Abstract
Macrophages are key players in the immune response and have been implicated in various human diseases, including atherosclerosis, cancer, and chronic inflammatory disorders. While numerous studies have delved into the nuances of macrophage behavior in these conditions, there remains a gap in understanding the specific role of Delta-like ligand 4 (Dll4)-expressing macrophages and their overarching implications across these diseases. Among the plethora of factors expressed by macrophages, Dll4 has emerged as a molecule of particular interest. Recent studies have highlighted its unique role in modulating macrophage functions and its potential implications in various diseases. This review seeks to consolidate existing knowledge, address this gap, and present a comprehensive overview of Dll4-expressing macrophages in the context of these disorders and highlight their potential as therapeutic targets. We examined the involvement of Dll4-expressing macrophages in multiple human diseases such as atherosclerosis, cancer and chronic inflammatory diseases, emphasizing their influence on disease progression. We also discussed the challenges, limitations, and emerging research areas in targeting Dll4-expressing macrophages and provide an outlook on potential therapeutic strategies for the treatment of these diseases. By addressing the previously existing research gap, we've provided a roadmap that brings together fragmented insights, paving the way for more holistic research and potentially more effective therapeutic strategies centered on Dll4-expressing macrophages.
Keywords: Macrophages, Delta-like ligand 4, Atherosclerosis, Cancer, Chronic inflammation
1. Introduction
Macrophages are versatile immune cells that play critical roles in both the innate and adaptive immune responses [1]. As the primary phagocytic cells, macrophages are responsible for the clearance of pathogens, apoptotic cells, and cellular debris, as well as the modulation of tissue repair and remodeling processes [2]. These functions are essential for maintaining homeostasis and defending the host against a wide range of infectious and non-infectious threats [3].
Increasing evidence suggests that the polarization of macrophages into distinct functional phenotypes is a crucial determinant of their roles in various human diseases [[4], [5], [6], [7], [8]]. Classically activated M1 macrophages are characterized by their pro-inflammatory and antimicrobial functions, while alternatively activated M2 macrophages exhibit immunoregulatory and tissue repair properties [9]. The balance between M1 and M2 polarization states is essential for maintaining immune homeostasis, and its dysregulation has been implicated in the pathogenesis of numerous pathological conditions, including atherosclerosis, cancer, and chronic inflammatory diseases [10,11].
Delta-like ligand 4 (Dll4), a transmembrane protein, is a crucial ligand in the Notch signaling pathway [12]. Dll4-Notch signaling has been shown to regulate a variety of cellular processes, including cell differentiation, proliferation, and survival, as well as angiogenesis and vascular development [13]. Recent studies implemented by our team and others have revealed that Dll4's vital roles in modulating macrophage polarization, function and mediating behaviors of other cells upon cell-to-cell communications [[14], [15], [16], [17], [18]].
We conducted a comprehensive literature search using databases such as PubMed, Scopus, and Web of Science. The main search terms included “Delta-Like Ligand 4″, “Macrophages”, “Inflammation”, “Polarization”, “Atherosclerosis”, “Notch”, “Tumor” and “Targeted therapy”. These terms were used in various combinations using Boolean operators to identify relevant articles. In this review, we aim to examine the role of Dll4-expressing macrophages in various human diseases, highlighting their involvement in the pathophysiology of these conditions and exploring the therapeutic opportunities that arise from understanding the complex interplay between Dll4, macrophage polarization, and disease progression.
2. Macrophage polarization and Dll4 expression
Macrophages are highly plastic cells, capable of adopting distinct functional phenotypes in response to various environmental cues [19]. Classically activated M1 macrophages are induced by pro-inflammatory stimuli, such as interferon-gamma (IFN-γ) and lipopolysaccharide (LPS), and produce pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin-1beta (IL-1β), and interleukin-6 (IL-6) [20]. M1 macrophages are crucial for host defense against intracellular pathogens and contribute to tissue damage in inflammatory diseases. In contrast, M2 macrophages are polarized by anti-inflammatory cytokines, such as interleukin-4 (IL-4) and interleukin-13 (IL-13), and exhibit immunoregulatory and tissue repair properties [21]. M2 macrophages can be further subdivided into M2a, M2b, M2c, and M2d subtypes, each with distinct functional characteristics [3]. Characteristics of these macrophage subtypes were listed in Table 1.
Table 1.
Types and subtypes of macrophages.
| Subtype | Activation signals | Function/characteristics | Markers |
|---|---|---|---|
| M1 [22] | IFNγ, LPS |
|
Nitric oxide synthase (NOS) |
| M2a [22] | IL-4, IL-13 |
|
|
| M2b [23] | Immune complexes, LPS |
|
|
| M2c [24] | IL-10, glucocorticoids |
|
|
| M2d [25] | Adenosine, IL-6 |
|
|
Macrophage polarization is regulated by a complex interplay of cytokines, chemokines, and signaling pathways. Transcription factors, such as signal transducer and activator of transcription (STAT) proteins and nuclear factor kappa B (NF-κB), play essential roles in orchestrating M1/M2 polarization [26]. In addition to cytokine-mediated signaling, cell-cell interactions, extracellular matrix components, and metabolic cues also contribute to shaping macrophage phenotype and function [27,28].
Recent studies have revealed that Dll4 is preferentially expressed in M1 macrophages [17,29]. Dll4 expression is upregulated by pro-inflammatory stimuli, such as LPS and IFN-γ, and is further enhanced by the activation of the Toll-like receptor (TLR) signaling pathway and NF-κB [30]. Dll4 expression has been found to be positively correlated with the expression of M1-associated markers, such as inducible nitric oxide synthase (iNOS) and IL-6 [31].
