Abstract
A 46-year-old woman was referred for hypertension and a right adrenal tumor. Primary aldosteronism (PA) was suspected because of the high plasma aldosterone concentration-to-plasma renin activity ratio. However, a subsequent evaluation revealed coexistent PA and pheochromocytoma. We performed laparoscopic right adrenalectomy. Histology of the resected adrenal gland confirmed pheochromocytoma and multiple aldosterone-producing adrenocortical micronodules. Following adrenalectomy, the urinary catecholamine levels normalized, and hyperaldosteronism improved but persisted. Hypertension also improved but persisted and was normalized with spironolactone. The clinical course indicated that the PA lesions were likely bilateral. This was a histologically proven case of coexistent pheochromocytoma and PA due to multiple aldosterone-producing micronodules.
Keywords: pheochromocytoma, primary aldosteronism, multiple aldosterone-producing micronodules, CYP11B2, coexistence
Introduction
Primary aldosteronism (PA) is one of the most frequent causes of secondary hypertension, with a reported prevalence of 5-10% in hypertensive patients (1). In contrast, pheochromocytoma is a relatively rare adrenal tumor that may be potentially lethal if left undiagnosed. Therefore, the clinical evaluation of secondary hypertension must be performed with caution.
The simultaneous occurrence of PA and pheochromocytoma is generally considered extremely rare. This is because these two lesions arise from different origins: adrenocortical cells (PA) and chromaffin cells of the medulla (pheochromocytoma). To our knowledge, very few cases of the coexistence of these two diseases have been reported (2-9).
We herein report a case of the coexistence of PA and pheochromocytoma considered to be present in the ipsilateral adrenal glands. A detailed histological examination of the resected adrenal gland confirmed the presence of pheochromocytoma and multiple aldosterone-producing micronodules.
Case Report
A 46-year-old woman was referred to our endocrinology clinic for the further clinical investigation of hypertension and a right adrenal tumor. She had been healthy and had no medication history prior to presentation. The hypertension had been noted during an annual health checkup three years earlier. When she was admitted for surgical removal of an adenomatous goiter of the thyroid, her blood pressure was 150/95 mmHg, and abdominal computed tomography revealed a right adrenal tumor measuring 20 mm in its greatest dimension (Fig. 1A). Amlodipine, 5 mg, was subsequently administered. She did not have a family history of hypertension or endocrine diseases. When she came to our clinic, her blood pressure was 157/105 mmHg, and her pulse rate was 76 bpm with 5 mg of amlodipine administration.
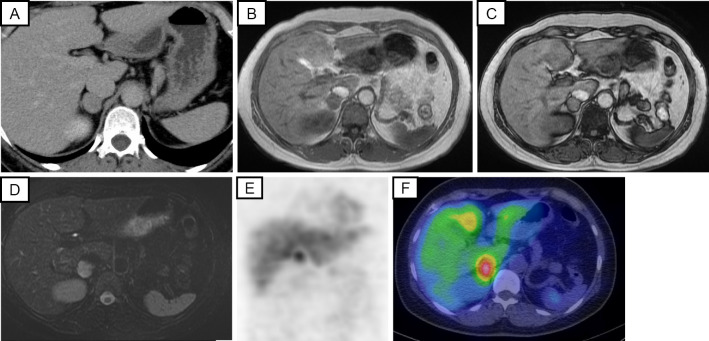
Figure 1.
