Summary
Lung cancer (LC) and tuberculosis (TB) are two major global public health problems, and the incidence of LC-TB is currently on the rise. Therefore effective clinical interventions are crucial for LC-TB. The aim of this review is to provide up-to-date information on the immunological profile and therapeutic biomarkers in patients with LC-TB. We discuss the immune mechanisms involved, including the immune checkpoints that play an important role in the treatment of patients with LC-TB. In addition, we explore the susceptibility of patients with LC to TB and summarise the latest research on LC-TB. Finally, we discuss future prospects in this field, including the identification of potential targets for immune intervention. In conclusion, this review provides important insights into the complex relationship between LC and TB and highlights new advances in the detection and treatment of both diseases.
Subject areas: Immunology, Microbiology, Cancer
Graphical abstract
Immunology; Microbiology; Cancer
Introduction
Cancer is a major global health threat to humanity, and patients with malignant tumors have a poor prognosis, with a less than 15% overall survival rate.1 According to statistical data, in 2022, there were 1,918,030 new cancer cases and 609,360 deaths in the United States.2 Surprisingly, about 350 people die from LC daily, making it the leading cause of cancer death in the United States.2 In 2022, cancer survey data from China and the United States showed that compared with the United States, the incidence and mortality of LC in China have significantly increased.3 In addition, according to GLOBO-CAN2020 data, the incidence and mortality of LC in China accounted for 37.0% and 39.8% of the global total, respectively.4
Tuberculosis (TB) is a global infection caused by Mycobacterium tuberculosis (MTB). TB has become one of the critical health concerns of the World Health Organization (WHO). Before the COVID-19 pandemic, TB was the leading cause of death from a single infectious agent worldwide, with a mortality rate higher than that of human immunodeficiency virus (HIV) infection, TB is the leading cause of death from a single infectious agent worldwide.5 According to the 2022 Global Tuberculosis Report, approximately one-quarter of the global population is infected with MTB. In 2021, a staggering 10.6 million new TB cases were reported worldwide, with 1.6 million resulting in death, representing a 4.5% and 6.7% increase, respectively, compared to the previous year.6 China is a high-burden country, with the second highest number of new cases after India and Indonesia. It ranks third in the world, accounting for 7.4% of the global total.6
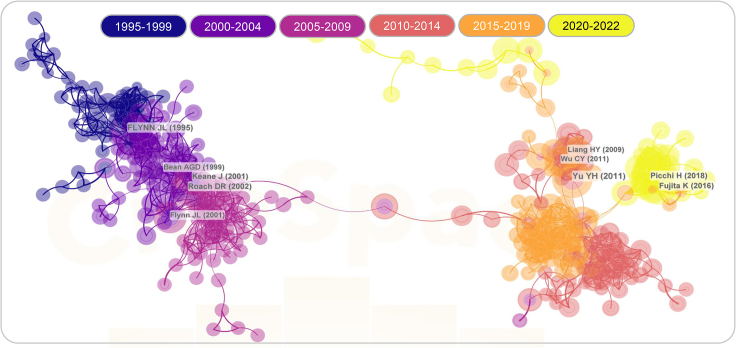
Even more concerning, there has been a significant increase in the incidence of TB in patients with LC in recent years,7 and the number of patients with TB with LC complicated by tuberculosis (LC-TB) has also markedly risen8 (Figure 1). Several studies have indicated that patients with different types of tumors face varying degrees of increased risk for MTB infection. Specifically, individuals with hematological or solid tumors have approximately double the risk of MTB infection when compared to the normal healthy population. Moreover, patients with LC face a significantly higher risk, approximately 6-fold, of MTB infection compared to those with non-LC.9,10 A retrospective clinical study exploring the relationship between LC and TB found that out of 2,608 patients with cancer receiving anti-LC treatment, 34 cases (1.3% of the total) were infected with MTB.11 Furthermore, studies have found that patients with LC-TB have an 8-fold higher mortality rate than patients with LC alone.12 Meanwhile, TB, particularly pulmonary tuberculosis (PTB), as an independent risk factor for LC, dramatically increases the risk of cancer in patients with TB.10 A retrospective study has revealed that among 3,776 patients with TB who were followed up, 86 patients (2.3% of the total population) developed LC.13
Figure 1.
Bibliometric analysis of studies involving patients with lung cancer complicated by pulmonary tuberculosis
The PubMed, Web of Science, Medline, and Chinese National Knowledge Infrastructure (CNKI) databases were utilized with the search formula "(((TS=(tuberculosis)) OR (TS=(TB))) AND ((TS=(lung cancer)) OR TS=(lung tumor)))" to retrieve and export the full record results. Further search parameters were set to DT=("ARTICLE" OR "REVIEW") and SILOID=("WOS") AND LA=("ENGLISH"), resulting in the inclusion of 2,705 unique publications in this study. Among these, 2,321 publications were categorized as articles, and 384 publications were categorized as reviews. Additionally, CiteSpace 6.2.R2 (64-bit) Basic (citespace.podia.com) was employed to perform citation-based visualization of the data derived from Web of Science.
The aforementioned evidence suggests a close correlation between the occurrence and development of LC and TB, and the innate and adaptive immunity of patients with LC-TB plays a crucial role in this process.14,15 However, little is currently known about the immunological characteristics and mechanisms of patients with both LC and TB. Therefore, this review takes the related immunological features of both diseases as a starting point and systematically reviews the pathogenesis of the co-occurrence of these two diseases. The aim is to identify effective immunological intervention targets that could provide valuable references for the clinical treatment of patients with LC-TB.
Immunological characteristics of patients with lung cancer-tuberculosis
Patients with LC-TB exhibit a unique immunological profile due to the coexistence of lung cancer and tuberculosis. The immune system in these patients is affected by both diseases, leading to a complex interplay of immune responses. However, this interplay can compromise the immune system’s ability to mount an effective anti-cancer response, as TB has immunosuppressive effects.16,17,18 Patients with LC often experience immune dysregulation, characterized by an impaired immune response against cancer cells.19 Tumor cells in LC create an immunosuppressive microenvironment by upregulating various immunosuppressive molecules, such as PD-1, CTLA-4, lag-3, TIM-3, and GITR, to evade immune surveillance. On the other hand, TB primarily affects the lung and triggers a robust immune response. Innate immune cells, including macrophages and dendritic cells, are activated to initiate a response against MTB. However, in some cases, the immune response fails to control the infection, leading to the formation of granulomas and the subsequent reactivation of latent TB in the presence of immunosuppression, as seen in patients with LC-TB. The immune responses of the host to LC and MTB infection are complex, and they play decisive roles in the immunological recognition, immune response, and immune evasion processes that lead to the disease’s onset, development, and outcomes.20
Immune recognition
The immune system of the host can recognize self and non-self material components. In addition to naturally inducing immune tolerance to self-material components, it can also eliminate non-self-material components to maintain the stability of the internal environment.21 When normal cells in the body undergo mutations and begin to multiply indefinitely, it can lead to the development of cancer. Cancer cells have a high degree of homology with normal cells and can often not be distinguished from them, which limits the ability of the immune system to recognize cancer cells.22,23 Unlike tumor cells, MTB bacteria and their surface-secreted antigens are the primary factors involved in manipulating host immune recognition, and the primary requirement for initiating innate and adaptive immune responses is that immune cells must recognize specific components of antigenic microorganisms through pattern recognition receptors.24 Toll-like receptors (TLRs) are essential receptors that are exposed on the surface and inside cells in innate immune responses, and their activation can trigger immune responses against pathogens.24,25 The interaction between mycobacterial EsxL and TLR2 leads to the activation of the macrophage NF-κB pathway, which facilitates the production of IL-12 and inducible nitric oxide synthase (iNOS), ultimately enhancing the immune system’s ability to clear mycobacterial infection.26,27 TLR9 recognizes the 5-CG-3 (CPG) structure present in MTB, promoting the production of inflammatory cytokines such as IL-12, TNF, and IFN-α, which are closely associated with the regulation of Th1/Th2 immune balance and the progression of tuberculosis granulomas.28,29
Immune responses
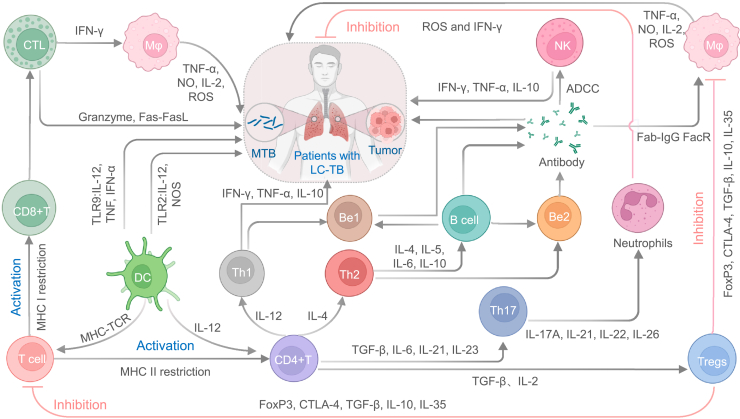
As is well-known, the immune response function of the body’s immune system is mediated by the coordinated action of innate and adaptive immune responses (Figure 2). Among them, immune cells such as macrophages, natural killer (NK) cells, dendritic cells (DCs), and effector T cells play a crucial role in eradicating tumor cells and mycobacterial infection.30 During the early stages of LC or mycobacterial disease, powerful innate immune cells such as macrophages, NK cells, dendritic cells, γδT cells, neutrophils, and other phagocytic cells play an important role.31 In contrast, in the advanced stages of tumor development or mycobacterial infection, T and B cell populations carry out most antigen-specific responses initiated by the adaptive immune response.
Figure 2.
Immune responses in patients with LC-TB
The immune responses against mutant tumor cells or invading bacteria involve the rapid activation of macrophages, NK cells, and DCs within the body. These immune cells act upon encountering the threat. The tumor or bacterial antigens are captured by APCs, notably DCs, which then migrate to the draining lymph node. In this location, the DCs activate immature T cells that subsequently differentiate into effector T cells. This activation occurs as the DCs present the captured antigens using MHC class I and II molecules on their surface. Activated T cells then enter the bloodstream and infiltrate the tumor or infection site, carrying out their function of eliminating tumor cells or MTB by releasing cytotoxic cytokines. APCs: Antigen-presenting cells; CTL: Cytotoxic T lymphocytes; DCs: Dendritic cells; IFN-γ: Interferon gamma; MHC: major histocompatibility complex class; NK cells: Natural killer cells; ROS: Reactive oxygen species; TGF-β: Transforming growth factor β; TLR: Toll-like receptors; TNF-α: Tumor necrosis factor alpha.
Innate immunity
Macrophages
Macrophages are a ubiquitous cellular component of the body, and the phagocytic function of the macrophage lineage is the first line of defense against exogenous microbial pathogens.32 In addition, macrophages not only express a rich repertoire of innate immune receptors such as TLRs, inflammasomes, and lectin-like receptors, but also engage in collaborative interactions with other innate immune cells, including neutrophils and innate lymphocytes, particularly NK cells, to collectively mediate the activation of the adaptive immune system.33 Whenever cancer cells invade the body’s tissues, the mutated cells activate macrophages in the lungs, which, in turn, differentiate into M1 and M2 macrophages in response to the environmental cues and signals encountered.34 M1 macrophages possess anti-microbial and anti-tumor activities and are typically induced to produce pro-inflammatory cytokines such as TNF-α, IL-6, IL-1, and Cox-2, as well as lower levels of IL-10, by Th1-type cytokines such as IFN-γ, TNF-α, and LPS.35 These pro-inflammatory cytokines mediate the clearance of tumor cells in the early stage of anti-tumor immunity by activating the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase reaction system and subsequent oxidative reactions (ROS).35 However, this oxidative clearance can lead to tissue damage and impair tissue regeneration and wound healing. To prevent such tissue damage, macrophages will polarize into M2 macrophages to suppress chronic inflammatory responses.35 M2 macrophages are induced by Th2-secreted cytokines, such as IL-4 and IL-13, which activate the STAT6 signaling pathway upon binding to their receptors. On the one hand, M2 macrophages suppress inflammatory responses in late-stage MTB infection. CD16+ monocytes isolated from patients with TB exhibit a further shift toward an M2-like activation state in the late stage of MTB infection, secreting IL-10 and TGF-β to suppress the production of pro-inflammatory cytokines,36 thus limiting the inflammatory response and promoting the survival of MTB. On the other hand, M2 macrophages also promote the formation of tumor microvessels in the tumor environment, creating a favorable environment for tumor occurrence, development, and metastasis.35 These findings have been demonstrated in the treatment of cervical and ovarian cancer cells, where the use of platinum-based chemotherapeutic drugs has been found to induce monocyte differentiation into M2 macrophages and release of the cytokine VEGF-A, promoting angiogenesis and tumor metastasis.37
Interestingly, 50% of macrophages in the tumor microenvironment are also known as tumor-associated macrophages (TAMs), which are important immunosuppressive cells in the LC immune microenvironment.34 When patients with LC are co-infected with TB, TAMs not only bind with T cell surface inhibitory receptor PD-1 through high expression of programmed cell death ligand 1 (PD-L1),38 causing the exhaustion of effector T cells but also reduce the infiltration of CD8+ T cells through the secretion of immunosuppressive cytokines such as IL-10, TGF-β and CXCL8,39 thus inhibiting CTL-mediated anti-MTB infection response and resulting in suppression of adaptive immune response. In addition, studies have found that MTB can prevent the fusion of phagosomes with lysosomes inside macrophages, thus allowing it to survive and replicate within macrophages,40 ultimately leading to the development of active TB in patients with LC.
