Abstract
Ponatinib is a novel multi-tyrosine kinase inhibitor (TKI) with potent inhibitory activity against refractory chronic myeloid leukemia (CML). Despite its high clinical efficacy, ponatinib induces various adverse events due to its multi-target characteristic. However, renal complications associated with ponatinib are rare. A 76-year-old woman had a history of chronic myeloid leukemia (CML) resistant to imatinib and nilotinib. Our patient developed proteinuria and renal function deterioration during treatment with ponatinib but not with imatinib or nilotinib. We herein report the first case of a patient with secondary focal segmental glomerulosclerosis (FSGS) with partial glomerular collapse induced by ponatinib treatment.
Keywords: focal segmental glomerulosclerosis, ponatinib, tyrosine kinase inhibitor, endothelial injury, vascular endothelial growth factor receptor (VEGFR)
Introduction
Tyrosine kinase inhibitors (TKIs) prevent the activity of one or more receptor tyrosine kinase (RTK) families and exert diverse effects depending upon their specificity (1). TKIs are commonly used as anti-cancer drugs, and they substantially improve the outcome in multiple types of cancer. Due to their multi-targeted characteristics, however, TKIs are associated with various adverse effects in several organs.
Ponatinib is an orally administered TKI that exerts potent inhibitory activity against native and mutant breakpoint cluster region-Abelson (BCR-ABL), including the gatekeeper mutant T315I (2). Recent studies have demonstrated that ponatinib shows high efficacy in heavily pretreated patients with chronic myeloid leukemia (CML) and Philadelphia chromosome-positive acute lymphoblastic leukemia (2-4). Ponatinib has diverse target molecules, such as BCR-ABL, fibroblast growth factor receptors (FGFRs) 1-4, vascular endothelial growth factor receptor (VEGFR) 2, platelet derived growth factor receptor-α (PDGFRα), RET, and c-kit (4). Common side effects of ponatinib include rash, abdominal pain, thrombocytopenia, hypertension, and arterial occlusive events (3,5-8). However, glomerular lesions associated with ponatinib have not been reported.
We herein report a patient with secondary focal segmental sclerosis (FSGS) concurrent with endothelial injury that developed after initiating ponatinib during the CML treatment course. To our knowledge, this is the first report of secondary FSGS associated with ponatinib.
Case Report
A 76-year-old woman was referred to our department due to severe proteinuria and renal function deterioration. She had been diagnosed with CML five years earlier, and TKI therapy was initiated. Her status was refractory to imatinib and nilotinib, and ponatinib had been initiated three years before admission. Under imatinib and nilotinib treatment, her blood pressure and renal function were normal, and proteinuria was negative. Although she achieved a satisfactory hematologic response under ponatinib therapy, a complete cytogenetic response had not been achieved. After initiating ponatinib, she developed severe hypertension, for which several anti-hypertensive medications were prescribed. On a urinalysis, her urinary protein levels had ranged from (-) to (±) for two years after the initiation of ponatinib. One year before admission, however, her urinary protein levels gradually increased, ranging from (1+) to (2+). Three months before admission, she noticed pitting edema on her lower extremities, and 1 month before admission, the serum albumin level had decreased to 2.4 g/dL with a urinary protein level of 8.2 g/gCre. The serum creatinine level had increased from 0.8 mg/dL at baseline to 1.3 mg/dL. She was therefore diagnosed with nephrotic syndrome and admitted to our department for a further evaluation and treatment.
On admission, she was on ponatinib, nifedipine 20 mg, candesartan 8 mg, and furosemide 40 mg. Her family history and social history were unremarkable. On a physical examination, her height was 147.4 cm, and her body weight was 36.0 kg. Vital sign test results were as follows: blood pressure, 170/78 mmHg; pulse rate, 95 beats/min; temperature, 36.2°C; and respiratory rate, 20 breaths/min. Bilateral pitting edema on the lower extremities was evident.