Radiological findings of the adrenal tumor: (A) abdominal CT, (B) abdominal MRI T1WI (in phase), (C) MRI T1WI (out of phase), (D) MRI T2WI, (E) 123I-MIBG scintigraphy, (F) single-photon emission computed tomography (SPECT). CT: computed tomography, 123I-MIBG: 123I-metaiodobenzylguanidine, MRI: magnetic resonance imaging, T1WI: T1-weighted imaging, T2WI: T2-weighted imaging
The patient's laboratory findings are shown in Table 1. She did not have hypokalemia or renal dysfunction. The plasma aldosterone concentration (PAC) was 139 pg/mL; plasma renin activity (PRA), 0.5 ng/mL/h; and PAC-PRA ratio (ARR), 278 (Table 2). Therefore, clinically, primary aldosteronism was suspected, and a further examination was subsequently performed, which revealed an ARR of 247 after the captopril challenge test (normal: <200), PRA of 1.0 ng/mL/h after the furosemide upright test (normal: >2.0 ng/mL/h), and PAC of 159 pg/mL following the administration of 2 L of saline (normal: <60 pg/mL) (Table 2). All results met the positive criteria in accordance with the guidelines for the diagnosis and treatment of primary aldosteronism of the Japan Endocrine Society (10); therefore, PA was diagnosed.
Table 1.
Laboratory Findings.
| WBC | 4,400 | /μL |
| RBC | 4.43×104 | /μL |
| Hb | 13.1 | g/dL |
| Hct | 39.1 | % |
| Plt | 290×103 | /μL |
| T-Bil | 0.51 | mg/dL |
| AST | 16 | U/L |
| ALT | 11 | U/L |
| ALP | 149 | U/L |
| LDH | 164 | U/L |
| TP | 7.1 | g/dL |
| Albumin | 4.3 | g/dL |
| Na | 142 | mmol/L |
| K | 4.7 | mmol/L |
| Cl | 107 | mmol/L |
| Ca | 9.2 | mg/dL |
| P | 3.1 | mg/dL |
| T-Cho | 157 | mg/dL |
| HDL-C | 52 | mg/dL |
| Triglyceride | 42 | mg/dL |
| BUN | 12.1 | mg/dL |
| Creatinine | 0.8 | mg/dL |
| Glucose | 99 | mg/dL |
| HbA1c | 5.4 | % |
WBC: white blood cell count, RBC: red blood cell count, Hb: hemoglobin, Hct: hematocrit, Plt: platelet count, T-Bil: total bilirubin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, LDH: lactate dehydrogenase, Na: sodium, K: potassium, Cl: chloride, Ca: calcium, P: phosphorous, TP: total protein, T-Cho: total cholesterol, HDL-C: high-density lipoprotein cholesterol, BUN: blood urea nitrogen, HbA1c: hemoglobin A1c
Table 2.
Endocrinological Findings before and after Right Adrenalectomy.
| Test | Before surgery | After 2 months | After 8 months | ||
|---|---|---|---|---|---|
| Resting blood sample | Baseline | Baseline | Baseline | ||
| PAC, pg/mL | 139 | 143 | 104 | ||
| PRA, ng/mL/h | 0.5 | 0.8 | 0.4 | ||
| ARR | 278 | 179 | 259 | ||
| Adrenaline, pg/mL | 38 | ||||
| Noradrenaline, pg/mL | 564 | ||||
| Captopril challenge test | |||||
| Time | Baseline | 90min | Baseline | 90min | |
| PAC, pg/mL | 170 | 148 | 82.1 | 109 | |
| PRA, ng/mL/h | 0.5 | 0.6 | 0.5 | 0.5 | |
| ARR | 340 | 247 | 164 | 218 | |
| Frosemide upright test | |||||
| Time | Baseline | 120min | |||
| PAC, pg/mL | 224 | 541 | |||
| PRA, ng/mL/h | 0.5 | 1 | |||
| Saline infusion test | |||||
| Time | Baseline | 240min | |||
| PAC, pg/mL | 0.7 | 0.7 | |||
| PRA, ng/mL/h | 221 | 159 | |||
| Urinary catecholamine | |||||
| MN, mg/day | 0.76 | 0.1 | |||
| NMN, mg/day | 0.37 | 0.21 | |||
ARR: aldosterone-renin ratio, PAC: plasma aldosterone concentration, PRA: plasma renin activity, MN: metanephrine, NMN: normetanephrine
PAC was measured by radioimmunoassay.
