Abstract
The contributions of 23 insertion, deletion, or missense mutations within an 81-bp fragment of rpoB, the gene encoding the β-subunit of the DNA-dependent RNA polymerase of Mycobacterium tuberculosis, to the development of resistance to rifamycins (rifampin, rifabutin, rifapentine, and KRM-1648) in 29 rifampin-resistant clinical isolates were defined. Specific mutant rpoB alleles led to the development of cross-resistance to all rifamycins tested, while a subset of mutations were associated with resistance to rifampin and rifapentine but not to KRM-1648 or rifabutin. To further study the impact of specific rpoB mutant alleles on the development of rifamycin resistance, mutations were incorporated into the rpoB gene of M. tuberculosis H37Rv, contained on a mycobacterial shuttle plasmid, by in vitro mutagenesis. Recombinant M. tuberculosis clones containing plasmids with specific mutations in either codon 531 or 526 of rpoB exhibited high-level resistance to all rifamycins tested, whereas clones containing a plasmid with a mutation in codon 516 exhibited high-level resistance to rifampin and rifapentine but were susceptible to both rifabutin and KRM-1648. These results provided additional proof of the association of specific rpoB mutations with the development of rifamycin resistance and corroborate previous reports of the usefulness of rpoB genotyping for predicting rifamycin-resistant phenotypes.
The emergence of rifampin-resistant strains of Mycobacterium tuberculosis has led to an increased interest in the antimycobacterial actions of rifamycin S analogs (rifamycins) (3–5, 8, 13, 14). Rifampin resistance has been shown to be associated with genetic alterations in an 81-bp region of the rpoB gene encoding the DNA-dependent RNA polymerase β-subunit (9, 16, 17). The association of rpoB mutations with the development of rifampin resistance has been further supported by the use of genetic complementation studies in which a mutant rpoB allele of M. tuberculosis H37Rv was transferred into Mycobacterium smegmatis LR222 on a mycobacterial shuttle vector (7). Resultant clones were resistant to high levels of rifampin. Similar studies with M. tuberculosis, using mutant rpoB alleles and rifampin or other rifamycins that demonstrate potent in vitro activity against M. tuberculosis, have not been conducted.
The antimycobacterial activities of several rifamycins against rifampin-resistant M. tuberculosis clinical isolates with known rpoB mutations have been analyzed to correlate the levels of rifamycin resistance with specific rpoB genotypes (1, 2, 8, 11, 18). These in vitro studies indicated that a subset of mutations appeared to be associated with the development of high-level rifamycin cross-resistance.
We have previously described mutations within an 81-bp fragment of the rpoB genes of a large collection of rifampin-resistant clinical isolates (n = 177) of M. tuberculosis from 11 countries and 12 laboratories within the United States (17–20). Resistance to rifampin was associated with altered rpoB alleles in 96% of these strains (Table 1). Twenty-three distinct missense, deletion, or insertion mutations were present in these strains, and mutations in either codon 531, 526, or 516 were present in 86% of all of the strains analyzed. No mutations were observed in 50 susceptible isolates.
TABLE 1.