Natural killer cells
NK cells are a subset of innate lymphoid cells that mediate anti-tumor and anti-microbial responses, serving as the first line of defense in monitoring and clearing infected and tumor cells.41 Initially thought to only develop in the bone marrow, NK cells have recently been found to grow and mature in secondary lymphoid tissues (including the tonsils, spleen, and lymph nodes) in both humans and mice.42 The predominant type of NK cells in lymph nodes is CD56 bright NK cells,43 which are known to control tumor growth and metastasis, with a particularly prominent role in lung metastasis.44 When host cells are invaded by tumor cells, their cell surface major histocompatibility complex class I (MHC I) molecule expression decreases, making them vulnerable to attack by NK cells.45 Upon activation, CD56-expressing NK cells produce high-affinity chemokine CXCL2, which signals through CXCR2 to recruit more NK cells into the tumor microenvironment and promotes NK cell proliferation and production of cytolytic molecules, thereby acquiring anti-tumor cell toxicity.45,46 In addition, activated NK cells produce a variety of cytokines such as IFN-γ, TNF-α, IL-10, and GM-CSF.47 Among these cytokines, IFN-γ can activate monocytes and macrophages to enhance the expression of MHC molecules on antigen-presenting cells, thus improving antigen-presentation ability48 and activating the adaptive immune system to exert anti-tumor effects. However, NK cells in patients with LC often experience functional dysregulation or reduced frequency,49 increasing susceptibility to active TB in patients with LC.
Furthermore, this functional dysregulation may be related to proteins expressed on the surface of LC cells.50 Studies have found that LC cells in patients receiving radiation therapy exhibit high expression of programmed cell death ligand 1 (PD-L1) on their surface, but signal transduction of IL-6-MEK/ERK in LC cell lines decreases expression of NK cell activating ligands,51 thereby inhibiting the toxic effect of NK cells against infection. According to research, a decrease in NK cell-mediated cytotoxic activity was observed in the peripheral blood of patients with TB.52 Moreover, Schierloh et al. demonstrated that patients with TB pleurisy are more susceptible to apoptosis in the predominant NK cell subset (CD56dimCD3- cells) in the pleural fluid.53 In conclusion, in patients with LC-TB, the dysregulation or decreased frequency of NK cells can significantly increase the risk of reactivation of latent tuberculosis infection (LTBI) or active TB.
Dendritic cells
DCs, composed of a group of myeloid-derived antigen-presenting cells (APCs), are the most effective cells for antigen presentation. They can orchestrate antigen-specific immunity and tolerance, including immunity against cancer and self-tolerance.54 DCs play a crucial role in innate and adaptive immunity against tumors and MTB infection. DCs can be divided into two categories based on their developmental stages: immature DCs (iDCs) and mature DCs (mDCs).55 In the anticancer mechanism, iDCs lack the ability to secrete cytokines and express lower levels of MHC molecules, T cell co-stimulatory factors, and adhesion molecules.56 However, they have a strong ability to capture antigens. After capturing antigens, iDCs migrate to secondary draining lymph nodes and present the captured antigens to effector T cells, thereby initiating a cytotoxic response.57 Interestingly, iDCs gradually transform into mDCs during their migration process. mDCs not only upregulate co-stimulatory molecules, CD80/CD86, but also increase the production of pro-inflammatory and chemotactic cytokines,58 thereby activating T cell-mediated adaptive immune responses. However, when patients with LC are infected with MTB, not only does the TGF-β decrease the recruitment of DCs,59 but also the tumor-derived factor, versican, inhibits DC activation and reduces cytokine production.60 Moreover, studies have found that patients infected with MTB have a certain degree of DC deficiency and that the number of DCs is only about half that of a normal healthy population,61 which may be due to the activation of the NF-κB pathway, leading to DC apoptosis and damage after infection with the highly virulent strains of MTB in patients.62
Adaptive immunity
T cell population
As it is widely acknowledged, APCs activate T cells by presenting tumor or bacterial antigens in lymph nodes. The activation of T cells depends on the recognition of antigenic peptides presented by MHC molecules and co-stimulation through binding CD28 to CD80/CD86, which is expressed on the surface of APCs.63 The highly polymorphic set of MHC class I and class II molecules present on the surface of cells mediate the MHC control system.64 Activated T cells can be classified into two subtypes based on their surface molecular characteristics: CD4+ and CD8+ T cells, which play a vital role in anti-tumor and anti-TB immunity. Upon activation by tumor antigen-specific T cells, CD4+ T cells primarily interact with MHC class II molecules. In the presence of APC-derived IL-12, CD4+ T cells differentiate into helper T (Th) cells, which are further differentiated into Th1, Th2, and Th17 cells under the regulation of different cytokines.65 Th1 cells are mainly involved in anti-tumor and MTB immunity, producing cytokines such as IFN-γ, IL-2, and TNF-α.66 These cytokines can recruit monocytes and granulocytes and directly regulate the macrophage-mediated cytotoxic effect, leading to the synthesis of inflammatory mediators such as lysosomes, reactive oxygen species, and nitric oxide, ultimately inducing apoptosis of tumor cells or killing of MTB.67,68,69 In addition,Th17 cells secrete cytokines such as IL-17A, IL-21, IL-22, and IL-26, which recruit neutrophils and facilitate their trafficking to the site of infection, thereby enhancing the host defense.70,71
Conversely, CD8+ T cells mainly bind to MHC class I molecules. Upon TCR binding to the tumor antigen complex on LC cells, CD8+ T cells are activated into CTLs, which mediate the fragmentation and lysis of tumor cells as described later in discussion. In a state of invasion by LC cells, the inhibitory ligands expressed on the tumor cell surface bind to the receptors on the surface of T cells, leading to T cell inhibition, reduced host clearance, and killing of MTB. Besides, a high correlation exists between the number of bacterial colonies in draining lymph nodes and the percentage of proliferating T cells, signifying that the activation of T cells coincides with bacterial spread.72 Additionally, MTB-activated T cells differentiate into Foxp3 or CD25+ regulatory T cells, attenuating the early response of CD4+ T cells.73 Therefore, the active suppression of T cells in the tumor microenvironment increases the amount of bacteria in the draining lymph nodes, leading to TB in patients with LC.
Among the CD8+ T cells, Cytotoxic T lymphocytes (CTLs) are differentiated from activated T cells and, together with NK cells, are the primary cytotoxic effector cells of the body’s immune system in clearing pathogens and tumor cells. CTLs and NK cells have similar mechanisms for killing target cells.74,75,76 Upon invasion of tumor cells into the body, APCs, primarily DCs, phagocytose them and subsequently process them into tumor-associated antigens (TAAs). These TAAs are then transported to draining lymph nodes, where they bind to MHC class-I molecules to form MHC class-I antigen complexes. They are presented to helper T lymphocytes, promoting T cell activation and differentiation into CTLs.57 Next, when the T cell receptor (TCR) of CTLs binds to the antigen complex of LC cells, the perforin and granzymes in their cells enter the immunological synapse of the cell through exocytosis.77 The perforin polymerizes and inserts into the membrane of the tumor cells, mediating the granzymes and other soluble factors to disrupt normal cell gradients through small pores and lead to tumor cell dissolution.78 Finally, the death receptor (Fas) on the surface of CTLs binds to the Fas ligand (FasL) on the surface of tumor cells, initiating a cell death signal and inducing apoptosis in tumor cells.79 Fas, a surface protein of the tumor necrosis factor (TNF) receptor family, exerts its mechanism of action through the recruitment of the Fas-associated death domain protein (FADD) into the death domain (DD) of the Fas intracellular region. The Fas ligand on the tumor’s surface initiates this process, subsequently forming a complex signal that leads to cell death by inducing caspase-8 cleavage, ultimately activating downstream effector caspase-3 and inducing apoptosis in tumor cells.80,81
In addition to inducing apoptosis in LC cells through oxygen-dependent or oxygen-independent pathways, activated CTLs also secrete IFN-γ, which activates monocytes and macrophages to express direct killing products such as reactive oxygen species (ROS) and reactive nitrogen intermediates (RNI).82,83 Interestingly, patients with LC-TB often exhibit suppressed CTL function. Tumor-associated macrophages (TAMs) can suppress T cell activation within the tumor microenvironment by secreting immune-inhibitory factors such as IL-10, TGF-β, and CXCL8.39 Additionally, myeloid-derived suppressor cells (MDSCs) can hinder T cell differentiation by expressing indoleamine 2,3-dioxygenase (IDO) and arginase-1 (Arg-1).84 Alternatively, T cells activated by MTB can differentiate into regulatory T cells with immune-suppressive effects, which express inhibitory receptor ligands and bind to inhibitory receptors present on the surface of T cells, ultimately leading to active TB development.
B-cell population
The specific humoral immune response of the body is primarily mediated by B lymphocytes, which play a crucial role in immune protection and vaccination against tumors and various pathogenic microorganisms.64 For patients with LC, tumor-infiltrating B lymphocytes (TIBs) of different histological subtypes can appear at any stage of LC development.85,86 Approximately 35% of patients with LC in various stages of tumor development demonstrate B cell proliferation,87 highlighting the pivotal role of B cells in LC progression. In addition, B cells can act as specialized antigen-presenting cells and interact with T cells to exert immune response functions when cells within the body undergo mutation.88 A study investigating whether B cells can stimulate T cells found that anti-CD40-activated B lymphocytes in patients with tumor can trigger secondary T cell responses.89 Activated B cells can also mediate antibody-dependent cellular cytotoxicity (ADCC) by releasing antibodies.90 Specific antibodies' Fab segment can bind to antigenic epitopes on tumor cells to form immune complexes, capable of binding to the Fc receptors on the surface of cytotoxic cells (NK cells or macrophages) using their Fc segment,91 which ultimately enhances the direct cytotoxic effect of the cytotoxic cells.92
Conversely, immune complexes formed by some antibodies produced by plasma cells in response to tumor-associated antigens (TAAs) may induce the production of MDSCs, suppressing T cell-mediated adaptive immune responses.93 B cells can develop into different subpopulations, including B effector-1 (Be1), B effector-2 (Be2), and regulatory B cells (Bregs), based on the immune microenvironment. Bregs secrete IL-10 and TGF-β, which possess inhibitory effects and can suppress T cell activation.94 In addition, recent studies have also shown that B cells can synthesize and secrete the neurotransmitter gamma-aminobutyric acid (GABA), inducing macrophages to secrete IL-10 and suppress T cell immune response.95 Thus, patients with pulmonary tumors are more susceptible to developing TB.
Immune escape in patients with lung cancer-tuberculosis
Immune escape of tumor cells
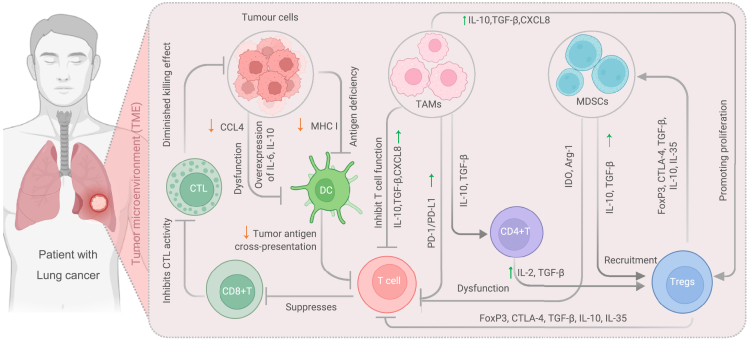
Tumor cells can evade the immune system at every stage of LC development,96 and the immune escape strategies utilized by tumors are vary (Figure 3). These strategies include tumor antigen deficiency, APC-mediated effector T cell aberrations, and the formation of suppressive tumor microenvironments.97
Figure 3.