The patient had no symptoms suggestive of catecholamine excess, such as palpitations, headache, or sweating. The plasma adrenaline level was 38 pg/mL (normal: <170 pg/mL), and the noradrenaline level was 564 pg/mL (normal: 150-570 pg/mL) (Table 2). However, magnetic resonance imaging (MRI) revealed a 20-mm right adrenal tumor with low fat content and low intensity on T1-weighted imaging and high intensity on T2-weighted imaging (Fig. 1B-D). However, these MRI findings were more consistent with pheochromocytoma. The urinary fractional metanephrine level with 24-h urine monitoring was 0.76 mg/day (normal: 0.04-0.2 mg/day), and the normetanephrine level was 0.37 mg/day (normal: 0.1-0.3 mg/day) (Table 2). 123I-metaiodobenzylguanidine scintigraphy revealed a marked accumulation in the right adrenal gland (Fig. 1E, F). Therefore, the patient was diagnosed with pheochromocytoma as well as PA.
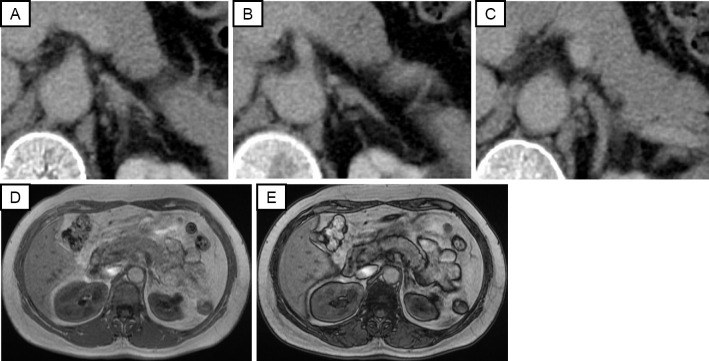
No abnormal findings were detected in the left adrenal gland by computed tomography or MRI (Fig. 2). We performed a 1 mg dexamethasone suppression test with caution (11), and the cortisol concentration was suppressed to 0.1 μg/dL after the test. These hormonal findings led to the diagnosis of the coexistence of PA and pheochromocytoma.
Figure 2.
Radiological findings of the left adrenal gland: (A-C) Three slices of the abdominal CT images; (D) abdominal MRI T1WI (in phase); (E) MRI T1WI (out of phase). CT: computed tomography, MRI: magnetic resonance imaging, T1WI: T1-weighted image
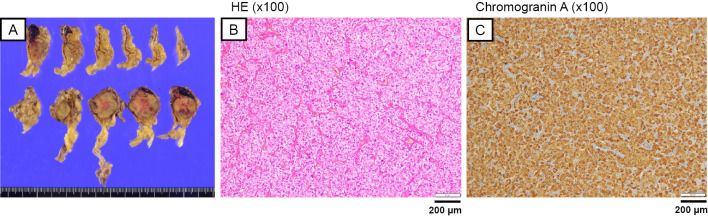
We added doxazosin to the amlodipine, and laparoscopic right adrenalectomy was performed. The resected right adrenal gland contained a 23×20×28-mm brown, well-defined, round, medullary mass containing intratumoral hemorrhaging (Fig. 3A). Hematoxylin and Eosin (HE) staining subsequently revealed that the cells had abundant eosinophilic cytoplasm and that the cells were arranged in an alveolar pattern (Zellballen pattern) with rich vascularity and intratumoral hemorrhaging (Fig. 3B). These tumor cells were diffusely positive for chromogranin A immunoreactivity (Fig. 3C), and the Pheochromocytoma of the Adrenal gland Scaled Score was 1. Therefore, the histopathological diagnosis of the mass lesion was pheochromocytoma.
Figure 3.
Histopathological findings of the resected tumor consistent with pheochromocytoma. (A) Cross section of the adrenal gland showing a 50×40×23-mm brown, well defined, round, lesion; (B) Hematoxylin and Eosin staining of the mass lesion. Cells have abundant eosinophilic cytoplasm and are arranged in an alveolar pattern with rich vascularity (×100); (C) positive staining for chromogranin A (×100).
