Abstract
Objective
The treatment background, as well as the frequency and type of complications, in autologous (auto-) and allogeneic (allo-) hematopoietic stem cell transplantation (HSCT) survivors influence the appearance of moderate to vigorous physical activity (MVPA) or sedentary behavior. We therefore assessed differences in the MVPA and sedentary behavior between auto- and allo-HSCT survivors.
Methods
This prospective observational study included 13 auto- and 36 allo-HSCT survivors (approximately 4 years after HSCT). The MVPA and sedentary behavior were assessed using a triaxial accelerometer.
Results
There were no significant between-group differences in the MVPA or sedentary behavior (p=0.768 and 0.739, respectively). In allo-HSCT survivors, the MVPA was negatively correlated with the Hospital Anxiety and Depression Scale score (r=-0.358, p=0.032). A stepwise multiple regression analysis showed that age was a significant predictor of sedentary behavior in allo-HSCT survivors (β=0.400, p=0.016).
Conclusion
We observed no significant between-group differences in the MVPA or sedentary behavior. Our results suggest that it may be unnecessary to change the rehabilitation program according to the donor type in interventions for promoting MVPA and reducing sedentary behavior in long-term HSCT survivors.
Keywords: allogeneic hematopoietic stem cell transplantation, autologous hematopoietic stem cell transplantation, physical activity, sedentary behavior
Introduction
There has been a recent increase in the number of hematopoietic stem cell transplantation (HSCT) procedures in Japan (1). In addition, there have been improvements in conditioning, immunosuppressive drugs, and supportive care for complications, with an increased long-term survival noted (2,3). However, there is a high risk of mortality from late complications during the first five years after HSCT.
The causes of late non-relapse mortality within two years after HSCT include infectious diseases, respiratory complications, secondary malignancies, and organ disorders (4). Late complications may reduce the quality of life (QOL) in survivors after HSCT. For cancer survivors other than those receiving HSCT, there has been an annual increase in the relative five-year survival rate due to advances in the early detection and treatment of diseases, with a concomitant increase in the number of patients returning to society. A Japanese study that conducted a follow-up of cases between 2009 and 2011 reported a relative 5-year survival rate of ≥60% for both men and women (5).
Given the increasing number of cancer survivors, there has been extensive research on physical activity, including moderate to vigorous physical activity (MVPA), and sedentary behavior in cancer survivors. Previous studies on cancer survivors, including survivors of breast and colorectal cancer, have reported correlations between the MVPA and the overall survival (OS), QOL, and treatment-related adverse effects (6-8). Furthermore, among cancer survivors, increased sedentary behavior is associated with an increased risk of mortality (9), the development of ischemic heart disease (10), and a decreased QOL (11).
Sedentary behavior is defined as waking behavior characterized by an energy expenditure of ≤1.5 metabolic equivalents (METs) while in a sitting or reclining posture (12). Studies in cancer survivors have examined the correlation of MVPA and sedentary behavior assessed using an accelerometer, which can objectively measure physical activity; however, few studies have objectively measured the physical activity in HSCT survivors. While several studies have investigated physical activity using questionnaires (13-15), measurement errors are likely to occur with this self-report method (16-18).
No studies have compared the MVPA and sedentary behavior between autologous (auto-) and allogeneic (allo-) HSCT survivors. Differences in treatment background as well as the frequency and type of complications between auto- and allo-HSCT survivors may result in differences in the MVPA and sedentary behavior. The present study therefore assessed the differences in the MVPA and sedentary behavior between auto- and allo-HSCT survivors.
Materials and Methods
Study design
This prospective observational study investigated whether or not there were differences in the MVPA and sedentary behavior between auto- and allo-HSCT survivors. In addition, we investigated the associations of the MVPA and sedentary behavior with demographics and clinical characteristics, the physical function, psychological health, and the QOL.
Participants
We included patients who underwent auto- or allo-HSCT (≥18 years old) at the Hamamatsu Medical Center in Japan, visited an outpatient department of hematology from March 2019 to March 2020, and were cognitively capable of handling accelerometers. We excluded patients who relapsed after HSCT or those who received treatment for recurring cancer. This study was approved by the Hamamatsu Medical Center Institutional Committee on Human Research. All participants provided their written informed consent before the study.
