Abstract
The bioavailabilities and bioequivalences of single 200-mg doses of itraconazole solution and two capsule formulations were evaluated in a crossover study of 30 male volunteers. The two capsule formulations were bioequivalent. The bioavailabilities of the solutions itraconazole and hydroxyitraconazole were 30 to 33% and 35 to 37% greater, respectively, than those of either capsule. However, the maximum concentrations of the drug in plasma (Cmax), the times to Cmax, and the terminal half-lives were comparable for all three formulations. These data indicate that the bioavailabilities of itraconazole and hydroxyitraconazole are enhanced when administered as an oral solution instead of capsules.
Itraconazole (ITR) (Sporanox; Janssen Pharmaceutica, Titusville, N.J.) is a broad-spectrum triazole agent available for the treatment of histoplasmosis, blastomycosis, onychomycosis, and amphotericin B-refractory aspergillosis (7, 8, 10, 15, 19, 20, 27). ITR is highly effective in vitro against Candida albicans and other Candida species, including many resistant to fluconazole (1, 4). To achieve maximum absorption, the ITR capsule formulation should be taken with food and in the presence of an acidic gastric environment (2, 14, 20).
An oral solution formulation of ITR (Sporanox oral solution [SOS]) containing hydroxypropyl-β-cyclodextrin is approved and has greater bioavailability when given in the fasted state than in the nonfasted state to healthy volunteers (3). Previously, single 100-mg doses of two formulations of ITR capsules (F05 and F12) were found to be bioequivalent (18). The objectives of the present trial were to compare the pharmacokinetics of these two capsule formulations when given at the recommended therapeutic dose (200 mg/day) and to determine the bioavailabilities of ITR and its active metabolite, hydroxyitraconazole (OH-ITR), by comparing SOS with both capsule formulations.
Patients.
Healthy male volunteers at least 18 years of age who were nonsmokers and who weighed within 10% of the normal body weights for their heights were eligible for study inclusion. Patients could have no clinically significant abnormalities on physical examination or in blood counts, biochemistries, or urinalyses, and a negative urine drug screen was required. Patients with a significant concurrent illness, a history of barbiturate, amphetamine, or narcotic abuse, an inability to swallow capsules, or a history of hypersensitivity to imidazole or azole compounds or who had participated in an investigational study or used an investigational drug within the previous month were not entered. Institutional review board approval was obtained and each subject gave written informed consent before entry.
Study design.
This was an open-label, single-dose, crossover study with three phases separated by 2-week washout intervals. Patients were randomized to one of six sequence groups (F05-F12-SOS, F05-SOS-F12, F12-F05-SOS, F12-SOS-F05, SOS-F05-F12, or SOS-F12-F05) and received one of the following three treatments with 200 ml of water during each phase: formula F05 (two 100-mg ITR capsules), formula F12 (two 100-mg ITR capsules), or SOS (20 ml, containing 200 mg of ITR).
Physical examinations and clinical laboratory tests (hematologies, biochemistries, urinalyses, and urine drug screens) were performed within 2 weeks of the start of dosing and again at the 96-h blood sampling for phase 3 (end of study). Subjects were admitted to the study unit the evening before dosing. No food or beverages (except water) were permitted after midnight. In the morning, subjects received a standard breakfast (fried egg and bacon, toast with butter and jam, whole milk, orange juice, and banana) followed immediately by the study medication; subjects were not permitted to drink until 2 h after dosing or to eat until 4 h after dosing. Patients remained at the study site through the 36-h blood collection and then returned for the 48-, 72-, and 96-h blood collections. No medications except analgesics were permitted during the study. Adverse events (AEs) were monitored by nondirected interviews conducted before each dosing and at 4, 12, 24, 72, and 96 h after each dosing. Information included date of onset, duration, intensity, frequency, action taken, relationship to study medication, and outcome.
Pharmacokinetic determinations.
During each of the three crossover phases, 10-ml blood samples were obtained immediately prior to dosing (time zero) and at 0.5, 1, 2, 3, 4, 5, 6, 8, 12, 24, 36, 48, 72, and 96 h after dosing. Blood was collected in heparinized tubes, centrifuged within 1 h of sampling, pipetted into labeled containers, and stored at ≤−20°C. The frozen plasma samples were sent to Janssen Research Foundation, where they were analyzed for concentrations of ITR and OH-ITR by high-performance liquid chromatography by the method of Woestenborghs et al. (30). The limits of quantification were 1 ng/ml for ITR and 2.5 ng/ml for OH-ITR. The relative errors of the assay method were +9% at 1 ng/ml and +0.2% at 500 ng/ml.