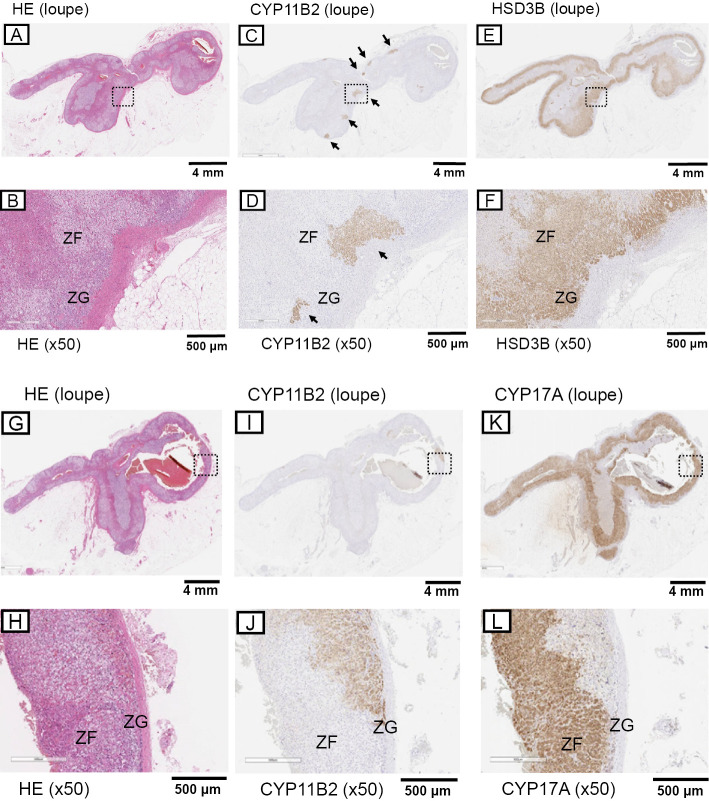
In comparison, there were no significant pathological abnormalities detected in the adrenal cortex with HE staining at ×100 magnification. However, histologically, the zona glomerulosa demonstrated hyperplasia with no CYP11B2 or HSD3β immunoreactivity (Fig. 4B, D, F), which was consistent with the findings of paradoxical hyperplasia associated with aldosterone overproduction (12,13). A subsequent immunohistochemical analysis demonstrated multiple CYP11B2- and HSD3β-positive nodules (measuring up to 1,000 μm) in the zona glomerulosa (Fig. 4C, D), which was consistent with multiple aldosterone-producing micronodules (13,14).
Figure 4.
Histopathological findings of the resected tumor consistent with multiple aldosterone-producing micronodules. (A, B, G, H) Hematoxylin and Eosin staining with low-power magnification and high-power magnification (×50) in the image in the box in (A) and (G) (respectively); (C, D, I, J) CYP11B2 staining with low-power magnification and high-power magnification (×50) in the image in the box in (C) and (I) (respectively). The arrows indicate multiple aldosterone-producing micronodules. (E, F) HSD3B staining with low-power magnification and high-power magnification (×50) in the image in the box in (E); (K, L) CYP17A staining with low-power magnification and high-power magnification (×50) in the image in the box in (K). HSD3B: 3β-hydroxysteroid dehydrogenase, ZF: zona fasciculata, ZG: zona glomerulosa
Two months after adrenalectomy, the urinary fractional metanephrine level on 24-h urine monitoring was 0.10 mg/day, and the normetanephrine level was 0.21 mg/day, suggesting that the catecholamine overproduction had been corrected (Table 2). The ARR was within the normal range, but the PAC was still high (PAC, 143 pg/mL; PRA, 0.8 ng/mL/h; ARR, 179) (Table 2). The patient's blood pressure without medications was 129/83 mmHg 1 month after surgery but gradually increased to 136/94 and 140/90 mmHg at 2 and 3 months following surgery, respectively. Three months following surgery, the captopril challenge test was repeated, indicating an ARR of 218 (normal: <200) (Table 2). Eight months after surgery, PAC was 103.7 pg/mL with a PRA of 0.4 ng/mL/h and ARR of 259. Therefore, we concluded that PA had not been clinically cured and initiated therapy with 25 mg spironolactone, which decreased her blood pressure to 110/70 mmHg. MRI revealed no recurrence two years after the surgery.
