Abstract
Background:
Pregnant women have historically been excluded from most medical research, including human challenge studies. The proof-of-concept Lactamica 9 human challenge study investigated whether nasal inoculation of pregnant women with commensal bacteria leads to horizontal transmission to the neonate. Given the unique practical and ethical considerations of both human challenge studies and interventional research involving pregnant women and their newborns, we sought to investigate the motivations, concerns and experiences of these volunteers.
Methods:
Pre- and post-participation questionnaires were given to all participants in the Lactamica 9 study. These fully anonymized qualitative and Semi-quantitative questionnaires used forced Likert scales, word association and free-text questions.
Results:
Pre- and post-participation questionnaires were completed by 87.1% (27/31) and 62.5% (15/24) of eligible participants, respectively. Almost all pre-participation respondents agreed with altruistic motivations for participation, and most concerns were related to discomfort from study procedures, with few concerned about the theoretical risks of inoculation to themselves (5/27; 18.5%) or their baby (6/27; 22.2%). Participants most frequently associated the study intervention with the terms “bacteria,” “natural,” “protective” and “safe.” For the post-participation questionnaire, 93.3% (14/15) found all study procedures acceptable, and qualitative feedback was almost entirely positive, with particular emphasis on the research team’s flexibility, approachability and friendliness.
Conclusions:
The successful completion of the Lactamica 9 study demonstrates that human challenge research in healthy pregnant women can be acceptable and feasible. Participants’ initial concerns of potential discomfort were outweighed by predominantly altruistic motivations and perception of the intervention as “natural.”
Keywords: participant perspectives, research in pregnancy, human challenge, microbiome
Human challenge trials involve the intentional exposure of healthy participants to an infectious agent. When conducted appropriately, human challenge research has proven to be a safe and valuable approach to investigating the pathophysiology, prevention and management of infectious diseases. These studies have been useful in expediting efficacy testing of immunizations against influenza,1 typhoid2 and cholera.3 The recent development of human challenge models for Bordetella pertussis, SARS-CoV-2, malaria, and group A streptococcus offers exciting prospects for novel vaccine development.4
Human challenge research, however, suffers from a controversial history of abhorrent unethical practice, including infection of unknowing or even unwilling vulnerable volunteers with anthrax, chlamydia, cholera, malaria, tetanus, tuberculosis, typhoid and viral hepatitis.5 The latter half of the twentieth century saw widespread introduction of research ethics legislation,6 and the World Health Organization recently introduced guidance on ethical conduct of human challenge trials,7 with additional international standards currently in development regarding the manufacture of infections agents.8 Thus, recent, and ongoing human challenge models have been developed under ethical scrutiny, with participant and public safety of paramount consideration.
As with human challenge trials, research involving pregnant women and their neonates presents unique ethical and practical considerations. Following several high-profile catastrophes related to untested medications and research trials in pregnant women in the 1960s and 1970s,9–11 pregnant women were labeled as a “vulnerable group” and subsequently largely excluded from medical research.12 Some have argued, however, that prolonged exclusion from research has paradoxically left women at greater risk, with safety and efficacy data in pregnancy lacking for up to 91% of available medications.13,14 As such, there has been a recent trend in the scientific community toward greater inclusion of pregnant women in research.15,16
The Lactamica 9 study is the first ever respiratory human challenge trial performed in pregnancy, in which pregnant women were inoculated nasally with Neisseria lactamica, and mother-infant pairs were followed up until 15-week postpartum.17 Nasal inoculation with N. lactamica, a non-pathogenic commensal of the upper respiratory tract, is a safe and well-characterized human challenge model.18 N. lactamica colonization kinetics,19,20 cellular and humoral immune responses,21 and genomic microevolution have been investigated previously,22 with no serious adverse reactions to date following inoculation of over 400 healthy nonpregnant adults. N. lactamica has an inverse relationship with N. meningitidis carriage and invasive disease,23,24 and N. lactamica inoculation reduces N. meningitidis carriage in human challenge volunteers from 18% to 8%,19 raising the question of whether it could be used clinically to reduce N. meningitidis carriage and even disease. As invasive meningococcal disease is most common in the first year of life25 and as infant upper respiratory microbes are derived at least in part from their mother’s upper respiratory tract,26 the Lactamica 9 study aimed to establish if nasal inoculation in pregnancy results in neonatal N. lactamica colonization after birth.17
Given the historical context and ongoing ethical and practical considerations of both human challenge trials and research in pregnancy. The aim of this questionnaire-based study was to investigate participant motivations, concerns and experiences, and to assess the acceptability of human challenge research in this group. By expanding our understanding of these issues, we hope to improve recruitment, communication and study conduct in similar future research.