Dll4 plays a critical role in modulating macrophage function through its involvement in the Notch signaling pathway. Upon binding to its cognate Notch receptors, Dll4 triggers the proteolytic cleavage and nuclear translocation of the Notch intracellular domain (NICD), leading to the activation of downstream target genes [32]. In macrophages, Dll4-Notch signaling has been shown to promote M1 polarization and enhance pro-inflammatory cytokine production, while suppressing M2-associated gene expression [18]. Furthermore, Dll4-Notch signaling is implicated in modulating macrophage-mediated angiogenesis and tissue remodeling processes [33,34]. The features of M1/M2 macrophages are demonstrated in Table 2 and Dll4's unique roles in M1 macrophages were showed in Fig. 1.
Table 2.
Macrophage polarization and Dll4 expression.
| Features | M1 macrophages | M2 macrophages |
|---|---|---|
| General Functions [35,36] | Pro-inflammatory, anti-tumor, antimicrobial | Anti-inflammatory, pro-tumor, tissue repair and remodeling |
| Markers | CD80, CD86, MHC-II | CD163, CD206 |
| Cytokines/chemokines [36] | IL-1β, IL-6, IL-12, IL-23, TNF-α | IL-4, IL-10, IL-13, TGF-β |
| Polarizing factors [36] | IFN-γ, LPS, GM-CSF | IL-4, IL-13, M-CSF |
| Key transcription factors [36] | IRF5, STAT1 | IRF4, STAT6, PPARγ |
| Dll4 expression [37] | Higher expression | Lower or absent expression |
| Role of Dll4-expressing macrophages [37,38] | Modulation of inflammatory response, cancer, regulation of vascular function and plaque stability | Not well-defined |
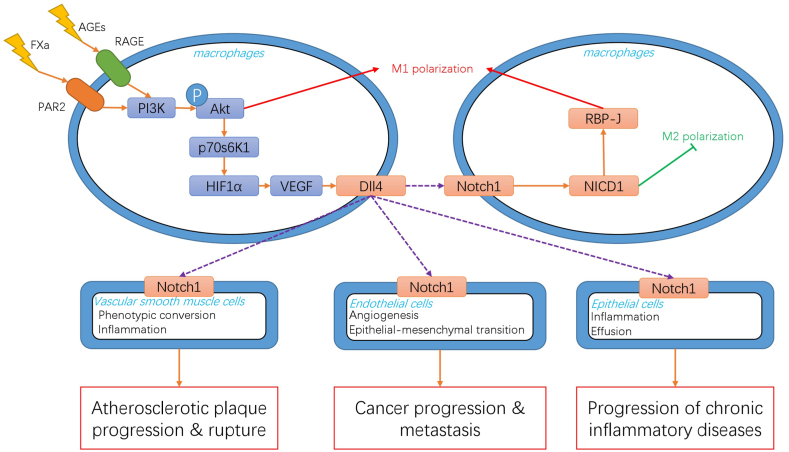
Fig. 1.
Schematic Overview of Dll4-Mediated Signaling in Macrophages and its Implications in Human Diseases
Dll4 plays a pivotal role in macrophage functions and is implicated in various human diseases. When macrophages are exposed to pathological stimuli, such as AGEs (advanced glycation end products) and Factor Xa, they activate their respective receptors, RAGE (receptor for advanced glycation end products) and protease-activated receptor 2 (PAR2). This activation triggers intracellular pathways, notably the phosphoinositide 3-kinase (PI3K)/Akt pathway, promoting the M1 polarization of macrophages. Concurrently, the PI3K/Akt pathway also induces the hypoxia inducible factor 1 (HIF1)α/vascular endothelial growth factor (VEGF) cascade, leading to an upregulation of Dll4 expression in macrophages. When macrophages expressing Dll4 come into contact with neighboring macrophages, it instigates the activation of the Notch signaling pathway in these neighboring cells. This promotes further M1 polarization while concurrently inhibiting M2 polarization. Moreover, when Dll4-mediated Notch pathway activation occurs in vascular smooth muscle cells (VSMCs), endothelial cells, and epithelial cells, it contributes to the progression of various diseases, including atherosclerosis, cancer, and chronic inflammatory conditions.
3. Dll4-expressing macrophages in human diseases and potential therapeutic approaches
3.1. Atherosclerosis
Atherosclerosis is a chronic inflammatory disease characterized by the accumulation of lipids, immune cells, and extracellular matrix components within the arterial wall, leading to the formation of plaques [39]. Macrophages play a central role in atherosclerosis development and progression, through the uptake of modified lipoproteins, such as oxidized low-density lipoprotein (oxLDL), resulting in the formation of foam cells [40]. Additionally, macrophages secrete various pro-inflammatory cytokines and matrix metalloproteinases (MMPs), contributing to plaque inflammation, matrix degradation, and eventual plaque rupture [41].
Our recent studies have demonstrated the presence of Dll4-expressing macrophages within atherosclerotic plaques [14]. Dll4 expression is predominantly localized to the plaque shoulder regions and areas of neovascularization, where it promotes endothelial cell activation and angiogenesis [32]. Activation of the Dll4-Notch signaling pathway in plaque macrophages has been associated with increased expression of pro-inflammatory cytokines, chemokines, and MMPs, exacerbating plaque inflammation and instability [42].
Dll4-expressing macrophages contribute to plaque instability by promoting a pro-inflammatory M1 phenotype and suppressing the anti-inflammatory M2 phenotype [43]. Dll4-Notch signaling has also been implicated in the regulation of vascular smooth muscle cell (VSMC) function within the plaque. Activation of Notch signaling in VSMCs by Dll4-expressing macrophages induces VSMC senescence and apoptosis, weakening the fibrous cap and increasing the risk of plaque rupture [44,45].
