Abstract
Finding the ideal balance between efficacy and safety of immunosuppression is challenging, particularly in cases of severe TAFRO syndrome. We herein report a 60-year-old man diagnosed with grade 5 TAFRO syndrome mimicking hepatorenal syndrome that was successfully treated by glucocorticoid, tocilizumab, and cyclosporin despite virus infection. Furthermore, by examining 14 peer-reviewed remission cases, we revealed that the recovery periods among inflammation, renal dysfunction, and thrombocytopenia were quite different, with recovery from thrombocytopenia notably slow. All patients requiring dialysis were successfully withdrawn from dialysis, and the reversibility from kidney injury was good. This clinical information will help clinicians plan treatments and tailor the intensity of immunosuppression.
Keywords: TAFRO syndrome, thrombocytopenia, dialysis, kidney injury, immunosuppression
Introduction
TAFRO syndrome, characterized by Thrombocytopenia, Anasarca, Fever, Reticulin myelofibrosis (or Renal insufficiency), and Organomegaly, is a rare systemic inflammatory disorder first proposed in Japan in 2010 (1,2). Because of its variety of symptoms, it is important for not only hematologists and gastroenterologists but also nephrologists to be aware of TAFRO syndrome. TAFRO syndrome has been categorized as a subtype of idiopathic multicentric Castleman disease, but its clinical features differ markedly from those of classical Castleman disease.
Most patients with TAFRO syndrome have a sub-acute onset and progressive clinical courses. Therefore, the prompt initiation of treatments and multiple immunosuppressants are required (3). However, striking the appropriate balance between efficacy and safety of immunosuppression is challenging. While insufficient immunosuppression leads to fatal conditions, such as hemorrhaging and multi-organ failure, over-immunosuppression triggers severe infection and drug-associated adverse events (4).
The present case suggests that the time required for recovery from various symptoms, such as systemic inflammation, renal dysfunction, thrombocytopenia, anasarca, and organomegaly, is different. An awareness of temporal lags in the recovery periods for various symptoms can aid clinicians in deciding the proper amounts and duration of immunosuppression.
We herein report a case of severe TAFRO syndrome that mimicked hepatorenal syndrome successfully treated with glucocorticoid, tocilizumab (TCZ: anti-IL-6 monoclonal antibody), and cyclosporin A (CsA). We also present a literature review on the renal reversibility and the time to recover from inflammation, renal dysfunction, and thrombocytopenia.
Case Report
A 60-year-old Japanese man was referred to our hospital for a fever, ascites, and general fatigue. He had a medical history of alcoholic fatty liver 20 years earlier. He had not been prescribed any medications. He regularly visited a doctor in the Department of Hepatology but continued to consume alcohol daily. Four months before admission, he had persistent cough and purulent rhinorrhea. He developed abdominal swelling, edema, and a decrease in appetite. He was therefore referred to our department for acute kidney injury.
The level of creatinine increased from 0.8 mg/dL to 2.8 mg/dL. On admission, his blood pressure was 124/71 mmHg, pulse rate was 100/min, temperature was 37.5°C, and body mass index was 26.5. A physical examination revealed abdominal fullness and leg edema without palpable lymph nodes, joint pain, or neurological disorders. Laboratory test results are shown in Table 1. The levels of creatinine, D-dimer, C-reactive protein (CRP), procalcitonin, total bilirubin, platelets, albumin, and cholinesterase were 2.8 mg/dL, 31 μg/mL, 9.2 mg/dL, 7.3 ng/mL, 1.3 mg/dL, 54×103/μL, 2.0 g/dL, and 89 IU/L on admission, respectively. The levels of interleukin-6 (IL-6) and vascular endothelial growth factor (VEGF) were also high. Anti SS-A antibody and SS-B antibody were positive, although he had no dry eyes and mouth, joint pain, or rash. Tests for infectious diseases were all negative, and none of the culture tests of blood, sputum, urine, feces, or ascites showed any evidence of infection. A test of the ascites showed high hyaluronic acid (10,400 ng/mL), IL-6 (1,430 pg/mL), and VEGF (112 pg/mL) values (Table 1). The leukocyte count in the ascites was 200 /μL (lymphocytes 21%, monocytes 79%).
Table 1.
Laboratory Data.