Mechanisms of immune evasion in the tumor microenvironment
Within the tumor microenvironment, tumor cells employ various mechanisms to evade immune surveillance and elude immune-mediated destruction. These mechanisms include: 1) Tumor-induced Dysfunction of Dendritic Cells: Tumor cells overexpress cytokines such as IL-6 and IL-10, while downregulating CCL4, which leads to the impairment of dendritic cell function. Additionally, tumor cells can weaken the antigen presentation ability of antigen-presenting cells by downregulating tumor antigen cross-presentation and MHC I expression. This inhibits T cell activation and suppresses the differentiation of CD8+ T lymphocytes into cytotoxic T lymphocytes, thereby reducing the killing effect. 2) Immunosuppressive Effects of TAMs: TAMs suppress T cell function by upregulating the expression of immune checkpoint molecules such as PD-1/PD-L1. They also secrete immunosuppressive cytokines like IL-10, TGF-β, and CXCL8, which not only regulate CD4+ T lymphocytes but also activate Tregs. TAMs can enhance Treg proliferation and inhibit T cell function through various mechanisms involving FoxP3, CTLA-4, TGF-β, IL-10, and IL-35. 3) MDSCs-Mediated Immune Suppression: MDSCs induce dysfunction in T cells by utilizing enzymes such as IDO and Arg-1. They also recruit Tregs by upregulating the expression of IL-10 and TGF-β. Additionally, Tregs activate MDSCs through the secretion of FoxP3, CTLA-4, TGF-β, IL-10, and IL-35, further contributing to immune suppression within the tumor microenvironment. Abbreviations: APC, Antigen-presenting cells; Arg-1, Arginase-1; CTL, Cytotoxic T lymphocytes; CCLA4, CC-chemokine ligand 4; CXCL8, also known as interleukin-8 (IL-8); DC, Dendritic cells; IDO, Indoleamine 2,3-dioxygenase; MDSCs, Myeloid-derived suppressor cells; TAMs, Tumor-associated macrophages; TGF-β, Transforming growth factor β.
Tumor antigen deficiency
Alterations in the expression and presentation of tumor antigens can profoundly affect the efficacy of cancer immunotherapy. These defects can be broadly categorized into two types: a decrease in tumor mutational burden (TMB) and the loss of tumor antigens. Here, we discuss the mechanisms behind these defects and how they influence the immune surveillance of tumor cells. Fristly, mutations in the host cancer genome can lead to the expression of tumor-specific antigens in tumor cells. These mutations can result in the expression of mutated proteins on abnormal cells known as neoantigens.98 Low tumor mutational burden is one of the significant sources of neoantigens, leading to the reduced expression of MHC-I in tumor cells, which hinders the activation of T cell-mediated cytotoxic function.99 Consequently, tumors with low TMB can evade immune surveillance by the body’s immune cells, thereby reducing the therapeutic efficacy of cancer immunotherapies. This was demonstrated in clinical studies evaluating the relationship between tumor mutational burden and the therapeutic efficacy of Nivolumab alone or in combination with Ipilimumab for small cell LC.100,101 Secondly, the loss of tumor antigens due to immune editing can also contribute to immune evasion in tumor cells. Tumor cells use poor immunogenicity for tumor antigen presentation, leading to immune escape during the process of immune editing.102 In studies of patients with melanoma, tumor cells lost neoantigen expression due to immune editing during the interaction between tumor cells and T cells, leading to altered immune surveillance of tumor cells and potentially resulting in immune escape of tumor cells.103 Studies on acquired resistance in patients with non-small cell LC receiving immunotherapy with checkpoint inhibitors have demonstrated the role of antigen loss in tumor cell immune evasion.104 The mechanisms underlying neoantigen loss remain unclear. Further investigation is required to elucidate the mechanisms behind the loss of tumor antigens and its impact on the immune surveillance of tumor cells. Such efforts will aid the development of effective cancer immunotherapies and potentially result in improved patient outcomes.
Abnormalities in antigen-presenting cell-mediated effector T cells
DCs play a crucial role in promoting immunity or tolerance by presenting antigens to T cells and providing immune modulatory signals through cell-cell contacts.105 Their function is influenced by various environmental signals.106 However, the phagocytosis of tumor cells typically induces the activation of conventional dendritic cells (cDCs) and the initiation of effector T cells, which can be suppressed in the tumor environment. Studies have illustrated that tumors with active β-catenin have reduced the expression of chemokine ligand 4 (CCL4), which can decrease cDC recruitment and promote tumor growth.59 Cancer cell-derived factors have been identified to restrict the production and release of pro-inflammatory cytokines mediated by DCs. For example, studies have shown that the tumor-derived TLR2 ligand Versican led to the overexpression of IL-10 and IL-6, resulting in immune suppression of cDCs through the over-phosphorylation of DC signal transducer and transcription activator 3 (STAT3).60
Furthermore, tumor cells can induce the immune suppression of effector T cells.107 They promote the aggregation of host immune suppressor cells around the tumor cells and upregulate the expression of immune checkpoint proteins, such as programmed death receptor-1 (PD-1), cytotoxic T lymphocyte antigen-4 (CTLA-4), and T cell immunoglobulin and mucin-domain containing-3 (TIM-3).108,109 Despite the potential activation of tumor-specific T cells, the effector functions of these T cells are significantly suppressed by the inhibitory interaction between checkpoint proteins and their ligands on the surface of cDCs. Consequently, effector T cells undergo functional inactivation, allowing tumor cells to undergo immune escape. Recent studies on immunotherapy for non-small cell LC and its metastasis have provided strong evidence of this immunological mechanism.110
These findings demonstrated that tumor cells can induce the dysfunction of dendritic cells and the immune suppression of effector T cells, thus promoting the development of cancer. Understanding these mechanisms is crucial for developing novel immunotherapies to overcome immune evasion and improve the therapeutic efficacy of cancer treatment. Further research is needed to explore these mechanisms and develop strategies to overcome tumor-induced immunosuppression.
Inhibition of the formation of suppressive tumor microenvironments
The tumor microenvironment (TME) comprises immune cells, blood vessels, extracellular matrix (ECM), lymphocytes, bone marrow-derived inflammatory cells, and signaling molecules.111 Among them, tumor-associated macrophages (TAMs) play a critical role as immunosuppressive cells in the immune microenvironment. TAMs can upregulate the expression of PD-1-related ligands, resulting in immune suppression of effector T cells.38 TAMs have also been found to secrete immunosuppressive cytokines, such as IL-10, TGF-β, and CXCL8,39,112 which inhibit T cell function and promote Treg proliferation. Blocking TAMs in preclinical studies on invasive bladder cancer has been shown to enhance CD8+ mediated CTL anti-tumor immunity and improve the efficacy of PD-1 inhibitors.113
Additionally, the tumor-related factor VEGF promotes the growth and survival of endothelial cells, leading to the formation of new tumor blood vessels and hindering the trafficking and extravasation of effector T cells.114 Myeloid-derived suppressor cells (MDSCs) also contribute to the immunosuppressive tumor microenvironment by enhancing the recruitment of Tregs via the secretion of immunosuppressive cytokines such as IL-10 and TGF-β.115 MDSCs also cause T cell dysfunction by degrading L-tryptophan and L-arginine via the expression of indoleamine 2,3-dioxygenase (IDO) and arginase-1.84,116 Studies have shown that blocking MDSCs can improve the antitumor effects of PD-1 inhibitors in animal models, which are associated with a reduction in the expression of immunosuppressive proteins by MDSCs.117
Taken together, TAMs, VEGF, and MDSCs contribute to the immunosuppressive microenvironment of tumors by inducing immune suppression of effector T cells and promoting the recruitment and proliferation of Tregs. Understanding the role of these factors is essential for developing effective immunotherapy strategies. Further research is required to identify other tumor-related factors and develop new therapeutic approaches to overcome immunosuppression in the TME.
Immune escape of Mycobacterium tuberculosis
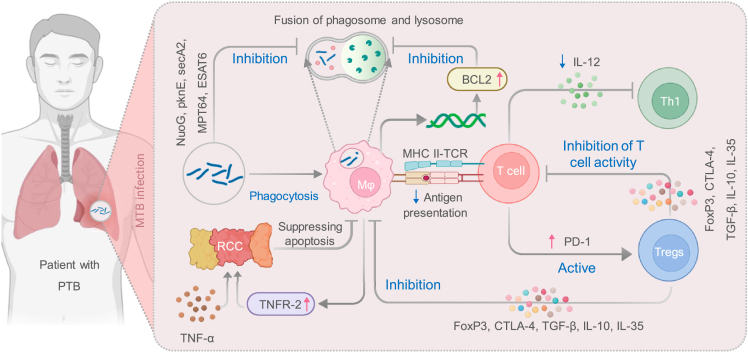
The immune system of the host plays a critical role in controlling infection by MTB. However, MTB has evolved numerous strategies to evade the host immune response and survive within host cells. This study highlights some of the mechanisms MTB employs to subvert host defenses and promote its survival118 (Figure 4). Firstly, MTB possesses virulence-associated anti-apoptotic genes or components such as the NuoG, pknE, secA2 genes, and MPT64 antigen.119 Riendau et al. reported that early secretory antigen target 6 (ESAT6), which is secreted by MTB during the early stage, can regulate cell apoptosis by activating caspase expression and induce THP-1 cell apoptosis.120 Moreover, the low-virulence MTB H37R strain and bovine bacillus Calmette-Guérin (BCG) strain can also generate substantial apoptosis in PMA-differentiated THP-1 cells.121 These studies suggest that MTB genes can transmit external signals of the bacteria to the cytoplasm, thus inhibiting cell apoptosis by blocking the signaling pathways of host cells. Secondly, infection of macrophages by a strong MTB strain activates the NF-κB pathway, leading to the upregulation of the expression of the apoptosis suppressor Bcl2, thereby preventing the fusion of phagosomes with lysosomes during organismal infection.62 In addition, the highly virulent strain of MTB also induces infected macrophages to release a significant amount of the cytokine IL-10, thereby promoting an increase in the release of TNFR2.122 TNFR2 can form a soluble receptor-chain complex with TNF-α, leading to the inactivation of TNF-α and the inhibition of its production and downregulation of TNFR expression,122 thereby suppressing apoptosis in macrophages. Conversely, when the body is chronically stimulated by bacterial, viral, and parasitic antigens, activated T cells upregulate the expression of the immune checkpoint receptor PD-1 and activate regulatory T cells.123 The latter exhibit surface expression of immunosuppressive proteins, including Foxp3, CTLA-4, and CCR4, thereby inducing immune suppression that ultimately leads to the evasion of MTB from immune surveillance in the host organism.124,125 LC increase susceptibility to TB.
Figure 4.
Strategies employed by MTB to evade host defenses and promote its survival
The immune system of the host plays a crucial role in controlling MTB infection. However, MTB has developed various mechanisms to subvert the host immune response and ensure its survival within host cells. This figure highlights the key strategies employed by MTB: 1) Inhibition of Host Cell Apoptosis: MTB possesses virulence-associated genes that hinder host cell apoptosis, preventing the elimination of infected cells and promoting its own survival. 2) Macrophage Activation and Immune Suppression: Infection with a virulent strain of MTB induces macrophage activation, resulting in the increased release of the cytokine IL-10. This promotes the release of TNFR2 and the inactivation of TNF-α, which impairs the inflammatory response. Additionally, chronic exposure to bacterial, viral, and parasitic antigens can lead to the upregulation of the immune checkpoint receptor PD-1 on activated T cells. This, in turn, activates Tregs and contributes to immune suppression, enabling MTB to evade host immune surveillance. By employing these strategies, MTB can evade host defenses, survive within host cells, and establish a persistent infection. Abbreviations: MHC, major histocompatibility complex class; RCC, Receptor-chain complex; TGF-β, Transforming growth factor β; TNF-α, Tumor necrosis factor alpha.
The susceptibility of individuals to TB can be influenced by the state of their immune system. In healthy individuals, innate immune cells recognize MTB through pattern recognition receptors, which leads to the activation of adaptive immune cells that can eliminate MTB. However, in certain environments, such as in the context of cancer, the immune system of the host may be suppressed or compromised. This can result in a favorable condition for the invasion and infection of MTB, leading to an increased susceptibility to TB.126 In this study, we searched PubMed for all experimental articles published between 1990 and 2023 and investigated the increased susceptibility to TB in LC (Table 1). By analyzing the results of these studies, we found that the risk of newly acquired TB infection or reactivation of LTBI is 1.7 times higher in male patients with LC than in female patients (95% CI: 1.5–2.0),127 and incidence of TB increases with the age of patients with LC. In cases where TB complicates LC, the average age of patients typically ranges from 60 to 69 years old.128 In addition, active TB that develops in patients with LC post-diagnosis is often a reactivation of LTBI infection, which typically occurs within the first five years of cancer diagnosis, with cumulative incidence rates of 0.65%, 1.15%, and 1.38% at six months, one year, and two years, respectively.129,130,131 Moreover, patients with LC with concurrent TB have a shorter survival time compared to those without TB (p = 0.007), and active TB may increase the mortality rate in patients with both conditions.132,133 Finally, compared to the control group, the incidence rate ratio (IRR) of active TB is 4.69 (95% CI: 1.52–14.46) in patients with LC,126 further highlighting the increased susceptibility to MTB infection in individuals with LC.
Table 1.