Given the pivotal role of Dll4-expressing macrophages in atherosclerosis progression and plaque instability, targeting Dll4 and its associated signaling pathways may represent a promising therapeutic strategy for the treatment of atherosclerosis. Results from an in vitro study suggested Dll4 blockade significantly attenuates the M1 polarizationof macrophages which expressed less proinflammatry mediators such as iNOS and TNF-α [46]. Preclinical studies have shown that blockade of Dll4-Notch signaling by neutralizing anti-Dll4 antibody administration reduced macrophages accumulation and atheroma in mice model of atherosclerosis and vein graft disease [47]. Furthermore, Dll4-Notch signaling suppression increased M2 marcrophages augmentation and reduce the risk of fatal ventricular arrhythmia post myocardial infarction [48].
3.2. Cancer
Tumor-associated macrophages (TAMs) are a major component of the tumor microenvironment and play a crucial role in cancer progression [49]. TAMs can exhibit both pro- and anti-tumoral functions, depending on their polarization state. M2-polarized TAMs are generally associated with tumor progression, promoting angiogenesis, immunosuppression, and tissue remodeling, while M1-polarized TAMs exhibit pro-inflammatory and anti-tumoral activities [11].
Dll4 expression has been observed in TAMs within various tumor types, including breast cancer, glioblastoma, and colorectal cancer [50,51]. Dll4-expressing TAMs predominantly exhibit an M2-like phenotype and contribute to the activation of the Notch signaling pathway in the tumor microenvironment, thereby modulating various cellular processes, including angiogenesis, cell proliferation, and migration [52].
Dll4-expressing TAMs play a pivotal role in promoting tumor angiogenesis by activating the Notch signaling pathway in endothelial cells, resulting in the formation of abnormal, leaky blood vessels [53]. This aberrant vascularization leads to an increase in tumor hypoxia, which further drives the recruitment of TAMs, perpetuating a cycle of tumor growth and angiogenesis [54,55]. Additionally, Dll4-expressing TAMs can promote the epithelial-mesenchymal transition (EMT) in tumor cells, enhancing their migratory and invasive capacities, and ultimately facilitating metastasis [56].
Given the crucial role of Dll4-expressing TAMs in tumor progression, angiogenesis, and metastasis, targeting Dll4 may offer a promising therapeutic strategy in cancer treatment. Disrupting Dll4-Notch signaling in TAMs was found to reduce microglia recruitment and results in abnormal angiogenesis [57]. It was reported that an engineered fully human IgG1 mAb named REGN421, which bind human Dll4 with sub-nanomolar affinity, inhibited human ovarian cancer in xenografts models [58]. Using the fusion protein Dll4-Fc as an inhibitor of Dll4-Notch signaling suppressed liver metastasis of small cell lung cancer [59]. Moreover, combining Dll4-targeted therapies with conventional chemotherapy, immunotherapy, or anti-angiogenic agents has shown synergistic effects in preclinical models, highlighting the potential of targeting Dll4 in combination with other therapeutic modalities for improved cancer treatment outcomes [60,61].
3.3. Chronic inflammatory diseases
Macrophages are key regulators of chronic inflammation, which is associated with various diseases, such as rheumatoid arthritis, inflammatory bowel disease, and multiple sclerosis [[62], [63], [64]]. Depending on their polarization state, macrophages can either promote or resolve inflammation. M1 macrophages exhibit pro-inflammatory activities, while M2 macrophages are involved in resolving inflammation and promoting tissue repair [20].
Dll4 expression has been detected in macrophages within the inflamed tissues of patients with chronic inflammatory diseases [65,66]. Activation of the Notch signaling pathway, in which Dll4 plays a crucial role, has been implicated in the pathogenesis of various inflammatory diseases by modulating the function and differentiation of immune cells, including macrophages, T cells, and dendritic cells [47,[67], [68], [69]]. Dll4/Notch pathway was found play important roles in inflammatory diseases such as rheumatoid arthritis and Crohn's disease. Increased expressions of Dll4 and NICD were found localized to perivascular/vascular regions in synovial tissue specimens from patients with rheumatoid arthritis [70]. Dll4/Notch pathway activation was suggested to participate in progression of rheumatoid arthritis by regulating inflammation responses through signal transducer and activator of transcription (STAT3) [71]. Notch ligands Jag1 and Dll4 were found high expressed in mucosa of Crohn's disease patients. Jag1 and Dll4-induced M1 macrophages-associated Notch pathway in epithelial cells was related with Crohn's disease [72]. Moreover, sponging miR-30 family-mediated Dll4 expression was found critical in pathogenesis of Crohn's disease [73].
Dll4-expressing macrophages contribute to the progression of chronic inflammatory diseases by promoting the M1 polarization state and enhancing the pro-inflammatory response [74]. In rheumatoid arthritis, for instance, Dll4-expressing macrophages are associated with increased production of pro-inflammatory cytokines, such as TNF-α and IL-6, which promote synovial inflammation and joint destruction [75]. Similarly, in inflammatory bowel disease, Dll4-expressing macrophages contribute to the maintenance of chronic intestinal inflammation through the activation of the Notch signaling pathway [76].
Given the role of Dll4-expressing macrophages in the pathogenesis of chronic inflammatory diseases, targeting Dll4 may represent a promising therapeutic strategy. Blockade of Dll4 suppressed macrophage accumulation and the expression of metaphase chromosome protein 1 (MCP1) in adipose tissue which alleviated non-alcoholic fatty liver disease in mice [47]. Moreover, combining Dll4-targeted therapies with existing immunomodulatory agents may provide synergistic effects and improve treatment outcomes in chronic inflammatory diseases [77].