The following pharmacokinetic parameters were evaluated for ITR and OH-ITR: the maximum concentration of the drug in plasma (Cmax), the time to Cmax (Tmax), the terminal half-life (t1/2), the area under the plasma concentration-time curve from 0 to 96 h postdose (AUC0–96), and the area under the plasma concentration-time curve from 0 to ∞ (AUC0–∞). The t1/2 was computed as ln 2/β, where β is the elimination rate constant determined by linear regression of the terminal points of the log-linear plasma concentration-time curves. AUCs were calculated via trapezoidal summation.
Statistical analysis.
SAS version 5.16 was used for all data calculations and statistical analyses. Demographic data were compared with a one-way analysis of variance for continuous variables and a chi-square test for categorical variables. Pharmacokinetic data were analyzed with an analysis of variance model appropriate for a three-treatment, three-period crossover design. Pairwise comparisons were carried out with t tests on the least-squares means. All P values were based on two-sided tests, with alpha equal to 0.05.
In order to determine bioequivalences, 90% confidence intervals (CI) were computed for each pharmacokinetic parameter for each pair of formulations (25). If the 90% CI of the test formulation was completely contained in the range of 80 to 120% of the reference formulation, the two formulations were considered bioequivalent for that pharmacokinetic parameter.
Thirty subjects were enrolled and all completed the study. Five subjects were randomized to each of the six sequence groups, which were comparable for demographic variables. The study population had a mean age of 24 years (range, 19 to 34) and a mean weight of 167 lb (range, 130 to 206).
Plasma ITR concentrations.
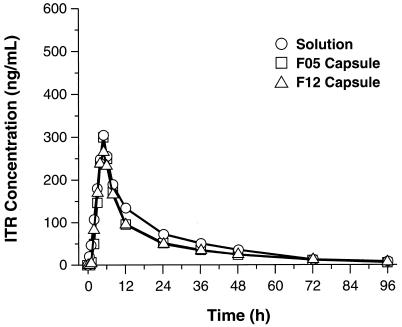
The bioequivalences of the three formulations are illustrated in Fig. 1. SOS and F05 were bioequivalent with regard to Cmax, Tmax, and t1/2 (Table 1). SOS and F12 also were bioequivalent with regard to Cmax, Tmax, and t1/2 (Table 2).
FIG. 1.
Mean plasma ITR concentrations versus time.
TABLE 1.
Pharmacokinetics of ITR: SOS versus F05a
| Form of ITR | Cmax (ng/ml) | Tmax (h) | t1/2 (h) | AUC0–96 (ng · h/ml) | AUC0–∞ (ng · h/ml) |
|---|---|---|---|---|---|
| SOS | 306.4 (97.4) | 5.0 | 22.1 | 5,550.2 (130.7) | 5,838.0 (130.4) |
| F05 | 314.7 | 5.0 | 22.0 | 4,247.5 | 4,475.8 |
| 90% CIb | 87.5–107.2 | 94.1–103.2 | 94.9–105.4 | 118.6–142.8 | 118.3–142.6 |
Reference is F05. All pharmacokinetic values except bioavailability are least-square means. Numbers in parentheses are bioavailabilities (indicated as percentages) of SOS relative to F05.
If the 90% CI is completely contained in the range of 80 to 120% of the reference formulation, bioequivalence can be declared with respect to the parameter tested. Thus, AUCs indicate that the formulations are not bioequivalent.
TABLE 2.
Pharmacokinetics of ITR: SOS versus F12a
| Form of ITR | Cmax (ng/ml) | Tmax (h) | t1/2 (h) | AUC0–96 (ng · h/ml) | AUC0–∞ (ng · h/ml) |
|---|---|---|---|---|---|
| SOS | 306.4 (101.5) | 5.0 | 22.1 | 5,550.2 (132.7) | 5,838.0 (131.4) |
| F12 | 301.9 | 4.9 | 22.9 | 4,183.4 | 4,441.3 |
| 90% CIb | 91.2–111.7 | 97.3–106.8 | 91.4–101.5 | 120.4–144.9 | 119.2–143.7 |
Reference is F12. All pharmacokinetic values except bioavailability are least-squares means. Numbers in parentheses are bioavailabilities (indicated as percentages) of SOS relative to F12.