| Laboratory tests | Laboratory tests | Laboratory tests | |||
|---|---|---|---|---|---|
| Urinalysis | Immunology | Infection | |||
| Specific gravity | 1.019 | Immunoglobulin G (mg/dL) | 2,239 | HBsAg | - |
| pH | 5 | Immunoglobulin G4 (mg/dL) | 44 | HBsAb | - |
| Protein | 1+ | Immunoglobulin A (mg/dL) | 654 | HbcAb | - |
| Occult blood | ± | Immunoglobulin M (mg/dL) | 62 | HCVAb | - |
| Glucose | - | Complement 3 (mg/dL) | 97 | TP Ab | - |
| NAG (IU/L) | 53.2 | Complement 4 (mg/dL) | 11 | T-SPOT | - |
| β2-microglobulin (μg/mL) | 0.015 | Rheumatoid factor (IU/mL) | 11 | HIV | - |
| Urin sediment | Antistreptolysin-O (IU/mL) | 298 | EB IgG | + | |
| Erythrocytes (dysmorphic)/HPF | 1-4 | Cryoglobulin | - | EB IgM | - |
| Leukocytes/HPF | 1-4 | Antinuclear antibody | 160 | Varicella IgG | - |
| Complete blood count | Anti-GBM antibody (U/mL) | <2.0 | Varicella IgM | - | |
| White blood cell (×103/μL) | 7.28 | MPO-ANCA (U/mL) | <1.0 | Herpes simplex virus IgG | - |
| Neutrophils (%) | 70.4 | PR3-ANCA (U/mL) | <1.0 | Herpes simplex virus IgM | - |
| Hematocrit (%) | 40.6 | Anti-SS-A/Ro antibody (IU/mL) | 299 | Human herpesvirus 8 DNA | - |
| Platelets (×103/μL) | 54 | Anti-SS-B/La antibody (IU/mL) | 59.1 | Cytomegalovirus antigenemia assay | - |
| Chemistry | ADAMTS13 activity (IU/mL) | 0.51 | Plasma cytokine | ||
| Aspartate aminotransferase (IU/L) | 42 | PAIgG (ng/107 cells) | 249.7 | Interleukin 6 (pg/mL) | 22 |
| Alanine aminotransferase (IU/L) | 23 | Lupus anticoagulant (U/mL) | 1.01 | Vascular endothelial growth factor (pg/mL) | 98.8 |
| γ-GTP (IU/L) | 72 | Cardiolipin β2 glicoprotein (U/mL) | 0.9 | Ascites tests | |
| Alkaline phosphatase (IU/L) | 263 | Cardiolipin Ab (U/mL) | 8.7 | White blood cell (/μL) | 200 |
| Lactate dehydrogenase (IU/L) | 227 | Coagulation system | Lymphocyte (%) | 21 | |
| Choline esterase (IU/L) | 89 | PT (%) | 60 | Monocyte (%) | 79 |
| Total bilirubin (mg/dL) | 1.3 | PT-INR | 1.34 | Lactate dehydrogenase (IU/L) | 227 |
| Total protein (g/dL) | 6.7 | APTT (s) | 46.8 | Total protein (g/dL) | 2.7 |
| Albumin (g/dL) | 2.5 | Fibrinogen (mg/dL) | 279 | Albumin (g/dL) | 1.2 |
| Blood urea nitrogen (mg/dL) | 65 | D-dimer (μg/mL) | 31.3 | Choline esterase (IU/L) | 89 |
| Creatinine (mg/dL) | 2.8 | AT-III (%) | 43 | Total cholesterol (mg/dL) | 26 |
| Sodium (mEq/L) | 137 | TAT (ng/dL) | 35 | Triglyceride (mg/dL) | 22 |
| Potassium (mEq/L) | 4.8 | PIC (μg/mL) | 7.4 | Lipase (U/L) | 63 |
| Chloride (mEq/L) | 110 | Tumor marker | Hyaluronic acid (ng/mL) | 10,400 | |
| Calcium (mEq/L) | 7.4 | Soluble interleukin-2 receptor (IU/mL) | 717 | Interleukin 6 (pg/mL) | 1,430 |
| Glucose (mg/dL) | 82 | Cancer antigen 125 (IU/mL) | 129.8 | Vascular endothelial growth factor (pg/mL) | 112 |
| C-reactive protein (mg/dL) | 9.2 | Cytokelatin 19 fragment (ng/mL) | 13.6 | ||
| Procalcitonin (ng/mL) | 7.3 | Carbohydrate antigen 19-9 (ng/mL) | 49.3 | ||
| Erythrocyte sedimentation rate (mm) | 78 | Carcinoembryonic antigen (ng/mL) | 1.9 | ||
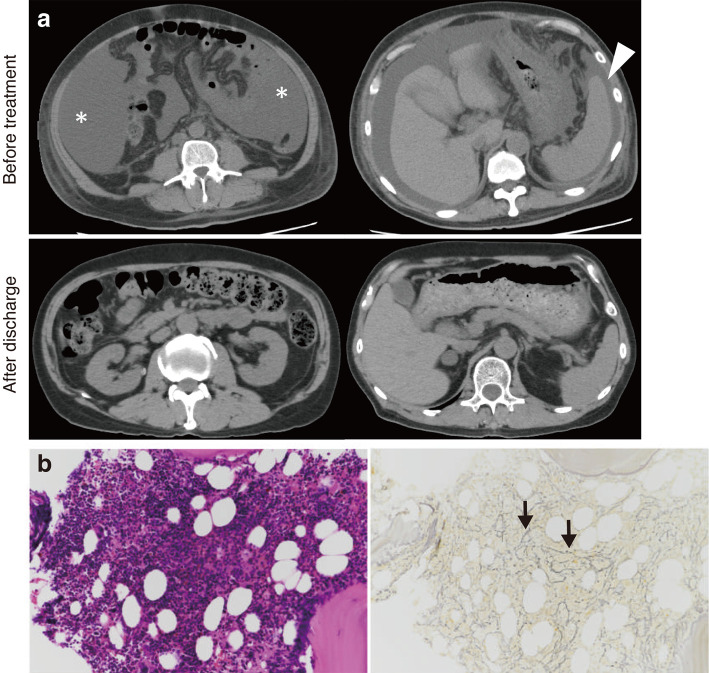
Abdominal computed tomography showed massive ascites, slight hepatic atrophy, and splenomegaly without periaortic lymphadenopathy of a size insufficient for a biopsy (Fig. 1a). A bone marrow biopsy revealed mildly hypercellular marrow (50% of cellularity) with mild reticulin fibrosis (Fig. 1b). A renal biopsy could not be performed because of severe thrombocytopenia. Positron emission tomography with 2-deoxy-2-fluoro-D-glucose, upper gastrointestinal endoscopy, colonoscopy, and a random skin biopsy showed no signs of neoplasm.