Mutant rpoB alleles in 177 rifampin-resistant clinical isolates of M. tuberculosis
| Isolate pheno- type | Amino acid(s) affecteda | Amino acid change(s) | No. of isolates (frequency [%]) | Origin of isolate(s) (no.) |
|---|---|---|---|---|
| Resistantb | 509, 526 | Ser→Thr, His→Asp | 1 (0.6) | Canada (1) |
| 511 | Leu→Pro | 1 (0.6) | U.S.c (1) | |
| 513 | Gln→Leu | 1 (0.6) | U.S. (1) | |
| 513 | Gln→Pro | 2 (1.1) | Philippines (1), U.S. (1) | |
| 514 | Phe insertion | 1 (0.6) | U.S. (1) | |
| 516 | Asp→Val | 13 (7.4) | U.S. (9), Switzerland (1), Peru (1), Yemen (2) | |
| 516 | Asp→Gly | 1 (0.6) | U.S. (1) | |
| 517 | Gln deletion | 1 (0.6) | U.S. (1) | |
| 518 | Asn deletion | 2 (1.1) | U.S. (2) | |
| 519 | Asn→Lys | 1 (0.6) | U.S. (1) | |
| 521 | Leu→Met | 1 (0.6) | U.S. (1) | |
| 522 | Ser→Leu | 2 (1.1) | U.S. (1), Japan (1) | |
| 522, 516 | Ser→Leu, Asp→Val | 1 (0.6) | Honduras (1) | |
| 526 | His→Pro | 4 (2.3) | Japan (1), Peru (1), U.S. (2) | |
| 526 | His→Leu | 5 (2.9) | Rwanda (1), U.S. (4) | |
| 526 | His→Asp | 8 (4.6) | Switzerland (1), U.S. (7) | |
| 526 | His→Arg | 9 (5.2) | Rwanda (2), Japan (4), U.S. (3) | |
| 526 | His→Tyr | 35 (20.0) | U.S. (30), Yemen (1), Peru (1), Philippines (1), Honduras (2) | |
| 527 | Lys→Gln | 1 (0.6) | U.S. (1) | |
| 527, 526 | Lys→Gln, His→Pro | 1 (0.6) | U.S. (1) | |
| 531 | Ser→Leu | 74 (42.0) | Belgium (1), Japan (5), Rwanda (9), Canada (2), Philippines (3), Vietnam (1), Yemen (4), U.S. (44), Peru (2), Honduras (3) | |
| 531 | Ser→Phe | 1 (0.6) | U.S. (1) | |
| 533 | Leu→Pro | 3 (1.7) | U.S. (2), Philippines (1) | |
| None | None | 8 (4.6) | Rwanda (2), Berundi (1), U.S. (5) | |
| Susceptible | None | None | 50 | U.S. (42), Honduras (8) |
Amino acid numbers correspond to the E. coli numbering system for the RNA polymerase β-subunit.
Resistant to rifampin at 2 μg/ml by the BACTEC radiometric method or at 1 μg/ml by the proportion method.
U.S., United States.
Our goal in the present study was to correlate the level of rifamycin resistance with specific rpoB genotypes and to study the direct effect of mutant rpoB alleles on the development of rifamycin cross-resistance in M. tuberculosis. To accomplish this, a collection of 31 isolates, consisting of 29 rifampin-resistant strains with 23 unique rpoB alleles and 2 susceptible strains, was selected from our strain collection and tested against rifampin, rifabutin, rifapentine, and KRM-1648 to determine the MICs of each of these drugs. In addition, the three most frequently encountered rpoB mutations associated with the development of the rifampin-resistant phenotype were independently incorporated into the M. tuberculosis rpoB gene, contained on the pLN-2 plasmid (7), by PCR–site-directed mutagenesis. Resultant M. tuberculosis transformants were analyzed for susceptibility to the four rifamycins.
Rifampin was purchased from Sigma Chemical Co., St. Louis, Mo.; rifabutin was obtained from Pharmacia-Upjohn, Dublin, Ohio; rifapentine was obtained from Hoechst Marion Roussel, Kansas City, Mo.; and KRM-1648 was obtained from Pathogenesis Corp., Seattle, Wash. Stock solutions of each drug were prepared in dimethyl sulfoxide (DMSO), and serial twofold dilutions were prepared in DMSO.
Strains were obtained for susceptibility testing by streaking each clinical isolate on Middlebrook 7H11 agar (Difco Laboratories, Detroit, Mich.) containing 1 μg of rifampin/ml. A single colony of each strain was added to Middlebrook 7H9 broth containing 1 μg of rifampin/ml and cultured for 8 to 12 days. Seed cultures of these strains were obtained by adding 100 μl of each culture to 4 ml of 7H12 broth (BACTEC 12B vials; Becton Dickinson, Towson, Md.) and incubating these cultures at 37°C until a growth index (GI) reading of 800 was obtained in a BACTEC 460 instrument (Becton Dickinson Diagnostic Instrument Systems, Sparks, Md.).
