Abstract
A 36-year-old man was diagnosed with multiple gastric polyps by esophagogastroduodenoscopy. Subsequent colonoscopy identified two tubular adenomas, and computed tomography revealed subcutaneous tumors. Based on these findings, we suspected that gastric polyposis was associated with the APC gene, either attenuated familial adenomatous polyposis (AFAP) or gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS). A genetic analysis demonstrated that he had a frameshift variant at codon 1928 of APC, suggesting AFAP. In this era of less Helicobacter pylori infection and frequent use of proton pump inhibitors, diagnoses of AFAP and GAPPS should be considered in patients with prominent gastric fundic gland polyposis.
Keywords: attenuated familial adenomatous polyposis, gastric adenocarcinoma and proximal polyposis of the stomach, desmoid tumor, APC-associated polyposis
Introduction
Fundic gland polyps are benign gastric epithelial lesions consisting of the hyperplastic expansion of the deep epithelial compartment of the mucosa (1). They are often detected at esophagogastroduodenoscopy (EGD) and regarded as characteristic of Helicobacter pylori uninfected gastric mucosa (2-4). Sporadic fundic gland polyposis may occur in H. pylori uninfected gastric mucosa and/or individuals with long-term use of proton pump inhibitors (PPI) (5).
Fundic gland polyposis occurs as a counterpart to APC-associated gastric polyposis. The underlying conditions of APC-associated gastric polyposis include classical familial adenomatous polyposis (FAP), attenuated FAP (AFAP) and gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS). Genotype-phenotype correlations between APC-associated gastric fundic gland polyposis and the APC gene have been reported (6-10).
We herein report a case of AFAP, in which the APC gene was diagnostic of the disease.
Case Report
A 36-year-old man was referred to our hospital for a detailed examination of multiple gastric polyps. He had a history of a back desmoid tumor at three years old, and his family history was remarkable for gastric cancer in his father but negative for colorectal cancer. There was no family history of cancer or gastrointestinal polyposis. A physical examination and laboratory data showed no abnormalities.
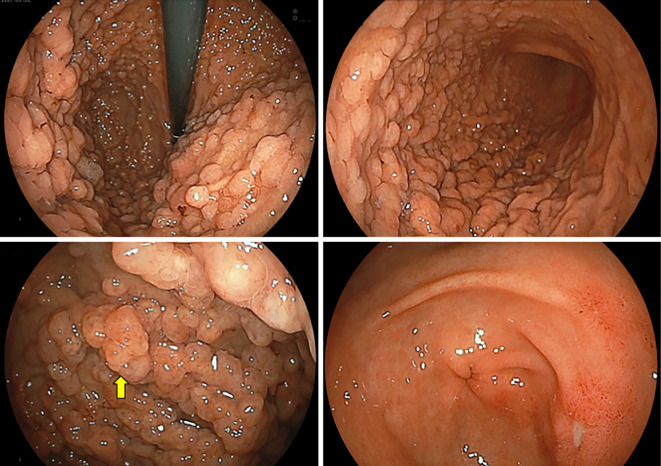
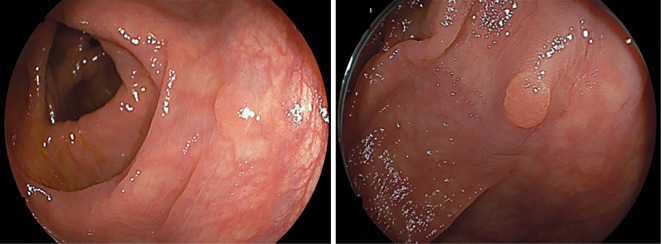
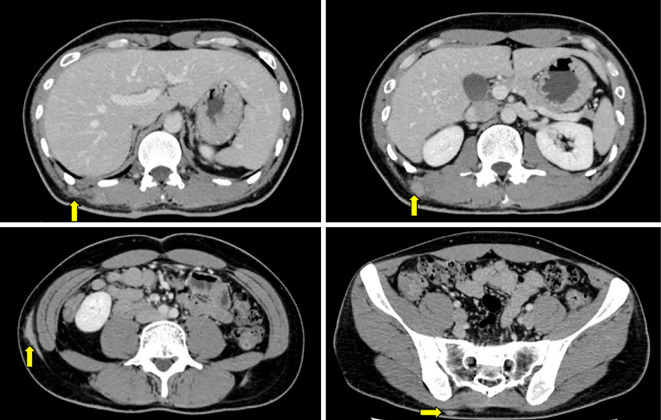
EGD showed multiple polyps in the fundus and body in non-atrophied background mucosa. The polyps were characterized by a sessile or semi-pedunculated configuration and were the same color as the background mucosa (Fig. 1). Biopsy specimens obtained from the polyps revealed dilated fundic glands with no cellular atypia and a partial increase in the density of the glands (Fig. 2). No abnormalities were found in the esophagus and duodenum. Two small polyps were found in the transverse colon during subsequent colonoscopy. Both polyps were removed by cold snare polypectomy and diagnosed as tubular adenomas (Fig. 3). Capsule endoscopy showed no abnormalities in the small intestine. Contrast-enhanced computed tomography (CT) of the neck, chest, abdomen, and pelvis showed scattered elevated soft tissue density in the patient's back, suggestive of desmoid tumors (Fig. 4). There were no signs of tumors in the thyroid, pancreas, liver, or adrenal glands.