METHODS
Lactamica 9 Study Overview
This questionnaire-based study was nested within the Lactamica 9 human challenge trial (ClinicalTrials.gov NCT04784845), the protocol for which has been published separately.17 Details of the questionnaires were incorporated into the Lactamica 9 trial’s ethical approval (London Central Research Ethics Committee, 21/RP/0373) and consent procedures (see consent form, Supplemental Digital Content 1 http://links.lww.com/INF/F169).
Recruitment to the single-center Lactamica 9 study was primarily through letters mailed by research midwives to potentially eligible women in the second and third trimester of pregnancy, as well as advertisements on social media and in the maternity hospital (Figure 1). Inclusion criteria were adult age (over 18 years old), singleton pregnancy, and absence of life-limiting, craniofacial or neuroanatomical anomalies on 20-week ultrasound scan. Individuals with significant immunosuppression, recent or planned use of antibiotics or immunosuppressants, or any serious pregnancy complications were excluded.
FIGURE 1.
Recruitment poster for the Lactamica 9 study. Ethically approved wording reflective of that used in all Lactamica 9 recruitment materials, including mailed letters, emails, and social media posts.
The Lactamica 9 trial involved 6 study visits: screening visit 1 (34–37 weeks gestation), inoculation visit 2 (36–38 weeks gestation), birth visit 3 (0–24 hours postpartum) and follow-up visits 4, 5 and 6 (at 1-, 4- and 15-week postpartum, respectively). Eligible participants were inoculated nasally with 105 colony-forming units N. lactamica; participants already naturally colonised with N. lactamica at screening were not inoculated but were followed up (visits 3–6) exactly as for inoculated volunteers. Maternal upper respiratory swabs were collected at all visits, as well as infant swabs at visits 3–6. Additional optional samples included breastmilk (visits 3–6), umbilical cord blood (visit 3), venous blood from the infant (visits 5 and 6) and mother (visit 6) and upper respiratory swabs from household contacts under 5 years old (visit 6). Participants were compensated up to £100 for participation, and visits were conducted at the participant’s home or the Clinical Research Facility, depending on participant preference.
Pre- and Post-participation Questionnaires
All Lactamica 9 study participants were asked to complete a questionnaire at screening visit 1 (34–37 weeks gestation) and follow-up visit 5 (4 weeks postpartum). Both questionnaires were optional, and failure to complete either or both did not affect study eligibility. Each questionnaire was designed to take less than 5 minutes to complete, and participants could complete questionnaires immediately after the visit or in their own time. To ensure anonymity, questionnaires were returned to the study team in blank sealed envelopes, and those returned via email had identifiable participant details removed by a study administrator before forwarding on to the study team.
The pre-participation questionnaire asked participants to rank their agreement (using a forced four-point Likert scale from strongly agree to strongly disagree) with a list of statements regarding their motivations and concerns about study participation. Participants were also asked which descriptive words and interventions they most closely associated with N. lactamica inoculation (see full questionnaire, Supplemental Digital Content 2, http://links.lww.com/INF/F170).
The post-participation questionnaire focused on the participants’ experiences during the Lactamica 9 trial, and the tolerability of study procedures. Participants were again asked to rank their agreement, this time with a list of statements regarding study design and conduct. This questionnaire also collected free-text qualitative feedback from participants.
Quantitative and qualitative questionnaire data were extracted in duplicate by 2 members of the research team from paper-based source forms to an electronic spreadsheet. Where respondents selected more answers than stated by the question, these were included in the analysis as non-integer values with relative apportionment applied for aggregation. For example, if a respondent selected 4 answers (rather than 3), or 2 options on a Likert scale (agree and disagree), each answer would be given a relative apportionment of 0.75 and 0.5 participant responses respectively. In cases where respondents did not answer questions, this was excluded from denominator values. Free-text qualitative responses were analyzed blindly by 2 authors, and recurring themes were agreed by consensus discussion.