The susceptibilities of these strains to rifamycins were determined by adding 100-μl aliquots of the seed cultures to BACTEC 12B vials containing DMSO alone or serial twofold dilutions of each rifamycin in DMSO, with concentrations ranging from 0.015 to 64 μg/ml for rifampin and rifabutin, from 0.031 to 64 μg/ml for rifapentine, and from 0.0156 to 10.24 μg/ml for KRM-1648. Drug-free controls consisted of a 1:100 dilution of the original inoculum added to BACTEC 12B vials (1:100 control). Cultures were incubated at 37°C, and GI readings were recorded daily with the BACTEC 460 instrument until the GI of the 1:100 control reached ≥30. The MIC for each rifamycin was defined as the lowest concentration that inhibited more than 99% of the bacterial population as assessed by GI readings in the BACTEC instrument.
Rifamycin MICs for rifampin-resistant clinical isolates of M. tuberculosis with unique rpoB mutant alleles are shown in Table 2. Eighteen of the rpoB mutations were associated with MICs of rifampin, rifabutin, rifapentine, and KRM-1648 above those obtained for susceptible strains. The remaining five rpoB mutations, in codons 511, 516, 519, and 522, were associated with resistance to rifampin and rifapentine but susceptibility to rifabutin and KRM-1648. These results confirmed earlier observations that strains with mutations in selected rpoB codons remain moderately susceptible to rifabutin or KRM-1648 or exhibit low-level resistance to these rifamycins (1, 8, 18). Strains with the same rpoB genotype but from different geographical locations showed similar patterns of resistance (Tables 1 and 2).
TABLE 2.
Rifamycin MICs for clinical isolates of M. tuberculosis with mutant rpoB alleles
| Clinical isolate | Affected amino acid(s) (change)b | MIC (μg/ml) ofa:
|
|||
|---|---|---|---|---|---|
| Rifam- pin | Rifa- butin | Rifa- pentine | KRM1648 | ||
| Resistantc | |||||
| TB54 | 511 (Leu→Pro) | 2 | 0.5 | 1 | <0.01 |
| TB022 | 513 (Gln→Pro) | 32 | 8.0 | 32 | 0.32 |
| TB872 | 513 (Gln→Pro) | 32 | 4.0 | 32 | 0.32 |
| TB713 | 513 (Gln→Leu) | 16 | 2.0 | 16 | 0.16 |
| TBU36 | 514 (Phe insertion) | 32 | <2.0 | 32 | <0.16 |
| TB3908 | 516 (Asp→Val) | 32 | <0.5 | 32 | 0.01 |
| TBYE-67 | 516 (Asp→Val) | 32 | 0.5 | 32 | 0.01 |
| TBGro-1 | 516 (Asp→Tyr) | 2 | <0.5 | 8 | 0.04 |
| TB33 | 517 (Gln deletion) | 32 | 8.0 | 64 | 2.56 |
| TB4183 | 518 (Asn deletion) | 64 | 64 | >32 | 5.12 |
| TB053 | 518–519 (Asn deletion) | >64 | >32 | >32 | >10.24 |
| TBRos-1 | 519 (Asn→Lys) | 32 | 0.5 | 32 | 0.01 |
| TB3505 | 521 (Leu→Met) | 32 | 8.0 | 32 | 2.56 |
| TBNor-1 | 522 (Ser→Leu) | 32 | 0.5 | 32 | 0.01 |
| TB2230 | 522 (Ser→Leu), 516 (Asp→Val) | >32 | >1.0 | 32 | 0.12 |
| TBRR-1 | 526 (His→Arg) | 64 | 64 | <32 | 5.12 |
| TB5095 | 526 (His→Leu) | 16 | 8 | 16 | 0.56 |
| TBRR-7 | 526 (His→Pro) | >64 | 32 | >64 | 2.56 |
| TB160 | 526 (His→Pro) | >64 | >32 | >64 | 5.12 |
| TBYE-68 | 526 (His→Tyr) | >64 | 32 | >64 | >2.56 |
| TB3081 | 526 (His→Tyr) | >64 | >32 | >64 | 5.12 |
| TB052 | 526 (His→Asp) | >64 | 64 | >32 | >2.56 |
| TB3140 | 526 (His→Asp) | >64 | 64 | >32 | 5.12 |
| TB91 | 526 (His→Asp), 509 (Ser→Leu) | >64 | 8 | >64 | 0.64 |
| TB023 | 527 (Lys→Gln) | >32 | 16 | 32 | 2.56 |
| TBYE-14 | 531 (Ser→Leu) | >64 | 32 | >64 | 10.24 |
| TB3241 | 531 (Ser→Leu) | >64 | 32 | >64 | >10.24 |
| TB019 | 531 (Ser→Phe) | 64 | 32 | >64 | 10.24 |
| TB3126 | 533 (Leu→Pro) | 64 | 8 | 64 | 2.56 |
| Susceptible | |||||
| TBSmi-1 | None | 0.5 | <0.5 | <0.12 | <0.01 |
| TBH1-7 | None | 0.5 | <0.5 | 0.12 | <0.01 |
MICs determined by the BACTEC radiometric method.
