Abstract
An 89-year-old woman with a giant hiatal hernia complained of persistent chest pain. An electrocardiogram (ECG) showed hyperacute T waves, suggesting the early phase of ST-elevation myocardial infarction. After endoscopic drainage for hiatal hernia, the chest pain disappeared, and the ECG abnormalities resolved. The present case illustrates that compression of the heart by a giant hiatal hernia can induce T wave elevation mimicking acute coronary syndrome.
Keywords: hiatal hernia, hyperacute T waves, acute coronary syndrome
Introduction
Hiatal hernia is one of common diseases, but it is not well known that it can cause abnormal electrocardiogram (ECG) findings. Hyperacute T waves have been recognized as the earliest ECG signs of ST-elevation myocardial infarction (1). We herein present a case that illustrates hyperacute T waves mimicking acute coronary syndrome (ACS) induced by a giant hiatal hernia.
Case Report
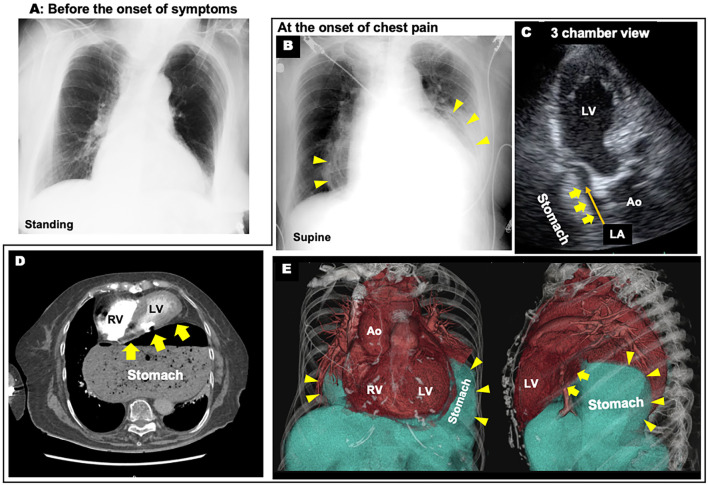
An 89-year-old woman complaining of persistent chest pain was referred to the department of cardiology at our hospital for suspected ACS. She had undergone surgery for distal radius fractures 4 days before the onset of chest pain. Acute chest pain with cold sweats and nausea occurred after defecation. The chest pain was persistent and did not improve with rest. The patient had hypertension and dyslipidemia as coronary risk factors. Her blood pressure was 179/97 mmHg, and her heart rate was 98 beats/min. A physical examination revealed no cardiac murmurs or gallops, and the lungs were clear with bilateral normal breath sounds on auscultation. An ECG at 1 hour after the onset of chest pain showed hyperacute T waves in anterior leads V1-4 (Fig. 1). A chest X-ray demonstrated an abnormal circular shadow behind the heart (Fig. 2B).
Figure 1.
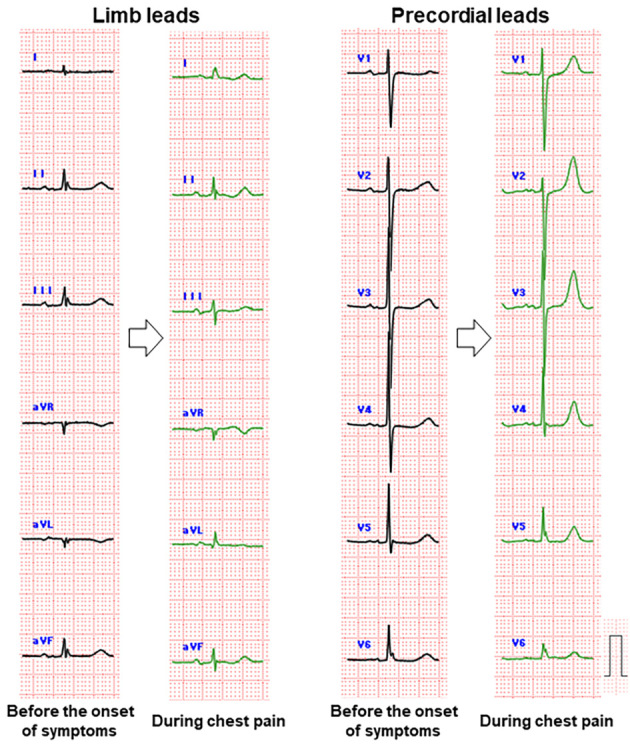
Comparison of electrocardiograms before the onset of symptoms (black lines) and during chest pain (green lines).