Laboratory data on admission revealed the following results: white blood cell count, 5,630 /μL; hemoglobin, 10.0 g/dL; hematocrit, 31.6%; platelet count, 135,000 /μL; creatinine, 1.6 mg/dL; blood urea nitrogen, 37 mg/dL; total protein, 5.2 g/dL; albumin, 2.4 g/dL; sodium, 144 mEq/L; potassium, 3.2 mEq/L; chloride, 106 mEq/L; calcium, 7.2 mg/dL; phosphate, 3.7 mg/dL; glucose, 113 mg/dL; C3, 138.9 mg/dL; and C4, 46.0 mg/dL. A urinalysis showed high proteinuria (7.9 g/gCre) and high urinary N-Acetyl-β-(D)-glucosaminidase (NAG) levels (23.2 U/L), without microscopic hematuria or Bence-Jones protein. Serum antibody test results, including anti-nuclear antibody, myeloperoxidase-antineutrophil cytoplasmic antibody (MPO-ANCA), proteinase-3-anti-neutrophil cytoplasmic antibody (PR3-ANCA), and anti-glomerular basement membrane (GBM) antibody, were negative. Chest X-ray and computed tomography revealed moderate pleural effusion.
Based on the clinical course and laboratory findings, we suspected ponatinib-induced nephrotic syndrome, but we needed to exclude other etiologies, such as primary glomerulonephritis. Because the patient had not achieved a complete cytogenic response, ponatinib treatment could not be discontinued without convincing clinical evidence. Therefore, to confirm the underlying etiology of nephrotic syndrome, we performed a percutaneous kidney biopsy on hospital day 2.
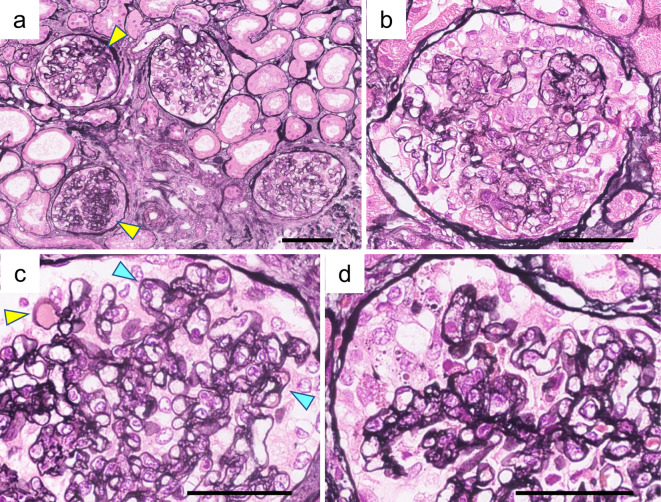
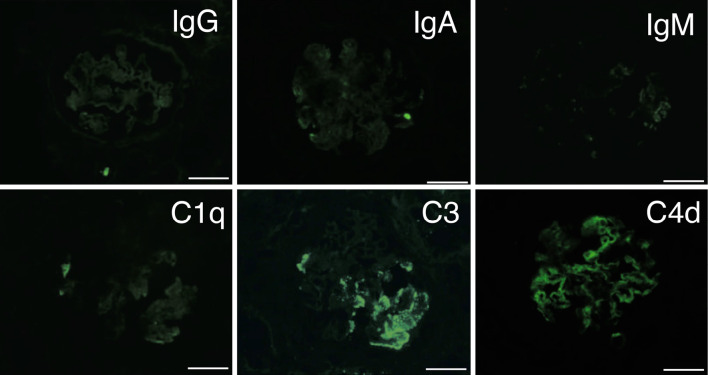
Light microscopy revealed 42 glomeruli, 14 of which had global sclerosis. There were nine segmental sclerotic glomeruli (Fig. 1a). Podocyte hypertrophy and hyperplasia were observed in most of the glomeruli. Glomeruli with prominent podocyte hyperplasia showed endocapillary hypercellularity (Fig. 1b). Hyalinosis and partial double contour were also found (Fig. 1c). Glomeruli with segmental sclerosis revealed collapsed capillaries covered with swelling podocytes involving droplets (Fig. 1d). Tubular atrophy and fibrosis were also evident. The grade of interstitial fibrosis/tubular atrophy was Grade II (moderate interstitial fibrosis and tubular atrophy), accounting for approximately 40% of the cortical area. Interlobular arteries showed arteriosclerosis without intimal hyperplasia or vascular luminal occlusion. Immunofluorescence revealed nonspecific granular deposits of C3 and C4d in the capillary loop and mesangial area (Fig. 2). Staining for C1q, IgG, IgA, and IgM was negative.
Figure 1.