Amino acid numbers correspond to the E. coli numbering system for the RNA polymerase β-subunit.
Clinical isolates of M. tuberculosis resistant to rifampin at ≥2 μg/ml by the BACTEC radiometric method or at 1 μg/ml by conventional agar plate testing.
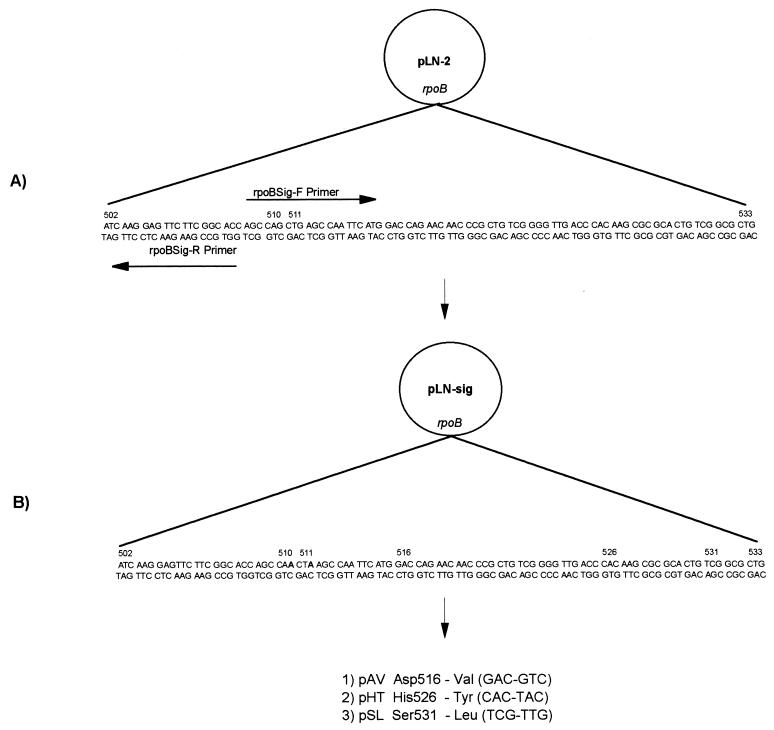
To further define the role of mutations in the development of rifamycin cross-resistance in M. tuberculosis, we employed a molecular genetic approach involving a PCR-based site-directed mutagenesis protocol. Mutagenesis was used to incorporate silent mutations (signature nucleotides) in codons 510 and 511 of the rpoB gene of pLN-2 (Fig. 1). These signature nucleotides would be used to identify the presence of the plasmid copy of rpoB and, therefore, eliminate the possibility that the rifampin-resistant phenotype of transformants resulted from the selection of spontaneously rifampin-resistant mutants. Briefly, 250 ng of pLN-2 was combined with XL-PCR reagents (Perkin-Elmer, Norwalk, Conn.) and primers rpoBSig-F and rpoBSig-R (Table 3) and amplified, using the manufacturer’s recommended protocol to incorporate nucleotide substitutions and produce linearized plasmid DNA. PCR products were treated with 5 U of Pfu DNA polymerase (Stratagene, La Jolla, Calif.) to polish the ends of the linear plasmid DNA and digested with 40 U of the restriction endonuclease DpnI (Stratagene) to eliminate parental plasmid DNA. The preparation was treated with 4 U of T4 ligase (Gibco-BRL, Gaithersburg, Md.), and recircularized plasmids were cloned into Escherichia coli XL1-Blue (Stratagene). Kanamycin-resistant colonies were analyzed for the presence of rpoB containing signature nucleotides by PCR-direct DNA sequencing using primers and conditions previously described (19). Plasmid (pLN-sig) DNA was purified from a positive clone by using a QIAquick plasmid kit (Qiagen Inc., Chatsworth, Calif.).