We collected the following information from the patients' medical records: age, sex, hematological diagnosis, stem cell source, donor type, clinical laboratory data, comorbidity, time elapsed after HSCT, acute and chronic graft-versus-host disease (GVHD), presence of comorbidity, corticosteroid dose, and ongoing maintenance treatment.
Measurements
•MVPA and sedentary behavior
We assessed the MVPA and sedentary behavior using a triaxial accelerometer (HJA-750C, Active style Pro; Omron Healthcare, Kyoto, Japan). The reliability and validity of the accelerometer have been previously confirmed (19). We investigated MVPA (≥3 METs) and sedentary behavior (≤1.5 METs); furthermore, we calculated the daily MVPA and sedentary behavior. The patients were instructed to wear the accelerometer on an elastic waistband for seven consecutive days, from their waking time in the morning to bedtime at night, with the accelerometer being removed during bathing. We used data on days when the accelerometer was worn for ≥10 hours for ≥4 days. Non-wear time was defined as an interval of ≥60 consecutive minutes of zero counts (20). We performed MET-based classification of the physical activity using the manufacturer-provided software program (HMS-HJA-IC01J; Omron Healthcare).
•The physical function
We used handgrip strength (kg) as an index of upper limb strength, which was evaluated using a standard adjustable-handle dynamometer (TKK5101; Takei Scientific Instruments, Niigata, Japan). Measurements were conducted twice using both hands, with the highest value being selected for the analysis. The knee extensor muscle strength (kg/kgwt) was measured as an index of lower limb strength using a handheld dynamometer (HHD; mobile MT-100; SAKAImed, Tokyo, Japan). All sessions used the same HHD equipped with a stabilizing belt to facilitate resistance application by the tester. We examined the knee extension force with the patients sitting with their knee flexed to approximately 60°. We used a dynamometer to the anterior surface of the tibia proximal to the malleoli. We recorded the maximum force developed during a 10-second static effort. Triplicate measurements were bilaterally recorded, with the highest value being used for analysis. The exercise capacity was evaluated using the six-minute walking test (6 MWT) following the guidelines of the American Thoracic Society (21). Specifically, patients walked at their own pace along a 20-m corridor for 6 minutes, with the distance traveled (in meters) during this period being measured.
•The QOL
The QOL was assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core-30 (EORTC QLQ C-30) version 3.0. This self-administered questionnaire incorporated five functional scales, nine symptom scales, and global health status. All item scores were transformed into values from 0 to 100. The values were positively correlated with the functional and health levels in functional scales, QOL in the global health status, and presence of symptoms in the symptom scales. The EORTC QLQ is valid and reliable for patients with cancer worldwide (22); furthermore, the reliability and validity of the Japanese-translated questionnaire have been confirmed (23).
•Psychological health
Psychological health was assessed using the Hospital Anxiety and Depression Scale (HADS), which assesses the frequency of depressive status (7 items) and anxiety (7 items) over the past week from 0 (not at all) to 3 (most of the time). Positively worded items were reverse-scored. A higher score indicated greater symptomology. The Japanese version of the HADS has been previously validated (24).
Statistical analyses
The Shapiro-Wilk test was used to evaluate the data distribution. Between-group comparisons of continuous variables were assessed using unpaired t-tests and Mann-Whitney U tests. Between-group comparisons of categorical variables were assessed using chi-squared tests. We calculated Pearson's product-moment correlation coefficients and Spearman's rank correlation coefficients to evaluate the relationships of MVPA and sedentary behavior with demographic and clinical characteristics. To identify variables independently associated with the MVPA and sedentary behavior in the included patients, we performed a stepwise multiple regression analysis. Factors correlated with the MVPA and sedentary behavior were considered to be predictors of the MVPA and sedentary behavior in auto- and allo-HSCT survivors.
Statistical analyses were performed using the SPSS 24.0 J software program (SPSS Japan, Tokyo, Japan). Statistical significance was set at p<0.05.