RESULTS
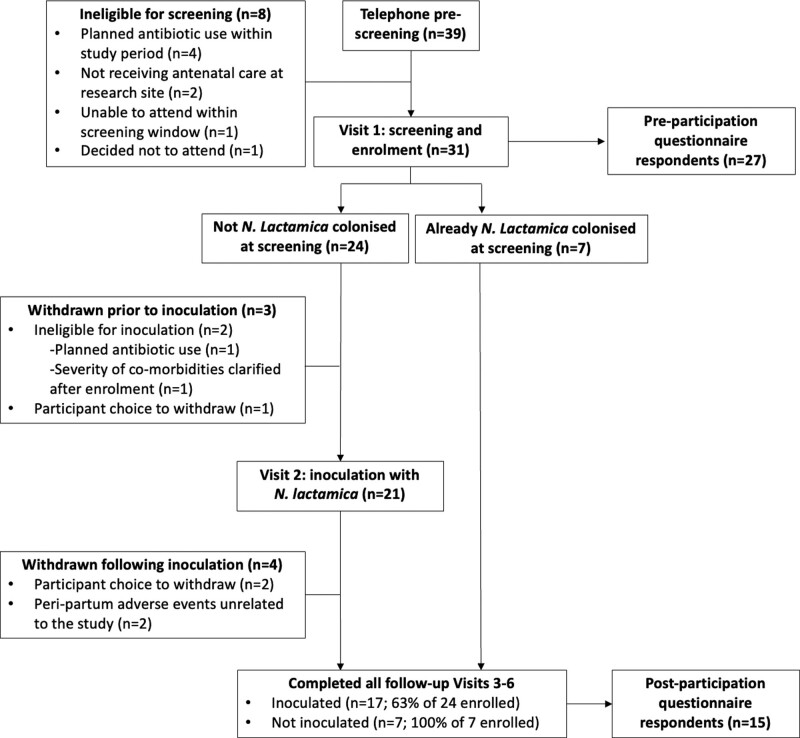
The Lactamica 9 study pre-screened 39 participants of which 31 participants were recruited, of whom 24 (77.4%) completed the study. Participants were withdrawn because of ineligibility for inoculation, unrelated peripartum complications, or participant choice to leave the study (Figure 2). Of those eligible to complete the questionnaires, 27/31 (87.1%) and 15/24 (62.5%) responded to the pre- and post-participation questionnaires, respectively. Most respondents 25/27 (92.6%) were recruited via the 1268 letters mailed by research midwives, with the remaining respondents recruited via word of mouth and another unspecified medium. Applying this ratio to all 39 pre-screened participants, it can be estimated that response rate from letters sent was 2.8%.
FIGURE 2.
Adjusted CONSORT flow diagram for single-arm clinical trial.
Because of anonymization, precise respondent demographics are unknown, and it was not possible to pair completed pre- and post-participation questionnaires. However, demographic, and clinical data from all 31 enrolled participants reveal median maternal age 33.5 years (range 23.1–39.9 years) and overwhelmingly White ethnicity (93.5%). Of the 28 participants that completed birth visit 3, 89.3% (25/28) delivered vaginally and 85.7% (24/28) initiated breastfeeding, while 87.5% (21/24) reported ongoing breastfeeding at study completion (15-week postpartum). Maternal venous blood samples were obtained from 75% of 24 women (Visit 6), compared with 58% of their infants (at either Visit 5 or 6 or both).
