Abstract
Background
Postpartum smoking relapse is a serious public health concern. Previous studies have identified several risk factors for postpartum smoking relapse; however, very little is known about the predictors of early postpartum smoking relapse. This study aimed to determine postpartum smoking relapse status and its associated risk factors at 1 month postpartum among Japanese women.
Methods
Data were obtained from 93,851 mothers with live births in an ongoing birth cohort study, the Japan Environment and Children’s Study. Data on smoking status and confounding variables were collected using self-administered questionnaires and medical record transcripts. Self-administered questionnaires were administered during the first trimester, second/third trimester, and 1 month after delivery. A multiple logistic regression analysis was performed.
Results
Among the 14,326 mothers who smoked during pregnancy, 10,917 (76.2%) quit smoking during pregnancy. Subsequently, 617 (5.7%) of the mothers who had quit relapsed smoking at 1 month postpartum. Maternal age (≤24, ≥35), maternal education (≤12 years), parity (≥Second), feeding method (Formula milk), partner smoking status during pregnancy (Smoker), number of cigarettes per day before the cessation of smoking (≥11), maternal alcohol consumption at 1-month postpartum (Drinker), postpartum depression (EPDS score ≥9), and spending time at the parents’ home after delivery (≥14 days) were associated with smoking relapse.
Conclusions
A certain number of mothers relapsed even 1 month postpartum. Besides mother's alcohol and smoking habit before pregnancy, breastfeeding and partner smoking are important factors in early postpartum smoking relapse in Japan.
Keywords: Postpartum smoking relapse, Maternal smoking, Smoking cessation during pregnancy, Factor of smoking relapse
Introduction
Maternal smoking during pregnancy is a critical public health issue. Active smoking increases the risk of some cancers [1–3] and other preventable diseases such as cardiovascular diseases, respiratory diseases, and diabetes mellitus [4, 5]. Smoking during pregnancy increases the risk of hypertensive disorders of pregnancy [6] and postpartum depression [7]. In addition, it is well known that maternal smoking during pregnancy increases not only the risk of adverse birth outcomes, such as low birth weight [8, 9], stillbirth, neonatal death, perinatal death [10], and preterm birth [11], but also their health risks such as atopic eczema [12], wheezing, and asthma [13]. Under these circumstances, pregnancy is an opportunity for women to quit smoking. Previous studies reported that 33.9–53.0% of women who smoked quit smoking when they become pregnant [14–16] and it is 66.5–77.7% in Japan [13, 17, 18].
However, some mothers who quit then resume smoking after giving birth, and this relapse causes postnatal exposure to tobacco smoke among infants. Postnatal secondhand exposure to tobacco smoke increases the risk for various childhood illnesses, including asthma [19, 20], severe bronchiolitis [21] and attention-deficit/hyperactivity disorder symptoms [22]. It has been reported that 22.5% of women who quit smoking during their pregnancy relapsed at 3–4 months postpartum and 43.4–70.3% of that after 18 months postpartum in Japan [17, 18]. Therefore, efforts to keep mothers who quit smoking abstinent after childbirth are also extremely important.
Several previous studies have described the risk factors for maternal smoking relapse. According to a systematic review conducted by Orton et al. [23], being less educated, younger maternal age, multiparity, living with a partner or household member who smoked, experiencing higher stress, depression or anxiety, not breastfeeding, intending to quit only for pregnancy, and low confidence to remain abstinent postpartum were significant predictors of postpartum smoking relapse. Similarly, a shorter breastfeeding period, younger maternal age, women who were employed at the time of survey, women having a partner who smoked after giving birth were positive predictors for maternal postpartum relapse, and women spending time with their child in a relaxed mood and women having someone to talk to on the Internet about childrearing were negative predictors in the Japanese studies [17, 18].
Furthermore, early postpartum smoking relapse immediately after childbirth is an additional serious issue because of the exposure of infants to tobacco-derived chemical compounds through breastfeeding. Indeed, nicotine, cotinine, and 3-hydroxycotinine have been detected in smoking mothers’ breast milk [24]. Maternal smoking during lactation increases the risk of sudden infant death syndrome, neurodevelopmental and behavioral disorders, and sleep disruption in offspring as short-term health outcomes [25]. Previous studies conducted in the United Kingdom and United States reported that 46.5% of women who quit smoking during pregnancy relapsed smoking at 6 weeks postpartum in the United Kingdom, and 27.2% at 1 month postpartum in the United States [26, 27]. In those studies, women who lived in deprived urban areas, had three or more children, had other smokers in the household, not intending to remain abstinent and lower quitting confidence were included as predictor of early postpartum smoking relapse. In addition, breastfeeding-related variables are also important factors; women who were breastfed were significantly less likely to relapse in the United Kingdom, and women not planning to breastfeed at 1 month postpartum were significantly more likely to relapse in the United States [26, 27].
However, the smoking relapse rate in the early postpartum period and its predictors have not yet been clarified in Japan. Therefore, the first purpose of the present study was to determine the rate of postpartum smoking relapse at 1 month postpartum in Japan using a nationwide large birth cohort study. The second purpose was to clarify the risk factors associated with smoking relapse at 1 month postpartum. This information will be useful to keep mothers abstinent from smoking relapse in the early postpartum period.