Results
Participants
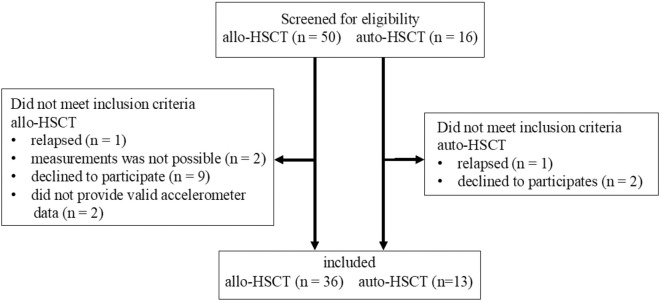
We screened 50 allo- and 16 auto-HSCT survivors (Figure). Among them, 14 allo- and 3 auto-HSCT survivors dropped out of the study, with the reasons for dropping out being noted. We ultimately included and compared 36 allo- and 13 auto-HSCT patients. Table 1 shows the sample demographic and clinical characteristics. The mean ages of allo- and auto-HSCT survivors were 49.4 and 54.8 years old, respectively, and 41.7% and 61.5% of the participants were men, respectively. The hematological diagnoses included acute leukemia (55.5%), myelodysplastic syndrome (25.0%), malignant lymphoma (5.6%), and others (13.9%). Among auto-HSCT survivors, seven and six patients had multiple myeloma and malignant lymphoma, respectively. Among allo- and auto-HSCT survivors, the mean time elapsed after HSCT was 49.5 months (4.1 years) and 43.0 months (3.6 years), respectively. The proportion of comorbidities, including late complications, was 41.7% and 38.5% in allo- and auto-HSCT survivors, respectively. In addition, 15 (41.7%) and 8 (22.2%) patients had a history of and current chronic GVHD, respectively. Five patients (38.5%) with multiple myeloma following auto-HSCT were placed on maintenance treatment. There were no significant between-group differences in demographic or clinical characteristics.
Figure.
Study flow diagram.
Table 1.
Demographic and Clinical Characteristics of Auto- and Allo-HSCT Survivors.
| Allo-HSCT (n=36) | Auto-HSCT (n=13) | p | |
|---|---|---|---|
| Age, years | 49.4 (12.8) | 54.8 (11.5) | 0.197 |
| Female/male | 21 (58.7%)/15 (41.7%) | 5 (38.5%)/8 (61.5%) | 0.218 |
| Time elapsed from HSCT | 49.5 (8-199) | 43 (1-121) | 0.150 |
| Diagnosis | |||
| Acute leukemia | 20 (55.5%) | 0 | |
| Myelodysplastic syndrome | 9 (25.0%) | 0 | |
| Multiple myeloma | 0 | 7 (53.8%) | |
| Malignant lymphoma | 2 (5.6%) | 6 (46.2%) | |
| Other | 5 (13.9%) | 0 | |
| Clinical laboratory data | |||
| CRP (mg/dL) | 0.1 (±0.1) | 0.1 (±0.1) | 0.658 |
| Albumin (g/dL) | 4.1 (±0.3) | 4.3 (±0.2) | 0.189 |
| Total protein (g/dL) | 6.7 (±0.4) | 6.7 (±0.5) | 0.695 |
| Hemoglobin (g/dL) | 12.7 (±1.3) | 12.5 (±2.2) | 0.76 |
| White cell (number, 103/μL) | 5.6 (±1.9) | 4.7 (±1.9) | 0.138 |
| Neutrophils (number, 103/μL) | 3.1 (±1.4) | 2.3 (±1.3) | 0.081 |
| Lymphocytes (number, 103/μL) | 1.9 (±0.8) | 1.9 (±0.7) | 0.859 |
| Platelet (number, 103/μL) | 205.0 (±55.8) | 184.7 (±53.5) | 0.271 |
| Comorbidity | 0.84 | ||
| None | 21 (58.3%) | 8 (61.5%) | |
| Yes | 15 (41.7%) | 5 (38.5%) | |
| Stem cell source | |||
| Bone marrow | 23 (63.9%) | NA | |
| Cord blood | 9 (25.0%) | NA | |
| Peripheral blood stem cell | 4 (11.1%) | 13 (100%) | |
| Donor type | NA | ||
| HLA-mismatched/unrelated | 13 (36.1%) | ||
| HLA-matched/related | 11 (30.6%) | ||
| HLA-matched/unrelated | 10 (27.8%) | ||
| HLA-mismatched/related | 1 (2.8%) | ||
| History of aGVHD | NA | ||
| Yes | 26 (72.2%) | ||
| No | 10 (27.8%) | ||
| cGVHD at survey | NA | ||
| Yes | 8 (22.2%) | ||
| No | 28 (77.8%) | ||
| History of cGVHD | NA | ||
| Yes | 15 (41.7%) | ||
| No | 21 (58.3%) | ||
| On maintenance treatment | NA | ||
| Yes | 5 (38.5%) | ||
| No | 8 (61.5%) |