List of studies in which LC increases the risk of developing TB
| Reference | Years | Countries | Experiment type | Sample size | LC-TB number | Time of follow-up | Contents |
|---|---|---|---|---|---|---|---|
| Aoki et al.128 | 1991 | Japan | Retrospective | 442 | 5 | \ | Five of 442 patients with LC developed active TB during LC treatment despite having latent TB. |
| Chen et al.132 | 1996 | China | Retrospective | 3928 | 31 | 2 | Out of 3928 patients with LC, 31 individuals developed TB (tuberculosis). The risk of developing active TB was higher in patients with LC compared to the general population. Additionally, patients who developed active TB before or at the same time as their LC diagnosis experienced shorter survival times compared to those who did not develop TB (p = 0.007). |
| Tamura et al.185 | 1999 | Japan | Retrospective | 25a | 3 | \ | Out of 25 patients with LC, one individual developed TB, resulting in a 1.9% incidence of active TB in patients with untreated LC. |
| Watanabe et al.186 | 1999 | Japan | Retrospective | 758 | 6 | \ | Out of the 758 patients with cancer, 16 individuals had combined TB. Among these patients, 6 were found to have both TB and cancer. |
| Remiszewski et al.133 | 2001 | Poland | Retrospective | 854 | 60 | \ | 60 of 845 patients with small cell LC died from active TB. |
| Cicenas and Vencevicius187 | 2007 | Lithuania | Retrospective | 2218 | 46 | \ | Out of the 2,218 patients with LC, 46 individuals (2.1%) had coexisting LC and TB. |
| Kim et al.126 | 2008 | Korea | Case-control | 3618 | 9 | 3 | The incidence of active TB was found to be 3.07 cases per 1000 person-years in patients with cancer, while it was 0.77 cases in controls. This difference was statistically significant with a p value of 0.009. Patients with cancer had an increased risk of developing TB compared to controls, with an incidence rate ratio (IRR) of 4.69 (95% CI: 1.52–14.46). |
| Cha et al.130 | 2009 | Korea | Retrospective | 108 | 36 | \ | Out of the patients with LC, 10 individuals (27.8%) were diagnosed with TB at the same time as the diagnosis of LC, while 26 individuals (72.2%) were diagnosed with TB after the diagnosis of LC. The median time from LC diagnosis to TB diagnosis was 4 months, with a range of 1–47 months. |
| Silva et al.129 | 2013 | Portugal | Retrospective cross-sectional | 24b | 10 | \ | Out of the cases diagnosed with both TB and LC, 10 were diagnosed simultaneously, meaning they received the diagnoses at the same time. In 14 cases, TB was diagnosed before the diagnosis of LC. The median time between the diagnoses of TB and LC was 5 years, with an interquartile range of 1–30 years. |
| Suzuki et al.131 | 2016 | Japan | Observational | 904 | 9 | 2 | During the observation period, 9 out of 904 patients with cancer (1.00%) developed TB. In all cases, the occurrence of TB disease was within 2 years of the cancer diagnosis. The cumulative incidence of TB disease at 6 months was 0.65%, at 1 year it was 1.15%, and at 2 years it was 1.38%. |
| Şimşek et al.188 | 2017 | turkey | Retrospective | 3101 | 11 | \ | Out of the total cases of LC and TB, 17 cases were diagnosed with both combined MTB or NTM and LC. These cases accounted for 1.2% of the total LC cases and 0.9% of the total TB cases. Among these 17 cases, in 11 of them (64.8%), MTB was identified as Mycobacterium bovis. |
| Cukic V.11 | 2017 | Bosnia | Retrospective | 2608 | 34 | \ | 34 of 2,608 patients with LC, or 1.3%, were diagnosed with tb alone. |
| Tamura et al.189 | 2020 | Japan | Retrospective | 1450 | 7 | 2 | Among the patients with LC, seven had active TB, 45 had previous TB, and 1,398 had neither active nor previous TB. Among the 1,398 patients without active or previous TB, 795 (57%) underwent an IGRA test, and of those, 120 (15%) were diagnosed with LTBI. This data indeed suggests that active TB is an important complication among patients with Japanese LC. |
| Liao et al.127 | 2023 | China | Retrospective cohort | 71793 | 1335 | 1,335 cases of TB were identified among 71,793 patients with LC. The incidence of TB increased with age, and men had a significantly higher risk of developing TB than women, with an incidence rate ratio of 1.7 (95% CI: 1.5–2.0). Patients aged 60–69 years (HR: 1.4; 95% CI: 1.1–1.8) and those ≥70 years (HR: 1.9; 95% CI: 1.5–2.4) had a higher risk of TB than those younger than 50 years. Patients with a history of pneumoconiosis and those treated with surgery and chemotherapy also had a significantly increased risk of developing TB. |
The sample size consisted of the number of cases in clinical records with lung cancer complicated with pulmonary tuberculosis. However, there were 2 cases of active pulmonary tuberculosis diagnosed concurrently with lung cancer, and only 1 case of active pulmonary tuberculosis was found after the diagnosis of lung cancer.
The sample size consisted of the number of cases in clinical records with lung cancer complicated with pulmonary tuberculosis. However, only 10 cases of active pulmonary tuberculosis were found after the diagnosis of lung cancer, and the rest were discovered before the diagnosis of lung cancer.
The role of immune checkpoints in the treatment of patients with lung cancer with tuberculosis
The treatment of patients with LC-TB requires a multidisciplinary approach, addressing both diseases simultaneously. This involves a combination of LC treatment, such as surgery, chemotherapy, radiation therapy, targeted therapy, or immunotherapy, and TB treatment through the use of anti-tuberculosis drugs. Close monitoring of treatment response and potential drug-drug interactions is crucial. For patients with LC, the treatment approach depends on factors such as the stage and histological subtype of the cancer. Options may include surgery, chemotherapy, radiation therapy, targeted therapy (e.g., EGFR inhibitors or ALK inhibitors), and immunotherapy (e.g., PD-1/PD-L1 inhibitors). Personalized treatment strategies based on the tumor’s molecular characteristics are also gaining importance.
In recent years, immune checkpoint inhibitors (ICIs) have played a critical role in the immune therapy of advanced LC.134,135 They have also gained attention in TB treatment, providing new opportunities for patients with LC-TB.136 Currently, clinically studied immune checkpoint proteins include programmed death receptor 1 (PD-1), cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), lymphocyte activation gene-3 (LAG-3), T cell immunoglobulin domain and mucin domain-3 (TIM-3), and glucocorticoid-induced TNF receptor (GITR) (Table 2).97 PD-1/PD-L1 inhibitors have been a research hotspot in immune therapy for LC combined with TB, while CTLA-4, LAG-3, TIM-3, and GITR are expected to become the next generation of immune therapeutic targets.137
Table 2.
Immune checkpoint proteins in patients with LC-TB
| Target | Biological functions | Expression position | Effector cells | Route of action | Mechanism of action | Significance |
|---|---|---|---|---|---|---|
| Programmed death receptor 1 (PD-1) | Co-inhibitory receptors | Activated T and B cells and myeloid cells138 | PD-L1 on the surface of T cells, B cells, dendritic cells and macrophages, and tumor cells1139 | PD-1 and PD-L1 binding pathway | In the LC microenvironment, the interaction between the heightened expression of PD-L1 and PD-1 obstructs the activation of T-cells,140 while simultaneously promoting the differentiation and proliferation of Tregs.144 | By inducing T cell exhaustion, the evasive mechanism of tumor cells or MTB-infected cells from the host immune system’s surveillance and clearance is facilitated, subsequently fostering the progression and manifestation of diseases. |
| Cytotoxic T lymphocyte antigen 4 (CTLA-4) | Co-inhibitory receptors | Lym T phosphate cells149 | CD80/CD86 on the surface of antigen-presenting cells or tumor cells150 | CTLA4 binding pathway to CD80/CD86 | CTLA4 demonstrates a competitive and high-affinity binding to CD80/CD86 on antigen-presenting cells or tumor cells, consequently impeding the activation signal transmitted through the interaction of TCR and CD28. This ultimately results in the suppression of T cell activity.149,150,151 | The induction of T cell exhaustion enables the evasion of tumor cells or MTB-infected cells from the surveillance and clearance mechanisms of the host immune system. This evasion subsequently promotes the occurrence and progression of diseases. |
| Lymphocyte Activating Gene 3 (LAG-3) | Co-inhibitory receptors | Activated CD4+ and CD8+ T cells154 | MHC class II molecules presented by antigen-presenting cells156 | LAG-3 and MHCII binding pathway | The initial domain of LAG-3 exhibits greater activity compared to CD4, and it selectively binds to MHC II, leading to a reduction in T cell proliferation and cytokine secretion.156 | Inducing T cell exhaustion can enable evasion of the surveillance and clearance of tumor cells or MTB-infected cells by the host immune system, thus promoting the occurrence and development of diseases. |
| T cell immunoglobulin structural domain and mucin structural domain 3 (TIM-3) | Co-inhibitory receptors | monocytes (highest expression in dendritic cells), Th1-type cells164 | Binding of galactose lectin-9 (galectin-9) expressed on the surface of tumor cells165 | The binding pathway of TIM-3 with galectin-9. | The binding of TIM-3 with galectin-9 on the surface of tumor cells leads to the death of Th1-type T cells, resulting in a decrease in their secretion of the cytokine IFN-γ.164,165 | Inducing T cell exhaustion can evade the surveillance and clearance of tumor cells or MTB-infected cells by the host immune system, thus promoting the occurrence and development of diseases. |
| Glucocorticoid-induced TNF receptor (GITR) | Co-stimulatory receptors | Activation of T cells induces the upregulation of expression in different cells and tissues.167,172 | GITRL on the surface of antigen-presenting or tumor cells.190 | The binding pathway of GITR with GITRL. | The binding of GITR with GITRL on the surface of APCs can increase T lymphocyte proliferation and cytokine production, as well as promote the differentiation and expansion of Th17 cells and Tfh.167,172,174 When binding with GITRL in the tumor microenvironment, it inhibits the suppressive function of Tregs.190 | Enhancing T cell immune function can boost the longevity of inflammatory cytokines and strengthen the adaptive immune response mediated by T cells in the body. |
LC, Lung cancer; TCR,T cell receptor; APCs, antigen-presenting cells; Tfh, T follicular helper cells.
Programmed cell death ligand 1
PD-1 is an inhibitory cell surface receptor expressed on activated T, B, and myeloid cells but is expressed most prominently on the surface of T cells.138 PD-L1, the ligand for PD-1, is expressed on the surface of T cells, B cells, dendritic cells, macrophages, and several non-hematopoietic cell types and tumor cells.139 In the microenvironment of LC, the high expression of PD-L1 on the surface of tumor cells interacts with PD-1 on the surface of T lymphocytes, antagonizing T cell effector function in an antigen-specific manner, thereby inhibiting T cell activation,140 ultimately leading to immune escape of tumor cells. Interestingly, MTB can also induce high expression of PD-L1, thus evading immune surveillance through the PD-1/PD-L1 pathway.141 Its mechanism of action can be divided into two aspects: On one hand, PD-1 inhibits T cell activation when it binds with the T cell receptor (TCR).142 This is because PD-1 contains two tyrosine residues in its intracellular domain, which participate in the formation of the N-terminal immunoreceptor tyrosine-based inhibitory motif (ITIM) and the C-terminal immunoreceptor tyrosine-based switch motif (ITSM).143 The extracellular domain of PD-1 contains an IgV-like domain that possesses multiple glycosylation sites and is heavily glycosylated. This domain can bind to its ligands, leading to the recruitment of tyrosine phosphatases (SHP)-1 and SHP-2, which contain homologous domains within the ITSM motif. SHP-2 can then exert its function of inhibiting T cell activation through various mechanisms.143 On the other hand, similar to CTLA-4, PD-1/PD-L1 also promotes the differentiation and amplification of Treg cells, thereby suppressing the immune response of the body.144 In clinical treatment, many studies have shown that blocking the PD-1/PD-L1 signaling pathway has achieved good results in non-small cell LC.145 Moreover, PD-1 inhibitors can enhance the function of T lymphocytes in active TB, promote the cytotoxicity of CD8+ T lymphocytes, and increase the release of IFN-γ and TNF-α, thereby reducing macrophage necrosis and controlling infection with MTB.141,146 Therefore, the PD-1/PD-L1 signaling pathway may become a target for the treatment of LC combined with TB in the future.