For explanation of 90% CI, see Table 1, footnote b.
Bioavailability, as measured by the AUC0–96 and AUC0–∞, was greater for SOS than for either capsule formulation. The AUC0–96 and AUC0–∞ were 30.7 and 30.4% higher, respectively, for SOS than for F05. The AUC0–96 and AUC0–∞ for SOS were also higher (32.7 and 31.4%, respectively) than those for F12. The two capsule formulations were bioequivalent with regard to all parameters.
Plasma OH-ITR concentrations.
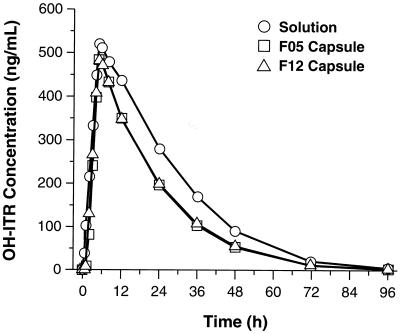
Overall, mean plasma OH-ITR concentrations were substantially higher than ITR concentrations with both capsule formulations and with SOS. As with ITR, the mean AUCs for OH-ITR were similar for F05, F12, and SOS from 0 to 96 h postdose (Fig. 2). SOS and F05 were bioequivalent with regard to Cmax, Tmax, and t1/2 but not with regard to AUC0–96 and AUC0–∞ (Table 3). The overall systemic exposure to OH-ITR was enhanced with SOS (compared to the exposure with F05) based on the AUC0–96 and AUC0–∞, which were 37.3 and 37.4% higher, respectively. SOS and F12 were bioequivalent with regard to Cmax, Tmax, and t1/2, but the AUC0–96 and AUC0–∞ were higher (34.9 and 34.8%, respectively) for SOS (Table 4). The two capsule formulations were bioequivalent with regard to all parameters.
FIG. 2.
Mean plasma OH-ITR concentrations versus time.
TABLE 3.
Pharmacokinetics of OH-ITR: SOS versus F05a
| Form of OH-ITR | Cmax (ng/ml) | Tmax (h) | t1/2 (h) | AUC0–96 (ng · h/ml) | AUC0–∞ (ng · h/ml) |
|---|---|---|---|---|---|
| SOS | 527.0 (105.2) | 5.7 | 10.7 | 14,889.3 (137.3) | 15,025.5 (137.4) |
| F05 | 501.0 | 5.5 | 10.3 | 10,847.8 | 10,937.8 |
| 90% CIb | 97.1–113.3 | 94.3–113.0 | 97.0–110.5 | 124.9–149.6 | 124.9–149.9 |
Reference is F05. All pharmacokinetic values except bioavailability are least-squares means. Numbers in parentheses are bioavailabilities (expressed as percentages) of SOS relative to F05.
For explanation of 90% CI, see Table 1, footnote b.
TABLE 4.
Pharmacokinetics of OH-ITR: SOS versus F12a
| Form of OH-ITR | Cmax (ng/ml) | Tmax (h) | t1/2 (h) | AUC0–96 (ng · h/ml) | AUC0–∞ (ng · h/ml) |
|---|---|---|---|---|---|
| SOS | 527.0 (104.5) | 5.7 | 10.7 | 14,889.3 (134.9) | 15,025.5 (134.8) |
| F12 | 504.3 | 5.3 | 10.7 | 11,033.3 | 11,148.1 |
| 90% CI | 96.5–112.5 | 96.7–115.8 | 93.3–106.3 | 122.8–147.1 | 122.5–147.0 |
Reference is F12. All pharmacokinetic values except bioavailability are least-squares means. Numbers in parentheses are bioavailabilities (indicated as percentages) of SOS relative to F12.
For explanation of 90% CI, see Table 1, footnote b.
Treatment sequence and phase effects.
For both ITR and OH-ITR, there were no significant differences among the six sequences with regard to carryover effects (P ≥ 0.31) or phase effects.