FIG. 1.
Nucleotide substitutions were incorporated into the rpoB gene of M. tuberculosis H37Rv contained on pLN-2, using mutagenic primers (Table 3) and long-fragment PCR–site-directed mutagenesis. (A) Signature nucleotides were incorporated into pLN-2 at codons 510 (CAG→CAA) and 511 (CTG→CTA) to yield the pLN-sig plasmid. (B) Mutant rpoB alleles were incorporated into pLN-sig as follows: 1) a GAC→GTC nucleotide substitution in codon 516 of pLN-sig to yield plasmid pAV, 2) a CAC→TAC nucleotide substitution in codon 526 of pLN-sig to yield plasmid pHT, and 3) a TCG→TTG nucleotide substitution in codon 531 of pLN-sig to yield plasmid pSL.
TABLE 3.
Primers for PCR–site-directed mutagenesis of M. tuberculosis rpoB in plasmid pLN-2
| Primer | Primer sequencea | Mutationb |
|---|---|---|
| rpoBSig-F | 5′ CAGCCAACTAAGCCAATTC 3′ | Signature nucleotides |
| rpoBSig-R | 5′ GTGCCGAAGAACTCCTTGAT 3′ | |
| rpoBVal516-F | 5′ TCATGGTCCAGAACAACCCG 3′ | Asp 516→Val (GAC→GTC) |
| rpoBVal516-R | 5′ ATTGGCTTAGTTGGCTG 3′ | |
| rpoBTyr526-F | 5′ GTTGACCTACAAGCGCCGA 3′ | His 526→Tyr (CAC→TAC) |
| rpoBTyr526-R | 5′ CCCGACAGCGGGTTGTTCTGGTC 3′ | |
| rpoBLeu531-F | 5′ GACTGTTGGCGCTGG 3′ | Ser 531→Leu (TCG→TTG) |
| rpoBLeu531-R | 5′ GGCGCTTGTGGGTCAA 3′ |
All primers were phosphorylated on the 5′ end.
Mutations in the rpoB of M. tuberculosis H37Rv contained on plasmid pLN-2 by use of PCR–site-directed mutagenesis.
Specific rpoB mutant alleles (531 Leu, 526 Tyr, and 516 Val), associated with the rifampin-resistant phenotype of M. tuberculosis (Table 1), were independently incorporated into pLN-sig by PCR–site-directed mutagenesis (Fig. 1) with the primers described in Table 3. All resultant plasmids were cloned into E. coli XL1-Blue, and recombinant clones with signature nucleotides and mutant rpoB alleles were identified by PCR-direct DNA sequencing.
Plasmid DNA of one clone for each mutant allele was purified by using a large-scale plasmid DNA preparation protocol (6). The presence of signature nucleotides and mutant rpoB alleles and the absence of spurious mutations in rpoB in these plasmids were confirmed by PCR-DNA sequencing with primers that span the entire insert containing rpoB (data not shown). Next, using a Gene Pulser (Bio-Rad Laboratories, Hercules, Calif.) and a 0.2-cm-light-path cuvette, 1 to 2 μg of each plasmid was electroporated (at 2.5 kV, 25 μF, and 1,000 Ω) into electrocompetent (glycerol-treated) M. tuberculosis H37Rv (ATCC 27294) cells. Clones were selected on 7H11 agar containing 30 μg of kanamycin/ml.
Mutant β-subunits were expressed in these recombinants, and the susceptibilities of recombinant M. tuberculosis clones to rifamycins were determined radiometrically with the BACTEC instrument, using a liquid medium as indicated above. Rifampin-susceptible M. tuberculosis H37Rv (ATCC 27294) and rifampin-resistant M. tuberculosis H37Rv-Rif-r (ATCC 35838) were used as controls.