Cytotoxic T lymphocyte antigen-4
CTLA-4 was the first ICI to be discovered with its inhibitory function.147 CTLA-4 is a molecule that can inhibit TCR signaling in T cells, which are the main cytotoxic cells. Activation of T cells requires two signals: recognition of antigen peptides presented by MHC (as previously mentioned), and co-stimulation through CD28 after binding with CD80/CD86 expressed on the surface of APCs.63 Among these, CD28 induces T lymphocyte activation through the phosphatidylinositol 3-kinase (PI3K) and protein kinase B (Akt) pathways upon binding to CD80/CD86 and blocks the suppressive function of peripheral regulatory T lymphocytes by inhibiting class O forkhead transcription factors (FOXO).148 Interestingly, CTLA-4 shares high homology with CD28, both of which are expressed by Lym T phosphatase cells and can bind to CD80/CD86 expressed on the surface of APCs. However, CTLA-4 has a higher affinity for CD80/CD86 than CD28.149,150 In the tumor microenvironment, although CTLA-4 does not have an ITIM sequence like other co-inhibitory molecules, it can recruit protein phosphatase 2A (PP2A) and SHP-2 to inhibit the activation signals of TCR and CD28 signaling, thereby inhibiting T cell activity,148,151 ultimately causing immune escape of tumor cells. Similarly, it has been found that certain immune checkpoints restrict protective immune responses in many chronic infectious diseases, thereby inhibiting immune-mediated clearance of infections.136 Upon blocking these inhibitory immune proteins with inhibitors such as TIM-3 inhibitors, the body regains T cell-mediated immune clearance function.152 Therefore, CTLA-4 is expected to become a novel pathway for the treatment of LC in patients with concomitant TB.136
Lymphocyte activation gene-3
LAG-3 is an inhibitory co-receptor with a structure similar to CD4, containing four Ig-like domains (D1-4) in its extracellular (EC) region and about 60 amino acid residues in its intracellular (IC) region, but lacks a typical inhibitory motif.153 Like the immune checkpoint proteins PD-1 and CTLA-4, LAG-3 is not expressed on naive T cells, but can be induced on CD4 and CD8 T cells upon stimulation with tumor antigens or bacterial stimuli.154 Research has shown that LAG-3 can act alone or in synergy with other inhibitory co-receptors, including PD-1, to regulate autoimmune, cancer, and infection immunity negatively.155 When cells in the body undergo mutations, the extracellular domain of LAG-3 primarily binds to major histocompatibility complex class II (MHCII), thereby evading immune surveillance.156 On the one hand, LAG-3 can independently inhibit T cell activation, but the underlying mechanisms remain unclear.154 On the other hand, LAG-3 uses its first domain, which has a higher affinity than CD4, to specifically bind to MHCII, thus leading to the downregulation of proliferation and cytokine secretion of CD4+ antigen-specific T cells through negative MHCII signaling transduction.156 This ultimately leads to the immune escape of tumor cells. Moreover, the intracellular domain of LAG-3 contains three conserved regions: the KIEELE, and acidic glutamic acid-proline (EP) repeat sequences, as well as a conserved pseudo-tyrosine phosphorylation site.157 Workman et al. demonstrated that KIEELE is required for LAG-3 to inhibit T cell hybridoma activity.158 Interestingly, studies have shown that LAG-3 may decrease Th1-type helper cell-mediated immune responses and potentially lead to uncontrolled replication of MTB.159 Therefore, blocking the immunosuppressive effects of LAG-3 may become a novel therapeutic target for patients with LC with concurrent TB.
T cell immunoglobulin and mucin-domain containing-3
TIM-3 is a type I transmembrane protein that consists of an N-terminal immunoglobulin variable (IgV) domain, a mucin-like domain, a transmembrane domain, and a cytoplasmic tail.160 Although initially identified as a negative regulator of IFN-γ production by CD4 and CD8 T cells, recent studies have revealed that this immune checkpoint protein also has significant potential in regulating innate immune cells.161 Several studies have reported that TIM-3 is expressed on the surface of the mouse and human monocytes and is highly expressed on the surface of DCs.162 The elevated levels of TIM-3 on DCs synergize with TLR signaling on the cell surface, leading to DC activation, promotion of pro-inflammatory cytokine production, and activation of immunoreactive T cells.162,163 Activated CD4+ T cells further differentiate into Th1 and Th2 subsets, with TIM-3 expressed on the surface of differentiated Th1 cells. The binding of TIM-3 to its ligand galectin-9, which is expressed on the surface of tumor cells, leads to Th1 cell death, thereby suppressing the immune response of T cells.163,164,165 Furthermore, studies have demonstrated a decreased expression of TIM-3 on the surface of T cells in the cerebrospinal fluid of patients with multiple sclerosis, along with elevated production of the cytokine IFN-γ.166 These findings suggest a potential link between impaired T cell function and dysregulation of TIM-3 expression. An interesting observation was made in a study exploring the role of TIM-3 in MTB infection. It was found that during MTB infection, mice co-expressed other inhibitory receptors (including PD-1), and treatment with an anti-TIM-3 monoclonal antibody was effective in treating TB.152 These results suggest that targeting TIM-3 could have significant therapeutic benefits for patients with both LC and TB.
Glucocorticoid-induced tumor necrosis factor receptor
GITR, also known as glucocorticoid-induced TNFR family-related protein or activation-inducible TNFR family receptor (AITR/TNFRSF18), possesses a rich cysteine domain in its extracellular region and shares homology in its cytoplasmic domain with other TNFR family receptors, including 4-1BB, OX40, CD40, and CD27. All of these receptors are expressed on various cells and tissues and serve as co-stimulatory molecules.167 In addition to blocking co-inhibitory pathways such as PD-1, CTLA-4, LAG-3, and TIM-3, therapies that activate co-stimulatory pathways also hold significant research interest in anti-tumor treatments.168 Therefore, GITR has emerged as a promising target for cancer immunotherapy, and clinical trials targeting this receptor are currently underway.169,170 In the tumor microenvironment, GITR is expressed at a low level on the surface of CD4, and CD8 T cells, as well as various types of APCs, but its expression is upregulated upon T cell activation by TCR binding to tumor antigens.171 The binding of GITR to its ligand GITRL, expressed on the surface of tumor cells, can increase TCR-induced T lymphocyte proliferation and cytokine production,172 which can enhance the immune response in the body and potentially lead to tumor regression. Thus, targeting GITR signaling holds great promise for cancer immunotherapy. Studies suggest that the GITR/GITRL regulatory system promotes the differentiation and expansion of Th17 cells and T follicular helper cells (Tfh), thereby enhancing T cell-mediated adaptive immune responses.173,174 Interestingly, research has found that Tregs cells also enhance their cell differentiation by upregulating the expression of tumor necrosis factor receptor superfamily (TNFRSF) members on their surface, but the binding of GITR to its ligand GITRL interferes with the development of Treg cells.175 For example, in Ptpn22-deficient mice studied by Nowakowska and Kissler, although the number of Treg cells increased, the regulation of the GITR/GITRL system blocked the expansion of Treg cells mediated by Ptpn22, revealing the important role of the GITR/GITRL system in inhibiting Treg cell function.176 However, research has found that when the body is infected with MTB, the expression of Treg cells is significantly higher than normal levels, indicating that Treg cells may play a critical role in controlling cellular aspects of TB infection.125 Therefore, when GITR antibodies are applied to LC, their inhibitory effect on Treg cells may also be beneficial for the body to control MTB infection. However, further research is needed to determine the optimal use and efficacy of GITR-targeting strategies in treating LC and TB co-infection.
Tuberculosis reactivation after immune checkpoint inhibitors
In the treatment of LC, significant progress has been made with the use of immune checkpoint inhibitors, which have shown remarkable efficacy in tumor treatment. However, it is surprising that the use of ICIs for immune activation therapy may lead to immune-related adverse events (irAEs), with an incidence rate ranging from 54% to 76%.177
Recent evidence has shown that patients with tumor may experience TB reactivation or rapid progression of TB infection after receiving ICI treatment.178 A systematic review published in the European Journal of Cancer in 2020 evaluated the relationship between ICI treatment and TB reactivation, and found that 16 cases of active TB occurred in patients with LC during PD-L1 inhibitor treatment, with a median time of 6.3 months.179 Similarly, a recent systematic review and meta-analysis that included 27 studies explored the incidence of TB caused by PD-1/PD-L1 immune blockade therapy.180 The results showed that among patients receiving PD-1/PD-L1 blockade treatment, there were a total of 35 cases of TB, resulting in an incidence rate of 2,000 cases per 100,000 people, which was 35 times higher than the general population.180 These data suggest that the clinical use of PD-1/PD-L1 inhibitors may significantly increase the risk of TB reactivation, and the mortality rate caused by TB is also extremely high.
We cannot help but ask: why does the use of ICI therapy in tumor patients lead to TB reactivation? Although the current research on this question is not yet deep enough, from the existing literature, we can summarize two potential mechanisms: (1) Immunotherapy infections due to immunosuppression (ITI-IS). The balance between host immune response and pathogen invasiveness is crucial for disease outcomes and progression after MTB infection.17,18,181 ICI therapy inhibits the immune response of patients, especially the function of T lymphocytes. Day, C.L. et al. analyzed peripheral blood samples from patients with LTBI and PTB and found an association between the expression level of PD-1 on MTB-specific T cells and MTB loads.182 The results showed that the expression of PD-1 on Th1+ CD4 T cells of patients with smear-positive PTB was significantly higher than that of patients with smear-negative PTB and LTBI, and the expression level of PD-1 on Th1+ CD4 T cells decreased after completing anti-tuberculosis treatment. These data suggest that PD-1 expression on MTB-specific CD4 T cells is closely related to MTB infection. Therefore, after using ICI therapy, the function of Th1+ CD4 T cells in patients with tumor is inhibited, providing favorable conditions for the reactivation of latent MTB infection. (2) Immunotherapy infections due to dysregulated immunity (ITI-DI). ITI-DI is a completely different mechanism of TB reactivation from ITI-IS, which paradoxically benefits the pathogen due to excessive host immune response caused by immune checkpoint inhibition.178 Tezera, L.B. et al. studied how immune checkpoint therapy for cancer leads to the activation of TB infection using a three-dimensional cell culture model, and found that PD-1 plays a regulatory role in the immune response to TB, and inhibiting PD-1 accelerates the growth of MTB through excessive secretion of TNF-α,183 indicating that ITI-DI caused by immune checkpoint inhibition benefits the survival of MTB. Further research on these mechanisms is crucial in preventing and managing the risk of TB reactivation under ICI therapy. Moreover, clinicians need to closely monitor patients for TB infection and provide prevention and treatment when necessary when using ICI therapy for patients with tumor. Combining immune therapy with TB management contributes to maximizing treatment efficacy and safety for patients.
Future perspectives
Globally, LC has emerged as a leading cause of cancer-related deaths, while TB remains one of the leading causes of death among infectious diseases. Consequently, both cancer and TB are two global public health issues that severely impact human health. This study offers a comprehensive review and analysis of current research reports, providing the latest evidence to clarify the significant correlation between LC and TB.
Elucidating the complex relationship between lung cancer and tuberculosis through immune system analysis
From the perspective of the immune system, it is believed that the immune response caused by MTB infection (such as inflammation, scar formation, and gene mutations) and drug therapy may be associated with an increased risk of LC. Furthermore, as LC develops and the immune system is damaged, there may be an increased risk of activating LTBI or acquiring new MTB infections. However, despite the mounting evidence of the correlation between these two diseases, their underlying mechanisms and interaction are not yet fully understood. Hence, further research is required to elucidate the complex relationship between LC and TB and develop effective diagnostic and therapeutic strategies to address this public health challenge.
Immunotherapy targeting programmed death receptor-1 as a potential novel treatment strategy for lung cancer and tuberculosis: Clinical implications
The recognition and surveillance functions of the immune system play a crucial role in the host’s clearance of tumor cells and MTB. Immunotherapies targeting immune evasion by tumor cells and MTB have gained increasing attention in recent years. Previous studies have shown that clinical pathological slides from patients with LC and TB both exhibit high expression of PD-1, a checkpoint receptor expressed on activated T cells that can inhibit T cell function,15 suggesting that PD-1 may have potential value in the early clinical diagnosis, prevention, and treatment of LC combined with TB. Specifically, blocking PD-1 may reinvigorate T cell function and enhance the immune response against both tumors and MTB. Hence, PD-1 blockade therapy may have significant clinical implications for improving the outcomes of patients with LC and TB.
Exploring the potential of immune checkpoint inhibitors in managing lung cancer and tuberculosis: Current limitations and future possibilities
ICIs have been shown to alleviate T cell exhaustion and reduce immunosuppressive cells, thereby improving the prognosis of patients with late-stage LC, especially patients with non-small cell LC. Moreover, ICIs enhance the immune system, leading to T cell proliferation and increased cytokine production, which can effectively eliminate MTB infection and induce its dormancy. However, while PD-1/PD-L1 inhibitors are effective in treating non-small cell LC, studies suggest that they may not be suitable for enhancing TB immunity.136,184 It remains to be studied whether the combination of PD-1/PD-L1 inhibitors and traditional anti-tuberculosis drugs can achieve the expected results. Therefore, applying ICIs such as PD-1, CTLA-4, LAG-3, and TIM-3 in preventing, diagnosing, and treating LC with concurrent TB requires extensive experimental verification.
Conclusions
In summary, the complex relationship between LC and TB has increasingly attracted attention in recent years. As far as the immune system is concerned, it plays a critically important role in both protecting the host’s immunity against TB and eliminating the tumor cells. Immunotherapies including ICIs show promise in preventing late-stage LC and improving the prognosis of patients with non-small cell LC. However, it remains unclear whether these inhibitors are suitable for enhancing TB immunity. With the pressing challenge posed by LC and TB, further research, testing, and analysis are required to better understand the immune system’s mechanisms and identify effective strategies for preventing, diagnosing, and treating these diseases. The ICIs' potential in such treatments remains an exciting avenue for exploration in clinical settings. By continuing to explore the efficacy and limitations of these inhibitors in managing LC and TB, researchers can develop more successful and comprehensive treatment strategies to improve patient outcomes and alleviate morbidity and mortality associated with these diseases.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (Grant No. 81801643) and Eighth Medical Center of PLA General Hospital (grant number MS202211002).