Renal histological findings on light microscopy. A renal biopsy revealed the collapsing FSGS variant with concurrent endothelial damage. Periodic acid-methenamine-silver staining (PAM) revealed the following: (a) Focal segmental sclerotic lesion (yellow arrowheads). (b) Podocyte hyperplasia and endocapillary hypercellularity. (c) Hyalinosis (yellow arrowhead) and double contour (blue arrowheads). (d) Segmental collapsed capillary with podocyte hyperplasia. (a) Bars=100 μm; (b-d) Bars=50 μm.
Figure 2.
Immunofluorescence study. Immunofluorescence revealed focal granular staining of C3 and C4d in the capillary loop and mesangial area. Immunofluorescence for C1q, IgG, IgA, and IgM was negative. Bars = 50 μm
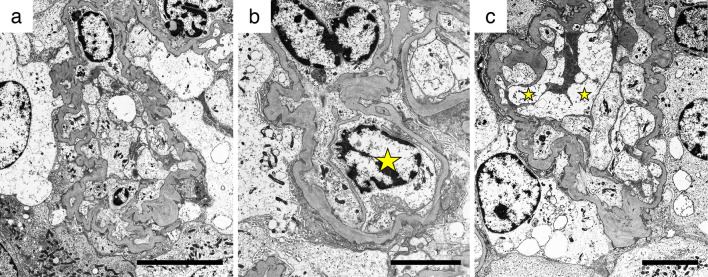
Next, we performed an electron microscopic analysis. Diffuse foot process effacement was observed (Fig. 3a, b). Capillaries were collapsed with podocyte hypertrophy (Fig. 3a). Endothelial cell swelling with double contour and mesangial interposition was shown in Fig. 3b. Enlargement of the subendothelial space was also observed (Fig. 3c). Villi formation of the podocytes (Fig. 3b) and vacuolar degeneration of swelling podocytes (Fig. 3c) were also shown. No dense deposition was observed under electron microscopy. These findings indicated both podocyte and endothelial injury. Finally, the pathological diagnosis was the collapsing FSGS variant, in accordance with the Columbia classification criteria.
Figure 3.
Images on electron microscopy. Electron microscopy revealed podocyte injury and endothelial damage, as follows: (a) Diffuse foot process effacement, podocyte hypertrophy, and collapsed capillaries. (b) Endothelial cell swelling (star), double contour, and mesangial interposition. (c) Podocyte hypertrophy, endothelial cell swelling (star), and enlargement of the subendothelial space. (a) Bars=10 μm; (b, C) Bars=5 μm.
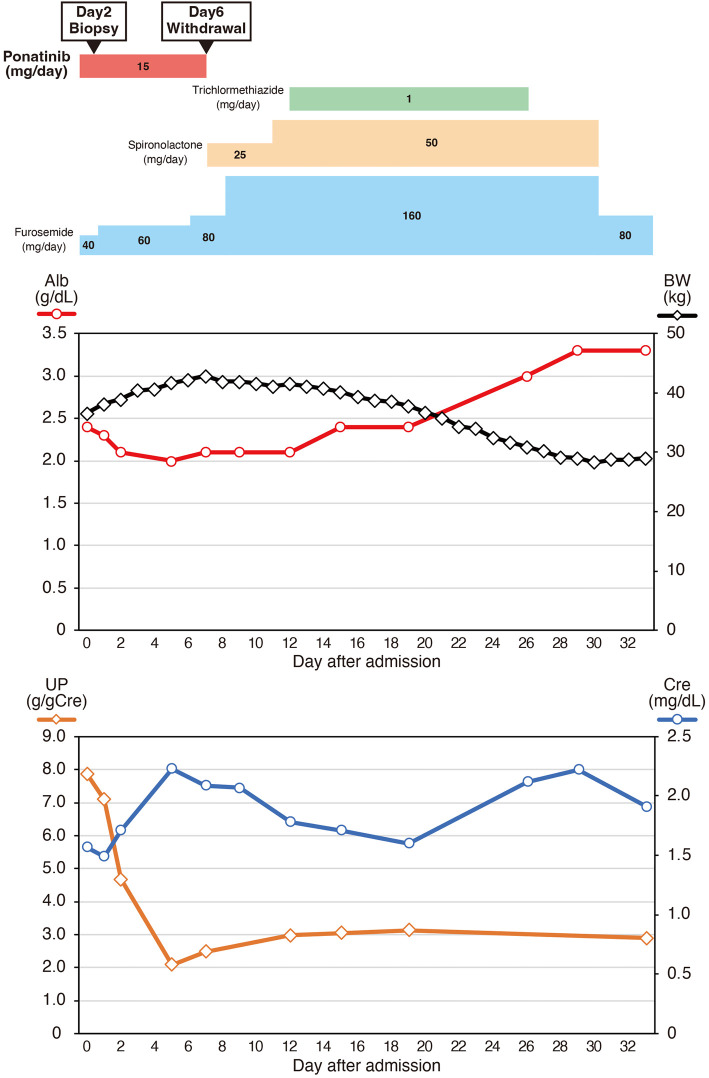
Based on the clinical course and pathological findings, we confirmed a diagnosis of secondary FSGS concurrent with endothelial injury induced by ponatinib treatment. We discontinued ponatinib on hospital day 6 after hematologist consultation and carefully obtaining informed consent (Fig. 4). We also increased the furosemide dose and administered spironolactone and trichlormethiazide. The serum albumin levels gradually increased above 3.0 g/dL, and the patient's body weight recovered to the baseline weight on hospitalization day 26. Her blood pressure decreased to 130/70 mmHg, and the proteinuria levels decreased from 8.2 to 2.0-3.0 g/gCre. The serum creatinine level varied between 1.5 and 2.2 mg/dL during hospitalization. Because the patient's serum albumin levels and volume status improved, she was discharged on hospital day 34. After 2 years of outpatient follow-up, the urinary protein excretion had gradually decreased to 1.0-1.5 g/gCre with stable serum creatinine levels of 1.0 mg/dL. Even without ponatinib treatment, her CML clinical status was stable.
Figure 4.
Clinical course after admission. Ponatinib was discontinued on day 6 after admission. Although proteinuria persisted in the range of 2.0-3.0 g/gCre, serum albumin levels gradually increased, and fluid retention improved after increasing the diuretic dose. The patient was discharged on hospital day 34.
Discussion