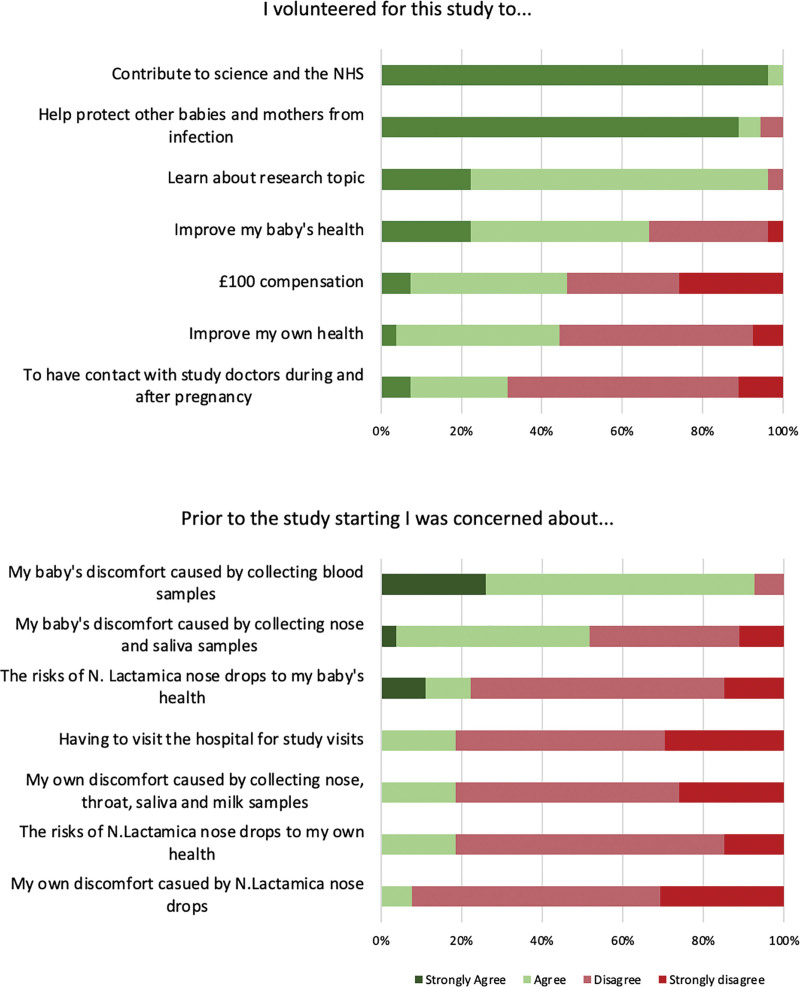
Respondents mostly agreed with altruistic statements (Figure 3), with 100% (27/27) reporting motivation to contribute to science and the NHS, and 97% (25.5/27) to help protect other babies and mothers from infection. In addition, 97% (26/27) agreed that learning about the research topic was part of their motivation for participating. There was less agreement with statements of self-interest that referred to financial and health benefits to the participant and their neonate.
FIGURE 3.
Pre-participation participant motivations and concerns assessed using forced 4-point Likert scale.
Pre-participation Concerns About Volunteering
Respondents reported most concern about the discomfort sampling would cause to their baby, with 92.6% (25/27) and 51.9% (14/27) agreeing that collecting blood and respiratory swabs was a concern, respectively (Figure 3). Few agreed with statements of perceived health risks of N. lactamica to themselves (18.5; 5/27) or their baby (22.2%; 6/27). Only 7.7% (2/26) of respondents were concerned about potential discomfort to themselves of the inoculation procedure itself.
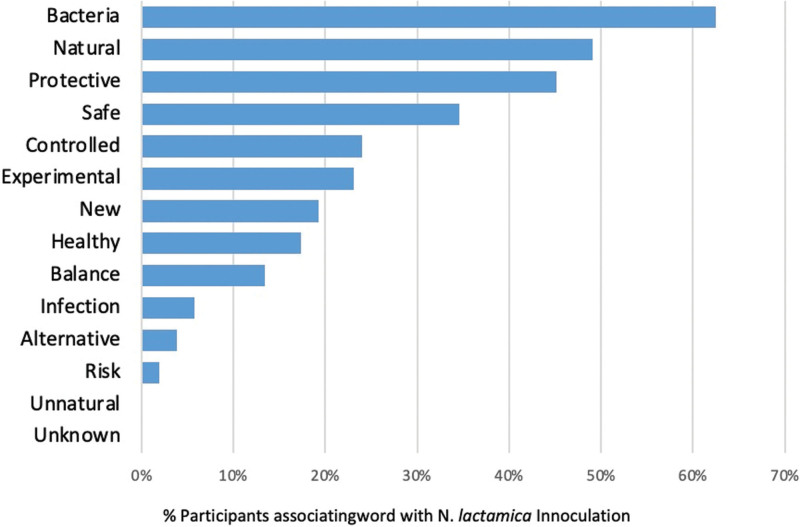
When asked to select 3 words, from an unranked list of fourteen words, to describe N. lactamica inoculation, most respondents selected “bacteria” (62.5%; 16.25/26), “natural” (49.0%; 12.75/26), “protective” (45.2%; 11.75/26) and “safe” (34.6%; 9/26). No respondents selected the words “unnatural” or “unknown” (Figure 4).
FIGURE 4.
Word association between N. lactamica inoculation and an unranked list of words.
When asked to select which medical intervention, they felt N. lactamica inoculation was most like from 4 unranked options provided, the most popular was “probiotic supplements that you can buy over-the-counter” (42.3%; 11/26), followed by “bacteria used in laboratory experiments” (30.8%; 8/26) and “vaccinations in a national public health programme” (23.1%; 6/26). Only 1 respondent (3.8%; 1/26) viewed “antibiotics prescribed by a doctor” as the intervention most like N. lactamica inoculation.