Figure 1.
(a) Plain CT shows the massive ascites (asterisk) and splenomegaly (arrowhead) before treatment. The splenomegaly and ascites improved after discharge. (b) The findings of the marrow biopsy show mildly hypercellular marrow (50% cellularity) with mild reticulin fibrosis (black arrow) (left panel: Hematoxylin and Eosin staining; right panel: silver stain, ×200).
Regarding the diagnostic criteria of TAFRO syndrome (5), the patient met all three major categories (anasarca, thrombocytopenia, and systemic inflammation) and three of the four minor categories (reticulin myelofibrosis, organomegaly, and progressive renal insufficiency). Thrombocytopenia and positive platelet associated IgG are findings associated with idiopathic thrombocytopenic purpura (ITP), but complications of enlarged lymph nodes, splenomegaly, and renal failure were not consistent with ITP. The finding of reticulin myelofibrosis on a bone marrow examination is typical of TAFRO syndrome and is generally uncommon in ITP. Other potential etiologies, including malignant tumor, sepsis, collagen diseases, and IgG4-related disease, with the exception of hepatorenal syndrome, were incompatible with the examinations and clinical findings.
Because the diagnosis of spontaneous bacterial peritonitis (SBP) is based on positive ascitic fluid bacterial cultures and the detection of an elevated polymorphonuclear neutrophil count in the ascites (>250 /μL), the patient was deemed to not be complicated with SBP. No triggers for hepatorenal syndrome, such as SBP, were found. Hypercytokinemia and the absence of portosystemic collateral pathways were suggestive of TAFRO syndrome rather than hepatorenal syndrome. We concluded that the hypergammaglobulinemia and high hyaluronic acid levels in ascites on admission had been caused by exacerbation of chronic liver disease. Based on these findings, we made a diagnosis of TAFRO syndrome complicated with liver injury.
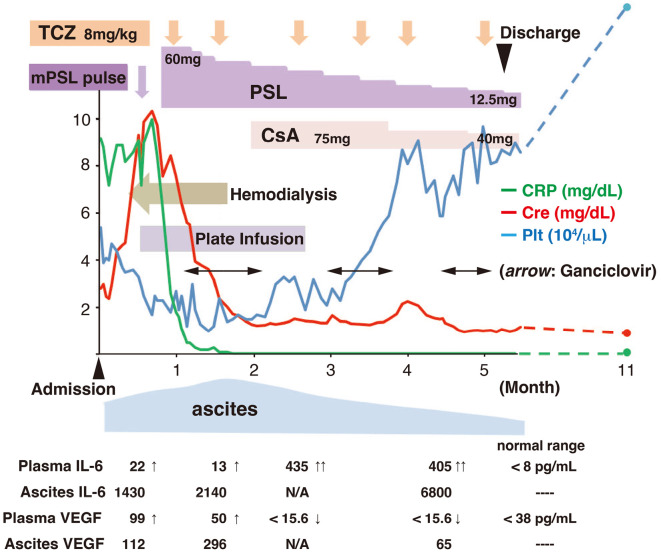
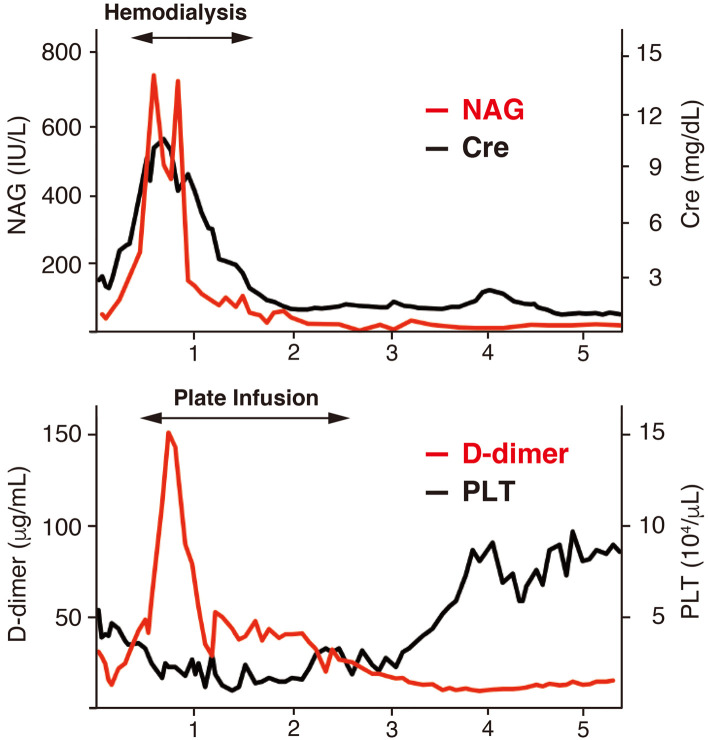
The clinical course of the patient is shown in Fig. 2. The maximum levels of creatinine, D-dimer, CRP, procalcitonin, and total bilirubin were increased remarkably up to 10.3 mg/dL, 151 μg/mL, 10.0 mg/dL, 18.7 ng/mL, and 6.7 mg/dL after admission, respectively. The minimum levels of platelets, albumin, and cholinesterase were 10×103/μL, 2.0 g/dL, and 52 IU/L, respectively. These findings indicated a clinically serious case. Hemodialysis and platelet transfusion were initiated on days 10 and 13 of admission, respectively. Intravenous methylprednisolone pulse therapy (1,000 mg/day ×3 days) and oral prednisolone (60 mg/day) after the pulse therapy were started on day 16 of admission. After the initiation of glucocorticoid therapy, the level of CRP decreased rapidly. We added TCZ (8 mg/kg) on day 30 of admission. Subsequently, the renal function improved gradually, and hemodialysis was withdrawn 33 days after the initiation of immunosuppressive therapy (on day 48 of admission). The level of urinary N-acetyl-β-(D)-glucosaminidase (NAG) declined sensitively, reflecting an improvement in the renal function (Fig. 3). However, severe thrombocytopenia persisted after the second administration of TCZ. We therefore added a further 75 mg of CsA per day on day 60 of admission. When the level of plasma D-dimer fell below 20 μg/mL, the platelet count conversely started to increase (Fig. 3). Transfusion was discontinued on day 79 of admission, and the platelet count remarkably increased in the following month. Massive ascites was still present, despite the improvement in spleen organomegaly on day 63 after admission. Cytomegalovirus (CMV) infection was detected from the very early period (16 days after the initiation of glucocorticoid therapy) and required the administration of ganciclovir. An increase in serum creatinine was observed again on day 120 of admission. A high CsA concentration and late peak (C2, 389 ng/mL; C4, 1,350 ng/mL) were detected, which might have been caused by complications of liver damage. The emergence of decoy cells in the urine was also found.