The results of these experiments indicated that M. tuberculosis recombinant clones containing plasmids with specific rpoB mutant alleles affecting either codon 531 or 526 showed high-level resistance to all rifamycins analyzed (Table 4). Clones containing a mutation within codon 516, resulting in the substitution of a valine for an aspartic acid residue in the β-subunit, showed a differential susceptibility to rifamycins, with KRM-1648 and rifabutin being the most active against this mutant. Clones containing either pLN-2 or pLN-sig (i.e., containing wild-type rpoB or rpoB with signature nucleotides, respectively) demonstrated rifamycin MICs consistent with the rifampin-susceptible phenotype of M. tuberculosis (Table 4). In addition, it was previously shown that the same vector with lacZ (not rpoB) as the insert (pMV261::lacZ) did not affect the rifamycin phenotype of either a rifampin-susceptible or a rifampin-resistant strain of M. smegmatis (7). Therefore, these results demonstrated that expression of the plasmid-encoded β-subunit was responsible for the alteration of the rifamycin susceptibility of recombinant clones.
TABLE 4.
Rifamycin resistance of M. tuberculosis recombinant clones
| Straina | Mutated amino acidb | MIC (μg/ml) ofc:
|
|||
|---|---|---|---|---|---|
| Rifampin | Rifabutin | Rifapentine | KRM-1648 | ||
| H37Rv | None | 0.25 | ≤0.015 | 0.031 | ≤0.015 |
| H37Rv-pLN-2 | None | 0.25 | ≤0.015 | 0.031 | ≤0.015 |
| H37Rv-pLN-sig | None | 0.25 | ≤0.015 | ≤0.031 | ≤0.015 |
| H37Rv-pAV | Val 516 | 32 | ≤0.015 | 16 | ≤0.015 |
| H37Rv-pHT | Tyr 526 | 64 | 16 | 16 | 8 |
| H37Rv-pSL | Leu 531 | >64 | 16 | >64 | 8 |
| H37Rv-Rif-r | Leu 531 | >64 | 16 | >64 | 8 |
H37Rv, M. tuberculosis H37Rv (ATCC 27294); H37Rv-pLN-2, M. tuberculosis H37Rv clone containing plasmid pLN-2 with wild-type rpoB; H37Rv-pLN-sig, M. tuberculosis H37Rv clone containing pLN-sig with signature nucleotides in rpoB; H37Rv-pAV, M. tuberculosis H37Rv clone containing plasmid pAV with Val 516 mutant allele in rpoB; H37Rv-pHT, M. tuberculosis H37Rv clone containing plasmid pHT with Tyr 526 mutant allele in rpoB; H37Rv-pSL, M. tuberculosis H37Rv clone containing plasmid pSL with Leu 531 mutant allele in rpoB; H37Rv-Rif-r, M. tuberculosis H37Rv (ATCC 35838).
Amino acid numbering corresponds to that of the E. coli RNA polymerase β-subunit.
MICs determined by the BACTEC radiometric method.
The lower MICs of some of the recombinant clones compared to those of clinical isolates most likely resulted from the merodiploid state of rpoB in these cells (wild-type rpoB and rpoB containing mutant alleles). However, expression of plasmid-encoded β-subunits containing mutant rpoB alleles dramatically altered the rifampin phenotype in most of the recombinants. The most plausible explanation for this is as follows. pLN-2 is a multicopy vector, a derivative of pMV261, which has been shown to efficiently transform mycobacterial species and to maintain about five copies per genome equivalent in these mycobacterial cells (15). Therefore, the plasmid-encoded β-subunit is produced in abundance and thereby effectively outcompetes the single chromosomally encoded copy to make a mutant RNA polymerase holoenzyme. This altered enzyme results in the development of rifampin resistance.
Previously, we demonstrated that transformation of M. smegmatis LR222 with these plasmids resulted in the conversion of the rifampin-susceptible phenotype to the resistant phenotype (18). The present study extended these observations by including the performance of complementation studies in M. tuberculosis and analysis of three of the most frequently occurring rpoB mutant alleles of clinical isolates. In addition, the contribution of specific rpoB mutations in the development of rifamycin cross-resistance in M. tuberculosis was further defined by using this approach, with the results demonstrating that rpoB mutations present in rifampin-resistant clinical isolates are solely responsible for the development of the rifamycin-resistant phenotype.