Post-participation Perceptions of Study Conduct and Acceptability
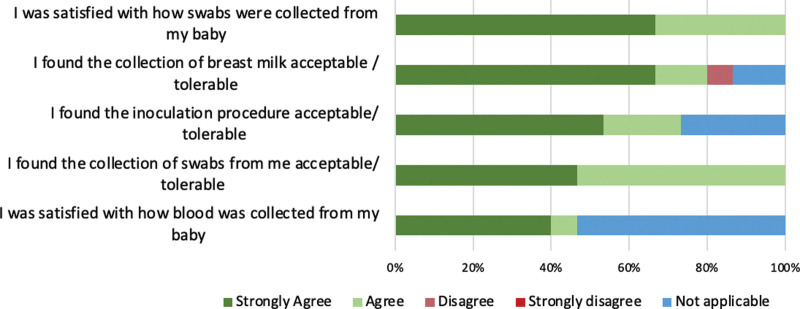
All 15 post-participation questionnaire respondents agreed that the study procedures, risks and aims were explained in sufficient detail, and that the study team were easy to contact and approachable with concerns. All respondents were satisfied with the collection of samples from their babies, and found their own swabs and inoculation acceptable, while only 1 participant disagreed that the collection of breast milk was tolerable (Figure 5).
FIGURE 5.
Post-participation perceptions of study acceptability assessed using forced 4-point Likert scale.
Free-text qualitative feedback with regards to study conduct and participant experience was overall very positive. Respondents were complimentary about the research team’s approachability and friendliness, as well as the flexibility of study visit scheduling and location:
“Very approachable staff and easy appointment. All the team made it easy to ask questions. Felt very at ease”
“Good communication, friendly staff”
“How lovely everyone has been, so friendly and approachable. Thank you. Also, how flexible appointments were.”
When asked to state 1 thing that respondents did not like about the study design and conduct, only 2 negative comments were provided:
“Length of time of 2 antenatal hospital visits”
“Compensation for mileage”
DISCUSSION
Ethical approval and institutional sponsorship of the Lactamica 9 study indicate that human challenge trials in pregnancy can be scientifically and ethically acceptable. Moreover, overwhelmingly positive qualitative and quantitative feedback and high participant retention suggest that such studies can also be acceptable to healthy pregnant women. Participants’ initial concerns appeared to focus on direct discomfort to the neonate arising from study procedures but were seemingly outweighed by predominantly altruistic motivations for volunteering, research team conduct and aspects of study design. A “natural” or “probiotic” perception of the N. lactamica inoculum was common within the group. Additionally, the estimated response rate from recruitment letters aligns closely to those reported in the literature,27–29 indicating a reasonable level of acceptability among the target population.
Well-cited barriers to retention in clinical trials in pregnancy and childhood include parental concerns regarding discomfort of study procedures, mistrust in researchers and logistical difficulties with participation.30,31 It is possible that the Lactamica 9 study’s design and conduct may have helped allay such concerns: potentially uncomfortable blood taking was optional; most study visits were conducted at the participants’ preferred location and time; and the inoculum was delivered intranasally rather than intramuscularly, which is known to carry higher levels of acceptability in vaccine trials.32,33 Study conduct was also clearly important, with respondents citing the approachability, communication and flexibility of the research team as important in making participation a positive experience.
Perceived acceptability of participation is also closely tied to an individual’s initial motivations and is thought to derive from a complex relationship between altruism and self-interest.34 Emotional drivers are known to facilitate participation in research, and the research context of serious childhood illness (in this case infant meningococcal disease) may have been particularly emotive to pregnant prospective participants.35
The perception of the intervention itself may have been an additional facilitator of acceptability, with around half of the participants viewing N. lactamica inoculation as “natural” or akin to probiotics. As the research team were careful not to use these terms in recruitment materials (Figure 1) or during the screening visit before pre-participation questionnaire completion, it appears that participants may have had preconceived ideas about the nature of the intervention, and an awareness of probiotics as a concept. Indeed, self-selection of a population already primed to view the intervention as “natural” may have facilitated their overall impression of study acceptability. “Naturalness” is known to be viewed positively by patients and study participants: individuals are more likely to take medications that are seen as natural rather than synthetic36,37 and perceive natural interventions as safer than synthetic ones.38 A 2016 series of 5 large studies posing hypothetical choices between identically safe and efficacious synthetic and natural drugs found that 79% of participants would prefer to take the natural option and would consider it safer, and 20% preferred a natural drug even if it were less safe or effective than a synthetic alternative.39 This bias towards “naturalness” may partly explain the low safety concerns from respondents in this trial.