Values are mean±SD, n (%), or median (range). HSCT: hematopoietic stem cell transplantation, SD: standard deviation, CRP: C-reactive protein, NA: not applicable, HLA: human leukocyte antigen, GVHD: graft versus host disease
Between-group comparisons of the MVPA, sedentary behavior, physical function, psychological health, and QOL
Table 2 shows the between-group comparison of the MVPA, sedentary behavior, physical function, psychological health, and QOL. There were no significant between-group differences in the MVPA and sedentary behavior p=0.768 and 0.739, respectively). The mean duration of accelerometer wear time in allo- and auto-HSCT survivors was 903.2 and 870.8 min/day, respectively. Allo-HSCT survivors had 539.1 minutes of sedentary behavior per day [9.0 hours; standard deviation (SD)=106.9 minutes] and performed 40.4 minutes of MVPA per day (SD=32.8 minutes). Contrastingly, auto-HSCT survivors had 526.7 minutes of sedentary behavior per day (8.8 hours; SD=123.0 minutes) and performed 34.9 minutes of MVPA per day. There was a significant between-group difference in dyspnea, which is the symptom scale of the EORTC QLQ C-30 p=0.011). There were no significant between-group differences in the other measurements.
Table 2.
Between-group Comparison of Measurements.
| Variables | Allo-HSCT | Auto-HSCT | p |
|---|---|---|---|
| Mean (SD) | |||
| Accelerometer wear time, min/day | 903.2±79.5 | 870.8±97.7 | 0.254 |
| MVPA, min/day | 40.4 (32.8) | 34.9 (26.5) | 0.768 |
| Sedentary behavior, min/day | 539.1 (106.9) | 526.7 (123.0) | 0.739 |
| Handgrip, kg | 29.7 (9.5) | 33.0 (8.9) | 0.262 |
| Knee extension force, kgf/kgw | 0.53 (0.16) | 0.58 (0.18) | 0.460 |
| 6MWT, m | 481.7 (60.7) | 457.9 (73.4) | 0.270 |
| HADS | |||
| Anxiety | 3.2 (2.9) | 3.9 (3.6) | 0.521 |
| Depression | 4.3 (3.0) | 5 (2.2) | 0.264 |
| EORTC QLQ C-30 | |||
| Global health status | 78.9 (15.7) | 69.1 (20.3) | 0.208 |
| Physical function | 87.8 (14.5) | 86.2 (12.5) | 0.473 |
| Role function | 88.5 (22.4) | 88.5 (17.7) | 0.798 |
| Emotional function | 90.6 (13.1) | 90.6 (11.4) | 0.718 |
| Cognitive function | 82.9 (19.7) | 85.8 (8.9) | 0.838 |
| Social function | 87.2 (24.1) | 89.7 (19.2) | 0.977 |
| Fatigue | 27.7 (19.7) | 27.8 (21.3) | 0.898 |
| Nausea/vomiting | 2.8 (7.4) | 0 | 0.161 |
| Pain | 15.3 (20.1) | 16.6 (27.7) | 0.738 |
| Dyspnea | 7.4 (19.4) | 20.4 (20.8) | 0.011* |
| Insomnia | 14.7 (21.3) | 10.2 (15.2) | 0.571 |
| Appetite loss | 12.0 (19.5) | 12.8±20.8 | 0.955 |
| Constipation | 10.1 (17.2) | 23±30.3 | 0.164 |
| Diarrhea | 13.8 (21.2) | 10.2±15.2 | 0.692 |
| Financial difficulties | 19.4 (31.8) | 12.7±16.1 | 0.936 |
MVPA: moderate-to-vigorous intensity physical activity, 6MWT: six minutes walking test, HADS: Hospital Anxiety and Depression Scale, EORTC QLQ C-30: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core-30
*p<0.05
On a comparison of MVPA and sedentary behavior between allo-HSCT survivors with or without chronic GVHD in a survey, a history of chronic GVHD, or undergoing maintenance treatment, there was no significant difference between the MVPA or sedentary behavior in all comparisons (p=0.985 and 0.243, 0.835 and 0.565, 0.488 and 0.919 respectively).