Figure 2.
Images at the onset of chest pain. A, B: Chest X-ray: Comparison between before the onset of symptoms (A) and at the onset of chest pain (B), C: Echocardiogram showing a compressed left atrium (LA), D: Computed tomography showing a heart compressed by a giant hiatal hernia, E: Volume-rendering reconstruction CT images (Yellow arrowheads indicate stomach borders and yellow arrows indicate cardiac compression by the giant hiatal hernia). Ao: aorta, LV: left ventricle, RV: right ventricle
Hyperacute T waves with chest pain was the first suspicious finding in the early phase of ACS. Important diagnostic considerations in the case of this elderly woman with chest pain after surgery included pulmonary artery thromboembolism and Takotsubo syndrome. High-sensitivity cardiac troponin I showed no significant elevation and dynamic changes were not observed over several hours. An echocardiogram 2.5 hours after the onset of chest pain showed no abnormal left ventricular wall motion suggestive of ACS or Takotsubo syndrome, and no right ventricular dysfunction secondary to a pulmonary embolism. However, flattening of the left atrium, with compression from the dorsal side was found (Fig. 2C). Computed tomography revealed a giant hiatal hernia involving translocation of the stomach to the thoracic cavity leading to compression of the heart (Fig. 2D, E). Therefore, we thought that cardiac compression by the giant hiatal hernia might be the origin of the chest pain and T wave elevation. Preoperative cardiac computed tomography revealed no coronary artery lesions that could cause ACS.
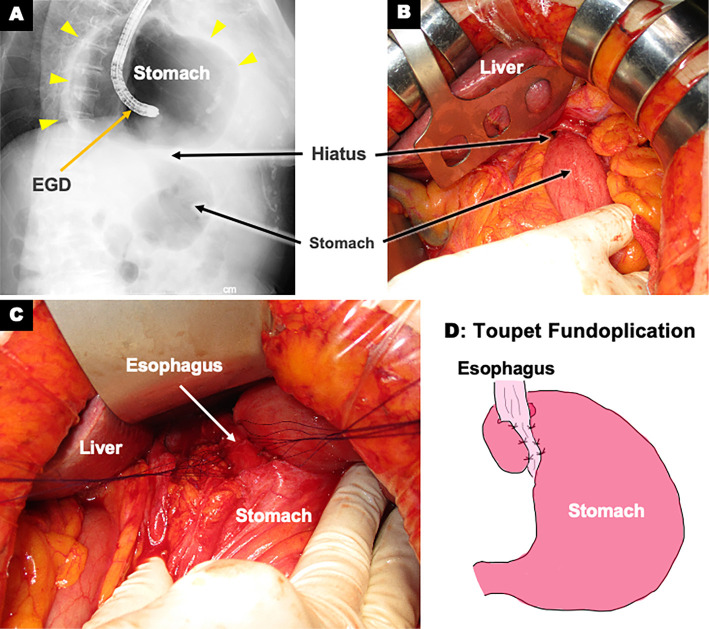
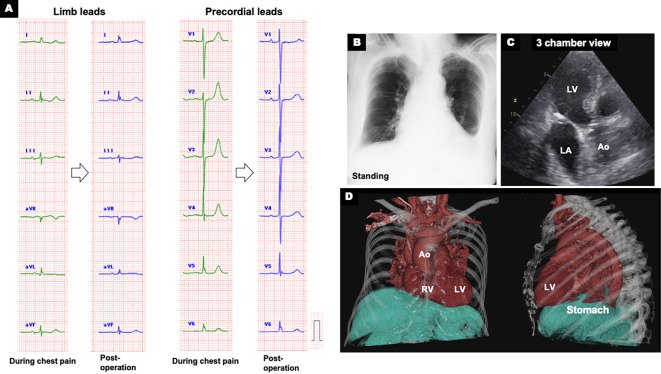
We referred the patient to the department of gastroenterology for endoscopic drainage. As a first aid measure, suctioning through a nasal transfer tube slightly improved the chest pain. Immediately after the subsequent endoscopic drainage, her chest pain disappeared with improvement of the ECG abnormalities five hours after the onset of chest pain. Based on the endoscopic findings, she was diagnosed with type 1 (sliding hiatal hernias). Esophagogastroduodenoscopy was performed three times for drainage and hiatal hernia repair (Fig. 3A). However, the repair was unsuccessful and was accompanied by gastric stenosis at the hiatus. Therefore, we selected surgical repair. Intraoperative findings showed that most of the stomach remained in the mediastinum (Fig. 3B). The stomach was manually evacuated into the abdominal cavity, and Toupet fundoplication was successfully performed for radical surgery (Fig. 3C, D). After surgery, the ECG normalized (Fig. 4A) and the circular shadow behind the heart on chest X-ray disappeared (Fig. 4B). Echocardiography and computed tomography confirmed the resolution of the left atrium compression by the giant hiatal hernia (Fig. 4C, D). The patient was discharged 18 days after surgery without any recurrence of chest pain. At the 3-month follow-up examination, the patient was in a healthy condition without any recurrence of hiatal hernia or chest pain.