While the prevailing view throughout most of the last century was that bacteria are harmful, a more nuanced scientific and social discourse has emerged recognizing that bacteria can be both detrimental and beneficial to human health.40 This shift is highlighted by recent exponential growth in interest and investment in probiotics (live microorganisms that, when administered in adequate amounts, aim to confer a health benefit on the host).41,42 There have also been calls for greater engagement of social scientists in research focused on probiotics and human microbiota (the overall community of living microorganisms on and inside a host43).44,45 The potential for impressions of naturalness to impact acceptability of research and even clinical interventions must be considered when communicating with stakeholders, to ensure that risk and consent discussions are not unduly influenced (inadvertently or disingenuously) by preconceived ideas.
To our knowledge, this is the first study investigating the acceptability of pregnant women participating in a human challenge trial. The study design allowed for assessment of motivations and concerns both before and after study participation, facilitating distinction between preconceived ideas and those based on study participation and childbirth. The questionnaires were limited principally by the relatively small sample of demographically similar participants. Indeed, it is worth noting that maternal age at birth was greater than for mothers in the general UK population (33.5 years compared with 30.7 years46), and breastfeeding rates were higher than the UK average (87.5% reported any breastfeeding at 15-week postpartum, compared with only 55% at 6 weeks47), although it is not clear if these differences were statistically significant. Because of the self-selected nature of the respondents, their acceptance of the intervention and overall study may not be generalizable to other pregnant women in the United Kingdom or internationally, or even the whole cohort of study participants, as not all women responded to the post-participation questionnaire. Further work is required to understand how cultural differences may affect the acceptability of such studies. In addition, participants’ responses to questions using Likert scales were limited to the options provided, although free-text qualitative feedback provided additional valuable insights. Further work would be required in a demographically diverse group to assess the wider acceptability of such trials in pregnant women.
CONCLUSION
This human challenge study in pregnancy has been deemed acceptable by the ethics committee, academic sponsor and research participants. The women participating in the study were predominantly concerned about direct discomfort to their neonate rather than the perceived risks of bacterial inoculation. The acceptability of the study may be partly because of perceptions of the intervention as “natural” or akin to probiotics. These findings reaffirm the need for clinical and social scientists to consider the acceptability of research and clinical interventions involving live biological products and particularly the role of “naturalness” as a facilitator.
Supplementary Material
Footnotes
This work was supported by a Medical Research Council Clinical Research Training Fellowship awarded to Dr Anastasia Theodosiou, grant number MR/V002015/1.
The authors have no conflicts of interest to disclose.
J.H.J.B. and A.A.T. are cofirst authors.
A.T., C.J. and R.R. designed and supervised the overall Lactamica 9 study. J.C. and A.T. designed the pre- and post-participation questionnaires. R.D., A.T. and J.B. collected the questionnaire responses. J.B. and A.T. analyzed the study data. J.B. prepared the first draft of the article. All authors reviewed and edited the article before submission.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
Contributor Information
Anastasia A. Theodosiou, Email: A.Theodosiou@soton.ac.uk.
Robert B. Dorey, Email: R.Dorey@soton.ac.uk.
Robert C. Read, Email: R.C.Read@soton.ac.uk.
Christine E. Jones, Email: C.E.Jones@soton.ac.uk.