Discussion
We presented a rare case of the coexistence of PA and pheochromocytoma, which were present in ipsilateral adrenal glands. PA was clinically diagnosed by endocrinological examinations. The results of three different tests met the criteria for PA; however, the patient's urinary catecholamine levels were high. MRI suggested pheochromocytoma, and 123I-metaiodobenzylguanidine scintigraphy demonstrated an increased uptake in the adrenal tumor. Therefore, the patient was also diagnosed with pheochromocytoma. She was clinically considered to have coexistent pheochromocytoma and PA. A subsequent detailed histological examination of the resected tumor clearly demonstrated pheochromocytoma. However, HE staining at ×100 magnification showed no abnormalities responsible for PA in her adrenal gland, although the presence of paradoxical hyperplasia of the zona glomerulosa was consistent with the clinical findings of PA.
PA has been clinically classified into two subtypes: aldosterone-producing adenoma and idiopathic hyperaldosteronism (IHA). IHA is considered to be caused by hyperplasia of the zona glomerulosa (15). However, the detailed pathological characteristics of hyperplasia remain unknown. Recently, a specific monoclonal antibody against aldosterone synthase (CYP11B2) was developed (16). Using this antibody, it has become possible to detect possible responsible adrenocortical lesions involved in aldosterone excess (14,17). Yamazaki et al. reviewed cases of non-neoplastic hyperaldosteronism and classified them into two histologically distinct subtypes based on the distribution of CYP11B2-positive cells in hyperaldosteronism: multiple adrenocortical micronodules and diffuse hyperplasia of the zona glomerulosa (13). Somatic mutations were frequently detected in CYP11B2-positive cortical micronodules, indicating that the lesions were related to hyperaldosteronism (13,18). The prevalence of multiple adrenocortical micronodules is considered by no means uncommon and is reported in approximately 10% of surgically removed adrenal glands harboring PA (19).
The International Histopathology Consensus for Unilateral Primary Aldosteronism was published recently (14). These consensus guidelines were developed by 11 pathologists worldwide and provide recommended criteria for the histopathological diagnosis of unilateral PA. The histopathological findings in our case were consistent with multiple aldosterone-producing micronodules (14), so we considered this condition to be the responsible lesion causing our patient's primary aldosteronism.
Cases of the coexistence of PA and pheochromocytoma are very rare. Few cases have been reported, to our knowledge, but we found 15 cases among 8 English-language reports (2-9). Among cases with histologically proven lesions responsible for PA, five involved the coexistence of an aldosterone-producing adenoma and pheochromocytoma, and two involved coexistent IHA and pheochromocytoma (2-6,8,9). Recently, Mao et al. reported five cases of coexistent pheochromocytoma and PA by conducting a retrospective case series at the Mayo clinic (8). In their report, the authors first encountered a case in which coexistent PA was thought to be caused by multiple aldosterone-producing micronodules (8). Therefore, ours would be the second report of coexistent pheochromocytoma and PA caused by multiple aldosterone-producing micronodules.