Associations of the MVPA and sedentary behavior with demographic and clinical characteristics, the physical function, psychological health, and the QOL among auto- and allo-HSCT survivors
Table 3 shows the associations of MVPA and sedentary behavior with demographic and clinical characteristics, the physical function, psychological health, and the QOL in allo-HSCT survivors. There was a negative correlation between MVPA and HADS (depression) in allo-HSCT survivors (r=-0.358, p=0.032). Similarly, there was a negative correlation of sedentary behavior with HADS (anxiety) (r=-0.408, p=0.014), age (r=0.400, p=0.016), and EORTC QLQ-C30 (emotion) (r=0.356, p=0.033) in allo-HSCT survivors. In contrast, the MVPA and sedentary behavior were not associated with demographic or clinical characteristics or measurements in auto-HSCT survivors (results not shown). A stepwise multiple regression analysis revealed that age was a significant predictor of sedentary behavior (β=0.400, p=0.016, Table 4).
Table 3.
Associations of the MVAP and Sedentary Behavior with Demographic and Clinical Characteristics, the Physical Function, Psychological Health, and the QOL in Allo-HSCT Survivors.
| Variables | MVPA | sedentary behavior | ||
|---|---|---|---|---|
| Correlation coefficient (r) | p | Correlation coefficient (r) | p | |
| Age | -0.194 | 0.099 | 0.400 | 0.016* |
| BMI | 0.035 | 0.764 | 0.212 | 0.214 |
| Comorbidity | -0.051 | 0.707 | 0.211 | 0.217 |
| Time elapsed from HSCT | 0.166 | 0.156 | -0.160 | 0.352 |
| Handgrip | 0.025 | 0.827 | 0.205 | 0.230 |
| Knee extension force | 0.035 | 0.764 | 0.269 | 0.112 |
| 6MWT | 0.013 | 0.913 | -0.16 | 0.927 |
| HADS (anxiety) | 0.084 | 0.490 | -0.408 | 0.014* |
| HADS (depression) | -0.358 | 0.032* | 0.071 | 0.680 |
| EORTC QLQ-C30 | ||||
| Global health status | 0.053 | 0.667 | 0.075 | 0.665 |
| Physical function | 0.079 | 0.529 | 0.055 | 0.749 |
| Role function | 0.042 | 0.755 | -0.171 | 0.319 |
| Emotional function | -0.057 | 0.661 | 0.356 | 0.033 |
| Cognitive function | 0.079 | 0.544 | 0.110 | 0.521 |
| Social function | -0.021 | 0.876 | -0.106 | 0.539 |
| Fatigue | -0.062 | 0.621 | -0.301 | 0.075 |
| Nausea/vomiting | 0.022 | 0.873 | -0.173 | 0.314 |
| Pain | -0.066 | 0.615 | 0.018 | 0.919 |
| Dyspnea | 0.059 | 0.672 | 0.278 | 0.100 |
| Insomnia | 0.040 | 0.772 | -0.277 | 0.102 |
| Appetite loss | -0.226 | 0.099 | 0.327 | 0.052 |
| Constipation | -0.112 | 0.419 | 0.025 | 0.886 |
| Diarrhea | -0.177 | 0.202 | 0.165 | 0.355 |
| Financial difficulties | -0.037 | 0.781 | 0.148 | 0.388 |
MVPA: moderate-to-vigorous intensity physical activity, BMI: body mass index, HSCT: hematopoietic stem cell transplantation, 6MWT: six minutes walking test, HADS: Hospital Anxiety and Depression Scale, EORTC QLQ C-30: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core-30
*p<0.05
Table 4.
A Stepwise Multiple Regression Analysis of Predictive Factors for Sedentary Behavior in Allo-HSCT Survivors.
| Variables | β | 95%CI | p |
|---|---|---|---|
| Age | 0.400 | 0.673-6.023 | 0.016* |
| HADS (anxiety) | -0.311 | -22.828-0.201 | 0.054 |
CI: confidence interval, HADS: Hospital Anxiety and Depression Scale
*p<0.05
Discussion
This is the first study to compare objectively measured MVPA and sedentary behavior among auto- and allo-HSCT survivors. We observed no significant between-group differences in the MVPA and sedentary behavior. In addition, we found that depression and age were associated with the MVPA and sedentary behavior, respectively, in allo-HSCT survivors. The lack of a between-group difference in the MVPA and sedentary behavior may be due to the low prevalence of chronic GVHD among allo-HSCT survivors, as well as the lack of a between-group difference in the prevalence of complications. The cumulative incidence of chronic GVHD among allo-HSCT survivors was reported to be 47% (25); in addition, the incidence of chronic GVHD at 2 years after allo-HSCT in Japan was 37% (26). Furthermore, HSCT survivors with chronic GVHD have lower physical and social functioning than those without chronic GVHD (27).