REFERENCES
- 1.Liebowitz D, Gottlieb K, Kolhatkar NS, et al. Efficacy, immunogenicity, and safety of an oral influenza vaccine: a placebo-controlled and active-controlled phase 2 human challenge study. Lancet Infect Dis. 2020;20:435–444. [DOI] [PubMed] [Google Scholar]
- 2.Waddington CS, Darton TC, Jones C, et al. An outpatient, ambulant-design, controlled human infection model using escalating doses of salmonella typhi challenge delivered in sodium bicarbonate solution. Clin Infect Dis. 2014;58:1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shirley DA, McArthur MA. The utility of human challenge studies in vaccine development: lessons learned from cholera. Vaccine Dev Ther. 2011;2011:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sekhar A, Kang G. Human challenge trials in vaccine development. Semin Immunol. 2020;50:101429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jamrozik E, Selgelid MJ. History of Human Challenge Studies. In: Human Challenge Studies in Endemic Settings. SpringerBriefs in Ethics. Springer International Publishing; 2021:9–23. [Google Scholar]
- 6.Roestenberg M, Hoogerwerf MA, Ferreira DM, et al. Experimental infection of human volunteers. Lancet Infect Dis. 2018;18:e312–e322. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organisation. WHO Guidance on the Ethical Conduct of Controlled Human Infection Studies; 2021. [Google Scholar]
- 8.La C, Bell A, Trouvin J-H, et al. Considerations on the principles of development and manufacturing qualities of challenge agents for use in human infection models; Published online 2022:607393 Bytes. [Google Scholar]
- 9.Kim JH, Scialli AR. Thalidomide: the tragedy of birth defects and the effective treatment of disease. Toxicol Sci. 2011;122:1–6. [DOI] [PubMed] [Google Scholar]
- 10.Veurink M, Koster M, Berg LT. The History of DES, lessons to be learned. Pharm World Sci. 2005;27:139–143. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control (CDC). Elevated risk of pelvic inflammatory disease among women using the Dalkon Shield. MMWR Morb Mortal Wkly Rep. 1983;32:221–222. [PubMed] [Google Scholar]
- 12.US Department of Health and Human Services. Code of Federal Regulations Part 46: Protection of human subjects. Published online 2009. Available at: https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/index.html. Accessed March 21, 2023.
- 13.van der Zande ISE, van der Graaf R, Oudijk MA, et al. Vulnerability of pregnant women in clinical research. J Med Ethics. 2017;43:657–663. [DOI] [PubMed] [Google Scholar]
- 14.Adam MP, Polifka JE, Friedman JM. Evolving knowledge of the teratogenicity of medications in human pregnancy. Am J Med Genet C Semin Med Genet. 2011;157:175–182. [DOI] [PubMed] [Google Scholar]
- 15.Hunt A, Banner N, Littler K. The global forum on bioethics in research meeting, “ethics of research in pregnancy”: emerging consensus themes and outputs. Reprod Health. 2017;14:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saenz C, Cheah PY, van der Graaf R, et al. Ethics, regulation, and beyond: the landscape of research with pregnant women. Reprod Health. 2017;14:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Theodosiou AA, Laver JR, Dale AP, et al. Controlled human infection with Neisseria lactamica in late pregnancy to measure horizontal transmission and microbiome changes in mother-neonate pairs: a single-arm interventional pilot study protocol. BMJ Open. 2022;12:e056081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dale AP, Gbesemete DF, Read RC, et al. Neisseria lactamica controlled human infection model. Methods Mol Biol. 2022;2414:387–404. [DOI] [PubMed] [Google Scholar]
- 19.Deasy AM, Guccione E, Dale AP, et al. Nasal inoculation of the commensal neisseria lactamica inhibits carriage of neisseria meningitidis by young adults: a controlled human infection study. Clin Infect Dis. 2015;60:1512–1520. [DOI] [PubMed] [Google Scholar]
- 20.Evans CM, Pratt CB, Matheson M, et al. Nasopharyngeal colonization by neisseria lactamica and induction of protective immunity against neisseria meningitidis. Clin Infect Dis. 2011;52:70–77. [DOI] [PubMed] [Google Scholar]
- 21.Dale AP, Theodosiou AA, Gbesemete DF, et al. Effect of colonisation with Neisseria lactamica on cross-reactive anti-meningococcal B-cell responses: a randomised, controlled, human infection trial. Lancet Microbe. 2022;3:e931–e943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pandey A, Cleary DW, Laver JR, et al. Microevolution of Neisseria lactamica during nasopharyngeal colonisation induced by controlled human infection. Nat Commun. 2018;9:4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gold R, Goldschneider I, Lepow ML, et al. Carriage of neisseria meningitidis and neisseria lactamica in infants and children. J Infect Dis. 1978;137:112–121. [DOI] [PubMed] [Google Scholar]