Methods
Study design and participants
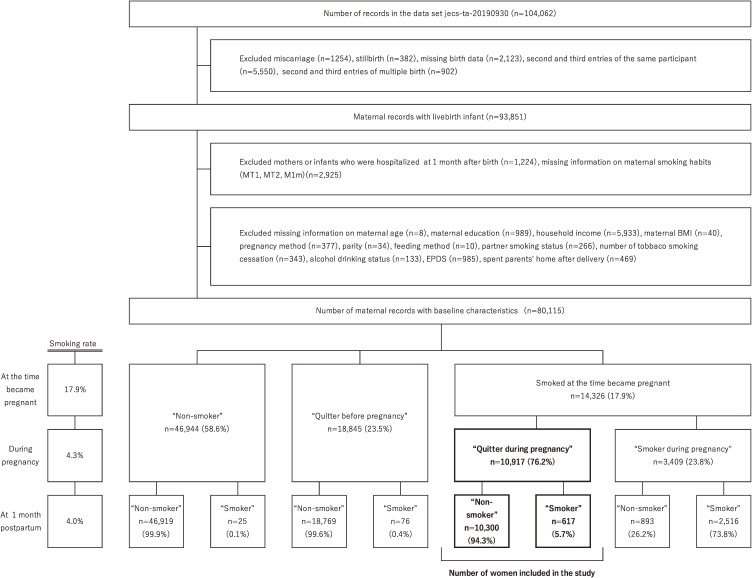
The methodology and protocol of the Japan Environment and Children’s Study (JECS) have been previously described in detail [28, 29]. The JECS is an ongoing nationwide birth cohort study, which is a national project funded directly by the Ministry of Environment to elucidate the influence of environmental factors on health. Between January 2011 and March 2014, we registered >100,000 pregnant women. Written informed consent was obtained from all the participants. The present study was based on the jecs-ta-20190930 dataset, which includes 104,062 fetal records, and was released in October 2019. From this dataset, women with miscarriage, stillbirth, missing birth data, second and third entries of the same participant, second and third entries of multiple births, mothers or infants hospitalized at 1 month after birth, missing information on maternal smoking habit during pregnancy, or self-administered questionnaires 1 month after delivery were excluded. We further excluded missing data on maternal age, maternal education, household income, maternal body mass index (BMI), fertility treatment status, parity, feeding method, partner smoking status during pregnancy, number of cigarettes smoked before quitting smoking, alcohol drinking status 1 month after delivery, score of Edinburgh Postnatal Depression Scale (EPDS) [30] and time spent at parents’ home after delivery. Women who quit smoking during pregnancy were included in this study, and 10,917 women were analyzed to determine the risk factors associated with postpartum smoking relapse 1 month after delivery (Fig. 1). The JECS protocol was reviewed and approved by the Ministry of the Environment’s Institutional Review Board on Epidemiological Studies and the Ethics Committees of all participating institutions (Ethical Number: No. 100910001).
Fig. 1.
Flow chart for selection of women from JECS and participants’ smoking status.
MT1, self-administered questionnaires conducted during the first trimester of pregnancy; MT2, self-administered questionnaires conducted during the second/third trimester of pregnancy; M1m, self-administered questionnaires conducted at one month after delivery; BMI, Body Mass Index; EPDS, Edinburgh Postnatal Depression Scale.
Data collection and variables
Outcome data and confounding variables were collected from self-administered questionnaires and medical record transcripts. Self-administered questionnaires were administered during the first trimester (MT1), second/third trimester (MT2), and one month after delivery (M1m).
Associated risk factors for postpartum smoking relapse which were reported by prior studies are included as independent variables; maternal age at delivery (<20, 20–24, 25–29, 30–34, 35–39, or ≥40), maternal education (≤12 years, or ≥13 years), annual household income (<2, 2–3.9, 4–5.9, 6–7.9, or ≥8 million JPY), pre-pregnancy BMI (<18.5, 18.5–24.9, or ≥25.0 kg/m2), parity (first, or ≥second), feeding method (breastmilk, breastmilk and formula milk, or formula milk), status of multiple birth (single birth or multiple birth), number of cigarettes smoked before cessation (1–10/day, ≥11/day), alcohol drinking status at 1 month after delivery (non-drinker, drinker), partner smoking status during pregnancy (Non-smoker, quitter before pregnancy, quitter during pregnancy, smoker) and maternal depression status after delivery (EPDS score <9, or ≥9). In addition to these factors, the status of spending time at parents’ home after delivery (did not spend at parents’ home or spent <14 days, or spent ≥14 days) was examined as a risk factor for postpartum smoking relapse. Finally, because women who underwent infertility treatment were less likely to smoke than those who did not [31], the status of infertility treatment (natural treatment, or infertility treatment) was included as an independent variable in this study.