Figure 2.
Clinical course after admission. TCZ: tocilizumab, mPSL: methylprednisolone, CsA: cyclosporin, IL-6: interleukin-6, VEGF: vascular endothelial growth factor
Figure 3.
The association between the levels of NAG and creatinine, and the inverse correlation between those of D-dimmer and platelet. NAG: N-acetyl-β-(D)-glucosaminidase, Cre: creatinine, PLT: platelet
After reducing the dose of CsA, the kidney function recovered quickly, and decoy cells disappeared. Although CMV infection and BK virus infection occurred, these complications were diagnosed in the early stages and successfully treated. Finally, combination treatment with glucocorticoid, TCZ, and CsA led to complete remission of TAFRO syndrome. The patient was discharged on day 160. Six months after discharge, the ascites had disappeared (Fig. 1a) with a creatinine level of 1.0 mg/dL, platelet count of 15×104/μL, and negative CRP values.
Discussion
We encountered a 60-year-old man diagnosed with grade 5 TAFRO syndrome and successfully treated by glucocorticoid, tocilizumab, and ciclosporin despite virus infection. The patient presented with severe renal dysfunction and prolonged thrombocytopenia. Notably, the time required for recovery from various symptoms, such as systemic inflammation, renal dysfunction, thrombocytopenia, anasarca, and organomegaly, was different.
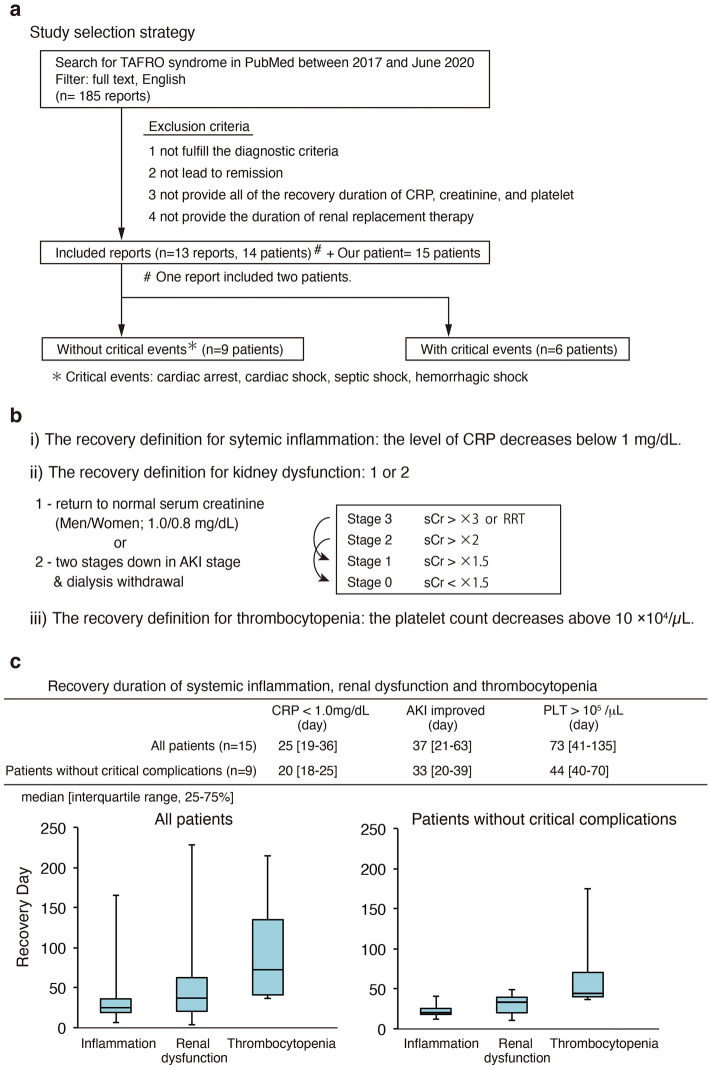
Information on the time required for recovery helps clinicians tailor the intensity of the treatment by assessing precisely the effectiveness of the drug against the dangers of excessive immunosuppression. Therefore, we reviewed remission cases reported previously, and analyzed the recovery durations of the various symptoms of TAFRO syndrome. We also examined the recovery of the renal function. We searched PubMed using the term TAFRO syndrome and the following additional filters: text availability, full text; publication date, from 2017/01/01 to 2020/06/30; and language, English (Fig. 4a). The exclusion criteria included 1) not consistent with the criteria proposed in 2019 by Masaki et al. (5); 2) a lack of full remission; 3) a lack of all recovery period data for systemic inflammation, renal dysfunction and thrombocytopenia; and 4) a lack of the duration of renal replacement therapy. After applying these criteria to our search results, we were left with 14 cases (13 reports) (6-18). For these cases, we first collected patient information, including the age, sex, race, TAFRO grade, complications, treatments, and trends in creatinine levels. We also investigated whether or not a kidney biopsy and dialysis had been performed. Next, we calculated the approximate number of days required for recovery from systemic inflammation, renal dysfunction, and thrombocytopenia, as well as dialysis duration, taking the data from texts, tables, and the graphs of clinical courses in these reports. We then divided the 15 cases (14 cases plus our present case) into 2 groups: cases with and without critical complications of cardiac, hemorrhagic or septic shock. Finally, in each group, we analyzed the dialysis duration and days required for recovery from systemic inflammation, renal dysfunction, and thrombocytopenia, as well as the level of creatinine at discharge. Data are shown as the median and interquartile range (IQR; 25-75%). We defined the recovery duration from systemic inflammation, thrombocytopenia, and renal dysfunction after the initiation of immunosuppression therapy as follows (Fig. 4b): 1) levels of CRP <1 mg/dL; 2) the platelet count >10×104/μL; 3) two-stage reduction from peak acute kidney injury (AKI) stage according to the KDIGO criteria, or recovery of serum creatinine to below 1.0 mg/dL for men and 0.8 mg/dL for women. To evaluate the AKI stage by KDIGO, the serum creatinine values before the onset of AKI caused by TAFRO syndrome were assumed to be 1.0 mg/dL for men and 0.8 mg/dL for women when the value was unavailable.