Figure 1.
EGD identified no atrophy of the gastric mucosa but detected multiple polyps in the fundus and body that were the same color as the background mucosa, some of which were slightly reddish (yellow arrow).
Figure 2.
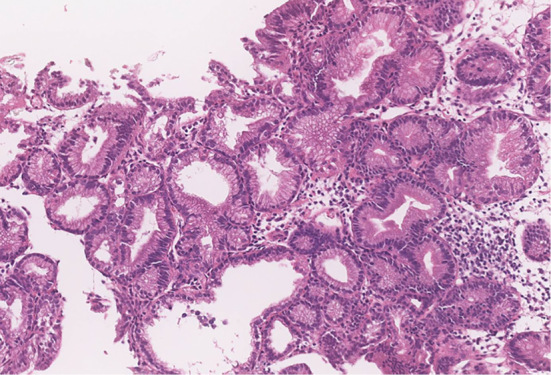
A biopsy of the reddish lesion revealed dilated fundic glands with no cellular atypia but a slight increase in the density of the glands.
Figure 3.
Colonoscopy identified two small polyps in the transverse colon. Cold snare polypectomy was performed, and both lesions were identified histologically as tubular adenomas.
Figure 4.
Contrast-enhanced computed tomography (CT) showed scattered, elevated masses with soft tissue density in the patient’s back, suggestive of desmoid tumors (yellow arrows).
Based on the presence of multiple fundic gland polyps, desmoid tumors, and a small number of colonic adenomas, we considered a diagnosis of APC-associated gastric polyposis, specifically AFAP and GAPPS, as differential diagnoses. We therefore screened the patient's APC gene after obtaining his written informed consent. As a result, a frameshift variant of codon 1928 (c.5282del; Gln>Arg) was found. Based on this result, we diagnosed the patient with AFAP. He has been under observation with annual EGD, colonoscopy (CS) and CT.
Discussion
To date, three categories of APC-associated fundic gland polyposis have been reported: classical FAP, AFAP and GAPPS (6). Spirio et al. reported AFAP as a family with few colorectal polyps and an older age at the onset of colon cancer than classical FAP, despite germline variants of APC (11). The reason the phenotype of AFAP is milder than that of FAP is not fully understood. It has been hypothesized that, in AFAP, mutant APC proteins with a mutation on the 5′ side of the APC gene can degrade β-catenin. In contrast, the variant APC proteins with 3′ variants found in AFAP families are partially functional, so the accumulation of intracellular β-catenin may be milder than in FAP. It has also been presumed that those 3′ and 5′ variants of APC are associated with a less dominant negative effect, which may be responsible for the phenotypic differences (12).
Burt et al. reported that the median number of colorectal polyps in AFAP was 25, with a range of 0 to 470 (13). However, the number of colorectal polyps in patients with FAP varies greatly with the colonoscopic procedure. It therefore seems possible that the actual number of colorectal polyps in patients diagnosed with AFAP may be greater than reported. However, as found in our patient, some cases of AFAP have an extraordinarily small number of colorectal polyps and desmoid tumors, most of which had 3′ variants of APC located distal to codon 1595 (14-16). In fact, our patient carried a variant at codon 1928. Prominent gastric fundic gland polyposis with very few colorectal adenomas may therefore be a specific clinical manifestation of AFAP due to 3′ variants of APC.
GAPPS is another condition that should be distinguished from AFAP. It is an autosomal dominant genetic disorder with variants in promoter 1B of the APC gene that cause gastric cancer to arise from fundic gland polyposis (10,17,18). The diagnostic criteria for GAPPS are dense gastric fundic gland polyposis without polyposis in the duodenum and colon, more than 30 gastric polyps in first-degree relatives, and pathologically verified dysplasia in fundic gland polyposis (9). In addition, Worthley et al. and McDuffie et al. reported that patients genetically diagnosed with GAPPS had 0-16 colorectal adenomas, suggesting difficulty distinguishing AFAP from GAPPS by clinical manifestations alone (10,19).
Neither the Japanese nor American guidelines (20,21) include any specific comments on APC gene testing for the differentiation of AFAP and GAPPS. However, we believe that AFAP and GAPPS should be differentiated genetically because of differences in the risk of gastric cancer (21,22). Because GAPPS is characterized by variants in the promoter region of APC, routine gene testing may fail to identify variants in APC in patients with the disease. Patients with profuse gastric polyposis without apparent APC mutations are therefore appropriate candidates for the investigation of the APC promoter region.