Maternal and partner smoking status during pregnancy (MT1 and MT2) were scored on the questionnaire as “Non-smoker,” “Quitter before pregnancy,” “Quitter during pregnancy” or “Smoker.” Data on maternal and partner smoking status during pregnancy were based on the MT2 questionnaire, with results from the MT1 questionnaire used to complement the maternal smoking status when it was missing from the MT2 questionnaire. Maternal smoking status at 1 month after childbirth was scored on the M1m questionnaire as “Non-smoker,” “Quitter before pregnancy,” “Quitter during pregnancy,” “Smoker (1–10 pieces per day),” “Smoker (11–20 pieces per day)” or “Smoker (21 pieces or more per day).” We combined “Non-smoker,” “Quitter before pregnancy” or “Quitter during pregnancy” as the “Non-smoker” after delivery, while others including “Smoker (1–10 cigarettes/day),” “Smoker (11–20 cigarettes/day)” or “Smoker (21 pieces or more per day)” were the “Smoker” at 1 month after delivery. Among “Quitter during pregnancy” women based on MT1 and MT2 questionnaires, those who were the “Non-smoker” at M1m were classified as the postpartum maintained cessation group, and those who were the “Smoker” at M1m classified as the postpartum smoking relapse group (Fig. 1).
Data on maternal education and annual household income were obtained using the MT2 questionnaire. Pre-pregnancy BMI was calculated using the formula: kg/m2. Data on parity and feeding methods were based on medical record transcripts, with results from the MT1 questionnaire for parity or the M1m questionnaire for feeding methods to complement when they were missing. Data on the number of cigarettes smoked before cessation were based on the MT2 questionnaire, with results from the MT1 questionnaire used to complement the maternal smoking status. Data on alcohol consumption at 1 month after delivery, status of spent parents’ home after delivery, and maternal depression after delivery were obtained from the M1m questionnaire. The Japanese version of the Edinburgh Postnatal Depression Scale (EPDS) was used to assess maternal postpartum depression [30]. The EPDS is a self-administered 10-item scale used to screen for postpartum depression [32]. In this study, an EPDS score of ≥9 was defined as postpartum depression [30, 33].
Data analysis
Univariate associations between maternal postpartum smoking status and variables were examined using the Chi-square test, as appropriate. Variables with a Chi-square test of p < 0.05 were selected as covariates for multiple logistic regression analyses to identify predictors of smoking relapse in the early postpartum period. All statistical analyses were performed using JMP® 16 software (SAS Institute Inc., Cary, NC, USA).
Results
Smoking rates at the time of pregnancy, during pregnancy, and after delivery were 17.9%, 4.3%, and 4.0%, respectively. Among women who smoked at the time of pregnancy, 76.2% quit smoking during pregnancy. Among these women, 94.3% maintained smoking cessation, and the remaining 5.7% relapsed smoking at 1 month postpartum (Fig. 1).
Table 1 shows the characteristics of the 10,917 women who quit smoking during pregnancy. The majority of the women were 25–34 years old (63.3%), and just over half of the women were first-time mothers (51.2%). More than half of the women had ≤12 years of education (56.6%), and the most frequent household income was 2–3 million JPY (44.9%). Thirty-six percent of the women had smoked ≥11 cigarettes/day before smoking cessation. In total, 7.3% of women had been drinking alcohol 1 month after delivery. The majority of women reported that their partner had been smoking during their current pregnancy (74.3%).
Table 1.
Characteristics of women who quit smoking during pregnancy.
|
Total
(n = 10,917) |
Cessation
(n = 10,300) |
Relapse
(n = 617) |
p | ||||
|
|
|
|
|||||
| n | % | n | % | n | % | ||
| Maternal age at delivery (yrs) | |||||||
| <20 | 145 | 1.3 | 127 | 1.2 | 18 | 2.9 | <0.0001 |
| 20–24 | 1726 | 15.8 | 1615 | 15.7 | 111 | 18.0 | |
| 25–29 | 3594 | 32.9 | 3403 | 33.0 | 191 | 31.0 | |
| 30–34 | 3321 | 30.4 | 3168 | 30.8 | 153 | 24.8 | |
| 35–39 | 1834 | 16.8 | 1716 | 16.7 | 118 | 19.1 | |
| ≥40 | 297 | 2.7 | 271 | 2.6 | 26 | 4.2 | |
| Maternal education (yrs) | |||||||
| ≤12 | 6183 | 56.6 | 5740 | 55.7 | 443 | 71.8 | <0.0001 |
| ≥13 | 4734 | 43.4 | 4560 | 44.3 | 174 | 28.2 | |
| Household income (million JPY) | |||||||
| <2 | 1040 | 9.5 | 960 | 9.3 | 80 | 13.0 | <0.0001 |
| 2–3 | 4902 | 44.9 | 4591 | 44.6 | 311 | 50.4 | |
| 4–5 | 3162 | 29.0 | 2999 | 29.1 | 163 | 26.4 | |
| 6–7 | 1136 | 10.4 | 1098 | 10.7 | 38 | 6.2 | |
