We have recently demonstrated excessive neutrophil accumulation in the airways of patients with diffuse panbronchiolitis (DPB) and have also described therapeutic benefits of low-dose, long-term administration of erythromycin which are due to antiinflammatory rather than bactericidal action (2, 4, 5, 8–10, 12). The accumulation of neutrophils in the airway might contribute to lung damage through the release of proteases, free oxygen radicals, and other degradative enzymes (1, 4, 7). In this study, therefore, we attempted to elucidate whether erythromycin has a direct inhibitory effect on superoxide anion production by N-formyl-methionyl-leucyl-phenylalanine (FMLP)-stimulated human neutrophils primed with granulocyte colony-stimulating factor (G-CSF).
Neutrophils were isolated from the blood of healthy volunteers with mono-poly resolving medium (M-PRM; Flow Laboratories, Irvine, Scotland) and density gradient centrifugation and were suspended in Hanks’ balanced salt solution (pH 7.4) (GIBCO, Grand Island, N.Y.) with 0.5% human serum at 107 cells/ml. O2− generation by FMLP-stimulated neutrophils was measured by determining the superoxide dismutase-inhibitible reduction of cytochrome c by a rapid microassay method (11). Neutrophils were incubated with various concentrations of erythromycin for 30 min at 37°C in a humidified atmosphere of 5% CO2 followed by addition of the desired dose of G-CSF (Chugai Pharmaceuticals, Tokyo, Japan) for 10 min. The cells were finally stimulated with 10−7 M FMLP for 10 min at 37°C, and then the absorbance changes were measured at a wavelength of 550 nm with a Multiskan instrument (Flow Laboratories, McLean, Va.). After each incubation, cell viability was confirmed to be >95% by the trypan blue dye exclusion method.
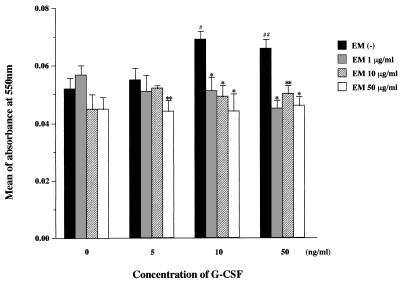
At concentrations of 10 and 50 ng/ml, G-CSF significantly primed the O2− generation by human neutrophils (Fig. 1), although G-CSF alone at 10 and 50 ng/ml induced no direct superoxide production by human neutrophils during a 3-h incubation period (data not shown). Erythromycin did not significantly affect O2− generation by unprimed neutrophils at any dose (Fig. 1). This confirmed previous observations that erythromycin at the clinically relevant dose of 1 μg/ml (8) had no direct inhibitory effect on unprimed neutrophils stimulated with FMLP (3, 6) and also implies that the drug neither interferes with binding of FMLP to its receptor on neutrophils nor acts as a free radical scavenger. When neutrophils were only slightly primed by 5 ng of G-CSF/ml, only a 50-μg/ml concentration of the drug significantly inhibited FMLP-stimulated O2− generation (P < 0.05). However, when human neutrophils were significantly primed by 10 or 50 ng of G-CSF/ml prior to FMLP stimulation to partly mimic the condition of the inflammatory site, the clinically relevant dose of erythromycin markedly suppressed O2− generation to the baseline levels observed for unprimed neutrophils stimulated with FMLP (P < 0.01), as did the higher doses of 10 and 50 μg/ml (Fig. 1). This result suggests that erythromycin acts to modulate the production of neutrophil-derived oxygen radicals at the inflammatory site from an excessive to a normal response rather than to suppress their production, ultimately reducing epithelial injury in the airways of patients with DPB.
FIG. 1.
Inhibitory effect of erythromycin on O2− generation by unprimed or G-CSF-primed human neutrophils stimulated with FMLP. Values are means (± standard errors of the means) of absorbance at 550 nm for three independent experiments. Statistical differences were determined by using the Student t test, and data were considered statistically significant when the P value was less than 0.05. Number signs indicate statistical significance of differences in O2− generation by primed versus unprimed neutrophils in the absence of erythromycin (EM) (#, P < 0.01; ##, P < 0.05). Asterisks indicate statistical significance of differences in O2− generation by G-CSF-primed neutrophils in the presence versus the absence of erythromycin at the indicated G-CSF concentrations (∗, P < 0.01; ∗∗, P < 0.05).