For ITR Tmaxs, the means were 4.9, 4.8, and 5.1 h for phases 1, 2, and 3, respectively (P = 0.06). For OH-ITR Tmaxs, the means were 5.3, 5.3, and 5.9 h for phases 1, 2, and 3, respectively (P = 0.08). These differences were not statistically significant and were not considered clinically meaningful.
Safety.
Of the 30 subjects who completed the study, 2 (7%) had one AE each, neither of which resulted in study discontinuation. One subject had a mild rash that began on the first day of SOS dosing and one subject reported mild headache that occurred the day after the first dose of F05. Both AEs resolved within 1 day, did not recur, and were judged to be possibly related to the study medication. No clinically significant changes from baseline in vital signs or laboratory parameters were observed.
The results of our study demonstrate that the bioavailability of ITR, as measured by the AUC, is enhanced with SOS. For ITR, bioavailability was 30 to 33% greater for SOS than for both capsule formulations, while the mean Cmaxs, Tmaxs, and t1/2s were comparable for all three formulations. For OH-ITR, overall systemic exposure was also higher (35 to 37%) for SOS than for both capsule formulations, and all three formulations had similar mean Cmaxs, Tmaxs, and t1/2s.
Capsule formulations F05 and F12 were bioequivalent. Mean peak plasma ITR concentrations were achieved within approximately 5 h after a 200-mg dose of each capsule formulation. Peak concentrations of OH-ITR were higher, with longer Tmaxs and shorter t1/2s. These data are consistent with those reported by Hardin et al. (14) for oral ITR capsules (200 mg/day).
ITR absorption from solid-dose forms is variable and can be enhanced by administration with food and in the presence of an acidic gastric environment (2, 12, 14, 26, 29). SOS has been shown to produce significantly higher plasma ITR levels in animal studies (16, 17), and when SOS is administered in the fasting state, the bioavailability of ITR is enhanced compared with that obtained with capsules (3).
Taken together, these data indicate that ITR has greater bioavailability in the form of SOS and may be effectively administered in that form without food, unlike ITR capsules. This has important implications for immunocompromised patients who are unable to take solid-dose forms or are unable to take medications with food (5). Studies have found SOS to be at least as effective as fluconazole tablets and clotrimazole troches in the treatment of oropharyngeal and esophageal candidiasis in immunocompromised patients, including those who are human immunodeficiency virus positive (13, 21, 28). SOS has also been shown to be effective for fluconazole-refractory oropharyngeal candidiasis in human immunodeficiency virus-positive patients (11, 22) and as antifungal prophylaxis in bone marrow autograft patients and in patients receiving chemotherapy for acute myeloid leukemia (23, 24).
No clear association between plasma ITR concentrations and clinical outcomes has been reported; however, data suggesting that undetectable plasma ITR concentrations are more often associated with therapeutic failure exist. In a study of ITR therapy for aspergillosis (9), higher mean trough concentrations were found among patients who had a response by 3 months than among those with stable diseases or treatment failures. No complete or partial responders had undetectable plasma ITR concentrations. Additionally, Cartledge et al. (6) showed that SOS achieves higher plasma ITR and OH-ITR concentrations than does the capsule in AIDS patients and that this is associated with improved efficacy.
In conclusion, the present study demonstrates that the Cmaxs, Tmaxs, and t1/2s for ITR and OH-ITR in the SOS formulation and the two capsule formulations are similar. However, the bioavailabilities of ITR and OH-ITR are significantly enhanced with SOS. Its effectiveness, coupled with ease of administration and enhanced ITR bioavailability, supports the use of SOS in immunocompromised patients.
Acknowledgments
This study was supported by Janssen Pharmaceutica.