| ≥8 | 677 | 6.2 | 652 | 6.3 | 25 | 4.1 | |
| Pre-pregnancy BMI (kg/m2) | |||||||
| <18.5 | 1865 | 17.1 | 1764 | 17.1 | 101 | 16.4 | 0.015 |
| 18.5–24.9 | 7675 | 70.3 | 7260 | 70.5 | 415 | 67.3 | |
| ≥25 | 1377 | 12.6 | 1276 | 12.4 | 101 | 16.4 | |
| Fertility treatment | |||||||
| No | 10568 | 96.8 | 9962 | 96.7 | 606 | 98.2 | 0.040 |
| Yes | 349 | 3.2 | 338 | 3.3 | 11 | 1.8 | |
| Status of multiple birth | |||||||
| Single birth | 10845 | 99.3 | 10232 | 99.3 | 613 | 99.4 | 0.972 |
| Multiple birth | 72 | 0.7 | 68 | 0.7 | 4 | 0.6 | |
| Parity | |||||||
| First | 5586 | 51.2 | 5369 | 52.1 | 217 | 35.2 | <0.0001 |
| ≥Second | 5331 | 48.8 | 4931 | 47.9 | 400 | 64.8 | |
| Feeding method at 1 month after delivery | |||||||
| Breast milk | 5150 | 47.2 | 4961 | 48.2 | 189 | 30.6 | <0.0001 |
| Breast and formula milk | 5071 | 46.5 | 4783 | 46.4 | 288 | 46.7 | |
| Formula milk | 696 | 6.4 | 556 | 5.4 | 140 | 22.7 | |
| Partner smoking status during pregnancy | |||||||
| Non-smoker | 1138 | 10.4 | 1085 | 10.5 | 53 | 8.6 | 0.001 |
| Quitter before pregnancy | 827 | 7.6 | 794 | 7.7 | 33 | 5.4 | |
| Quitter during pregnancy | 840 | 7.7 | 808 | 7.8 | 32 | 5.2 | |
| Smoker | 8112 | 74.3 | 7613 | 73.9 | 499 | 80.9 | |
| Number of cigarettes use per day | |||||||
| 1–10 | 7020 | 64.3 | 6716 | 65.2 | 304 | 49.3 | <0.0001 |
| ≥11 | 3897 | 35.7 | 3584 | 34.8 | 313 | 50.7 | |
| Maternal drinking status at 1 months after delivery | |||||||
| Non-drinker | 10122 | 92.7 | 9646 | 93.7 | 476 | 77.1 | <0.0001 |
| Drinker | 795 | 7.3 | 654 | 6.3 | 141 | 22.9 | |
| EPDS score | |||||||
| <9 | 8894 | 81.5 | 8420 | 81.7 | 474 | 76.8 | 0.002 |
| ≥9 | 2023 | 18.5 | 1880 | 18.3 | 143 | 23.2 | |
| Spent at parents’ home after delivery | |||||||
| Did not spend or <14 days |
6108 | 56.0 | 5691 | 55.3 | 417 | 67.6 | <0.0001 |
| ≥14 days | 4809 | 44.1 | 4609 | 44.8 | 200 | 32.4 | |
BMI, Body Mass Index; EPDS, Edinburgh Postnatal Depression Scale.
Table 2 shows results from logistic regression analyses examining the association between selected factors and smoking relapse at 1 month postpartum. In the crude model, statistically significant associations were observed for all selected factors; maternal age, maternal education, house hold income, pre-pregnancy BMI, fertility treatment, parity, feeding methods at 1 month after delivery, partner smoking status during pregnancy, number of cigarettes use per day, maternal drinking status at 1 month after delivery, score of EPDS and spent at parents’ home after delivery. In the adjusted model, women who feed formula milk were more likely to relapse than women who feed only breast milk (adjusted OR = 1.44, 95% CI: 1.19–1.75 for breast and formula milk; adjusted OR = 4.05, 95% CI: 3.15–5.22 for only formula milk). Women who have been drinking alcohol at 1 month after delivery were three times more likely to relapse than women who have not started drinking alcohol (adjusted OR = 3.19, 95% CI: 2.56–3.97). For other factors, women who are aged <20 years were twice as likely to relapse compared to those aged 30–34 years (adjusted OR = 2.77, 95% CI: 1.56–4.93). Significant increase of relapse risk was also observed aged 20–24, 30–34 and ≥40 years (adjusted OR = 1.45, 95% CI: 1.10–1.90 for 20–24 years; adjusted OR = 1.30, 95% CI: 1.01–1.68 for 35–39 years; adjusted OR = 1.68, 95% CI: 1.06–2.65 for ≥40 years). Women who had ≤12 years of education were more likely to relapse than those who had ≥13 years of education (adjusted OR = 1.27, 95% CI: 1.05–1.55). Women who had ≥second parity were more likely relapse than <first parity (adjusted OR = 1.84, 95% CI: 1.51–2.23). There were also significant positive associations between partner smoking status during pregnancy, the number of cigarettes smoked per day, and EPDS scores. Women having partner who had smoked during the current pregnancy were more likely to relapse than who have non-smoker partner (adjusted OR = 1.42, 95% CI: 1.05–1.92). Women who had smoked ≥11 cigarettes per day before their smoking cessation were more likely to relapse than women who had smoked <11 cigarettes per day (adjusted OR = 1.78, 95% CI: 1.50–2.11). Women who had an EPDS score ≥9 were more likely to relapse than those with a score <9 (adjusted OR = 1.28, 95% CI: 1.04–1.57). Spending ≥14 days at parents’ home after delivery was found to be a protective factor for postpartum smoking relapse (adjusted OR = 0.79, 95% CI: 0.66–0.96). Significant associations were not observed with household income, pre-pregnancy BMI and fertility treatment status at the current pregnancy in the adjusted model.
Table 2.