Author contributions
Conceptualization: WPG and JZG. Methodology: LY, LZ, ZYY, SLL, and WPG. Data Analysis: LY and LZ. Software: LY and WPG. Writing original article: LY. Review and revising article: WPG and JZG. Funding acquisition: WPG. All authors reviewed and approved the final article.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107881.
Contributor Information
Jingzhi Guan, Email: jzjz1970@hotmail.com.
Wenping Gong, Email: gwp891015@whu.edu.cn.
Supplemental information
References
- 1.Dela Cruz C.S., Tanoue L.T., Matthay R.A. Lung cancer: Epidemiology, etiology, and prevention. Clin. Chest Med. 2011;32:605–644. doi: 10.1016/j.ccm.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics. CA A Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Xia C., Dong X., Li H., Cao M., Sun D., He S., Yang F., Yan X., Zhang S., Li N., Chen W. Cancer statistics in china and united states, 2022: Profiles, trends, and determinants. Chin. Med. J. 2022;135:584–590. doi: 10.1097/CM9.0000000000002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 5.MacNeil A., Glaziou P., Sismanidis C., Date A., Maloney S., Floyd K. Global epidemiology of tuberculosis and progress toward meeting global targets - worldwide, 2018. MMWR Morb. Mortal. Wkly. Rep. 2020;69:281–285. doi: 10.15585/mmwr.mm6911a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagcchi S. Who's global tuberculosis report 2022. Lancet. Microbe. 2023;4:e20. doi: 10.1016/S2666-5247(22)00359-7. [DOI] [PubMed] [Google Scholar]
- 7.Liang H.Y., Li X.L., Yu X.S., Guan P., Yin Z.H., He Q.C., Zhou B.S. Facts and fiction of the relationship between preexisting tuberculosis and lung cancer risk: A systematic review. Int. J. Cancer. 2009;125:2936–2944. doi: 10.1002/ijc.24636. [DOI] [PubMed] [Google Scholar]
- 8.Dobler C.C., Cheung K., Nguyen J., Martin A. Risk of tuberculosis in patients with solid cancers and haematological malignancies: A systematic review and meta-analysis. Eur. Respir. J. 2017;50:1700157. doi: 10.1183/13993003.00157-2017. [DOI] [PubMed] [Google Scholar]
- 9.Cheng M.P., Abou Chakra C.N., Yansouni C.P., Cnossen S., Shrier I., Menzies D., Greenaway C. Risk of active tuberculosis in patients with cancer: A systematic review and meta-analysis. Clin. Infect. Dis. 2017;64:635–644. doi: 10.1093/cid/ciw838. [DOI] [PubMed] [Google Scholar]
- 10.Hwang S.Y., Kim J.Y., Lee H.S., Lee S., Kim D., Kim S., Hyun J.H., Shin J.I., Lee K.H., Han S.H., et al. Pulmonary tuberculosis and risk of lung cancer: A systematic review and meta-analysis. J. Clin. Med. 2022;11:765. doi: 10.3390/jcm11030765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cukic V. The association between lung carcinoma and tuberculosis. Med. Arch. 2017;71:212–214. doi: 10.5455/medarh.2017.71.212-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engels E.A., Shen M., Chapman R.S., Pfeiffer R.M., Yu Y.Y., He X., Lan Q. Tuberculosis and subsequent risk of lung cancer in xuanwei, china. Int. J. Cancer. 2009;124:1183–1187. doi: 10.1002/ijc.24042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.An S.J., Kim Y.J., Han S.S., Heo J. Effects of age on the association between pulmonary tuberculosis and lung cancer in a south korean cohort. J. Thorac. Dis. 2020;12:375–382. doi: 10.21037/jtd.2020.01.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langan E.A., Graetz V., Allerheiligen J., Zillikens D., Rupp J., Terheyden P. Immune checkpoint inhibitors and tuberculosis: An old disease in a new context. Lancet Oncol. 2020;21:e55–e65. doi: 10.1016/S1470-2045(19)30674-6. [DOI] [PubMed] [Google Scholar]
- 15.Shi J., Li J., Wang Q., Cheng X., Du H., Han R., Li X., Zhao C., Gao G., He Y., et al. The safety and efficacy of immunotherapy with anti-programmed cell death 1 monoclonal antibody for lung cancer complicated with mycobacterium tuberculosis infection. Transl. Lung Cancer Res. 2021;10:3929–3942. doi: 10.21037/tlcr-21-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong W., Liang Y., Wu X. Animal models of tuberculosis vaccine research: An important component in the fight against tuberculosis. BioMed Res. Int. 2020;2020:4263079. doi: 10.1155/2020/4263079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong W., Yang K., Zhao W., Zheng J., Yu J., Guo K., Sun X. Peptide-based vaccines for tuberculosis. Front. Immunol. 2022;13:1052111. doi: 10.3389/fimmu.2022.830497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong W., Wu X. Differential diagnosis of latent tuberculosis infection and active tuberculosis: A key to a successful tuberculosis control strategy. Front. Microbiol. 2021;12:745592. doi: 10.3389/fmicb.2021.745592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Domagala-Kulawik J. The role of the immune system in non-small cell lung carcinoma and potential for therapeutic intervention. Transl. Lung Cancer Res. 2015;4:177–190. doi: 10.3978/j.issn.2218-6751.2015.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mi J., Liang Y., Liang J., Gong W., Wang S., Zhang J., Li Z., Wu X. The research progress in immunotherapy of tuberculosis. Front. Cell. Infect. Microbiol. 2021;11:763591. doi: 10.3389/fcimb.2021.763591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaplin D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010;125:S3–S23. doi: 10.1016/j.jaci.2009.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sánchez-Danés A., Blanpain C. Deciphering the cells of origin of squamous cell carcinomas. Nat. Rev. Cancer. 2018;18:549–561. doi: 10.1038/s41568-018-0024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sia D., Villanueva A., Friedman S.L., Llovet J.M. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. 2017;152:745–761. doi: 10.1053/j.gastro.2016.11.048. [DOI] [PubMed] [Google Scholar]
- 24.Ishii K.J., Koyama S., Nakagawa A., Coban C., Akira S. Host innate immune receptors and beyond: Making sense of microbial infections. Cell Host Microbe. 2008;3:352–363. doi: 10.1016/j.chom.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Miggin S.M., O'Neill L.A.J. New insights into the regulation of tlr signaling. J. Leukoc. Biol. 2006;80:220–226. doi: 10.1189/jlb.1105672. [DOI] [PubMed] [Google Scholar]
- 26.Pattanaik K.P., Ganguli G., Naik S.K., Sonawane A. Mycobacterium tuberculosis esxl induces tnf-α secretion through activation of tlr2 dependent mapk and nf-κb pathways. Mol. Immunol. 2021;130:133–141. doi: 10.1016/j.molimm.2020.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Kawai T., Akira S. Tlr signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 28.Bafica A., Scanga C.A., Feng C.G., Leifer C., Cheever A., Sher A. Tlr9 regulates th1 responses and cooperates with tlr2 in mediating optimal resistance to mycobacterium tuberculosis. J. Exp. Med. 2005;202:1715–1724. doi: 10.1084/jem.20051782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orme I.M., Robinson R.T., Cooper A.M. The balance between protective and pathogenic immune responses in the tb-infected lung. Nat. Immunol. 2015;16:57–63. doi: 10.1038/ni.3048. [DOI] [PubMed] [Google Scholar]
- 30.Jaiswal S., Chao M.P., Majeti R., Weissman I.L. Macrophages as mediators of tumor immunosurveillance. Trends Immunol. 2010;31:212–219. doi: 10.1016/j.it.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sia J.K., Georgieva M., Rengarajan J. Innate immune defenses in human tuberculosis: An overview of the interactions between mycobacterium tuberculosis and innate immune cells. J. Immunol. Res. 2015;2015:747543. doi: 10.1155/2015/747543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wynn T.A., Chawla A., Pollard J.W. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molgora M., Supino D., Mavilio D., Santoni A., Moretta L., Mantovani A., Garlanda C. The yin-yang of the interaction between myelomonocytic cells and nk cells. Scand. J. Immunol. 2018;88:e12705. doi: 10.1111/sji.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo Q., Jin Z., Yuan Y., Liu R., Xu T., Wei H., Xu X., He S., Chen S., Shi Z., et al. New mechanisms of tumor-associated macrophages on promoting tumor progression: Recent research advances and potential targets for tumor immunotherapy. J. Immunol. Res. 2016;2016:9720912. doi: 10.1155/2016/9720912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shapouri-Moghaddam A., Mohammadian S., Vazini H., Taghadosi M., Esmaeili S.A., Mardani F., Seifi B., Mohammadi A., Afshari J.T., Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018;233:6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 36.Khan A., Singh V.K., Hunter R.L., Jagannath C. Macrophage heterogeneity and plasticity in tuberculosis. J. Leukoc. Biol. 2019;106:275–282. doi: 10.1002/JLB.MR0318-095RR. [DOI] [PubMed] [Google Scholar]
- 37.Dijkgraaf E.M., Heusinkveld M., Tummers B., Vogelpoel L.T.C., Goedemans R., Jha V., Nortier J.W.R., Welters M.J.P., Kroep J.R., van der Burg S.H. Chemotherapy alters monocyte differentiation to favor generation of cancer-supporting m2 macrophages in the tumor microenvironment. Cancer Res. 2013;73:2480–2492. doi: 10.1158/0008-5472.CAN-12-3542. [DOI] [PubMed] [Google Scholar]
- 38.Pu Y., Ji Q. Tumor-associated macrophages regulate pd-1/pd-l1 immunosuppression. Front. Immunol. 2022;13:874589. doi: 10.3389/fimmu.2022.874589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin C., He H., Liu H., Li R., Chen Y., Qi Y., Jiang Q., Chen L., Zhang P., Zhang H., et al. Tumour-associated macrophages-derived cxcl8 determines immune evasion through autonomous pd-l1 expression in gastric cancer. Gut. 2019;68:1764–1773. doi: 10.1136/gutjnl-2018-316324. [DOI] [PubMed] [Google Scholar]
- 40.Bhatt K., Salgame P. Host innate immune response to mycobacterium tuberculosis. J. Clin. Immunol. 2007;27:347–362. doi: 10.1007/s10875-007-9084-0. [DOI] [PubMed] [Google Scholar]
- 41.El Hage F., Abouzahr-Rifai S., Meslin F., Mami-Chouaib F., Chouaib S. [immune response and cancer] Bull. Cancer. 2008;95:57–67. doi: 10.1684/bdc.2008.0558. [DOI] [PubMed] [Google Scholar]
- 42.Scoville S.D., Freud A.G., Caligiuri M.A. Modeling human natural killer cell development in the era of innate lymphoid cells. Front. Immunol. 2017;8:360. doi: 10.3389/fimmu.2017.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lanier L.L., Testi R., Bindl J., Phillips J.H. Identity of leu-19 (cd56) leukocyte differentiation antigen and neural cell adhesion molecule. J. Exp. Med. 1989;169:2233–2238. doi: 10.1084/jem.169.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abel A.M., Yang C., Thakar M.S., Malarkannan S. Natural killer cells: Development, maturation, and clinical utilization. Front. Immunol. 2018;9:1869. doi: 10.3389/fimmu.2018.01869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bald T., Krummel M.F., Smyth M.J., Barry K.C. The nk cell-cancer cycle: Advances and new challenges in nk cell-based immunotherapies. Nat. Immunol. 2020;21:835–847. doi: 10.1038/s41590-020-0728-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skak K., Frederiksen K.S., Lundsgaard D. Interleukin-21 activates human natural killer cells and modulates their surface receptor expression. Immunology. 2008;123:575–583. doi: 10.1111/j.1365-2567.2007.02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gotthardt D., Trifinopoulos J., Sexl V., Putz E.M. Jak/stat cytokine signaling at the crossroad of nk cell development and maturation. Front. Immunol. 2019;10:2590. doi: 10.3389/fimmu.2019.02590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunn G.P., Old L.J., Schreiber R.D. The three es of cancer immunoediting. Annu. Rev. Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 49.Balch C.M., Tilden A.B., Dougherty P.A., Cloud G.A. Depressed levels of granular lymphocytes with natural killer (nk) cell function in 247 cancer patients. Ann. Surg. 1983;198:192–199. doi: 10.1097/00000658-198308000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cong J., Wang X., Zheng X., Wang D., Fu B., Sun R., Tian Z., Wei H. Dysfunction of natural killer cells by fbp1-induced inhibition of glycolysis during lung cancer progression. Cell Metabol. 2018;28:243–255.e5. doi: 10.1016/j.cmet.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 51.Shen M.J., Xu L.J., Yang L., Tsai Y., Keng P.C., Chen Y., Lee S.O., Chen Y. Radiation alters pd-l1/nkg2d ligand levels in lung cancer cells and leads to immune escape from nk cell cytotoxicity via il-6-mek/erk signaling pathway. Oncotarget. 2017;8:80506–80520. doi: 10.18632/oncotarget.19193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schierloh P., Alemán M., Yokobori N., Alves L., Roldán N., Abbate E., del C Sasiain M., de la Barrera S. Nk cell activity in tuberculosis is associated with impaired cd11a and icam-1 expression: A regulatory role of monocytes in nk activation. Immunology. 2005;116:541–552. doi: 10.1111/j.1365-2567.2005.02259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schierloh P., Yokobori N., Alemán M., Musella R.M., Beigier-Bompadre M., Saab M.A., Alves L., Abbate E., de la Barrera S.S., Sasiain M.C. Increased susceptibility to apoptosis of cd56dimcd16+ nk cells induces the enrichment of ifn-gamma-producing cd56bright cells in tuberculous pleurisy. J. Immunol. 2005;175:6852–6860. doi: 10.4049/jimmunol.175.10.6852. [DOI] [PubMed] [Google Scholar]