Conclusion
Our experience suggests that, in addition to FAP, AFAP should be considered seriously in patients with profuse gastric polyposis. Clinical guidelines for the clear distinction of AFAP from GAPPS must be established, because both conditions are characterized by no or very few colorectal adenomas.
notes
As this paper is a single case report, it has not been approved by the Ethics Committee. Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Genta RMG. Fundic gland polyps. WHO Classification of Tumours. In: Digestive System Tumours. 5th ed. Lyon, 2019: 67-68. [Google Scholar]
- 2. Burt R. Gastric fundic gland polyps. Gastroenterology 125: 1462-1469, 2003. [DOI] [PubMed] [Google Scholar]
- 3. Spiegel A, Stein P, Patel M, Patel R, Lebovics E. A report of gastric fundic gland polyps. Gastroenterol Hepatol (N Y) 6: 45-48, 2010. [PMC free article] [PubMed] [Google Scholar]
- 4. Lam SK, Lau GKK. Idiopathic multitudinous fundic gland polyposis: a new disease and a management dilemma. JGH Open 5: 525-527, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jalving M, Koornstra JJ, Wesseling J, Boezen HM, De Jong S, Kleibeuker JH. Increased risk of fundic gland polyps during long‐term proton pump inhibitor therapy. Aliment Pharmacol Ther 24: 1341-1348, 2006. [DOI] [PubMed] [Google Scholar]
- 6. Mahachai V, Graham DY, Odze RD. Gastric polyps [Internet]. 2022 [cited 2022 Nov 1]. Available from: https://www.uptodate.com/contents/gastric-polyps/print.
- 7. Abraham SC, Nobukawa B, Giardiello FM, Hamilton SR, Wu TT. Fundic gland polyps in familial adenomatous polyposis: neoplasms with frequent somatic adenomatous polyposis coli gene alterations. Am J Pathol 157: 747-754, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leone PJ, Mankaney G, Sarvapelli S, et al. Endoscopic and histologic features associated with gastric cancer in familial adenomatous polyposis. Gastrointest Endosc 89: 961-968, 2019. [DOI] [PubMed] [Google Scholar]
- 9. Yanaru-Fujisawa R, Nakamura S, Moriyama T, et al. Familial fundic gland polyposis with gastric cancer. Gut 61: 1103-1104, 2012. [DOI] [PubMed] [Google Scholar]
- 10. Worthley DL, Phillips KD, Wayte N, et al. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS): a new autosomal dominant syndrome. Gut 61: 774-779, 2012. [DOI] [PubMed] [Google Scholar]
- 11. Spirio L, Otterud B, Stauffer D, et al. Linkage of a variant or attenuated form of adenomatous polyposis coli to the adenomatous polyposis coli (APC) locus. Am J Hum Genet 51: 92-100, 1992. [PMC free article] [PubMed] [Google Scholar]
- 12. Su LK, Barnes CJ, Yao W, Qi Y, Lynch PM, Steinbach G. Inactivation of germline mutant APC alleles by attenuated somatic mutations: a molecular genetic mechanism for attenuated familial adenomatous polyposis. Am J Hum Genet 67: 582-590, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burt RW, Leppert MF, Slattery ML, et al. Genetic testing and phenotype in a large kindred with attenuated familial adenomatous polyposis. Gastroenterology 127: 444-451, 2004. [DOI] [PubMed] [Google Scholar]
- 14. Nieuwenhuis MH, Vasen HF. Correlations between mutation site in APC and phenotype of familial adenomatous polyposis (FAP): a review of the literature. Crit Rev Oncol Hematol 61: 153-161, 2007. [DOI] [PubMed] [Google Scholar]
- 15. Hernegger GS, Moore HG, Guillem JG. Attenuated familial adenomatous polyposis: an evolving and poorly understood entity. Dis Colon Rectum 45: 127-134, 2002. [DOI] [PubMed] [Google Scholar]
- 16. Leoz ML, Carballal S, Moreira L, Ocaña T, Balaguer F. The genetic basis of familial adenomatous polyposis and its implications for clinical practice and risk management. Appl Clin Genet 8: 95-107, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li J, Woods SL, Healey S, et al. Point mutations in exon 1B of APC reveal gastric adenocarcinoma and proximal polyposis of the stomach as a familial adenomatous polyposis variant. Am J Hum Genet 98: 830-842, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Repak R, Kohoutova D, Podhola M, et al. The first European family with gastric adenocarcinoma and proximal polyposis of the stomach: case report and review of the literature. Gastrointest Endosc 84: 718-725, 2016. [DOI] [PubMed] [Google Scholar]
- 19. McDuffie LA, Sabesan A, Allgäeuer M, et al. β-catenin activation in fundic gland polyps, gastric cancer and colonic polyps in families afflicted by ‘gastric adenocarcinoma and proximal polyposis of the stomach' (GAPPS). J Clin Pathol 69: 826-833, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tomita N, Ishida H, Tanakaya K, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2020 for the clinical practice of hereditary colorectal cancer. Int J Clin Oncol 26: 1353-1419, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Syngal S, Brand RE, Church JM, et al. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 110: 223-262, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Foretová L, Navrátilová M, Svoboda M, et al. GAPPS - gastric adenocarcinoma and proximal polyposis of the stomach syndrome in 8 families tested at Masaryk Memorial Cancer Institute - prevention and prophylactic gastrectomies. Klin Onkol 32: 109-117, 2019. [DOI] [PubMed] [Google Scholar]