These experiments define the role of specific rpoB mutant alleles that are involved in the development of selective susceptibility to rifampin analogs with specific chemical compositions. Rifamycin analogs that retain antituberculosis activity in the presence of specific mutant rpoB alleles have been identified. The ability of rifabutin and KRM-1648 to overcome rifampin resistance in vitro in a selected group of strains containing specific rpoB mutant alleles suggests that amino acid positions and specific substitutions in these regions of the β-subunit of the RNA polymerase are very important for the selective affinity and activity of specific rifamycin structural analogs for mutant β-subunits. Therefore, it appears that rifamycin structure can potentially be modified to circumvent rifampin resistance in some rifampin-resistant strains. However, this has not been proven in vivo in human clinical trials, in which issues such as solubility and attainable blood levels must be investigated.
The data obtained in the present study suggest that the detection of rpoB mutant alleles by several molecular genetic-based analyses, such as the line probe assay (2), PCR-heteroduplex formation (19, 20), PCR-SSCP (16), and PCR-direct DNA sequencing (10, 12), could be used to rapidly determine the susceptibility of a clinical strain to various rifamycins and provide information that may have an impact on treatment strategies and check further spread of drug-resistant mutants. In addition, this highly characterized collection of rifampin-resistant clinical isolates and genetically engineered M. tuberculosis clones expressing mutant β-subunits should be useful for studying specific structure-activity relationships between various structurally modified rifamycin analogs and wild-type and mutant β-subunits. Understanding how rifamycins kill M. tuberculosis will potentially provide information for the rational design of a rifamycin analog(s) or to identify existing analogs which can be used to circumvent rifampin resistance.
Acknowledgments
We thank Max Salfinger and Carlos Javier-Zepeda for contributing strains for this study.
This study was supported by grants from NIH/NIAID (AI35274) and Hoechst Marion Roussel.
REFERENCES
- 1.Bodmer T, Zurcher G, Imboden P, Telenti A. Mutation position and type of substitution in the β-subunit of the RNA polymerase influence in-vitro activity of rifamycins in rifampicin-resistant Mycobacterium tuberculosis. J Antimicrob Chemother. 1995;35:345–348. doi: 10.1093/jac/35.2.345. [DOI] [PubMed] [Google Scholar]
- 2.De Beenhouwer H, Lhiang Z, Jannes G, Mijs W, Machtelinckx L, Rossau R, Traore H, Portaels F. Rapid detection of rifampicin resistance in sputum and biopsy specimens from tuberculosis patients by PCR and line probe assay. Tubercle Lung Dis. 1995;76:425–430. doi: 10.1016/0962-8479(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 3.Heifets L B, Lindholm-Levy P J, Flory M A. Bactericidal activity in vitro of various rifamycins against Mycobacterium avium and Mycobacterium tuberculosis. Am Rev Respir Dis. 1990;141:626–630. doi: 10.1164/ajrccm/141.3.626. [DOI] [PubMed] [Google Scholar]
- 4.Hong Kong Chest Service and British Medical Research Council. A controlled study of rifabutin and an uncontrolled study of ofloxacin in the treatment of patients with pulmonary tuberculosis resistant to isoniazid, streptomycin and rifampicin. Tubercle Lung Dis. 1992;73:59–67. [PubMed] [Google Scholar]
- 5.Luna-Herrera J, Reddy M V, Gangadharam P R J. In vitro activity of the benzoxazinorifamycin KRM-1648 against drug-susceptible and multidrug-resistant tubercle bacilli. Antimicrob Agents Chemother. 1995;39:440–444. doi: 10.1128/aac.39.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maniatis T, Fritsch E F, Sambrook J. Molecular Cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1982. Large-scale isolation of plasmid DNA: lysis by alkali; pp. 90–91. [Google Scholar]