4. Future perspectives
Dll4 blockade or silencing would be promising strategy of targeted therapies. In one of our projects, we found that linage knockout of Dll4 in mono-macrophages lead to dramatic alleviation of formation, progression and vulnerability of atherosclerosis. While targeting Dll4-expressing macrophages holds promise for the treatment of various human diseases, there are challenges and limitations to be addressed. One of the main challenges is the potential off-target effects due to the broad involvement of Notch signaling in various cellular processes and tissue homeostasis [78]. Additionally, the complexity of the immune system and the diverse functions of macrophages in health and disease necessitate a thorough understanding of the context-dependent roles of Dll4-expressing macrophages [42].
Emerging research areas and potential new therapeutic strategies are being explored to increase the specificity of targeting. For instance, the development of more selective inhibitors targeting specific Notch ligands or receptors, or modulating specific downstream pathways, may help to overcome off-target effects and improve therapeutic efficacy [79]. Moreover, our team is developing a nanocarrier targeting specific high dll4 expressing- M1 macrophage subgroup. The increased cell targeting would be benefit in decreasing unexpected disruption of immune system function. Additionally, the potential role of Dll4-expressing macrophages in other diseases, such as neurodegenerative disorders, autoimmune diseases, and fibrosis, warrants further investigation [80]. Currently developing Dll4-involved therapeutic strategies in human diseases are demonstrated in Table 3.
Table 3.
Potential therapeutic approaches targeting Dll4 in human diseases.
| Disease | Therapeutic strategy | Mechanism of action |
|---|---|---|
| Atherosclerosis [81] | Anti-Dll4 antibodies | Inhibition of M1 macrophage polarization, reduction of inflammation, and promotion of plaque stability |
| Breast cancer [82] | Dll4-targeted therapies | Modulation of tumor-associated macrophages, inhibition of tumor angiogenesis, and suppression of metastasis |
| Rheumatoid arthritis [83] | Dll4 blockade or inhibition | Suppression of M1 macrophage polarization, reduction of inflammation, and attenuation of disease progression |
| Colorectal cancer [84] | Dll4-targeted therapies | Modulation of tumor-associated macrophages, inhibition of tumor angiogenesis, and suppression of metastasis |
| Multiple sclerosis [85] | Dll4 blockade or inhibition | Suppression of M1 macrophage polarization, reduction of inflammation, and attenuation of disease progression |
| Psoriasis [86] | Dll4 blockade or inhibition | Suppression of M1 macrophage polarization, reduction of inflammation, and attenuation of disease progression |
The role of Dll4 in disease pathology suggests its potential as a diagnostic or prognostic biomarker. Future research might delve into developing assays or tests that leverage Dll4 levels or activity to predict disease onset or progression. Moreover, understanding how Dll4 interacts with other signaling molecules or pathways can provide a holistic view of its role. This might lead to combined therapeutic strategies that target multiple pathways for enhanced efficacy.
5. Conclusion
In conclusion, our review brings together fragmented insights on Dll4-expressing macrophages and offers a fresh perspective on their multifaceted roles in human diseases. The study of in-depth molecular mechanisms has contributed to our understanding of disease progression and provided potential therapeutic opportunities by targeting these Dll4-expressing macrophages. Continued research in this area will be essential to fully elucidate the complex roles of Dll4-expressing macrophages in human diseases and to develop more effective therapeutic strategies.
Compliance with ethics guidelines
Wenya Yu, Xiqiang Wang, Jing Liu, Zhongwei Liu and Haitao Zhu declare that they have no conflict of interest. This review does not contain any studies with human or animal subjects performed by any of the authors.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by National Natural Scientific Foundation of China (82070858); Youth Scientific Research and Innovation Team Program of Shaanxi Province (2022-SLRH-LJ-014); SPPH Scientific Research Supporting Projects (2021BJ-02 and 2021YJY-02).
Contributor Information
Zhongwei Liu, Email: liuzhongwei@nwpu.edu.cn.
Haitao Zhu, Email: zht0915@163.com.