Figure 4.
(a) Study selection strategy. (b) The recovery definitions for inflammation, kidney dysfunction, and thrombocytopenia. (c) The recovery duration of inflammation, renal dysfunction, and thrombocytopenia in our reviewed patients. AKI: acute kidney injury, PLT: platelet
The clinical characteristics and recovery duration of all cases are shown in Table 2. All of the cases have been reported from Asia, and 13 of these were from Japan, as shown in Table 2. There were 9 men and 6 women, from 17 to 85 years old (median: 59 years old). There were two patients with TAFRO grade 2, three with grade 3, five patients with grade 4, and five patients with grade 5. Six patients presented with critical complications of cardiogenic, hemorrhagic or septic shock, and cardiac arrest. Four patients with grade 5 TAFRO, not including our own patient, experienced a critical complication. Various opportunistic infections occurred during treatment. In particular, CMV infection occurred most frequently (Table 2). As for the treatment of TAFRO syndrome, all patients were initially treated with glucocorticoids, but the combination of medications used varied (Table 2). Ten patients received renal replacement therapy (RRT) (Table 3). All patients with grade 5 disease, including our case, received RRT. Renal biopsies were performed in four patients, two of whom received the biopsy in the acute phase. No patients with grade 5 received a renal biopsy (Table 3).
Table 2.
Clinical Characteristics and Recovery Duration of Our Reviewed Patients. The Recovery Day of Thrombocytopenia in Case 11 Is Described as “NA” (*) because the Platelet Count Remained above 10×104/μL throughout the Entire Hospitalization Period. These Data are Not Included in the Analysis.
| Case | Race | Age/ Sex | TAFRO grade | Critical complications before remission | Infections | Treatment | CRP <1.0mg/dL (day) | AKI improved (day) | PLT >105 /L (day) | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| without critical complications | 1 | Japanese | 51 F | 2 | None | NA | mPSL pulse, PSL | 20 | 10 | 40 | 6 |
| 2 | Japanese | 38 M | 3 | None | NA | mPSL pulse, PSL, CsA, TCZ | 15 | 33 | 38 | 7 | |
| 3 | Japanese | 85 F | 3 | None | NA | PSL | 12 | 15 | 40 | 8 | |
| 4 | Japanese | 76 F | 4 | None | NA | PSL | 32 | 49 | 70 | 9 | |
| 5 | Japanese | 46 M | 4 | None | NA | mPSL pulse, PSL, TCZ | 25 | 34 | 36 | 10 | |
| 6 | Chinese | 52 F | 4 | None | Pneumonia before remission (unknown etiology) | mPSL, PSL, BOR, CPA, DEX | 25 | 20 | 44 | 11 | |
| 7 | Japanese | 69 M | 4 | None | CMV, cholecystitis after remission (unknown etiology), Mycobacterium tuberculosis after remission | mPSL pulse, PSL, CsA | 40 | 45 | 175 | 12 | |
| 8 | Japanese | 48 F | 4 | None | NA | mPSL pulse, PSL, TCZ, CsA | 20 | 21 | 51 | 13 | |
| 9 | Japanese | 60 M | 5 | None | CMV, BK virus | mPSL pulse, PSL, TCZ, CsA | 18 | 39 | 169 | Our case | |
| with critical complications | 10 | Japanese | 68 F | 2 | Septic shock | Staphylococcus aureus | PSL, TCZ, CsA, TAC | 131 | 221 | 120 | 14 |
| 11 | Japanese | 17 M | 3 | Cardiogenic shock | NA | mPSL pulse, PSL, TAC | 6 | 3 | NA | 14 | |
| 12 | Japanese | 72 M | 5 | Cardiac arrest | Corynebacterium | mPSL pulse, PSL, TCA, CPA | 28 | 120 | 140 | 15 | |
| 13 | Japanese | 59 M | 5 | Cardiac arrest | NA | mPSL pulse, PSL, TCZ, CsA | 23 | 37 | 88 | 16 | |
| 14 | Japanese | 25 M | 5 | Cardiac arrest | NA | PSL, CPA, TCZ | 49 | 76 | 75 | 17 | |
| 15 | Japanese | 59 M | 5 | Hemorrhagic shock | Pneumocystis jirovecii, Pseudomonas aeruginosa, Stenotrophomonas maltophilia, Clostridium difficile, Escherichia coli, CMV, Candida | mPSL pulse, PSL, TCZ | 166 | 228 | 215 | 18 |
AKI: acute kidney injury, PLT: platelet, CMV: cytomegalovirus, mPSL: methyl prednisolone, PSL: prednisolone, CsA: ciclosporin, TCZ: tocilizumab, BOR: bortezomib, CPA: cyclophosphamide, DEX: dexamethasone
Table 3.