Regarding the mechanisms underlying the simultaneous occurrence of PA and pheochromocytoma, several hypotheses have been proposed based on the functional correlation between hyperaldosteronism and pheochromocytoma. Pheochromocytomas secrete a variety of peptides, including adrenocorticotropic hormone, leading to hyperaldosteronism (7). However, PA and pheochromocytoma arise from different embryological origins: adrenocortical cells and chromaffin cells of the medulla, respectively. In addition, it is well known that germline gene mutations are deeply involved in the etiology of pheochromocytoma. In contrast, somatic mutations, such as in KCNJ5, have recently been implicated in the autonomous secretion of aldosterone in PA (20). Thus, gene mutations are involved in the onset of both PA and pheochromocytoma; however, the involved genes differ between the two conditions. Overall, these findings suggest that the two lesions in this case may have been coincidental; however, further investigations are required for confirmation.
After right adrenalectomy, our patient's urinary catecholamine levels normalized. However, her plasma aldosterone levels remained high, although they were lower than before surgery. In addition, the results of the captopril challenge test three months following surgery remained positive, and the ARR was also still high at eight months after surgery. Consistent with these results, her blood pressure levels decreased after surgery but gradually increased thereafter. Spironolactone effectively lowered her blood pressure. These results indicated the presence of a PA lesion in her contralateral adrenal gland. Among 13 previous multiple adrenocortical micronodules cases, 8 were bilateral, in accordance with the clinical diagnosis made by adrenal venous sampling (AVS) (13). The present case is also considered to have involved bilateral lesions.
The possibility that an aldosterone-producing adenoma existed in the left adrenal gland cannot be ruled out. We also cannot conclude there was bilateral PA based solely on an immunohistological analysis of one adrenal gland. AVS is necessary to distinguish unilateral or bilateral adrenal lesions in PA. However, right adrenalectomy was indicated in our patient because of the clear existence of a pheochromocytoma in the right adrenal gland. Because pheochromocytomas may be lethal, their removal should be prioritized over that of PA. Notably, because we wanted to avoid the potential risk of a hypertensive crisis induced by iodinated contrast media, we decided not to perform AVS. However, AVS has been performed in several cases of coexistent pheochromocytoma and PA (4-9). Interestingly, in a recent review of 15 cases of coexistent pheochromocytoma and PA (8), AVS was performed for 9 patients. Five (56%) of the 9 patients were diagnosed with bilateral PA, and the remaining 4 (44%) were diagnosed with unilateral PA, suggesting that PA in the setting of coexistent pheochromocytoma may present unilaterally or bilaterally. These results suggest that performing AVS to evaluate laterality before surgery may help predict the likelihood of resolving aldosterone overproduction by unilateral adrenalectomy and thus be important for ensuring appropriate management in cases with coexistent disease.
In conclusion, we reported a case of the coexistence of PA and pheochromocytoma. A detailed pathological evaluation, including CYP11B2 immunohistochemistry, revealed that the lesions responsible for the PA were multiple aldosterone-producing micronodules. We concluded that this was a histologically proven case of coexistent pheochromocytoma and PA due to multiple aldosterone-producing micronodules, which was proven only by a detailed histopathological evaluation of the resected adrenal gland. This case also highlights the importance of the histopathological evaluation of resected adrenal glands associated with secondary hypertension.
credit
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Jane Charbonneau, DVM, for editing a draft of this manuscript.