References
- 1.Ilinykh A., Pinto A.R. The role of cardiac tissue macrophages in homeostasis and disease. Adv. Exp. Med. Biol. 2017;1003:105–118. doi: 10.1007/978-3-319-57613-8_6. [DOI] [PubMed] [Google Scholar]
- 2.Gordon S., Plüddemann A. Tissue macrophages: heterogeneity and functions. BMC Biol. 2017;15:53. doi: 10.1186/s12915-017-0392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Italiani P., Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. Functional differentiation. Front. Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atri C., Guerfali F.Z., Laouini D. Role of human macrophage polarization in inflammation during infectious diseases. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19061801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang C., Ma C., Gong L., Guo Y., Fu K., Zhang Y., Zhou H., Li Y. Macrophage polarization and its role in liver disease. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.803037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kishore A., Petrek M. Roles of macrophage polarization and macrophage-derived miRNAs in pulmonary fibrosis. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.678457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang S., Yuan H.Q., Hao Y.M., Ren Z., Qu S.L., Liu L.S., Wei D.H., Tang Z.H., Zhang J.F., Jiang Z.S. Macrophage polarization in atherosclerosis. Clinica chimica acta. international journal of clinical chemistry. 2020;501:142–146. doi: 10.1016/j.cca.2019.10.034. [DOI] [PubMed] [Google Scholar]
- 8.Arora S., Dev K., Agarwal B., Das P., Syed M.A. Macrophages: their role, activation and polarization in pulmonary diseases. Immunobiology. 2018;223:383–396. doi: 10.1016/j.imbio.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapouri-Moghaddam A., Mohammadian S., Vazini H., Taghadosi M., Esmaeili S.A., Mardani F., Seifi B., Mohammadi A., Afshari J.T., Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018;233:6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 10.Tabas I., Bornfeldt K.E. Macrophage phenotype and function in different stages of atherosclerosis. Circ. Res. 2016;118:653–667. doi: 10.1161/circresaha.115.306256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noy R., Pollard J.W. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bray S.J. Notch signalling in context. Nat. Rev. Mol. Cell Biol. 2016;17:722–735. doi: 10.1038/nrm.2016.94. [DOI] [PubMed] [Google Scholar]
- 13.Hultgren N.W., Fang J.S., Ziegler M.E., Ramirez R.N., Phan D.T.T., Hatch M.M.S., Welch-Reardon K.M., Paniagua A.E., Kim L.S., Shon N.N., et al. Slug regulates the Dll4-Notch-VEGFR2 axis to control endothelial cell activation and angiogenesis. Nat. Commun. 2020;11:5400. doi: 10.1038/s41467-020-18633-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xing Y., Pan S., Zhu L., Cui Q., Tang Z., Liu Z., Liu F. Advanced glycation end products induce atherosclerosis via RAGE/TLR4 signaling mediated-M1 macrophage polarization-dependent vascular smooth muscle cell phenotypic conversion. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/9763377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang N., Liang X., Fan B., Zhao C., Shen B., Ji X., Liu Y. Endothelial-specific molecule 1 inhibition lessens productive angiogenesis and tumor metastasis to overcome bevacizumab resistance. Cancers. 2022;14 doi: 10.3390/cancers14225681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Šućur A., Filipović M., Flegar D., Kelava T., Šisl D., Lukač N., Kovačić N., Grčević D. Notch receptors and ligands in inflammatory arthritis - a systematic review. Immunol. Lett. 2020;223:106–114. doi: 10.1016/j.imlet.2020.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Pagie S., Gérard N., Charreau B. Notch signaling triggered via the ligand DLL4 impedes M2 macrophage differentiation and promotes their apoptosis. Cell Commun. Signal. : CCS. 2018;16:4. doi: 10.1186/s12964-017-0214-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pabois A., Pagie S., Gérard N., Laboisse C., Pattier S., Hulin P., Nedellec S., Toquet C., Charreau B. Notch signaling mediates crosstalk between endothelial cells and macrophages via Dll4 and IL6 in cardiac microvascular inflammation. Biochem. Pharmacol. 2016;104:95–107. doi: 10.1016/j.bcp.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Saha S., Shalova I.N., Biswas S.K. Metabolic regulation of macrophage phenotype and function. Immunol. Rev. 2017;280:102–111. doi: 10.1111/imr.12603. [DOI] [PubMed] [Google Scholar]
- 20.Murray P.J., Allen J.E., Biswas S.K., Fisher E.A., Gilroy D.W., Goerdt S., Gordon S., Hamilton J.A., Ivashkiv L.B., Lawrence T., et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yunna C., Mengru H., Lei W., Weidong C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020;877 doi: 10.1016/j.ejphar.2020.173090. [DOI] [PubMed] [Google Scholar]
- 22.Shapouri-Moghaddam A., Mohammadian S., Vazini H., Taghadosi M., Esmaeili S.A., Mardani F., Seifi B., Mohammadi A., Afshari J.T., Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018;233:6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 23.Funes S.C., Rios M., Escobar-Vera J., Kalergis A.M. Implications of macrophage polarization in autoimmunity. Immunology. 2018;154:186–195. doi: 10.1111/imm.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Väyrynen J.P., Haruki K., Lau M.C., Väyrynen S.A., Zhong R., Dias Costa A., Borowsky J., Zhao M., Fujiyoshi K., Arima K., et al. The prognostic role of macrophage polarization in the colorectal cancer microenvironment. Cancer Immunol. Res. 2021;9:8–19. doi: 10.1158/2326-6066.Cir-20-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tarique A.A., Logan J., Thomas E., Holt P.G., Sly P.D., Fantino E. Phenotypic, functional, and plasticity features of classical and alternatively activated human macrophages. Am. J. Respir. Cell Mol. Biol. 2015;53:676–688. doi: 10.1165/rcmb.2015-0012OC. [DOI] [PubMed] [Google Scholar]