Trends in the Renal Function in Our Reviewed Patients.
| Case | Age/sex | TAFRO grade | Renal biopsy | RRT duration (day) | AKI improved (day) | AKI recovery definition (#) | Creatinine before the onset of TAFRO syndrome | Creatinine on admission | Max creatinine after treatment | Minimum creatinine after treatment | Creatinine at dischage | AKI to CKD | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| without critical complications | 1 | 51 F | 2 | Acute phase | None | 10 | 1 | NA | 1 | 1.2 | 0.6 | 0.8 | Unidentified |
| 2 | 38 M | 3 | No | 31 | 33 | 2 | NA | 2.8 | 5.0 | 0.8 | 0.8 | Unidentified | |
| 3 | 85 F | 3 | No | None | 15 | 1 | NA | 1.1 | 1.6 | 0.8 | 1.2 | Probably yes * | |
| 4 | 76 F | 4 | Chronic phase | 40 | 49 | 2 | NA | 3.0 | 6.5 | 1.4 | 1.5 | Probably yes * | |
| 5 | 46 M | 4 | No | None | 34 | 1 | NA | 0.9 | 1.5 | 0.9 | 0.9 | Unidentified | |
| 6 | 52 F | 4 | No | 13 | 20 | 2 | NA | 1.9 | 7.2 | 0.4 | 0.5 | Unidentified | |
| 7 | 69 M | 4 | Chronic phase | 17 | 45 | 2 | NA | 2.2 | 4.3 | 1.2 | 1.7 | Probably yes * | |
| 8 | 48 F | 4 | Acute phase | 17 | 21 | 2 | NA | 1.3 | 4.2 | 0.6 | 0.6 | Unidentified | |
| 9 (Our case) | 60 M | 5 | No | 39 | 39 | 2 | 0.8 | 2.8 | 10.4 | 1.1 | 1.2 | Yes | |
| with critical complications | 10 | 68 F | 2 | No | None | 221 | 1 | NA | 1.6 | 1.5 | 0.7 | 0.7 | Unidentified |
| 11 | 17 M | 3 | No | None | 3 | 1 | NA | 1.6 | 2.7 | 0.6 | 0.8 | Unidentified | |
| 12 | 72 M | 5 | No | 106 | 120 | 2 | NA | 1.3 | 6.0 | 0.9 | 1.5 | Probably yes * | |
| 13 | 59 M | 5 | No | 19 | 37 | 2 | NA | 1.3 | 2.2 | 0.9 | 1 | Unidentified | |
| 14 | 25 M | 5 | No | 76 | 76 | 2 | NA | 1.0 | 4.2 | 0.8 | 0.8 | Unidentified | |
| 15 | 59 M | 5 | No | 151 | 228 | 2 | NA | 1.4 | 4.5 | 0.8 | 1 | Unidentified | |
| Dialysis duration | Cre on admission | Maximum Cre | Cre at discharge | ||||||||||
| All patients | 35 [18-67] | 1.4 [1.2-2.1] | 4.2 [1.9-5.5] | 0.9 [0.8-1.2] | |||||||||
| Patients without critical complications median [interquartile range, 25-75%] | 24 [17-37] | 1.9 [1.1-2.8] | 4.3 [1.6-6.5] | 0.9 [0.8-1.2] | |||||||||
The cases described as “probably Yes” (*) have no record of creatinine before the onset of TAFRO syndrome. The AKI recovery definition (#) is shown in Fig. 4b.
RRT: renal replacement therapy, AKI: acute kidney injury, CKD: chronic kidney disease, Cre: creatinine
The median recovery periods for inflammation, renal dysfunction, and thrombocytopenia were 25 [19-36], 37 [21-63], and 73 [41-135] days, respectively (Fig. 4c). Excluding the patients with critical complications, the median recovery periods for inflammation, renal dysfunction, and thrombocytopenia were 20 (18-25), 33 (20-39), and 44 (40-70) days, respectively (Fig. 4c). In cases where the recovery from thrombocytopenia required more than 120 days, CMV infection, bacterial infection, and drug-induced thrombocytopenia were detected.