Crude and adjusted odds ratios for factors associated with postpartum smoking relapse.
| Relapsed | Crude | Adjusted | |||
|
|
|
|
|||
| % | OR | 95%CI | OR | 95%CI | |
| Maternal age at delivery (yrs) | |||||
| <20 | 12.4 | 2.93 | 1.75–4.93 | 2.77 | 1.56–4.93 |
| 20–24 | 6.4 | 1.42 | 1.11–1.83 | 1.45 | 1.10–1.90 |
| 25–29 | 5.3 | 1.16 | 0.93–1.45 | 1.22 | 0.97–1.54 |
| 30–34 | 4.6 | reference | reference | ||
| 35–39 | 6.4 | 1.42 | 1.11–1.82 | 1.30 | 1.01–1.68 |
| ≥40 | 8.8 | 1.99 | 1.29–3.07 | 1.68 | 1.06–2.65 |
| Maternal education (yrs) | |||||
| ≤12 years | 7.2 | 2.02 | 1.69–2.42 | 1.27 | 1.05–1.55 |
| ≥13 years | 3.7 | reference | reference | ||
| Household income (million JPY) | |||||
| <2 | 7.7 | 1.53 | 1.16–2.02 | 1.22 | 0.91–1.64 |
| 2–3 | 6.3 | 1.25 | 1.03–1.51 | 1.14 | 0.93–1.40 |
| 4–5 | 5.2 | reference | reference | ||
| 6–7 | 3.3 | 0.64 | 0.44–0.91 | 0.75 | 0.52–1.08 |
| ≥8 | 3.7 | 0.71 | 0.46–1.08 | 0.78 | 0.50–1.22 |
| Pre-pregnancy BMI (kg/m2) | |||||
| <18.5 | 5.4 | 1.00 | 0.80–1.25 | 0.97 | 0.77–1.23 |
| 18.5–24.9 | 5.4 | reference | reference | ||
| ≥25 | 7.3 | 1.38 | 1.11–1.73 | 1.17 | 0.92–1.48 |
| Fertility treatment | |||||
| No | 5.7 | reference | reference | ||
| Yes | 3.2 | 0.53 | 0.29–0.98 | 0.75 | 0.40–1.41 |
| Parity | |||||
| First | 3.9 | reference | reference | ||
| ≥Second | 7.5 | 2.01 | 1.69–2.38 | 1.84 | 1.51–2.23 |
| Feeding method at 1 month after delivery | |||||
| Breast milk | 3.7 | reference | reference | ||
| Breast and formula milk | 5.7 | 1.58 | 1.31–1.91 | 1.44 | 1.19–1.75 |
| Formula milk | 20.1 | 6.61 | 5.22–8.36 | 4.05 | 3.15–5.22 |
| Partner smoking status during pregnancy | |||||
| Non-smoker | 4.7 | reference | reference | ||
| Quitter before pregnancy | 4.0 | 0.85 | 0.55–1.33 | 1.01 | 0.64–1.60 |
| Quitter during pregnancy | 3.8 | 0.81 | 0.52–1.27 | 0.93 | 0.59–1.48 |
| Smoker | 6.2 | 1.49 | 1.22–1.83 | 1.42 | 1.05–1.92 |
| Number of cigarettes use per day | |||||
| 1–10 | 4.3 | reference | reference | ||
| ≥11 | 8.0 | 1.93 | 1.64–2.27 | 1.78 | 1.50–2.11 |
| Maternal drinking status at 1 months after delivery | |||||
| Non-drinker | 4.7 | reference | reference | ||
| Drinker | 17.7 | 4.37 | 3.56–5.36 | 3.19 | 2.56–3.97 |
| EPDS score | |||||
| <9 | 5.3 | reference | reference | ||
| ≥9 | 7.1 | 1.35 | 1.11–1.64 | 1.28 | 1.04–1.57 |
| Spent at parents’ home after delivery | |||||
| Did not spend or <14 days | 6.8 | reference | reference | ||
| ≥14 days | 4.2 | 0.59 | 0.50–0.70 | 0.79 | 0.66–0.96 |
OR, Odds ratio; 95% CI, 95% Confidence Interval; BMI, Body Mass Index; EPDS, Edinburgh Postnatal Depression Scale.
Discussion
This study examined the smoking relapse rate and its associated risk factors during the early postpartum period using Japanese nationwide data. In this study, 5.7% of women who quit smoking during pregnancy relapsed at 1 month postpartum. Women who were feeding formula milk, drinking alcohol at 1 month postpartum, aged ≤24 or ≥35, had ≤12 years of education, had ≥second parity, had a partner who had smoked during the current pregnancy, had smoked ≥11 cigarettes per day before their smoking cessation, and had an EPDS score ≥9 were more likely to relapse smoking. Women spending ≥14 days at their parents’ home after delivery were less likely to relapse smoking in the early postpartum period.
In this study, 14,326 (17.9%) women smoked at the time they became pregnant, and 76.2% quit smoking during pregnancy (Fig. 1). Although this number is slightly higher than that reported in previous studies in Japan (76.2% in this study, 66.5–68.9 in previous studies) [17, 18], our study results support the view of previous studies that pregnancy highly motivated mothers to quit smoking [17, 18]. The smoking relapse rate at 1 month postpartum in this study was 5.6%, which is lower than that reported in previous studies in the US (27.2%) and the UK (46.5%) [26, 27]. Although this number of relapsed women was smaller than that in other countries, it was confirmed that a certain number of women relapsed in the early postpartum period in Japan.