- 26.Lv R., Bao Q., Li Y. Regulation of M1-type and M2-type macrophage polarization in RAW264.7 cells by Galectin-9. Mol. Med. Rep. 2017;16:9111–9119. doi: 10.3892/mmr.2017.7719. [DOI] [PubMed] [Google Scholar]
- 27.Van den Bossche J., O'Neill L.A., Menon D. Macrophage immunometabolism: where are we (going)? Trends Immunol. 2017;38:395–406. doi: 10.1016/j.it.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Hulsmans M., Clauss S., Xiao L., Aguirre A.D., King K.R., Hanley A., Hucker W.J., Wülfers E.M., Seemann G., Courties G., et al. Macrophages facilitate electrical conduction in the heart. Cell. 2017;169:510–522.e520. doi: 10.1016/j.cell.2017.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J.L., Zhang L., Huang Y., Li X.H., Liu Y.F., Zhang S.M., Zhao Y.E., Chen X.J., Liu Y., He L.Y., et al. Epsin1-mediated exosomal sorting of Dll4 modulates the tubular-macrophage crosstalk in diabetic nephropathy. Mol. Ther. : the journal of the American Society of Gene Therapy. 2023;31:1451–1467. doi: 10.1016/j.ymthe.2023.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foldi J., Chung A.Y., Xu H., Zhu J., Outtz H.H., Kitajewski J., Li Y., Hu X., Ivashkiv L.B. Autoamplification of Notch signaling in macrophages by TLR-induced and RBP-J-dependent induction of Jagged1. J. Immunol. 2010;185:5023–5031. doi: 10.4049/jimmunol.1001544. (Baltimore, Md. : 1950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yurdagul A., Jr., Subramanian M., Wang X., Crown S.B., Ilkayeva O.R., Darville L., Kolluru G.K., Rymond C.C., Gerlach B.D., Zheng Z., et al. Macrophage metabolism of apoptotic cell-derived arginine promotes continual efferocytosis and resolution of injury. Cell Metabol. 2020;31:518–533.e510. doi: 10.1016/j.cmet.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakano T., Katsuki S., Chen M., Decano J.L., Halu A., Lee L.H., Pestana D.V.S., Kum A.S.T., Kuromoto R.K., Golden W.S., et al. Uremic toxin indoxyl sulfate promotes proinflammatory macrophage activation via the interplay of OATP2B1 and dll4-notch signaling. Circulation. 2019;139:78–96. doi: 10.1161/circulationaha.118.034588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kofler N.M., Shawber C.J., Kangsamaksin T., Reed H.O., Galatioto J., Kitajewski J. Notch signaling in developmental and tumor angiogenesis. Genes & cancer. 2011;2:1106–1116. doi: 10.1177/1947601911423030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koga J.I., Nakano T., Dahlman J.E., Figueiredo J.L., Zhang H., Decano J., Khan O.F., Niida T., Iwata H., Aster J.C., et al. Macrophage notch ligand delta-like 4 promotes vein graft lesion development: implications for the treatment of vein graft failure. Arterioscler. Thromb. Vasc. Biol. 2015;35:2343–2353. doi: 10.1161/atvbaha.115.305516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez F.O., Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000prime reports. 2014;6:13. doi: 10.12703/p6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xing Y., Pan S., Zhu L., Cui Q., Tang Z., Liu Z., Liu F. Advanced glycation end products induce atherosclerosis via RAGE/TLR4 signaling mediated-M1 macrophage polarization-dependent vascular smooth muscle cell phenotypic conversion. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/9763377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han C., Yang Y., Sheng Y., Wang J., Li W., Zhou X., Guo L. The mechanism of lncRNA-CRNDE in regulating tumour-associated macrophage M2 polarization and promoting tumour angiogenesis. J. Cell Mol. Med. 2021;25:4235–4247. doi: 10.1111/jcmm.16477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Libby P., Buring J.E., Badimon L., Hansson G.K., Deanfield J., Bittencourt M.S., Tokgözoğlu L., Lewis E.F. Atherosclerosis. Nat. Rev. Dis. Prim. 2019;5:56. doi: 10.1038/s41572-019-0106-z. [DOI] [PubMed] [Google Scholar]
- 40.Moore K.J., Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seifert R., Kuhlmann M.T., Eligehausen S., Kiefer F., Hermann S., Schäfers M. Molecular imaging of MMP activity discriminates unstable from stable plaque phenotypes in shear-stress induced murine atherosclerosis. PLoS One. 2018;13 doi: 10.1371/journal.pone.0204305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukuda D., Aikawa M. Expanding role of delta-like 4 mediated notch signaling in cardiovascular and metabolic diseases. Circ. J. : official journal of the Japanese Circulation Society. 2013;77:2462–2468. doi: 10.1253/circj.cj-13-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu H., Zhu J., Smith S., Foldi J., Zhao B., Chung A.Y., Outtz H., Kitajewski J., Shi C., Weber S., et al. Notch-RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nat. Immunol. 2012;13:642–650. doi: 10.1038/ni.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grootaert M.O., da Costa Martins P.A., Bitsch N., Pintelon I., De Meyer G.R., Martinet W., Schrijvers D.M. Defective autophagy in vascular smooth muscle cells accelerates senescence and promotes neointima formation and atherogenesis. Autophagy. 2015;11:2014–2032. doi: 10.1080/15548627.2015.1096485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clarke M.C., Figg N., Maguire J.J., Davenport A.P., Goddard M., Littlewood T.D., Bennett M.R. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat. Med. 2006;12:1075–1080. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- 46.Fukuda D., Aikawa E., Swirski F.K., Novobrantseva T.I., Kotelianski V., Gorgun C.Z., Chudnovskiy A., Yamazaki H., Croce K., Weissleder R., et al. Notch ligand delta-like 4 blockade attenuates atherosclerosis and metabolic disorders. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E1868–E1877. doi: 10.1073/pnas.1116889109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakano T., Fukuda D., Koga J., Aikawa M. Delta-like ligand 4-notch signaling in macrophage activation. Arterioscler. Thromb. Vasc. Biol. 2016;36:2038–2047. doi: 10.1161/atvbaha.116.306926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yin J., Hu H., Li X., Xue M., Cheng W., Wang Y., Xuan Y., Li X., Yang N., Shi Y., Yan S. Inhibition of Notch signaling pathway attenuates sympathetic hyperinnervation together with the augmentation of M2 macrophages in rats post-myocardial infarction. Am. J. Physiol. Cell Physiol. 2016;310:C41–C53. doi: 10.1152/ajpcell.00163. 2015. [DOI] [PubMed] [Google Scholar]
- 49.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoey T., Yen W.C., Axelrod F., Basi J., Donigian L., Dylla S., Fitch-Bruhns M., Lazetic S., Park I.K., Sato A., et al. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell. 2009;5:168–177. doi: 10.1016/j.stem.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 51.Zhu T.S., Costello M.A., Talsma C.E., Flack C.G., Crowley J.G., Hamm L.L., He X., Hervey-Jumper S.L., Heth J.A., Muraszko K.M., et al. Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self-renewal of cancer stem-like cells. Cancer Res. 2011;71:6061–6072. doi: 10.1158/0008-5472.Can-10-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han C., Yang Y., Sheng Y., Wang J., Li W., Zhou X., Guo L. The mechanism of lncRNA-CRNDE in regulating tumour-associated macrophage M2 polarization and promoting tumour angiogenesis. J. Cell Mol. Med. 2021;25:4235–4247. doi: 10.1111/jcmm.16477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El Hindy N., Keyvani K., Pagenstecher A., Dammann P., Sandalcioglu I.E., Sure U., Zhu Y. Implications of Dll4-Notch signaling activation in primary glioblastoma multiforme. Neuro Oncol. 2013;15:1366–1378. doi: 10.1093/neuonc/not071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Munir M.T., Kay M.K., Kang M.H., Rahman M.M., Al-Harrasi A., Choudhury M., Moustaid-Moussa N., Hussain F., Rahman S.M. Tumor-associated macrophages as multifaceted regulators of breast tumor growth. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22126526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fu L.Q., Du W.L., Cai M.H., Yao J.Y., Zhao Y.Y., Mou X.Z. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell. Immunol. 2020;353 doi: 10.1016/j.cellimm.2020.104119. [DOI] [PubMed] [Google Scholar]
- 56.Lu J., Ye X., Fan F., Xia L., Bhattacharya R., Bellister S., Tozzi F., Sceusi E., Zhou Y., Tachibana I., et al. Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1. Cancer Cell. 2013;23:171–185. doi: 10.1016/j.ccr.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kangsamaksin T., Tattersall I.W., Kitajewski J. Notch functions in developmental and tumour angiogenesis by diverse mechanisms. Biochem. Soc. Trans. 2014;42:1563–1568. doi: 10.1042/bst20140233. [DOI] [PubMed] [Google Scholar]
- 58.Kuhnert F., Chen G., Coetzee S., Thambi N., Hickey C., Shan J., Kovalenko P., Noguera-Troise I., Smith E., Fairhurst J., et al. Dll4 blockade in stromal cells mediates antitumor effects in preclinical models of ovarian cancer. Cancer Res. 2015;75:4086–4096. doi: 10.1158/0008-5472.Can-14-3773. [DOI] [PubMed] [Google Scholar]
- 59.Kuramoto T., Goto H., Mitsuhashi A., Tabata S., Ogawa H., Uehara H., Saijo A., Kakiuchi S., Maekawa Y., Yasutomo K., et al. Dll4-Fc, an inhibitor of Dll4-notch signaling, suppresses liver metastasis of small cell lung cancer cells through the downregulation of the NF-κB activity. Mol. Cancer Therapeut. 2012;11:2578–2587. doi: 10.1158/1535-7163.Mct-12-0640. [DOI] [PubMed] [Google Scholar]
- 60.Yan M., Callahan C.A., Beyer J.C., Allamneni K.P., Zhang G., Ridgway J.B., Niessen K., Plowman G.D. Chronic DLL4 blockade induces vascular neoplasms. Nature. 2010;463:E6–E7. doi: 10.1038/nature08751. [DOI] [PubMed] [Google Scholar]
- 61.Chiorean E.G., LoRusso P., Strother R.M., Diamond J.R., Younger A., Messersmith W.A., Adriaens L., Liu L., Kao R.J., DiCioccio A.T., et al. A phase I first-in-human study of enoticumab (REGN421), a fully human delta-like ligand 4 (Dll4) monoclonal antibody in patients with advanced solid tumors. Clin. Cancer Res. : an official journal of the American Association for Cancer Research. 2015;21:2695–2703. doi: 10.1158/1078-0432.Ccr-14-2797. [DOI] [PubMed] [Google Scholar]
- 62.Udalova I.A., Mantovani A., Feldmann M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat. Rev. Rheumatol. 2016;12:472–485. doi: 10.1038/nrrheum.2016.91. [DOI] [PubMed] [Google Scholar]
- 63.Zhou X., Li W., Wang S., Zhang P., Wang Q., Xiao J., Zhang C., Zheng X., Xu X., Xue S., et al. YAP aggravates inflammatory bowel disease by regulating M1/M2 macrophage polarization and gut microbial homeostasis. Cell Rep. 2019;27:1176–1189.e1175. doi: 10.1016/j.celrep.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 64.Donninelli G., Saraf-Sinik I., Mazziotti V., Capone A., Grasso M.G., Battistini L., Reynolds R., Magliozzi R., Volpe E. Interleukin-9 regulates macrophage activation in the progressive multiple sclerosis brain. J. Neuroinflammation. 2020;17:149. doi: 10.1186/s12974-020-01770-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kijanka G., Hector S., Kay E.W., Murray F., Cummins R., Murphy D., MacCraith B.D., Prehn J.H., Kenny D. Human IgG antibody profiles differentiate between symptomatic patients with and without colorectal cancer. Gut. 2010;59:69–78. doi: 10.1136/gut.2009.178574. [DOI] [PubMed] [Google Scholar]