Regarding the renal function (Table 3), the median levels of creatinine on admission and at discharge were 1.4 (1.2-2.1) and 0.9 (0.8-1.2) mg/dL, respectively. The maximum level of creatinine after initiation of treatment was 4.2 (1.9-5.5) mg/dL. The maximum creatinine in our case was 10.4 mg/dL, which was the highest among all patients. All 10 patients who required dialysis were successfully withdrawn from dialysis, and their median level of creatinine was 1.0 (0.8-1.4) mg/dL at discharge, which indicates the reversibility of renal function impairment in TAFRO syndrome. In addition, all patients who developed chronic kidney disease after remission were over 60 years old.
Our literature review and analysis directly demonstrated crucial differences in the recovery periods of various symptoms and prolonged thrombocytopenia in TAFRO syndrome, and these findings are interesting features of TAFRO syndrome. The cause of the temporal lag between symptoms might be explained by different organ-specific recovery capacity and etiologies, as follows: 1) systemic inflammation caused by autoimmunity or undetermined infection; 2) renal dysfunction caused by endothelial injury; 3) thrombocytopenia caused by autoimmunity, coagulative disorder, or megakaryocyte dysfunction; and 4) ascites and hepatic dysfunction caused by the elevation of vascular permeability or hepatobiliary infection with an undetermined pathogen (19,20).
Previous studies have also reported on prolonged thrombocytopenia in patients with TAFRO syndrome (21,22). Yamaguchi et al. also showed that the median recovery periods from prolonged thrombocytopenia in TAFRO syndrome patients were 25 and 50 days for patients treated with mono-therapy and combination therapy, respectively (defined as the time to increase the platelet count above 5×104/μL) (22). In typical ITP, platelet counts recover to above 5×104/μL in 1-2 weeks with glucocorticoid or IVIG treatment (23-25). Therefore, platelet recovery takes longer with TAFRO syndrome than with ITP. In the present case, it is notable that the recovery of platelets took an extremely long time - much longer than the median duration in our reviewed cases. We assumed that persistent thrombocytopenia was mainly due to the severity of TAFRO syndrome and partly to chronic liver injury, CMV virus infection, and administration of ganciclovir.
One of the remarkable features of the present case was the dramatic improvement from very severe kidney dysfunction. Previous studies have reported that the pathological diagnosis of patients with TAFRO syndrome involves thrombotic microangiopathy or membranoproliferative glomerulonephritis, including endothelial cell swelling, mesangiolysis, and double contours of glomerular basement membrane, which cause abnormal urinalysis findings (13,20,26). Tubulointerstitial injury accompanied with glomerular lesions was also reported in some cases (13,26). Our patient had only mild proteinuria and a low fractional excretion of sodium (FENa) on admission. As the kidney function declined, glomerular hematuria appeared. The level of urinary NAG, a marker of tubular injury, elevated sharply to 735 IU/L, but improved rapidly. We suspect that the reduction in glomerular perfusion and micro-hemodynamic alterations may induce extremely low FENa and oliguric AKI, ultimately leading to acute tubular cell injury. Taken together, these findings indicate that ischemic acute tubular injury also plays an important role in the pathogenesis of AKI in TAFRO syndrome, especially in the most severe cases that require dialysis, and this might have contributed to the dramatic and rapid recovery of the renal function upon treatment in our patient. Our investigation also suggested that the renal recovery rate of remission patients with TAFRO syndrome was good and that an advanced age might be a risk factor for the AKI-CKD transition (Table 3).
The limitations of our analysis are its small size and bias in race and severity of cases. Since we needed to collect cases with complete data, the number of cases was small, and the reviewed cases included many severe patients. In addition, the duration of recovery will vary depending on the definition of recovery, so it is necessary to be careful in interpreting the recovery periods.
Our analysis of the recovery duration might aid clinicians in deciding the intensity of immunosuppression. Furthermore, our analysis of peer-reviewed cases demonstrated crucial differences in the recovery durations of the various symptoms of TAFRO syndrome. We also found that the renal impairments seen in TAFRO syndrome can be reversed with appropriate treatment.
Conclusion
We experienced a case of severe TAFRO syndrome with exacerbation of chronic liver disease successfully treated by a multidisciplinary approach. Our investigation also suggested that the renal recovery rate of remission patients with TAFRO syndrome was good. Furthermore, our findings on the crucial differences in recovery periods of various symptoms might help clinicians plan treatments and tailor the intensity of immunosuppression.
Written consent for publication of this case report was obtained from the patient.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors would like to thank Dr. Muso for the valuable discussion regarding the manuscript.