Although previous reports suggest that ponatinib is associated with hypertension (5-8), renal complications associated with ponatinib are rare. To our knowledge, this is the first case report that describes pathological findings of renal complications associated with ponatinib treatment. In the present case, prominent podocyte injury and endothelial cell damage were evident using both light and electron microscopy, which is consistent with endothelial injury with concurrent FSGS. Although endothelial injury was present, typical pathological findings related to thrombotic microangiopathy (TMA), such as luminal occlusion with fibrin thrombi, were not observed. In addition to acute lesions, chronic lesions were also observed. Fourteen of 42 glomeruli showed global sclerosis, and interlobular artery arteriosclerosis was observed. Because this patient had concomitant hypertension after ponatinib was initiated, these pathological findings are presumably induced by chronic hypertension and prolonged vascular endothelial damage. Despite both severe endothelial and podocyte injury with chronic lesions, her renal function improved gradually after ponatinib withdrawal, which suggests that glomerular disorder induced by ponatinib might recover to some extent.
In the present case, three years elapsed between ponatinib initiation and nephrotic syndrome onset. The duration between initiating TKIs and the renal complication onset is variable (9-11). In a single-center retrospective study evaluating patients with renal complications associated with TKIs or anti-VEGF therapy, the duration before the onset of renal adverse events ranged from 0.25 to 30 months in 21 patients with minimal change in nephrotic syndrome or FSGS (11). The rationale for the large variety in latent periods in TKI-related renal complications remains unclear. In addition, the TKI-related differences of latent periods have not been investigated. A greater number of cases is necessary to clarify typical clinical presentations of ponatinib-induced nephropathy.
One of the most interesting points in our case is that the patient did not exhibit renal complications under treatment with the conventional TKIs imatinib and nilotinib, instead developing nephrotic syndrome exclusively under ponatinib treatment. This may be attributed to the multi-target characteristic of ponatinib. Unlike imatinib or nilotinib, ponatinib has inhibitory activity against VEGFR2 (4,12), which can induce both podocyte and endothelial injury. The underlying mechanism of glomerular injury under TKI treatment remains the focus of vigorous research, but studies indicate that VEGF antibody (VEGFA)-VEGFR2 signaling plays a crucial role (13). VEGFA is expressed on podocytes and contributes to the development and maintenance of the normal glomerular structure (14,15). VEGFA binds to the RTKs VEGFR2 and VEGFR1, and VEGFR2, which is primarily expressed on glomerular endothelial cells (16), is responsible for most VEGFA signaling. VEGFA produced by podocytes presumably travels across the GBM and affects endothelial cells in a paracrine manner, which contributes to the development and maintenance of the glomerular filtration barrier (15,17,18). Inducible podocyte-specific deletion of VEGFA in adult mice leads to endothelial injury and TMA, which is consistent with renal pathological findings in patients under anti-VEGF therapy (18). In addition to the paracrine effect of VEGFA, autocrine VEGFA-VEGFR2 signaling in the podocyte might also play a role in maintaining the podocyte function and normal glomerular structure (19,20). In our case, VEGFR2 inhibition specifically induced by ponatinib might have disrupted the VEGFA-VEGFR2 signaling in glomeruli, resulting in podocyte injury and endothelial cell damage.