Figure 3.
Images during Esophagogastroduodenoscopy (EGD) and surgical hiatal hernia repair. A: Gastrofluoroscopic image during EGD showing most of the stomach in the mediastinum and gastric stenosis at the hiatus, B: Intraoperative image after laparotomy showing only part of the stomach in the abdominal cavity, C: Toupet fundoplication, D: Simplified diagram of Toupet fundoplication in which the stomach was wrapped around the esophagus to prevent reflux (Yellow arrowheads indicate stomach borders).
Figure 4.
Post-operative electrocardiogram and images. A: Electrocardiogram (ECG) showing normalization of ECG abnormalities (green lines: during chest pain; blue lines: post-operation), B: Chest X-ray after operation, C: Post-operative echocardiogram showing the resolution of left atrium (LA) compression by the giant hiatal hernia, D: Volume-rendering reconstruction CT images. Ao: aorta, LV: left ventricle, RV: right ventricle
Discussion
The patient in the present case suddenly complained of chest pain with T wave elevation on her ECG; thus, we first suspected a fatal ACS. However, there were no other findings specific to ACS (i.e., time-related troponin I increase or ventricular asynergy). Immediately after endoscopic drainage for the hiatal hernia, the patient's chest pain disappeared, and a decrease in elevated T waves was observed. Therefore, we concluded that the cardiac symptoms and abnormal ECG changes mimicking ACS were due to cardiac compression by the giant hiatal hernia.
A hiatal hernia with intrathoracic acute gastric volvulus may cause the sudden onset of chest pain (2). However, there was no gastric volvulus in this case. Therefore, the cause of her chest pain was thought to be myocardial ischemia. It has been reported that hiatal hernia can cause various ECG changes (3). To date, the mechanism of the ECG changes in patients with hiatal hernia is not completely understood, however, the following hypotheses have been proposed: 1) absolute ischemia induced by mechanical compression of the coronary arteries; 2) relative ischemia caused by increased intraventricular pressure in a compressed heart; and 3) pericarditis due to spillover of inflammation in reflux esophagitis (3-5). Another possible mechanism of ECG changes due to hiatal hernia may be the physical shortening of the distance between the precordial monopolar lead and the heart. However, in the present case, the change in the QRS wave voltage was poor in comparison to the T wave increase. Furthermore, decompression via endoscopic drainage resulted in the resolution of the ECG abnormalities. Therefore, mechanical compression - rather than the change in physical distance - may have caused pseudo-hyperacute T waves via myocardial ischemia.
Compression of the heart by a hiatal hernia can cause hyperacute T waves mimicking ACS. When patients present hyperacute T waves, hiatal hernia should be considered in the differential diagnosis, together with ACS.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Thygesen K, Alpert JS, Jaffe AS, Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 72: 2231-2264, 2018.30153967 [Google Scholar]
- 2. Kohn GP, Price RR, DeMeester SR, et al. SAGES Guidelines Committee. Guidelines for the management of hiatal hernia. Surg Endosc 27: 4409-4428, 2013. [DOI] [PubMed] [Google Scholar]
- 3. Krawiec K, Szczasny M, Kadej A, Piasecka M, Blaszczak P, Głowniak A. Hiatal hernia as a rare cause of cardiac complications - case-based review of the literature. Ann Agric Environ Med 28: 20-26, 2021. [DOI] [PubMed] [Google Scholar]
- 4. Rubini Gimenez M, Gonzalez Jurka L, Zellweger MJ, Haaf P. A case report of a giant hiatal hernia mimicking an ST-elevation myocardial infarction. Eur Heart J Case Rep 3: ytz138, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hokamaki J, Kawano H, Miyamoto S, et al. Dynamic electrocardiographic changes due to cardiac compression by a giant hiatal hernia. Intern Med 44: 136-140, 2005. [DOI] [PubMed] [Google Scholar]