REFERENCES
- 1.Barchiesi F, Colombo A L, McGough D A, Fothergill A W, Rinaldi M G. In vitro activity of itraconazole against fluconazole-susceptible and resistant Candida albicans isolates from oral cavities of patients infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1994;38:1530–1533. doi: 10.1128/aac.38.7.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barone J A, Koh J G, Bierman R H, Colaizzi J L, Swanson K A, Gaffar M C, Moskovitz B L, Mechlinski W, Van De Velde V. Food interaction and steady-state pharmacokinetics of itraconazole capsules in healthy male volunteers. Antimicrob Agents Chemother. 1993;37:778–784. doi: 10.1128/aac.37.4.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barone J A, Moskovitz B L, Guarnieri J, Hassell A E, Colaizzi J L, Bierman R H, Jessen L. Food interaction and steady-state pharmacokinetics of itraconazole (ITR) oral solution in healthy volunteers. Pharmacotherapy. 1997;17:195. . (Abstract 76.) [PubMed] [Google Scholar]
- 4.Blatchford N R. Treatment of oral candidosis with itraconazole: a review. J Am Acad Dermatol. 1990;23:565–567. doi: 10.1016/0190-9622(90)70256-h. [DOI] [PubMed] [Google Scholar]
- 5.Bradford C R, Prentice K A G, Warnock D W, Copplestone J A. Comparison of the multiple dose pharmacokinetics of two formulations of itraconazole during remission induction for acute myeloblastic leukaemia. J Antimicrob Chemother. 1991;28:555–560. doi: 10.1093/jac/28.4.555. [DOI] [PubMed] [Google Scholar]
- 6.Cartledge J D, Midgely J, Gazzard B G. Itraconazole solution: higher serum drug concentrations and better clinical response rates than the capsule formulation in acquired immunodeficiency syndrome patients with candidosis. J Clin Pathol. 1997;50:477–480. doi: 10.1136/jcp.50.6.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleary J D, Taylor J W, Chapman S W. Itraconazole in antifungal therapy. Ann Pharmacother. 1992;26:502–509. doi: 10.1177/106002809202600411. [DOI] [PubMed] [Google Scholar]
- 8.De Doncker P, Decrois J, Piérard G E, Roelant D, Woestenborghs R, Jacqmin P, Odds F, Heremans A, Dockx P, Roseeuw D. Antifungal pulse therapy for onychomycosis. Arch Dermatol. 1996;132:34–41. doi: 10.1001/archderm.132.1.34. [DOI] [PubMed] [Google Scholar]
- 9.Denning D W, Lee J Y, Hostetler J S, Pappas P, Kauffman C A, Dewsnup D H, Galgiani J N, Graybill J R, Sugar A M, Catanzaro A, Gallis H, Perfect J R, Dockery B, Diskmukes W E, Stevens D A. NIAID Mycoses Study Group multicenter trial of oral itraconazole therapy for invasive aspergillosis. Am J Med. 1994;97:135–144. doi: 10.1016/0002-9343(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 10.Denning D W, Tucker R M, Hanson L H, Stevens D A. Treatment of invasive aspergillosis with itraconazole. Am J Med. 1989;86:791–800. doi: 10.1016/0002-9343(89)90475-0. [DOI] [PubMed] [Google Scholar]
- 11.Fessel W J, Merrill K W, Ward D J, Moskovitz B L, Oleka N, Guarnieri J A, Brenner-Gati L, Klausner M. Program and abstracts of the 4th National Conference on Human Retroviruses. 1997. An open-label evaluation of itraconazole oral solution in the treatment of HIV-positive or AIDS patients with fluconazole-refractory oropharyngeal candidiasis; p. 124. [Google Scholar]
- 12.Grant S M, Clissold S P. Itraconazole: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in superficial and systemic mycoses. Drugs. 1989;37:310–344. doi: 10.2165/00003495-198937030-00003. [DOI] [PubMed] [Google Scholar]
- 13.Graybill J R, Vazquez J, Darouiche R, Morhart R, Greenspan D, Tuazon C, Wheat L J, Carey J, Leviton I, Hewitt R G, MacGregor R R, Valenti W, Restrepo M, Moskovitz B L. Randomized trial of itraconazole oral solution for oropharyngeal candidiasis in HIV/AIDS patients. Am J Med. 1998;104:33–39. doi: 10.1016/s0002-9343(97)00307-0. [DOI] [PubMed] [Google Scholar]
- 14.Hardin T C, Graybill J R, Fetchick R, Woestenborghs R, Rinaldi M G, Kuhn J G. Pharmacokinetics of itraconazole following oral administration to normal volunteers. Antimicrob Agents Chemother. 1988;32:1310–1313. doi: 10.1128/aac.32.9.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hay, R. J., B. Dupont, and J. R. Graybill. 1987. First International Symposium on Itraconazole: a summary. Rev. Infect. Dis. 9(Suppl. 1):S1–S3. [PubMed]
- 16.Hostetler J S, Hanson L H, Stevens D A. Effect of cyclodextrin on the pharmacology of antifungal oral azoles. Antimicrob Agents Chemother. 1992;36:477–480. doi: 10.1128/aac.36.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hostetler J S, Hanson L H, Stevens D A. Effect of hydroxypropyl-beta-cyclodextrin on efficacy of oral itraconazole in disseminated murine cryptococcosis. J Antimicrob Chemother. 1993;32:459–463. doi: 10.1093/jac/32.3.459. [DOI] [PubMed] [Google Scholar]
- 18.Janssen Pharmaceutica. Data on file. Janssen Pharmaceutica, Titusville, N.J.