References
- 1. Brown JM, Siddiqui M, Calhoun DA, et al. The unrecognized prevalence of primary aldosteronism: a cross-sectional study. Ann Intern Med 173: 10-20, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilkins GE, Schmidt N, Lee-Son L. Coexistence of pheochromocytoma, adrenal adenoma and hypokalemia. Can Med Assoc J 116: 360-362, 1977. [PMC free article] [PubMed] [Google Scholar]
- 3. Wajiki M, Ogawa A, Fukui J, Komiya I, Yamada T, Maruyama Y. Coexistence of aldosteronoma and pheochromocytoma in an adrenal gland. J Surg Oncol 28: 75-78, 1985. [DOI] [PubMed] [Google Scholar]
- 4. Gordon RD, Bachmann AW, Klemm SA, et al. An association of primary aldosteronism and adrenaline-secreting phaeochromocytoma. Clin Exp Pharmacol Physiol 21: 219-222, 1994. [DOI] [PubMed] [Google Scholar]
- 5. Sakamoto N, Tojo K, Saito T, et al. Coexistence of aldosterone-producing adrenocortical adenoma and pheochromocytoma in an ipsilateral adrenal gland. Endocr J 56: 213-219, 2009. [DOI] [PubMed] [Google Scholar]
- 6. Ohta Y, Sakata S, Miyata E, Iguchi A, Momosaki S, Tsuchihashi T. Case report: coexistence of pheochromocytoma and bilateral aldosterone-producing adenomas in a 36-year-old woman. J Hum Hypertens 24: 555-557, 2010. [DOI] [PubMed] [Google Scholar]
- 7. Tan GH, Carney JA, Grant CS, Young WF. Coexistence of bilateral adrenal phaeochromocytoma and idiopathic hyperaldosteronism. Clin Endocrinol (Oxf) 44: 603-609, 1996. [DOI] [PubMed] [Google Scholar]
- 8. Mao JJ, Baker JE, Rainey WE, Young WF, Bancos I. Concomitant pheochromocytoma and primary aldosteronism: a case series and literature review. J Endocr Soc 5: bvab107, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miyazawa K, Kigoshi T, Nakano S, et al. Hypertension due to coexisting pheochromocytoma and aldosterone-producing adrenal cortical adenoma. Am J Nephrol 18: 547-550, 1998. [DOI] [PubMed] [Google Scholar]
- 10. Nishikawa T, Omura M, Satoh F, et al. Guidelines for the diagnosis and treatment of primary aldosteronism - the Japan Endocrine Society 2009 -. Endocr J 58: 711-721, 2011. [DOI] [PubMed] [Google Scholar]
- 11. Rosas AL, Kasperlik-Zaluska AA, Papierska L, Bass BL, Pacak K, Eisenhofer G. Pheochromocytoma crisis induced by glucocorticoids: a report of four cases and review of the literature. Eur J Endocrinol 158: 423-429, 2008. [DOI] [PubMed] [Google Scholar]
- 12. Sasano H. Localization of steroidogenic enzymes in adrenal cortex and its disorders. Endocr J 41: 471-482, 1994. [DOI] [PubMed] [Google Scholar]
- 13. Yamazaki Y, Nakamura Y, Omata K, et al. Histopathological classification of cross-sectional image-negative hyperaldosteronism. J Clin Endocrinol Metab 102: 1182-1192, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Williams TA, Gomez-Sanchez CE, Rainey WE, et al. International histopathology consensus for unilateral primary aldosteronism. J Clin Endocrinol Metab 106: 42-54, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reincke M, Bancos I, Mulatero P, Scholl UI, Stowasser M, Williams TA. Diagnosis and treatment of primary aldosteronism. Lancet Diabetes Endocrinol 9: 876-892, 2021. [DOI] [PubMed] [Google Scholar]
- 16. Gomez-Sanchez CE, Qi X, Velarde-Miranda C, et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol 383: 111-117, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Omura M, Sasano H, Fujiwara T, Yamaguchi K, Nishikawa T. Unique cases of unilateral hyperaldosteronemia due to multiple adrenocortical micronodules, which can only be detected by selective adrenal venous sampling. Metabolism 51: 350-355, 2002. [DOI] [PubMed] [Google Scholar]
- 18. Yamazaki Y, Omata K, Tezuka Y, et al. Non-neoplastic/hyperplastic primary aldosteronism - its histopathology and genotype. Curr Opin Endocr Metab Res 8: 122-131, 2019. [Google Scholar]
- 19. Meyer LS, Wang X, Sušnik E, et al. Immunohistopathology and steroid profiles associated with biochemical outcomes after adrenalectomy for unilateral primary aldosteronism. Hypertension 72: 650-657, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nishimoto K, Tomlins SA, Kuick R, et al. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci U S A 112: E4591-E4599, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]