Previous studies have suggested that breastfeeding protects against smoking relapse [27, 34]. Maternal smoking also increases the risk of early weaning [35]. Our findings are consistent with those of previous studies; the relapse rate of women who exclusively breastfed and partially breastfed was lower than that of women who did not breastfeed (Table 1). As a reason for not resuming smoking while breastfeeding, smoking mothers dislike the transfer of tobacco-derived chemicals to their infants. Nicotine, cotinine, and 3-hydroxycotinine have been detected in smoking mothers’ breastmilk [24], and nicotine and cotinine were detected in infant serum and urine from mothers who smoked through breastfeeding [36]. These circumstances are considered to be important obstacles to maternal relapse. The reason of lower smoking relapse rate in this study compere to US and UK may be related to breastfeeding status of subjects. Compared to previous studies, the proportion of breastfeeding among the subjects of this study was overwhelmingly high; 93.6% in this study, 50% in the study of US, 29.4% in the study of UK [26, 27]. Based on these findings, promotion of breastfeeding during maternity courses or prenatal checkups by local governments or clinics may be effective in preventing postpartum smoking relapse. An interventional study conducted by Logan et al. (2017) indicated that promoting breastfeeding is effective in maintaining smoking cessation prior to weaning [37]. Needless to say, exclusive breastfeeding of infants for the first 6 months of their life is recommended by the World Health Organization [38], since breastfeeding has many benefits.
Partner smoking is a predictor of postpartum smoking relapse [16, 17]. A similar result was observed in this study; women with a partner who smoked during pregnancy had a higher risk of postpartum smoking relapse than women with a partner who did not smoke. Our study additionally shows that having partners who quit smoking before or during pregnancy did not increase the risk of postpartum smoking relapse (Table 2). Therefore, the absence of smoking by partners during pregnancy is an important factor for preventing postpartum smoking relapse. Unfortunately, the number of partners who quit smoking during pregnancy is small, with only 9.4% of partners quitting smoking during pregnancy. Further efforts are required to help partners quit smoking before or during pregnancy. Considering that paternal smoking is also related to neonatal secondhand smoke, intervention for a partner smoking is an important issue to prevent maternal postpartum smoking relapse.
The strength of this study lies in its large sample size compared to previous studies. The limitations of this study include the fact that data on smoking status were obtained from self-administered questionnaire surveys instead of biological monitoring. In addition, because this was an observational study, causal relationships could not be concluded. This study indicated that not/less breastfeeding is a predictor of postpartum smoking relapse. However, it is unknown whether breastfeeding prevents maternal smoking relapse or if they do not breastfeed because they want to smoke.
Conclusions
This is the first study that examined the smoking relapse status during the early postpartum period in Japan using nationwide data. Among the women who smoked at the time they became pregnant, 76.2% quit smoking during pregnancy, and among these women, 5.6% had relapsed smoking at 1 month postpartum. Maternal age (≤24, ≥35), maternal education (≤12 years), parity (≥Second), feeding method (Formula milk), partner smoking status during pregnancy (Smoker), number of cigarettes per day before the cessation of smoking (≥11), maternal alcohol consumption at 1-month postpartum (Drinker), postpartum depression (EPDS score ≥9), and spending time at the parents’ home after delivery (≥14 days) were associated with smoking relapse. Among those predictors associated with maternal postpartum smoking relapse at 1 month postpartum, breastfeeding and partner smoking are important in preventing early postpartum smoking relapse in Japan.
Abbreviations
- JECS
the Japan Environment and Children’s Study
- BMI
body mass index
- EPDS
Edinburgh Postnatal Depression Scale
- MT1
first trimester
- MT2
second/third trimester
- M1m
one month after delivery
Declarations
Ethics approval and consent to participate
The JECS protocol was reviewed and approved by the Institutional Review Board on Epidemiological Studies of the Ministry of the Environment and the ethics committees of all participating institutions. The JECS was conducted in accordance with the Declaration of Helsinki and other internationally recognized regulations. Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Availability of data and material
Data are unsuitable for public deposition due to ethical restrictions and legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of 30 May 2003, amendment on 9 September 2015) to publicly deposit the data containing personal information. Ethical Guidelines for Medical and Health Research Involving Human Subjects enforced by the Japan Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare also restricts the open sharing of the epidemiologic data. All inquiries about access to data should be sent to: jecs-en@nies.go.jp. The person responsible for handling enquiries sent to this e-mail address is Dr Shoji F. Nakayama, JECS Programme Office, National Institute for Environmental Studies.
Competing of interests
The authors declare no conflicts of interest associated with this manuscript.
Funding
This study was funded by the Ministry of the Environment, Japan. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the above government.
Authors’ contributions
AA, NT and KN designed the study and wrote the draft. KS, SK, TA and KN contributed data curation, and investigation. AA, KA, NT and KN contributed statistical analysis. AA, KA, NT, KS, CO, JS, TA, NY and KN contributed Writing – review & editing. All authors contributed to and have approved the final manuscript.