- 66.Xu J., Chi F., Guo T., Punj V., Lee W.N., French S.W., Tsukamoto H. NOTCH reprograms mitochondrial metabolism for proinflammatory macrophage activation. J. Clin. Invest. 2015;125:1579–1590. doi: 10.1172/jci76468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meng L., Hu S., Wang J., He S., Zhang Y. DLL4(+) dendritic cells: key regulators of Notch Signaling in effector T cell responses. Pharmacol. Res. 2016;113:449–457. doi: 10.1016/j.phrs.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mitra A., Shanthalingam S., Sherman H.L., Singh K., Canakci M., Torres J.A., Lawlor R., Ran Y., Golde T.E., Miele L., et al. CD28 signaling drives notch ligand expression on CD4 T cells. Front. Immunol. 2020;11:735. doi: 10.3389/fimmu.2020.00735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laky K., Evans S., Perez-Diez A., Fowlkes B.J. Notch signaling regulates antigen sensitivity of naive CD4+ T cells by tuning co-stimulation. Immunity. 2015;42:80–94. doi: 10.1016/j.immuni.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao W., Sweeney C., Connolly M., Kennedy A., Ng C.T., McCormick J., Veale D.J., Fearon U. Notch-1 mediates hypoxia-induced angiogenesis in rheumatoid arthritis. Arthritis Rheum. 2012;64:2104–2113. doi: 10.1002/art.34397. [DOI] [PubMed] [Google Scholar]
- 71.Gao W., McCormick J., Connolly M., Balogh E., Veale D.J., Fearon U. Hypoxia and STAT3 signalling interactions regulate pro-inflammatory pathways in rheumatoid arthritis. Ann. Rheum. Dis. 2015;74:1275–1283. doi: 10.1136/annrheumdis-2013-204105. [DOI] [PubMed] [Google Scholar]
- 72.Ortiz-Masiá D., Cosín-Roger J., Calatayud S., Hernández C., Alós R., Hinojosa J., Esplugues J.V., Barrachina M.D. M1 macrophages activate notch signalling in epithelial cells: relevance in crohn's disease. Journal of Crohn's & colitis. 2016;10:582–592. doi: 10.1093/ecco-jcc/jjw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ye Y., Zhang L., Hu T., Yin J., Xu L., Pang Z., Chen W. CircRNA_103765 acts as a proinflammatory factor via sponging miR-30 family in Crohn's disease. Sci. Rep. 2021;11:565. doi: 10.1038/s41598-020-80663-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kang S., Tanaka T., Narazaki M., Kishimoto T. Targeting interleukin-6 signaling in clinic. Immunity. 2019;50:1007–1023. doi: 10.1016/j.immuni.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 75.Wang Y., Gao W., Shi X., Ding J., Liu W., He H., Wang K., Shao F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 76.Zundler S., Neurath M.F. Interleukin-12: functional activities and implications for disease. Cytokine Growth Factor Rev. 2015;26:559–568. doi: 10.1016/j.cytogfr.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 77.Perumalsamy L.R., Nagala M., Banerjee P., Sarin A. A hierarchical cascade activated by non-canonical Notch signaling and the mTOR-Rictor complex regulates neglect-induced death in mammalian cells. Cell Death Differ. 2009;16:879–889. doi: 10.1038/cdd.2009.20. [DOI] [PubMed] [Google Scholar]
- 78.Keewan E., Naser S.A. The role of notch signaling in macrophages during inflammation and infection: implication in rheumatoid arthritis? Cells. 2020;9 doi: 10.3390/cells9010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takebe N., Miele L., Harris P.J., Jeong W., Bando H., Kahn M., Yang S.X., Ivy S.P. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat. Rev. Clin. Oncol. 2015;12:445–464. doi: 10.1038/nrclinonc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rizzo P., Osipo C., Foreman K., Golde T., Osborne B., Miele L. Rational targeting of Notch signaling in cancer. Oncogene. 2008;27:5124–5131. doi: 10.1038/onc.2008.226. [DOI] [PubMed] [Google Scholar]
- 81.Fukuda D., Aikawa E., Swirski F.K., Novobrantseva T.I., Kotelianski V., Gorgun C.Z., Chudnovskiy A., Yamazaki H., Croce K., Weissleder R., et al. Notch ligand delta-like 4 blockade attenuates atherosclerosis and metabolic disorders. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E1868–E1877. doi: 10.1073/pnas.1116889109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoey T., Yen W.C., Axelrod F., Basi J., Donigian L., Dylla S., Fitch-Bruhns M., Lazetic S., Park I.K., Sato A., et al. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell. 2009;5:168–177. doi: 10.1016/j.stem.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 83.Gao W., McCormick J., Connolly M., Balogh E., Veale D.J., Fearon U. Hypoxia and STAT3 signalling interactions regulate pro-inflammatory pathways in rheumatoid arthritis. Ann. Rheum. Dis. 2015;74:1275–1283. doi: 10.1136/annrheumdis-2013-204105. [DOI] [PubMed] [Google Scholar]
- 84.Li J.L., Sainson R.C., Oon C.E., Turley H., Leek R., Sheldon H., Bridges E., Shi W., Snell C., Bowden E.T., et al. DLL4-Notch signaling mediates tumor resistance to anti-VEGF therapy in vivo. Cancer Res. 2011;71:6073–6083. doi: 10.1158/0008-5472.Can-11-1704. [DOI] [PubMed] [Google Scholar]
- 85.Bassil R., Zhu B., Lahoud Y., Riella L.V., Yagita H., Elyaman W., Khoury S.J. Notch ligand delta-like 4 blockade alleviates experimental autoimmune encephalomyelitis by promoting regulatory T cell development. J. Immunol. 2011;187:2322–2328. doi: 10.4049/jimmunol.1100725. (Baltimore, Md. : 1950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Piskin G., Sylva-Steenland R.M., Bos J.D., Teunissen M.B. In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin. J. Immunol. 2006;176:1908–1915. doi: 10.4049/jimmunol.176.3.1908. (Baltimore, Md. : 1950) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.