- 24.Olsen SF, Djurhuus B, Rasmussen K, et al. Pharyngeal carriage of Neisseria meningitidis and Neisseria lactamica in households with infants within areas with high and low incidences of meningococcal disease. Epidemiol Infect. 1991;106:445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabutti G, Stefanati A, Kuhdari P. Epidemiology of neisseria meningitidis infections: case distribution by age and relevance of carriage. J Prev Med Hyg. 2015;56:E116–E120. [PMC free article] [PubMed] [Google Scholar]
- 26.Ferretti P, Pasolli E, Tett A, et al. Mother-to-Infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe. 2018;24:133–145.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paquin RS, Lewis MA, Harper BA, et al. Outreach to new mothers through direct mail and email: recruitment in the early check research study. Clin Transl Sci. 2021;14:880–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baca-Motes K, Edwards AM, Waalen J, et al. Digital recruitment and enrollment in a remote nationwide trial of screening for undiagnosed atrial fibrillation: lessons from the randomized, controlled mSToPS trial. Contemp Clin Trials Commun. 2019;14:100318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crane MM, LaRose JG, Espeland MA, et al. Recruitment of young adults for weight gain prevention: randomized comparison of direct mail strategies. Trials. 2016;17:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watson SE, Smith P, Snowden J, et al. Facilitators and barriers to pediatric clinical trial recruitment and retention in rural and community settings: a scoping review of the literature. Clin Transl Sci. 2022;15:838–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barakat LP, Patterson CA, Mondestin V, et al. Initial development of a questionnaire evaluating perceived benefits and barriers to pediatric clinical trials participation. Contemp Clin Trials. 2013;34:218–226. [DOI] [PubMed] [Google Scholar]
- 32.Armitage EP, Camara J, Bah S, et al. Acceptability of intranasal live attenuated influenza vaccine, influenza knowledge and vaccine intent in the Gambia. Vaccine. 2018;36:1772–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flood EM, Ryan KJ, Rousculp MD, et al. A survey of children’s preferences for influenza vaccine attributes. Vaccine. 2011;29:4334–4340. [DOI] [PubMed] [Google Scholar]
- 34.Manton KJ, Gauld CS, White KM, et al. Qualitative study investigating the underlying motivations of healthy participants in phase I clinical trials. BMJ Open. 2019;9:e024224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheridan R, Martin-Kerry J, Hudson J, et al. Why do patients take part in research? an overview of systematic reviews of psychosocial barriers and facilitators. Trials. 2020;21:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rozin P, Spranca M, Krieger Z, et al. Preference for natural: instrumental and ideational/moral motivations, and the contrast between foods and medicines. Appetite. 2004;43:147–154. [DOI] [PubMed] [Google Scholar]
- 37.Li M, Chapman GB. Why do people like natural? instrumental and ideational bases for the naturalness preference: why do people like natural?. J Appl Soc Psychol. 2012;42:2859–2878. [Google Scholar]
- 38.Meier BP, Dillard AJ, Lappas CM. Naturally better? a review of the natural-is-better bias. Soc Personal Psychol Compass. 2019;13:e12494. [Google Scholar]
- 39.Meier BP, Lappas CM. The influence of safety, efficacy, and medical condition severity on natural versus synthetic drug preference. Med Decis Making. 2016;36:1011–1019. [DOI] [PubMed] [Google Scholar]
- 40.Lorimer J. Hookworms make Us human: the microbiome, eco-immunology, and a probiotic turn in western health care. Med Anthropol Q. 2019;33:60–79. [DOI] [PubMed] [Google Scholar]
- 41.Scott EA, Bruning E, Nims RW, et al. A 21st century view of infection control in everyday settings: moving from the germ theory of disease to the microbial theory of health. Am J Infect Control. 2020;48:1387–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swanson KS, Gibson GR, Hutkins R, et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. 2020;17:687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berg G, Rybakova D, Fischer D, et al. Microbiome definition re-visited: old concepts and new challenges. Microbiome. 2020;8:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marqual IT Solutions Pvt. Ltd (KBV Research). Global Probiotics Market (2018 - 2024); 2019.
- 45.Greenhough B, Read CJ, Lorimer J, et al. Setting the agenda for social science research on the human microbiome. Palgrave Commun. 2020;6:18. [Google Scholar]
- 46.Office for National Statistics (ONS). Birth characteristics in England and Wales: 2020, Birth characteristics in England and Wales. Office for National Statistics; 2022. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/bulletins/birthcharacteristicsinenglandandwales/2020 (Accessed: March 24, 2023). [Google Scholar]
- 47.Breastfeeding in the UK. Baby Friendly Initiative. UNICEF; 2022. Available at: https://www.unicef.org.uk/babyfriendly/about/breastfeeding-in-the-uk/. (Accessed: March 24, 2023). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.