Acknowledgements
The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the above government. We thank all participants and staff members of JECS for their cooperation to our study. Members of the JECS Group as of 2022: Michihiro Kamijima (principal investigator, Nagoya City University, Nagoya, Japan), Shin Yamazaki (National Institute for Environmental Studies, Tsukuba, Japan), Yukihiro Ohya (National Center for Child Health and Development, Tokyo, Japan), Reiko Kishi (Hokkaido University, Sapporo, Japan), Nobuo Yaegashi (Tohoku University, Sendai, Japan), Koichi Hashimoto (Fukushima Medical University, Fukushima, Japan), Chisato Mori (Chiba University, Chiba, Japan), Shuichi Ito (Yokohama City University, Yokohama, Japan), Zentaro Yamagata (University of Yamanashi, Chuo, Japan), Hidekuni Inadera (University of Toyama, Toyama, Japan), Takeo Nakayama (Kyoto University, Kyoto, Japan), Tomotaka Sobue (Osaka University, Suita, Japan), Masayuki Shima (Hyogo Medical University, Nishinomiya, Japan), Hiroshige Nakamura (Tottori University, Yonago, Japan), Narufumi Suganuma (Kochi University, Nankoku, Japan), Koichi Kusuhara (University of Occupational and Environmental Health, Kitakyushu, Japan), and Takahiko Katoh (Kumamoto University, Kumamoto, Japan).
References
- 1.Macacu A, Autier P, Boniol M, Boyle P. Active and passive smoking and risk of breast cancer: a meta-analysis. Breast Cancer Res Treat. 2015;154(2):213–24. [DOI] [PubMed] [Google Scholar]
- 2.Sugawara Y, Tsuji I, Mizoue T, Inoue M, Sawada N, Matsuo K, et al. Cigarette smoking and cervical cancer risk: an evaluation based on a systematic review and meta-analysis among Japanese women. Jpn J Clin Oncol. 2019;49(1):77–86. [DOI] [PubMed] [Google Scholar]
- 3.National Center for Chronic Disease P, Health Promotion Office on S, Health. Reports of the Surgeon General. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014.
- 4.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298(22):2654–64. [DOI] [PubMed] [Google Scholar]
- 5.Office of the Surgeon G, Office on S, Health. Reports of the Surgeon General. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2004. [PubMed]
- 6.Tanaka K, Nishigori H, Watanabe Z, Iwama N, Satoh M, Murakami T, et al. Higher prevalence of hypertensive disorders of pregnancy in women who smoke: the Japan environment and children’s study. Hypertens Res. 2019;42(4):558–66. [DOI] [PubMed] [Google Scholar]
- 7.Cui M, Kimura T, Ikehara S, Dong JY, Ueda K, Kawanishi Y, et al. Prenatal tobacco smoking is associated with postpartum depression in Japanese pregnant women: The japan environment and children’s study. J Affect Disord. 2020;264:76–81. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki K, Shinohara R, Sato M, Otawa S, Yamagata Z. Association Between Maternal Smoking During Pregnancy and Birth Weight: An Appropriately Adjusted Model From the Japan Environment and Children’s Study. J Epidemiol. 2016;26(7):371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereira PP, Da Mata FA, Figueiredo AC, de Andrade KR, Pereira MG. Maternal Active Smoking During Pregnancy and Low Birth Weight in the Americas: A Systematic Review and Meta-analysis. Nicotine Tob Res. 2017;19(5):497–505. [DOI] [PubMed] [Google Scholar]
- 10.Pineles BL, Hsu S, Park E, Samet JM. Systematic Review and Meta-Analyses of Perinatal Death and Maternal Exposure to Tobacco Smoke During Pregnancy. Am J Epidemiol. 2016;184(2):87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah NR, Bracken MB. A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. Am J Obstet Gynecol. 2000;182(2):465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka K, Miyake Y, Furukawa S, Arakawa M. Pre- and Postnatal Smoking Exposure and Risk of Atopic Eczema in Young Japanese Children: A Prospective Prebirth Cohort Study. Nicotine Tob Res. 2017;19(7):804–9. [DOI] [PubMed] [Google Scholar]
- 13.Wada T, Adachi Y, Murakami S, Ito Y, Itazawa T, Tsuchida A, et al. Maternal exposure to smoking and infant’s wheeze and asthma: Japan Environment and Children’s Study. Allergol Int. 2021;70(4):445–51. [DOI] [PubMed] [Google Scholar]
- 14.Carmichael SL, Ahluwalia IB. Correlates of postpartum smoking relapse. Results from the Pregnancy Risk Assessment Monitoring System (PRAMS). Am J Prev Med. 2000;19(3):193–6. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert NL, Nelson CR, Greaves L. Smoking cessation during pregnancy and relapse after childbirth in Canada. J Obstet Gynaecol Can. 2015;37(1):32–9. [DOI] [PubMed] [Google Scholar]
- 16.Lelong N, Kaminski M, Saurel-Cubizolles MJ, Bouvier-Colle MH. Postpartum return to smoking among usual smokers who quit during pregnancy. Eur J Public Health. 2001;11(3):334–9. [DOI] [PubMed] [Google Scholar]
- 17.Yasuda T, Ojima T, Nakamura M, Nagai A, Tanaka T, Kondo N, et al. Postpartum smoking relapse among women who quit during pregnancy: cross-sectional study in Japan. J Obstet Gynaecol Res. 2013;39(11):1505–12. [DOI] [PubMed] [Google Scholar]