Consistent with previous findings, the physical function may be decreased due to the effects of chronic GVHD and late complications, resulting in an increase and decrease in sedentary behavior and MVPA, respectively. The incidence of chronic GVHD was 41.7%, which was consistent with previous findings; however, there was a low prevalence during the survey. In our study, the mean time elapsed after HSCT was approximately four years, and the population was considered to have a low risk of developing chronic GVHD. In contrast, a previous study found that HSCT survivors with three or more late complications had a reduced physical function, restricted daily activities, and decreased full-time employment compared with HSCT survivors without late complications (28).
The cumulative incidence of late complications without malignancies at 5 years after HSCT was 44.8% and 79% among auto- and allo-HSCT patients, respectively (28). A previous study on chronic health disorders in survivors approximately 7 years after HSCT reported that the proportion of survivors with health disorders was 71% and 61% after allo-HSCT and auto-HSCT, respectively. Furthermore, allo-HSCT survivors had a significantly higher rate of health problems than auto-HSCT survivors (29). In our study, the proportion of complications, including late complications, was 38.5% and 41.7% among auto- and allo-HSCT survivors, respectively, with no significant between-group difference in the proportion of comorbidities. In addition, we observed no significant difference in the MVPA or sedentary behavior between allo-HSCT survivors with and without chronic GVHD on a survey, a history of chronic GVHD, or maintenance treatment.
As the number of long-term survivors after HSCT continues to increase, the importance of managing chronic GVHD and other late complications in long-term survivors is becoming increasingly important globally. Screening and prevention guidelines for late complications were developed by the Center for International Blood and Marrow Transplant Research, European Group for Blood and Marrow Transplantation, and American Society for Bone Marrow Transplantation in 2006 (30), and a revised version was published in 2012 in collaboration with other international HSCT societies (31). Consequently, a long-term follow-up system has been established in recent years facilitating appropriate medical management and supportive care for these patients. This has led to the alleviation of symptoms of chronic GVHD, and it is believed that there were minimal effects on the MVPA and sedentary behavior.
Previous meta-analyses have reported that maintenance treatment with lenalidomide following auto-HSCT prolongs not only the progression-free survival but also the overall survival (32). By prolonging the progression-free and overall survival, it is expected that patients' condition will stabilize, and their QOL will be maintained for a long period of time. It is further believed that the decrease in the MVPA and increase in sedentary behavior can be suppressed even with maintenance treatment.
We observed a negative correlation between the MVPA and depression in allo-HSCT survivors. Similarly, previous studies on breast and colorectal cancer survivors reported a negative correlation between the MVPA and depression (33,34). In addition, approximately 15% of auto- and allo-HSCT survivors have moderate-to-severe depression (35). Depression is associated with the severity of chronic GVHD in allo-HSCT survivors (36). Therefore, it is important to check for depression in the long-term follow-up of HSCT survivors. Depression should be considered when applying interventions for promoting MVPA in HSCT survivors who have returned to daily activities and work. Age was also significantly associated with sedentary behavior in allo-HSCT survivors. Previous studies on cancer survivors have reported a positive correlation between age and sedentary behavior (37,38). Similarly, a previous study on healthy adults reported that sedentary behavior tended to increase with age (39). This suggests that age should be considered when developing effective interventions for reducing sedentary behavior.
Several limitations associated with the present study warrant mention. First, the sample size was small; specifically, there was a small number of auto-HSCT survivors. The small number of auto-HSCT survivors may be due to some auto-HSCT survivors being classified as recurrent or intractable cases. Second, there was a large variation in the time elapsed after HSCT. This is because this was a cross-sectional study and there was no opportunity for outpatient visits during this study period, given the decreased frequency of outpatient visits upon stabilization of medical conditions after a long period following HSCT. Third, although we used an accelerometer to objectively measure physical activity, non-walking physical activity (e.g. swimming and cycling) could not be tracked in this way, which may have affected the results. However, in Japan, where medical fees are not granted for outpatient rehabilitation after HSCT, our findings may facilitate the introduction of outpatient rehabilitation after HSCT.