- 7.Miller L P, Crawford J T, Shinnick T M. The rpoB gene of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1994;38:805–811. doi: 10.1128/aac.38.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moghazeh S L, Pan X, Arain T, Stover C K, Musser J M, Kreiswirth B N. Comparative antimycobacterial activities of rifampin, rifapentine, and KRM-1648 against a collection of rifampin-resistant Mycobacterium tuberculosis isolates with known rpoB mutations. Antimicrob Agents Chemother. 1996;40:2655–2657. doi: 10.1128/aac.40.11.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Musser J M. Antimicrobial agent resistance in mycobacteria: molecular genetic insights. Clin Microbiol Rev. 1995;8:496–514. doi: 10.1128/cmr.8.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nachamkin I, Kang C, Weinstein M P. Detection of resistance to isoniazid, rifampin, and streptomycin in clinical isolates of Mycobacterium tuberculosis by molecular methods. Clin Infect Dis. 1997;24:894–900. doi: 10.1093/clinids/24.5.894. [DOI] [PubMed] [Google Scholar]
- 11.Ohno H, Koga H, Kohno S, Tashiro T, Hara K. Relationship between rifampin MICs for and rpoB mutations of Mycobacterium tuberculosis strains isolated in Japan. Antimicrob Agents Chemother. 1996;40:1053–1056. doi: 10.1128/aac.40.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pai S, Esen N, Pan X, Musser J M. Routine rapid Mycobacterium species assignment based on species-specific allelic variation in the 65-kilodalton heat shock protein gene (hsp65) Arch Pathol Lab Med. 1997;121:859–864. [PubMed] [Google Scholar]
- 13.Reddy M V, Luna-Herrera J, Daneluzzi D, Gangadharum P R. Chemotherapeutic activity of benzoxazinorifamycin KRM-1648 against Mycobacterium tuberculosis in C57BL/6 mice. Tubercle Lung Dis. 1996;77:154–159. doi: 10.1016/s0962-8479(96)90030-1. [DOI] [PubMed] [Google Scholar]
- 14.Sirgel D A, Botha F J, Parkin D P. The early bactericidal activity of rifabutin in patients with pulmonary tuberculosis measured by sputum viable counts: a new method of drug assessment. J Antimicrob Chemother. 1993;2:867–875. doi: 10.1093/jac/32.6.867. [DOI] [PubMed] [Google Scholar]
- 15.Stover C K, de la Cruz V F, Fuerst T R, Burlein J E, Benson L A, Bennett L T, Bansal G P, Young J F, Lee M H, Hatfull G F, Snapper S B, Barletta R G, Jacobs W R, Jr, Bloom B R. New use of BCG for recombinant vaccines. Nature. 1991;351:456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- 16.Telenti A, Imboden P, Marchesi F, Lowrie D, Cole S T, Colston M J, Matter L, Schopfer K, Bodmer T. Detection of rifampicin-resistance mutations in Mycobacterium tuberculosis. Lancet. 1993;341:647–650. doi: 10.1016/0140-6736(93)90417-f. [DOI] [PubMed] [Google Scholar]
- 17.Williams D L, Waguespack C, Eisenach K, Crawford J T, Portaels F, Salfinger M, Nolan C M, Abe C, Sticht-Groh V, Gillis T P. Characterization of rifampin resistance in pathogenic mycobacteria. Antimicrob Agents Chemother. 1994;38:2380–2386. doi: 10.1128/aac.38.10.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams D L, Spring L, Collins L, Miller L, Heifets L, Gillis T P. Program and abstracts of the 32nd Joint Leprosy/Tuberculosis Conference. U.S.-Japan Cooperative Medical Science Program, Cleveland, Ohio. 1997. Mutations in rpoB and rifamycin cross-resistance in Mycobacterium tuberculosis, abstr. 14; p. 226. [Google Scholar]
- 19.Williams D L, Limbers C W, Spring L, Jayachandra S, Gillis T P. PCR-heteroduplex detection of rifampin-resistant Mycobacterium tuberculosis. In: Persing D H, editor. PCR protocols for emerging infectious diseases: a supplement to diagnostic molecular microbiology: principles and applications. Washington, D.C: ASM Press; 1996. pp. 122–129. [Google Scholar]
- 20.Williams D L, Spring L, Salfinger M, Gillis T P, Persing D H. Evaluation of polymerase chain reaction-based universal heteroduplex generator assay for direct detection of rifampin susceptibility of Mycobacterium tuberculosis from sputum specimens. Clin Infect Dis. 1998;26:446–450. doi: 10.1086/516313. [DOI] [PubMed] [Google Scholar]