- 54.Steinman R.M. Decisions about dendritic cells: Past, present, and future. Annu. Rev. Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 55.Benteyn D., Heirman C., Bonehill A., Thielemans K., Breckpot K. Mrna-based dendritic cell vaccines. Expert Rev. Vaccines. 2015;14:161–176. doi: 10.1586/14760584.2014.957684. [DOI] [PubMed] [Google Scholar]
- 56.Bordon Y. Dendritic cells: Sorting, sorted. Nat. Rev. Immunol. 2016;16:657. doi: 10.1038/nri.2016.115. [DOI] [PubMed] [Google Scholar]
- 57.Gardner A., Ruffell B. Dendritic cells and cancer immunity. Trends Immunol. 2016;37:855–865. doi: 10.1016/j.it.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Domagala-Kulawik J., Osinska I., Hoser G. Mechanisms of immune response regulation in lung cancer. Transl. Lung Cancer Res. 2014;3:15–22. doi: 10.3978/j.issn.2218-6751.2013.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spranger S., Bao R., Gajewski T.F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 60.Tang M., Diao J., Gu H., Khatri I., Zhao J., Cattral M.S. Toll-like receptor 2 activation promotes tumor dendritic cell dysfunction by regulating il-6 and il-10 receptor signaling. Cell Rep. 2015;13:2851–2864. doi: 10.1016/j.celrep.2015.11.053. [DOI] [PubMed] [Google Scholar]
- 61.Lichtner M., Rossi R., Mengoni F., Vignoli S., Colacchia B., Massetti A.P., Kamga I., Hosmalin A., Vullo V., Mastroianni C.M. Circulating dendritic cells and interferon-alpha production in patients with tuberculosis: Correlation with clinical outcome and treatment response. Clin. Exp. Immunol. 2006;143:329–337. doi: 10.1111/j.1365-2249.2005.02994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gutierrez M.G., Mishra B.B., Jordao L., Elliott E., Anes E., Griffiths G. Nf-kappa b activation controls phagolysosome fusion-mediated killing of mycobacteria by macrophages. J. Immunol. 2008;181:2651–2663. doi: 10.4049/jimmunol.181.4.2651. [DOI] [PubMed] [Google Scholar]
- 63.Mueller D.L., Jenkins M.K., Schwartz R.H. Clonal expansion versus functional clonal inactivation: A costimulatory signalling pathway determines the outcome of t cell antigen receptor occupancy. Annu. Rev. Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 64.Chai Q., Lu Z., Liu C.H. Host defense mechanisms against mycobacterium tuberculosis. Cell. Mol. Life Sci. 2020;77:1859–1878. doi: 10.1007/s00018-019-03353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sekiya T., Yoshimura A. In vitro th differentiation protocol. Methods Mol. Biol. 2016;1344:183–191. doi: 10.1007/978-1-4939-2966-5_10. [DOI] [PubMed] [Google Scholar]
- 66.Geginat J., Paroni M., Maglie S., Alfen J.S., Kastirr I., Gruarin P., De Simone M., Pagani M., Abrignani S. Plasticity of human cd4 t cell subsets. Front. Immunol. 2014;5:630. doi: 10.3389/fimmu.2014.00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cowley S.C., Elkins K.L. Cd4+ t cells mediate ifn-gamma-independent control of mycobacterium tuberculosis infection both in vitro and in vivo. J. Immunol. 2003;171:4689–4699. doi: 10.4049/jimmunol.171.9.4689. [DOI] [PubMed] [Google Scholar]
- 68.Yahagi A., Umemura M., Tamura T., Kariyone A., Begum M.D., Kawakami K., Okamoto Y., Hamada S., Oshiro K., Kohama H., et al. Suppressed induction of mycobacterial antigen-specific th1-type cd4+ t cells in the lung after pulmonary mycobacterial infection. Int. Immunol. 2010;22:307–318. doi: 10.1093/intimm/dxq010. [DOI] [PubMed] [Google Scholar]
- 69.Green A.M., Difazio R., Flynn J.L. Ifn-γ from cd4 t cells is essential for host survival and enhances cd8 t cell function during mycobacterium tuberculosis infection. J. Immunol. 2013;190:270–277. doi: 10.4049/jimmunol.1200061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ouyang W., Kolls J.K., Zheng Y. The biological functions of t helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Knochelmann H.M., Dwyer C.J., Bailey S.R., Amaya S.M., Elston D.M., Mazza-McCrann J.M., Paulos C.M. When worlds collide: Th17 and treg cells in cancer and autoimmunity. Cell. Mol. Immunol. 2018;15:458–469. doi: 10.1038/s41423-018-0004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolf A.J., Desvignes L., Linas B., Banaiee N., Tamura T., Takatsu K., Ernst J.D. Initiation of the adaptive immune response to mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J. Exp. Med. 2008;205:105–115. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kursar M., Koch M., Mittrücker H.W., Nouailles G., Bonhagen K., Kamradt T., Kaufmann S.H.E. Cutting edge: Regulatory t cells prevent efficient clearance of mycobacterium tuberculosis. J. Immunol. 2007;178:2661–2665. doi: 10.4049/jimmunol.178.5.2661. [DOI] [PubMed] [Google Scholar]
- 74.Pearce E.J., Everts B. Dendritic cell metabolism. Nat. Rev. Immunol. 2015;15:18–29. doi: 10.1038/nri3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Motohashi S., Okamoto Y., Yoshino I., Nakayama T. Anti-tumor immune responses induced by inkt cell-based immunotherapy for lung cancer and head and neck cancer. Clin. Immunol. 2011;140:167–176. doi: 10.1016/j.clim.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 76.Salagianni M., Baxevanis C.N., Papamichail M., Perez S.A. New insights into the role of nk cells in cancer immunotherapy. OncoImmunology. 2012;1:205–207. doi: 10.4161/onci.1.2.18398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jenkins M.R., Griffiths G.M. The synapse and cytolytic machinery of cytotoxic t cells. Curr. Opin. Immunol. 2010;22:308–313. doi: 10.1016/j.coi.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lord S.J., Rajotte R.V., Korbutt G.S., Bleackley R.C. Granzyme b: A natural born killer. Immunol. Rev. 2003;193:31–38. doi: 10.1034/j.1600-065x.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- 79.Ashkenazi A., Dixit V.M. Death receptors: Signaling and modulation. Science (New York, N.Y.) 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 80.Thorburn A. Death receptor-induced cell killing. Cell. Signal. 2004;16:139–144. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 81.Saggioro F.P., Neder L., Stávale J.N., Paixão-Becker A.N.P., Malheiros S.M.F., Soares F.A., Pittella J.E.H., Matias C.C.M.S., Colli B.O., Carlotti C.G., Jr., Franco M. Fas, fasl, and cleaved caspases 8 and 3 in glioblastomas: A tissue microarray-based study. Pathol. Res. Pract. 2014;210:267–273. doi: 10.1016/j.prp.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 82.Nathan C.F., Murray H.W., Wiebe M.E., Rubin B.Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 1983;158:670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aqbi H.F., Wallace M., Sappal S., Payne K.K., Manjili M.H. Ifn-γ orchestrates tumor elimination, tumor dormancy, tumor escape, and progression. J. Leukoc. Biol. 2018;103:1219–1223. doi: 10.1002/JLB.5MIR0917-351R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li A., Barsoumian H.B., Schoenhals J.E., Cushman T.R., Caetano M.S., Wang X., Valdecanas D.R., Niknam S., Younes A.I., Li G., et al. Indoleamine 2,3-dioxygenase 1 inhibition targets anti-pd1-resistant lung tumors by blocking myeloid-derived suppressor cells. Cancer Lett. 2018;431:54–63. doi: 10.1016/j.canlet.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kurebayashi Y., Emoto K., Hayashi Y., Kamiyama I., Ohtsuka T., Asamura H., Sakamoto M. Comprehensive immune profiling of lung adenocarcinomas reveals four immunosubtypes with plasma cell subtype a negative indicator. Cancer Immunol. Res. 2016;4:234–247. doi: 10.1158/2326-6066.CIR-15-0214. [DOI] [PubMed] [Google Scholar]
- 86.Dieu-Nosjean M.C., Goc J., Giraldo N.A., Sautès-Fridman C., Fridman W.H. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 2014;35:571–580. doi: 10.1016/j.it.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 87.Gottlin E.B., Bentley R.C., Campa M.J., Pisetsky D.S., Herndon J.E., 2nd, Patz E.F., Jr. The association of intratumoral germinal centers with early-stage non-small cell lung cancer. J. Thorac. Oncol. 2011;6:1687–1690. doi: 10.1097/JTO.0b013e3182217bec. [DOI] [PubMed] [Google Scholar]
- 88.Hong S., Zhang Z., Liu H., Tian M., Zhu X., Zhang Z., Wang W., Zhou X., Zhang F., Ge Q., et al. B cells are the dominant antigen-presenting cells that activate naive cd4(+) t cells upon immunization with a virus-derived nanoparticle antigen. Immunity. 2018;49:695–708.e4. doi: 10.1016/j.immuni.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 89.Rossetti R.A.M., Lorenzi N.P.C., Yokochi K., Rosa M.B.S.d.F., Benevides L., Margarido P.F.R., Baracat E.C., Carvalho J.P., Villa L.L., Lepique A.P. B lymphocytes can be activated to act as antigen presenting cells to promote anti-tumor responses. PLoS One. 2018;13:e0199034. doi: 10.1371/journal.pone.0199034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.DeFalco J., Harbell M., Manning-Bog A., Baia G., Scholz A., Millare B., Sumi M., Zhang D., Chu F., Dowd C., et al. Non-progressing cancer patients have persistent b cell responses expressing shared antibody paratopes that target public tumor antigens. Clin. Immunol. 2018;187:37–45. doi: 10.1016/j.clim.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 91.Nimmerjahn F., Ravetch J.V. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 92.Bruno T.C. New predictors for immunotherapy responses sharpen our view of the tumour microenvironment. Nature. 2020;577:474–476. doi: 10.1038/d41586-019-03943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sharonov G.V., Serebrovskaya E.O., Yuzhakova D.V., Britanova O.V., Chudakov D.M. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat. Rev. Immunol. 2020;20:294–307. doi: 10.1038/s41577-019-0257-x. [DOI] [PubMed] [Google Scholar]
- 94.Shang J., Zha H., Sun Y. Phenotypes, functions, and clinical relevance of regulatory b cells in cancer. Front. Immunol. 2020;11:582657. doi: 10.3389/fimmu.2020.582657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang B., Vogelzang A., Miyajima M., Sugiura Y., Wu Y., Chamoto K., Nakano R., Hatae R., Menzies R.J., Sonomura K., et al. B cell-derived gaba elicits il-10(+) macrophages to limit anti-tumour immunity. Nature. 2021;599:471–476. doi: 10.1038/s41586-021-04082-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Anichini A., Perotti V.E., Sgambelluri F., Mortarini R. Immune escape mechanisms in non small cell lung cancer. Cancers. 2020;12:3605. doi: 10.3390/cancers12123605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jiang X., Wang J., Deng X., Xiong F., Ge J., Xiang B., Wu X., Ma J., Zhou M., Li X., et al. Role of the tumor microenvironment in pd-l1/pd-1-mediated tumor immune escape. Mol. Cancer. 2019;18:10. doi: 10.1186/s12943-018-0928-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yarchoan M., Johnson B.A., 3rd, Lutz E.R., Laheru D.A., Jaffee E.M. Targeting neoantigens to augment antitumour immunity. Nat. Rev. Cancer. 2017;17:569. doi: 10.1038/nrc.2017.74. [DOI] [PubMed] [Google Scholar]