References
- 1. Takai K, Nikkuni K, Shibuya H, Hashidate H. [Thrombocytopenia with mild bone marrow fibrosis accompanied by fever, pleural effusion, ascites and hepatosplenomegaly]. Rinsho Ketsueki Jpn (J Clin Hematol) 51: 320-325, 2010. [PubMed] [Google Scholar]
- 2. Masaki Y, Kawabata H, Fujimoto S, et al. Epidemiological analysis of multicentric and unicentric Castleman disease and TAFRO syndrome in Japan. J Clin Exp Hematop 59: 175-178, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fujimoto S, Sakai T, Kawabata H, et al. Is TAFRO syndrome a subtype of idiopathic multicentric Castleman disease? Am J Hematol 94: 975-983, 2019. [DOI] [PubMed] [Google Scholar]
- 4. Fujimoto S, Kawabata H, Sakai T, et al. Optimal treatments for TAFRO syndrome: a retrospective surveillance study in Japan. Int J Hematol 113: 73-80, 2021. [DOI] [PubMed] [Google Scholar]
- 5. Masaki Y, Kawabata H, Takai K, et al. 2019 updated diagnostic criteria and disease severity classification for TAFRO syndrome. Int J Hematol 111: 155-158, 2020. [DOI] [PubMed] [Google Scholar]
- 6. Ozeki T, Tsuji M, Yamamoto J, Shigematsu C, Maruyama S. Thrombotic microangiopathy on kidney biopsy in a patient with TAFRO syndrome. CEN Case Rep 7: 243-247, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takayama Y, Kubota T, Ogino Y, Ohnishi H, Togitani K, Yokoyama A. TAFRO syndrome with disseminated intravascular coagulation successfully treated with tocilizumab and recombinant thrombomodulin. Intern Med (Tokyo, Japan) 57: 1291-1296, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shimada Y, Adachi M, Kobayashi N, et al. A superelderly case of TAFRO syndrome treated effectively using corticosteroid hormones. Clin Case Rep 6: 638-643, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ito S, Uchida T, Itai H, et al. Serial manifestation of acute kidney injury and nephrotic syndrome in a patient with TAFRO syndrome. Intern Med 57: 3129-3133, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fujiwara Y, Ito K, Takamura A, Nagata K. The first case of thrombocytopenia, anasarca, fever, renal impairment or reticulin fibrosis, and organomegaly (TAFRO) syndrome with unilateral adrenal necrosis: a case report. J Med Case Rep 12: 295, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xia P, Zhang L, Zou M, et al. Acute kidney injury caused by TAFRO syndrome in a Chinese patient: efficacy of long-term corticosteroids combined with bortezomib and cyclophosphamide. Kidney Blood Press Res 45: 623-630, 2020. [DOI] [PubMed] [Google Scholar]
- 12. Hashimoto K, Sano T, Honma Y, et al. An autopsy case of TAFRO syndrome with membranoproliferative glomerulonephritis-like lesions. CEN Case Rep 8: 48-54, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nagayama Y, Yamano M, Yagame M, et al. TAFRO syndrome as a cause of glomerular microangiopathy: a case report and literature review. BMC Nephrol 20: 375, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shirai T, Onishi A, Waki D, Saegusa J, Morinobu A. Successful treatment with tacrolimus in TAFRO syndrome: two case reports and literature review. Medicine 97: e11045, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kikuchi T, Shimizu T, Toyama T, Abe R, Okamoto S. Successful treatment of TAFRO syndrome with tocilizumab, prednisone, and cyclophosphamide. Intern Med 56: 2205-2211, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okumura M, Ujiro A, Otsuka Y, et al. Cardiac arrest caused by rapidly increasing ascites in a patient with TAFRO syndrome: a case report. Acute Med Surg 4: 344-348, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suzuki K, Matsumoto T, Iwashita Y, et al. Clinicopathological features of TAFRO syndrome complicated by acquired hemophilia A and development of cardiopulmonary arrest that were successfully treated with VA-ECMO and tocilizumab. Int J Hematol 109: 737-743, 2019. [DOI] [PubMed] [Google Scholar]
- 18. Fujiki T, Hirasawa S, Watanabe S, Iwamoto S, Ando R. Successful treatment by tocilizumab without steroid in a very severe case of TAFRO syndrome. CEN Case Rep 6: 105-110, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iwaki N, Gion Y, Kondo E, et al. Elevated serum interferon γ-induced protein 10 kDa is associated with TAFRO syndrome. Sci Rep 7: 42316, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leurs A, Gnemmi V, Lionet A, et al. Renal pathologic findings in TAFRO syndrome: is there a continuum between thrombotic microangiopathy and membranoproliferative glomerulonephritis? A case report and literature review. Front Immunol 10: 1489, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsuhisa T, Takahashi N, Nakaguro M, et al. Fatal case of TAFRO syndrome associated with over-immunosuppression: a case report and review of the literature. Nagoya J Med Sci 81: 519-528, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamaguchi Y, Maeda Y, Shibahara T, et al. Recovery from prolonged thrombocytopenia in patients with TAFRO syndrome: case series and literature review. Mod Rheumatol Case Rep 4: 302-309, 2020. [DOI] [PubMed] [Google Scholar]
- 23. Godeau B, Chevret S, Varet B, et al. Intravenous immunoglobulin or high-dose methylprednisolone, with or without oral prednisone, for adults with untreated severe autoimmune thrombocytopenic purpura: a randomised, multicentre trial. Lancet 359: 23-29, 2002. [DOI] [PubMed] [Google Scholar]
- 24. George JN, El-Harake MA, Raskob GE. Chronic idiopathic thrombocytopenic purpura. N Engl J Med 331: 1207-1211, 1994. [DOI] [PubMed] [Google Scholar]
- 25. Cheng Y, Wong RS, Soo YO, et al. Initial treatment of immune thrombocytopenic purpura with high-dose dexamethasone. N Engl J Med 349: 831-836, 2003. [DOI] [PubMed] [Google Scholar]
- 26. Mizuno H, Sawa N, Watanabe S, et al. The clinical and histopathological feature of renal manifestation of TAFRO syndrome. Kidney Int Rep 5: 1172-1179, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]