood Rev. 2014. Mar;28(2):41–7. doi: 10.1016/j.blre.2013.12.003 [DOI] [PubMed] [Google Scholar]
- 425.Ansdell P, Thomas K, Hicks KM, Hunter SK, Howatson G, Goodall S. Physiological sex differences affect the integrative response to exercise: acute and chronic implications. Exp Physiol. 2020;105(12):2007–21. doi: 10.1113/EP088548 [DOI] [PubMed] [Google Scholar]
- 426.Joyner MJ. Physiological limits to endurance exercise performance: influence of sex. J Physiol. 2017. May 1;595(9):2949–54. doi: 10.1113/JP272268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Santisteban KJ, Lovering AT, Halliwill JR, Minson CT. Sex Differences in VO2max and the Impact on Endurance-Exercise Performance. Int J Environ Res Public Health. 2022. Apr 19;19(9):4946. doi: 10.3390/ijerph19094946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Young AJ, Karl JP, Berryman CE, Montain SJ, Beidleman BA, Pasiakos SM. Variability in human plasma volume responses during high‐altitude sojourn. Physiol Rep. 2019. Mar 28;7(6):e14051. doi: 10.14814/phy2.14051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Siebenmann C, Robach P, Lundby C. Regulation of blood volume in lowlanders exposed to high altitude. J Appl Physiol. 2017. Oct;123(4):957–66. doi: 10.1152/japplphysiol.00118.2017 [DOI] [PubMed] [Google Scholar]
- 430.Schmidt W, Prommer N. Effects of various training modalities on blood volume. Scand J Med Sci Sports. 2008;18(s1):57–69. doi: 10.1111/j.1600-0838.2008.00833.x [DOI] [PubMed] [Google Scholar]
- 431.Mairbäurl H. Red blood cells in sports: effects of exercise and training on oxygen supply by red blood cells. Front Physiol [Internet]. 2013. [cited 2020 Nov 20];4. Available from: http://journal.frontiersin.org/article/10.3389/fphys.2013.00332/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Sawka MN, Convertino VA, Eichner ER, Schnieder SM, Young AJ. Blood volume: importance and adaptations to exercise training, environmental stresses, and trauma/sickness: Med Sci Sports Exerc. 2000. Feb;32(2):332. [DOI] [PubMed] [Google Scholar]