- 18.Kaneko A, Kaneita Y, Yokoyama E, Miyake T, Harano S, Suzuki K, et al. Smoking trends before, during, and after pregnancy among women and their spouses. Pediatr Int. 2008;50(3):367–75. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida S, Mishina H, Takeuchi M, Kawakami K. [Association of prenatal maternal, prenatal secondhand, and postnatal secondhand smoking exposures with the incidence of asthma/atopic dermatitis in children: An epidemiological study using checkup data of mothers and children in Kobe city]. Nihon Koshu Eisei Zasshi. 2021;68(10):659–68. [DOI] [PubMed] [Google Scholar]
- 20.He Z, Wu H, Zhang S, Lin Y, Li R, Xie L, et al. The association between secondhand smoke and childhood asthma: A systematic review and meta-analysis. Pediatr Pulmonol. 2020;55(10):2518–31. [DOI] [PubMed] [Google Scholar]
- 21.Behrooz L, Balekian DS, Faridi MK, Espinola JA, Townley LP, Camargo CA Jr. Prenatal and postnatal tobacco smoke exposure and risk of severe bronchiolitis during infancy. Respir Med. 2018;140:21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin LZ, Xu SL, Wu QZ, Zhou Y, Ma HM, Chen DH, et al. Association of Prenatal, Early Postnatal, or Current Exposure to Secondhand Smoke With Attention-Deficit/Hyperactivity Disorder Symptoms in Children. JAMA Netw Open. 2021;4(5):e2110931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orton S, Coleman T, Coleman-Haynes T, Ussher M. Predictors of Postpartum Return to Smoking: A Systematic Review. Nicotine Tob Res. 2018;20(6):665–73. [DOI] [PubMed] [Google Scholar]
- 24.Nordenstam F, Lundell B, Edstedt Bonamy AK, Raaschou P, Wickström R. Snus users had high levels of nicotine, cotinine and 3-hydroxycotinine in their breastmilk, and the clearance was slower than in smoking mothers. Acta Paediatr. 2019;108(7):1250–5. [DOI] [PubMed] [Google Scholar]
- 25.Banderali G, Martelli A, Landi M, Moretti F, Betti F, Radaelli G, et al. Short and long term health effects of parental tobacco smoking during pregnancy and lactation: a descriptive review. J Transl Med. 2015;13:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simmons VN, Sutton SK, Quinn GP, Meade CD, Brandon TH. Prepartum and postpartum predictors of smoking. Nicotine Tob Res. 2014;16(4):461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harmer C, Memon A. Factors associated with smoking relapse in the postpartum period: an analysis of the child health surveillance system data in Southeast England. Nicotine Tob Res. 2013;15(5):904–9. [DOI] [PubMed] [Google Scholar]
- 28.Kawamoto T, Nitta H, Murata K, Toda E, Tsukamoto N, Hasegawa M, et al. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health. 2014;14:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michikawa T, Nitta H, Nakayama SF, Yamazaki S, Isobe T, Tamura K, et al. Baseline Profile of Participants in the Japan Environment and Children’s Study (JECS). J Epidemiol. 2018;28(2):99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okano T. Validation and reliability of the Japanese version of EPDS (Edinburgh Postnatal Depression Scale). Arch Psychiatr Diagn Clin Eval. 1996;7:525–33. [Google Scholar]
- 31.Yoshimasu K, Sato A, Miyauchi N, Tsuno K, Nishigori H, Nakai K, et al. Lack of association between receiving ART treatment and parental psychological distress during pregnancy: Preliminary findings of the Japan Environment and Children’s Study. Reprod Biomed Soc Online. 2018;5:5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–6. [DOI] [PubMed] [Google Scholar]
- 33.Yamashita H, Yoshida K, Nakano H, Tashiro N. Postnatal depression in Japanese women. Detecting the early onset of postnatal depression by closely monitoring the postpartum mood. J Affect Disord. 2000;58(2):145–54. [DOI] [PubMed] [Google Scholar]
- 34.Gyllstrom ME, Hellerstedt WL, Hennrikus D. The association of maternal mental health with prenatal smoking cessation and postpartum relapse in a population-based sample. Matern Child Health J. 2012;16(3):685–93. [DOI] [PubMed] [Google Scholar]
- 35.Horta BL, Kramer MS, Platt RW. Maternal smoking and the risk of early weaning: a meta-analysis. Am J Public Health. 2001;91(2):304–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luck W, Nau H. Nicotine and cotinine concentrations in serum and urine of infants exposed via passive smoking or milk from smoking mothers. J Pediatr. 1985;107(5):816–20. [DOI] [PubMed] [Google Scholar]
- 37.Logan CA, Rothenbacher D, Genuneit J. Postpartum Smoking Relapse and Breast Feeding: Defining the Window of Opportunity for Intervention. Nicotine Tob Res. 2017;19(3):367–72. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. Planning Guide for national implementation of the Global Strategy for Infant and Young Child Feeding. World Health Organization. 2007. Available from https://apps.who.int/iris/handle/10665/43619.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are unsuitable for public deposition due to ethical restrictions and legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of 30 May 2003, amendment on 9 September 2015) to publicly deposit the data containing personal information. Ethical Guidelines for Medical and Health Research Involving Human Subjects enforced by the Japan Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare also restricts the open sharing of the epidemiologic data. All inquiries about access to data should be sent to: jecs-en@nies.go.jp. The person responsible for handling enquiries sent to this e-mail address is Dr Shoji F. Nakayama, JECS Programme Office, National Institute for Environmental Studies.