In conclusion, we observed no significant between-group differences in the MVPA and sedentary behavior. Our results suggest that it may be unnecessary to change the rehabilitation program according to the donor type in interventions for promoting MVPA and reducing sedentary behavior in long-term survivors of HSCT. Although most of the results were negative in this study, previous studies in survivors of other cancer types reported benefits of an increased MVPA (6-8) and adverse effects of an increased sedentary behavior (9-11); an objective and quantitative evaluation would also be beneficial for HSCT survivors. Further studies are needed to explore the impact of the MVPA and sedentary behavior on the prognosis and health outcomes and to examine the effects of interventions aimed at promoting MVPA and reducing sedentary behavior in HSCT survivor populations.
The authors state that they have no Conflict of Interest (COI).
References
- 1.The Japanese Data Center for Hematopoietic Cell Transplantation (JDCHCT), the Japan Society for Transplantation and Cellular Therapy (JSTCT). Hematopoietic stem cell transplantation in Japan, a report from a nationwide survey in 2020. Ishoku (J Jpn Soc Transplant) 56: 273-282, 2021. (in Japanese). [Google Scholar]
- 2. Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med 363: 2091-2101, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yoshimi A, Suzuki R, Atsuta Y, et al. Hematopoietic SCT activity in Asia: a report from the Asia-Pacific Blood and Marrow Transplantation Group. Bone Marrow Transplant 45: 1682-1691, 2010. [DOI] [PubMed] [Google Scholar]
- 4. Atsuta Y, Hirakawa A, Nakasone H, et al. Late mortality and causes of death among long-term survivors after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 22: 1702-1709, 2016. [DOI] [PubMed] [Google Scholar]
- 5. Matsuda T, Ajiki W, Marugame T, et al. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol 41: 40-51, 2011. [DOI] [PubMed] [Google Scholar]
- 6. Lynch BM, Cerin E, Owen N, Hawkes AL, Aitken JF. Prospective relationships of physical activity with quality of life among colorectal cancer survivors. J Clin Oncol 26: 4480-4487, 2008. [DOI] [PubMed] [Google Scholar]
- 7. Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol 28: 753-765, 2011. [DOI] [PubMed] [Google Scholar]
- 8. Speck RM, Courneya KS, Mâsse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv 4: 87-100, 2010. [DOI] [PubMed] [Google Scholar]
- 9. Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol 31: 876-885, 2013. [DOI] [PubMed] [Google Scholar]
- 10. Hawkes AL, Lynch BM, Owen N, Aitken JF. Lifestyle factors associated concurrently and prospectively with co-morbid cardiovascular disease in a population-based cohort of colorectal cancer survivors. Eur J Cancer 47: 267-276, 2011. [DOI] [PubMed] [Google Scholar]
- 11. Leger KJ, Baker KS, Cushing-Haugen KL, et al. Lifestyle factors and subsequent ischemic heart disease risk after hematopoietic cell transplantation. Cancer 124: 1507-1515, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sedentary Behaviour Research Network. Letter to the editor: standardized use of the terms “sedentary” and “sedentary behaviours”. Appl Physiol Nutr Metab 37: 540-542, 2012. [DOI] [PubMed] [Google Scholar]
- 13. Bersvendsen HS, Haugnes HS, Fagerli UM, et al. Lifestyle behavior among lymphoma survivors after high-dose therapy with autologous hematopoietic stem cell transplantation, assessed by patient-reported outcomes. Acta Oncol 58: 690-699, 2019. [DOI] [PubMed] [Google Scholar]
- 14. Stenehjem JS, Smeland KB, Murbraech K, et al. Cardiorespiratory fitness in long-term lymphoma survivors after high-dose chemotherapy with autologous stem cell transplantation. Br J Cancer 115: 178-187, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ainsworth BE, Caspersen CJ, Matthews CE, Mâsse LC, Baranowski T, Zhu W. Recommendations to improve the accuracy of estimates of physical activity derived from self report. J Phys Act Health 9: S76-S84, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Douma JAJ, de Beaufort MB, Kampshoff CS, et al. Physical activity in patients with cancer: self-report versus accelerometer assessments. Support Care Cancer 28: 3701-3709, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Skender S, Ose J, Chang-Claude J, et al. Accelerometry and physical activity questionnaires - a systematic review. BMC Public Health 16: 515, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lynch BM, Cerin E, Owen N, Hawkes AL, Aitken JF. Television viewing time of colorectal cancer survivors is associated prospectively with quality of life. Cancer Causes Control 22: 1111-1120, 2011. [DOI] [PubMed] [Google Scholar]