Although VEGFR2 inhibition can directly induce podocyte injury, a recent study also highlighted the bidirectional crosstalk of podocyte and endothelial injury (21). Previous studies have reported that TKI-induced FSGS is sometimes accompanied by endothelial injury and TMA-like lesions, and it is difficult to completely distinguish between FSGS and TMA (22,23). Buob et al. investigated renal biopsy samples from 53 patients with histologically proven TMA and revealed that 33 cases (62.3%) had concurrent FSGS lesions (24). According to the Columbia classification criteria, the collapsing variant was the predominant variant, and it was present in 19 cases (58%). In addition to the podocyte-to-endothelial damage described above, endothelial-derived factors, such as endothelin-1 and plasminogen activator inhibitor-1, also induce podocyte injury (25,26). The effects of endothelial-derived factors on podocytes and ensuing podocyte injury are also reported in preeclampsia, the main etiology of which is endothelial cell injury (27,28). Overall, while VEGFA inhibition in podocytes induces endothelial injury (18), endothelial injury might also play a crucial role in the development of podocyte injury and FSGS. Our patient's clinical course suggests that TKI with VEGFR2 inhibitory capacity can induce both FSGS and endothelial injury in the kidney. In addition, due to the small weight of the patient, the relatively high dosage of ponatinib might have resulted in the accumulation of intermediate metabolites and subsequent induction of nephrotoxicity. Future studies are necessary to further elucidate the underlying mechanisms of ponatinib-induced nephropathy.
We herein report the first case of a patient with secondary FSGS concurrent with endothelial injury, which was induced by ponatinib treatment. Regular monitoring of the renal function and urinary findings is essential for the early detection of renal adverse events during the administration of ponatinib and other TKIs. In the present case, a renal biopsy was helpful in determining the treatment strategy. In the field of onco-nephrology, clinicians are sometimes confronted with conflicting demands, such as treating cancer and protecting the renal function. Close coordination between oncologists and nephrologists is required to provide optimal treatment for patients with cancer.
Written consent for the publication of this case report was obtained from the patient.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Estrada CC, Maldonado A, Mallipattu SK. Therapeutic inhibition of VEGF signaling and associated nephrotoxicities. J Am Soc Nephrol 30: 187-200, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cortes JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med 367: 2075-2088, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cortes JE, Kim DW, Pinilla-Ibarz J, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood 132: 393-404, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tan FH, Putoczki TL, Stylli SS, Luwor RB. Ponatinib: a novel multi-tyrosine kinase inhibitor against human malignancies. Onco Targets Ther 12: 635-645, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jhaveri KD, Wanchoo R, Sakhiya V, Ross DW, Fishbane S. Adverse renal effects of novel molecular oncologic targeted therapies: a narrative review. Kidney Int Rep 2: 108-123, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chan O, Talati C, Isenalumhe L, et al. Side-effects profile and outcomes of ponatinib in the treatment of chronic myeloid leukemia. Blood Adv 4: 530-538, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lipton JH, Chuah C, Guerci-Bresler A, et al. Ponatinib versus imatinib for newly diagnosed chronic myeloid leukaemia: an international, randomised, open-label, phase 3 trial. Lancet Oncol 17: 612-621, 2016. [DOI] [PubMed] [Google Scholar]