- 19.Lyman C A, Walsh T J. Systemically administered antifungal agents. A review of their clinical pharmacology and therapeutic applications. Drugs. 1992;44:9–35. doi: 10.2165/00003495-199244010-00002. [DOI] [PubMed] [Google Scholar]
- 20.Medical Economics Company. Physicians’ desk reference. 51st ed. Montvale, N.J: Medical Economics Company; 1997. pp. 1352–1354. [Google Scholar]
- 21.Murray P A, Koletar S L, Mallegol I, Wu J, Moskovitz B L. Itraconazole oral solution versus clotrimazole troches for the treatment of oropharyngeal candidiasis in immunocompromised patients. Clin Ther. 1997;19:471–480. doi: 10.1016/s0149-2918(97)80131-2. [DOI] [PubMed] [Google Scholar]
- 22.Phillips P, Zemcov J, Mahmood W, Montaner J S G, Craib K, Clarke A M. Itraconazole cyclodextrin solution for fluconazole-refractory oropharyngeal candidiasis in AIDS: correlation of clinical response with in vitro susceptibility. AIDS. 1996;10:1369–1376. doi: 10.1097/00002030-199610000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Prentice A G, Warnock D W, Johnson S A N, Phillips M J, Oliver D A. Multiple dose pharmacokinetics of an oral solution of itraconazole in autologous bone marrow transplant recipients. J Antimicrob Chemother. 1994;34:247–252. doi: 10.1093/jac/34.2.247. [DOI] [PubMed] [Google Scholar]
- 24.Prentice A G, Warnock D W, Johnson S A N, Taylor P C, Oliver D A. Multiple dose pharmacokinetics of an oral solution of itraconazole in patients receiving chemotherapy for acute myeloid leukaemia. J Antimicrob Chemother. 1995;36:657–663. doi: 10.1093/jac/36.4.657. [DOI] [PubMed] [Google Scholar]
- 25.Schuirmann D J. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm. 1987;15:657–680. doi: 10.1007/BF01068419. [DOI] [PubMed] [Google Scholar]
- 26.Van Peer A, Woestenborghs R, Heykants J, Gasparini R, Gauwenbergh G. The effects of food and dose on the oral systemic availability of itraconazole in healthy subjects. Eur J Clin Pharmacol. 1989;36:423–426. doi: 10.1007/BF00558308. [DOI] [PubMed] [Google Scholar]
- 27.Wheat J, Hafner R, Korzun A H, Limjoco M T, Spencer P, Larsen R A, Hecht F M, Powderly W the AIDS Clinical Trial Group. Itraconazole treatment of disseminated histoplasmosis in patients with the acquired immunodeficiency syndrome. Am J Med. 1995;98:336–342. doi: 10.1016/s0002-9343(99)80311-8. [DOI] [PubMed] [Google Scholar]
- 28.Wilcox C M, Darouiche R O, Laine L, Moskovitz B L, Mallegol I, Wu J. A randomized, double-blind comparison of itraconazole oral solution and fluconazole tablets in the treatment of esophageal candidiasis. J Infect Dis. 1997;176:227–232. doi: 10.1086/514028. [DOI] [PubMed] [Google Scholar]
- 29.Wishart J M. The influence of food on the pharmacokinetics of itraconazole in patients with superficial fungal infection. J Am Acad Dermatol. 1987;17:220–223. doi: 10.1016/s0190-9622(87)70194-7. [DOI] [PubMed] [Google Scholar]
- 30.Woestenborghs R, Lorreyne W, Heykants J. Determination of itraconazole in plasma and animal tissues by high performance liquid chromatography. J Chromatogr. 1987;413:332–337. doi: 10.1016/0378-4347(87)80249-9. [DOI] [PubMed] [Google Scholar]