- 19. Ohkawara K, Oshima Y, Hikihara Y, Ishikawa-Takata K, Tabata I, Tanaka S. Real-time estimation of daily physical activity intensity by a triaxial accelerometer and a gravity-removal classification algorithm. Br J Nutr 105: 1681-1691, 2011. [DOI] [PubMed] [Google Scholar]
- 20. Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003-2004. Am J Epidemiol 167: 875-881, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 166: 111-117, 2002. [DOI] [PubMed] [Google Scholar]
- 22. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85: 365-376, 1993. [DOI] [PubMed] [Google Scholar]
- 23. Kobayashi K, Takeda F, Teramukai S, et al. A cross-validation of the European Organization for Research and Treatment of Cancer QLQ-C30 (EORTC QLQ-C30) for Japanese with lung cancer. Eur J Cancer 34: 810-815, 1998. [DOI] [PubMed] [Google Scholar]
- 24. Motegi T, Watanabe Y, Fukui N, et al. Depression, anxiety and primiparity are negatively associated with mother-infant bonding in Japanese mothers. Neuropsychiatr Dis Treat 16: 3117-3122, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arora M, Cutler CS, Jagasia MH, et al. Late acute and chronic graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 22: 449-455, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kanda J, Nakasone H, Atsuta Y, et al. Risk factors and organ involvement of chronic GVHD in Japan. Bone Marrow Transplant 49: 228-235, 2014. [DOI] [PubMed] [Google Scholar]
- 27. Mitchell SA, Leidy NK, Mooney KH, et al. Determinants of functional performance in long-term survivors of allogeneic hematopoietic stem cell transplantation with chronic graft-versus-host disease (cGVHD). Bone Marrow Transplant 45: 762-769, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khera N, Storer B, Flowers ME, et al. Nonmalignant late effects and compromised functional status in survivors of hematopoietic cell transplantation. J Clin Oncol 30: 71-77, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun CL, Francisco L, Kawashima T, et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the bone marrow transplant survivor study. Blood 116: 3129-3139; quiz 3377, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rizzo JD, Wingard JR, Tichelli A, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, the Center for International Blood and Marrow Transplant Research, and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant 12: 138-151, 2006. [DOI] [PubMed] [Google Scholar]
- 31. Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant 18: 348-371, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol 35: 3279-3289, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brunet J, O'Loughlin JL, Gunnell KE, Sabiston CM. Physical activity and depressive symptoms after breast cancer: cross-sectional and longitudinal relationships. Health Psychol 37: 14-23, 2018. [DOI] [PubMed] [Google Scholar]
- 34. van Putten M, Husson O, Mols F, Luyer MDP, van de Poll-Franse LV, Ezendam NPM. Correlates of physical activity among colorectal cancer survivors: results from the longitudinal population-based profiles registry. Support Care Cancer 24: 573-583, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mosher CE, DuHamel KN, Rini C, Corner G, Lam J, Redd WH. Quality of life concerns and depression among hematopoietic stem cell transplant survivors. Support Care Cancer 19: 1357-1365, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jim HS, Sutton SK, Jacobsen PB, Martin PJ, Flowers ME, Lee SJ. Risk factors for depression and fatigue among survivors of hematopoietic cell transplantation. Cancer 122: 1290-1297, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thraen-Borowski KM, Gennuso KP, Cadmus-Bertram L. Accelerometer-derived physical activity and sedentary time by cancer type in the United States. PLOS ONE 12: e0182554, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sweegers MG, Boyle T, Vallance JK, et al. Which cancer survivors are at risk for a physically inactive and sedentary lifestyle? Results from pooled accelerometer data of 1447 cancer survivors. Int J Behav Nutr Phys Act 16: 66, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Healy GN, Clark BK, Winkler EA, Gardiner PA, Brown WJ, Matthews CE. Measurement of adults' sedentary time in population-based studies. Am J Prev Med 41: 216-227, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]