More adequate experimental designs mimicking conditions found at the inflammatory site are necessary to evaluate the possible beneficial nonantibiotic effect of erythromycin.
REFERENCES
- 1.Cantin A M, North S L, Fells G A, Hubbard R C, Crystal R G. Oxidant-mediated epithelial cell injury in idiopathic pulmonary fibrosis. J Clin Invest. 1987;79:1665–1673. doi: 10.1172/JCI113005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujii T, Kadota J, Kawakami K, Iida K, Shirai R, Kaseda M, Kawamoto S, Kohno S. Long term effect of erythromycin therapy in patients with chronic Pseudomonas aeruginosa infection. Thorax. 1995;50:1246–1252. doi: 10.1136/thx.50.12.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hojo M, Fujita I, Hamasaki Y, Miyazaki M, Miyazaki S. Erythromycin does not directly affect neutrophil functions. Chest. 1994;105:520–523. doi: 10.1378/chest.105.2.520. [DOI] [PubMed] [Google Scholar]
- 4.Ichikawa Y, Ninomiya H, Koga H, Tanaka M, Kinoshita M, Tokunaga N, Yano T, Oizumi K. Erythromycin reduces neutrophils and neutrophil-derived elastolytic-like activity in the lower respiratory tract of bronchiolitis patients. Am Rev Respir Dis. 1992;146:196–203. doi: 10.1164/ajrccm/146.1.196. [DOI] [PubMed] [Google Scholar]
- 5.Kadota J, Sakito O, Kohno S, Sawa H, Mukae H, Oda H, Kawakami K, Fukushima K, Hiratani K, Hara K. A mechanism of erythromycin treatment in patients with diffuse panbronchiolitis. Am Rev Respir Dis. 1993;147:153–159. doi: 10.1164/ajrccm/147.1.153. [DOI] [PubMed] [Google Scholar]
- 6.Mitsuyama T, Tanaka T, Hidaka K, Abe M, Hara N. Inhibition by erythromycin of superoxide anion production by human polymorphonuclear leukocytes through the action of cyclic AMP-dependent protein kinase. Respiration. 1995;62:269–273. doi: 10.1159/000196461. [DOI] [PubMed] [Google Scholar]
- 7.Mohammad J R, Mohammad B S, Pawluck L J, Bucci D M, Baker N R, Davis W B. Purification and cytotoxic potential of myeloperoxidase in cystic fibrosis sputum. J Lab Clin Med. 1988;112:711–720. [PubMed] [Google Scholar]
- 8.Nagai H, Shishido H, Yoneda R, Yamaguchi E, Tamura A, Kurashima A. Long-term low-dose administration of erythromycin to patients with diffuse panbronchiolitis. Respiration. 1991;58:145–149. doi: 10.1159/000195915. [DOI] [PubMed] [Google Scholar]
- 9.Oda H, Kadota J, Kohno S, Hara K. Leukotriene B4 in bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis. Chest. 1995;108:116–122. doi: 10.1378/chest.108.1.116. [DOI] [PubMed] [Google Scholar]
- 10.Oishi K, Sonoda F, Kobayashi S, Iwagaki A, Nagatake T, Matsushima K, Matsumoto K. Role of interleukin-8 (IL-8) and an inhibitory effect of erythromycin on IL-8 release in the airways of patients with chronic airway diseases. Infect Immun. 1994;62:4145–4152. doi: 10.1128/iai.62.10.4145-4152.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pick E, Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophage in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46:211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- 12.Sakito O, Kadota J, Kohno S, Abe K, Shirai R, Hara K. Interleukin-1β, tumor necrosis factor α and interleukin-8 in bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis: a potential mechanism of macrolide therapy. Respiration. 1996;63:42–48. doi: 10.1159/000196514. [DOI] [PubMed] [Google Scholar]