- 8. Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med 369: 1783-1796, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Furuto Y, Hashimoto H, Namikawa A, et al. Focal segmental glomerulosclerosis lesion associated with inhibition of tyrosine kinases by lenvatinib: a case report. BMC Nephrol 19: 273, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ruebner RL, Copelovitch L, Evageliou NF, Denburg MR, Belasco JB, Kaplan BS. Nephrotic syndrome associated with tyrosine kinase inhibitors for pediatric malignancy: case series and review of the literature. Pediatr Nephrol 29: 863-869, 2014. [DOI] [PubMed] [Google Scholar]
- 11. Izzedine H, Escudier B, Lhomme C, et al. Kidney diseases associated with anti-vascular endothelial growth factor (VEGF): an 8-year observational study at a single center. Medicine (Baltimore) 93: 333-339, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carneiro BA, Kaplan JB, Giles FJ. Tyrosine kinase inhibitor therapy in chronic myeloid leukemia: update on key adverse events. Expert Rev Hematol 8: 457-479, 2015. [DOI] [PubMed] [Google Scholar]
- 13. Kandula P, Agarwal R. Proteinuria and hypertension with tyrosine kinase inhibitors. Kidney Int 80: 1271-1277, 2011. [DOI] [PubMed] [Google Scholar]
- 14. Simon M, Gröne HJ, Jöhren O, et al. Expression of vascular endothelial growth factor and its receptors in human renal ontogenesis and in adult kidney. Am J Physiol 268: F240-F250, 1995. [DOI] [PubMed] [Google Scholar]
- 15. Eremina V, Sood M, Haigh J, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 111: 707-716, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dimke H, Sparks MA, Thomson BR, Frische S, Coffman TM, Quaggin SE. Tubulovascular cross-talk by vascular endothelial growth factor a maintains peritubular microvasculature in kidney. J Am Soc Nephrol 26: 1027-1038, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sison K, Eremina V, Baelde H, et al. Glomerular structure and function require paracrine, not autocrine, VEGF-VEGFR-2 signaling. J Am Soc Nephrol 21: 1691-1701, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129-1136, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guan F, Villegas G, Teichman J, Mundel P, Tufro A. Autocrine VEGF-A system in podocytes regulates podocin and its interaction with CD2AP. Am J Physiol Renal Physiol 291: F422-F428, 2006. [DOI] [PubMed] [Google Scholar]
- 20. Veron D, Reidy KJ, Bertuccio C, et al. Overexpression of VEGF-A in podocytes of adult mice causes glomerular disease. Kidney Int 77: 989-999, 2010. [DOI] [PubMed] [Google Scholar]
- 21. Fogo AB. Talking back: the podocytes and endothelial cells duke it out. Kidney Int 90: 1157-1159, 2016. [DOI] [PubMed] [Google Scholar]
- 22. Costero O, Picazo ML, Zamora P, Romero S, Martinez-Ara J, Selgas R. Inhibition of tyrosine kinases by sunitinib associated with focal segmental glomerulosclerosis lesion in addition to thrombotic microangiopathy. Nephrol Dial Transplant 25: 1001-1003, 2010. [DOI] [PubMed] [Google Scholar]
- 23. Takahashi D, Nagahama K, Tsuura Y, Tanaka H, Tamura T. Sunitinib-induced nephrotic syndrome and irreversible renal dysfunction. Clin Exp Nephrol 16: 310-315, 2012. [DOI] [PubMed] [Google Scholar]
- 24. Buob D, Decambron M, Gnemmi V, et al. Collapsing glomerulopathy is common in the setting of thrombotic microangiopathy of the native kidney. Kidney Int 90: 1321-1331, 2016. [DOI] [PubMed] [Google Scholar]
- 25. Kobayashi N, Ueno T, Ohashi K, et al. Podocyte injury-driven intracapillary plasminogen activator inhibitor type 1 accelerates podocyte loss via uPAR-mediated β1-integrin endocytosis. Am J Physiol Renal Physiol 308: F614-F626, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mahtal N, Lenoir O, Tharaux PL. Glomerular endothelial cell crosstalk with podocytes in diabetic kidney disease. Front Med (Lausanne) 8: 659013, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Collino F, Bussolati B, Gerbaudo E, et al. Preeclamptic sera induce nephrin shedding from podocytes through endothelin-1 release by endothelial glomerular cells. Am J Physiol Renal Physiol 294: F1185-F1194, 2008. [DOI] [PubMed] [Google Scholar]
- 28. Craici IM, Wagner SJ, Weissgerber TL, Grande JP, Garovic VD. Advances in the pathophysiology of pre-eclampsia and related podocyte injury. Kidney Int 86: 275-285, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]