- 99.Hellmann M.D., Nathanson T., Rizvi H., Creelan B.C., Sanchez-Vega F., Ahuja A., Ni A., Novik J.B., Mangarin L.M.B., Abu-Akeel M., et al. Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell. 2018;33:843–852.e4. doi: 10.1016/j.ccell.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ready N., Hellmann M.D., Awad M.M., Otterson G.A., Gutierrez M., Gainor J.F., Borghaei H., Jolivet J., Horn L., Mates M., et al. First-line nivolumab plus ipilimumab in advanced non-small-cell lung cancer (checkmate 568): Outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J. Clin. Oncol. 2019;37:992–1000. doi: 10.1200/JCO.18.01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hellmann M.D., Callahan M.K., Awad M.M., Calvo E., Ascierto P.A., Atmaca A., Rizvi N.A., Hirsch F.R., Selvaggi G., Szustakowski J.D., et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell. 2018;33:853–861.e4. doi: 10.1016/j.ccell.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mittal D., Gubin M.M., Schreiber R.D., Smyth M.J. New insights into cancer immunoediting and its three component phases--elimination, equilibrium and escape. Curr. Opin. Immunol. 2014;27:16–25. doi: 10.1016/j.coi.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Verdegaal E.M.E., de Miranda N.F.C.C., Visser M., Harryvan T., van Buuren M.M., Andersen R.S., Hadrup S.R., van der Minne C.E., Schotte R., Spits H., et al. Neoantigen landscape dynamics during human melanoma-t cell interactions. Nature. 2016;536:91–95. doi: 10.1038/nature18945. [DOI] [PubMed] [Google Scholar]
- 104.Anagnostou V., Smith K.N., Forde P.M., Niknafs N., Bhattacharya R., White J., Zhang T., Adleff V., Phallen J., Wali N., et al. Evolution of neoantigen landscape during immune checkpoint blockade in non-small cell lung cancer. Cancer Discov. 2017;7:264–276. doi: 10.1158/2159-8290.CD-16-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Merad M., Sathe P., Helft J., Miller J., Mortha A. The dendritic cell lineage: Ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schlitzer A., McGovern N., Ginhoux F. Dendritic cells and monocyte-derived cells: Two complementary and integrated functional systems. Semin. Cell Dev. Biol. 2015;41:9–22. doi: 10.1016/j.semcdb.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 107.Martínez-Reyes I., Chandel N.S. Cancer metabolism: Looking forward. Nat. Rev. Cancer. 2021;21:669–680. doi: 10.1038/s41568-021-00378-6. [DOI] [PubMed] [Google Scholar]
- 108.Kwiecien I., Stelmaszczyk-Emmel A., Polubiec-Kownacka M., Dziedzic D., Domagala-Kulawik J. Elevated regulatory t cells, surface and intracellular ctla-4 expression and interleukin-17 in the lung cancer microenvironment in humans. Cancer Immunol. Immunother. 2017;66:161–170. doi: 10.1007/s00262-016-1930-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aerts J.G., Hegmans J.P. Tumor-specific cytotoxic t cells are crucial for efficacy of immunomodulatory antibodies in patients with lung cancer. Cancer Res. 2013;73:2381–2388. doi: 10.1158/0008-5472.CAN-12-3932. [DOI] [PubMed] [Google Scholar]
- 110.Wang Y., Chen R., Wa Y., Ding S., Yang Y., Liao J., Tong L., Xiao G. Tumor immune microenvironment and immunotherapy in brain metastasis from non-small cell lung cancer. Front. Immunol. 2022;13:829451. doi: 10.3389/fimmu.2022.829451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fridman W.H., Pagès F., Sautès-Fridman C., Galon J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 112.Cassetta L., Pollard J.W. Targeting macrophages: Therapeutic approaches in cancer. Nat. Rev. Drug Discov. 2018;17:887–904. doi: 10.1038/nrd.2018.169. [DOI] [PubMed] [Google Scholar]
- 113.Hu B., Wang Z., Zeng H., Qi Y., Chen Y., Wang T., Wang J., Chang Y., Bai Q., Xia Y., et al. Blockade of dc-sign(+) tumor-associated macrophages reactivates antitumor immunity and improves immunotherapy in muscle-invasive bladder cancer. Cancer Res. 2020;80:1707–1719. doi: 10.1158/0008-5472.CAN-19-2254. [DOI] [PubMed] [Google Scholar]
- 114.Voron T., Colussi O., Marcheteau E., Pernot S., Nizard M., Pointet A.L., Latreche S., Bergaya S., Benhamouda N., Tanchot C., et al. Vegf-a modulates expression of inhibitory checkpoints on cd8+ t cells in tumors. J. Exp. Med. 2015;212:139–148. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Park M.J., Lee S.H., Kim E.K., Lee E.J., Baek J.A., Park S.H., Kwok S.K., Cho M.L. Interleukin-10 produced by myeloid-derived suppressor cells is critical for the induction of tregs and attenuation of rheumatoid inflammation in mice. Sci. Rep. 2018;8:3753. doi: 10.1038/s41598-018-21856-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vasquez-Dunddel D., Pan F., Zeng Q., Gorbounov M., Albesiano E., Fu J., Blosser R.L., Tam A.J., Bruno T., Zhang H., et al. Stat3 regulates arginase-i in myeloid-derived suppressor cells from cancer patients. J. Clin. Invest. 2013;123:1580–1589. doi: 10.1172/JCI60083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim S.H., Li M., Trousil S., Zhang Y., Pasca di Magliano M., Swanson K.D., Zheng B. Phenformin inhibits myeloid-derived suppressor cells and enhances the anti-tumor activity of pd-1 blockade in melanoma. J. Invest. Dermatol. 2017;137:1740–1748. doi: 10.1016/j.jid.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 118.Framework for conducting reviews of tuberculosis programmes. World Health Organization Copyright © World Health Organization 2014; 2014. Who guidelines approved by the guidelines review committee. [PubMed] [Google Scholar]
- 119.Briken V., Miller J.L. Living on the edge: Inhibition of host cell apoptosis by mycobacterium tuberculosis. Future Microbiol. 2008;3:415–422. doi: 10.2217/17460913.3.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Derrick S.C., Morris S.L. The esat6 protein of mycobacterium tuberculosis induces apoptosis of macrophages by activating caspase expression. Cell Microbiol. 2007;9:1547–1555. doi: 10.1111/j.1462-5822.2007.00892.x. [DOI] [PubMed] [Google Scholar]
- 121.Riendeau C.J., Kornfeld H. Thp-1 cell apoptosis in response to mycobacterial infection. Infect. Immun. 2003;71:254–259. doi: 10.1128/IAI.71.1.254-259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang N., Liang H., Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 2014;5:614. doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ogishi M., Yang R., Aytekin C., Langlais D., Bourgey M., Khan T., Ali F.A., Rahman M., Delmonte O.M., Chrabieh M., et al. Inherited pd-1 deficiency underlies tuberculosis and autoimmunity in a child. Nat. Med. 2021;27:1646–1654. doi: 10.1038/s41591-021-01388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen X., Zhou B., Li M., Deng Q., Wu X., Le X., Wu C., Larmonier N., Zhang W., Zhang H., et al. Cd4(+)cd25(+)foxp3(+) regulatory t cells suppress mycobacterium tuberculosis immunity in patients with active disease. Clin. Immunol. 2007;123:50–59. doi: 10.1016/j.clim.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 125.Li L., Lao S.H., Wu C.Y. Increased frequency of cd4(+)cd25(high) treg cells inhibit bcg-specific induction of ifn-gamma by cd4(+) t cells from tb patients. Tuberculosis. 2007;87:526–534. doi: 10.1016/j.tube.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 126.Kim H.R., Hwang S.S., Ro Y.K., Jeon C.H., Ha D.Y., Park S.J., Lee C.H., Lee S.M., Yoo C.G., Kim Y.W., et al. Solid-organ malignancy as a risk factor for tuberculosis. Respirology. 2008;13:413–419. doi: 10.1111/j.1440-1843.2008.01282.x. [DOI] [PubMed] [Google Scholar]
- 127.Liao K.M., Shu C.C., Liang F.W., Chen Y.C., Yu C.H., Wang J.J., Ho C.H. Risk factors for pulmonary tuberculosis in patients with lung cancer: A retrospective cohort study. J. Cancer. 2023;14:657–664. doi: 10.7150/jca.81616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Aoki Y., Kuroki S., Hiura K., Katoh O., Yamada H. [a clinical study of pulmonary tuberculosis in lung cancer patient] Kekkaku. 1991;66:727–732. [PubMed] [Google Scholar]
- 129.Silva D.R., Valentini D.F., Jr., Müller A.M., de Almeida C.P.B., Dalcin P.d.T.R. Pulmonary tuberculosis and lung cancer: Simultaneous and sequential occurrence. J. Bras. Pneumol. 2013;39:484–489. doi: 10.1590/S1806-37132013000400013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cha S.I., Shin K.M., Lee J.W., Lee S.Y., Kim C.H., Park J.Y., Jung T.H. The clinical course of respiratory tuberculosis in lung cancer patients. Int. J. Tubercul. Lung Dis. 2009;13:1002–1007. [PubMed] [Google Scholar]
- 131.Suzuki Y., Imokawa S., Sato J., Uto T., Suda T. Cumulative incidence of tuberculosis in lung cancer patients in japan: A 6-year observational study. Respir. Investig. 2016;54:179–183. doi: 10.1016/j.resinv.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 132.Chen Y.M., Chao J.Y., Tsai C.M., Lee P.Y., Perng R.P. Shortened survival of lung cancer patients initially presenting with pulmonary tuberculosis. Jpn. J. Clin. Oncol. 1996;26:322–327. doi: 10.1093/oxfordjournals.jjco.a023240. [DOI] [PubMed] [Google Scholar]
- 133.Remiszewski P., Słodkowska J., Wiatr E., Zych J., Radomski P., Rowińska-Zakrzewska E. Fatal infection in patients treated for small cell lung cancer in the institute of tuberculosis and chest diseases in the years 1980-1994. Lung Cancer. 2001;31:101–110. doi: 10.1016/s0169-5002(00)00185-9. [DOI] [PubMed] [Google Scholar]
- 134.Li B., Chan H.L., Chen P. Immune checkpoint inhibitors: Basics and challenges. Curr. Med. Chem. 2019;26:3009–3025. doi: 10.2174/0929867324666170804143706. [DOI] [PubMed] [Google Scholar]
- 135.Suresh K., Naidoo J., Lin C.T., Danoff S. Immune checkpoint immunotherapy for non-small cell lung cancer: Benefits and pulmonary toxicities. Chest. 2018;154:1416–1423. doi: 10.1016/j.chest.2018.08.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wykes M.N., Lewin S.R. Immune checkpoint blockade in infectious diseases. Nat. Rev. Immunol. 2018;18:91–104. doi: 10.1038/nri.2017.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hughes P.E., Caenepeel S., Wu L.C. Targeted therapy and checkpoint immunotherapy combinations for the treatment of cancer. Trends Immunol. 2016;37:462–476. doi: 10.1016/j.it.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 138.Ishida Y., Agata Y., Shibahara K., Honjo T. Induced expression of pd-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Keir M.E., Francisco L.M., Sharpe A.H. Pd-1 and its ligands in t-cell immunity. Curr. Opin. Immunol. 2007;19:309–314. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 140.Wu X., Gu Z., Chen Y., Chen B., Chen W., Weng L., Liu X. Application of pd-1 blockade in cancer immunotherapy. Comput. Struct. Biotechnol. J. 2019;17:661–674. doi: 10.1016/j.csbj.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Suarez G.V., Melucci Ganzarain C.D.C., Vecchione M.B., Trifone C.A., Marín Franco J.L., Genoula M., Moraña E.J., Balboa L., Quiroga M.F. Pd-1/pd-l1 pathway modulates macrophage susceptibility to mycobacterium tuberculosis specific cd8(+) t cell induced death. Sci. Rep. 2019;9:187. doi: 10.1038/s41598-018-36403-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Butte M.J., Keir M.E., Phamduy T.B., Sharpe A.H., Freeman G.J. Programmed death-1 ligand 1 interacts specifically with the b7-1 costimulatory molecule to inhibit t cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Riley J.L. Pd-1 signaling in primary t cells. Immunol. Rev. 2009;229:114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.van der Hoorn I.A.E., Flórez-Grau G., van den Heuvel M.M., de Vries I.J.M., Piet B. Recent advances and future perspective of dc-based therapy in nsclc. Front. Immunol. 2021;12:704776. doi: 10.3389/fimmu.2021.704776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cao S., Li J., Lu J., Zhong R., Zhong H. Mycobacterium tuberculosis antigens repress th1 immune response suppression and promotes lung cancer metastasis through pd-1/pdl-1 signaling pathway. Cell Death Dis. 2019;10:44. doi: 10.1038/s41419-018-1237-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kamboj D., Gupta P., Basil M.V., Mohan A., Guleria R., Bhatnagar A., Mehta G., Kumar P., Saurabh A., Deepak R., et al. Improved mycobacterium tuberculosis clearance after the restoration of ifn-γ(+) tnf-α(+) cd4(+) t cells: Impact of pd-1 inhibition in active tuberculosis patients. Eur. J. Immunol. 2020;50:736–747. doi: 10.1002/eji.201948283. [DOI] [PubMed] [Google Scholar]
- 147.Linsley P.S., Brady W., Urnes M., Grosmaire L.S., Damle N.K., Ledbetter J.A. Ctla-4 is a second receptor for the b cell activation antigen b7. J. Exp. Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bonnefoy N., Olive D., Vanhove B. [next generation of anti-immune checkpoints antibodies] Med. Sci. 2019;35:966–974. doi: 10.1051/medsci/2019193. [DOI] [PubMed] [Google Scholar]
- 149.van der Merwe P.A., Bodian D.L., Daenke S., Linsley P., Davis S.J. Cd80 (b7-1) binds both cd28 and ctla-4 with a low affinity and very fast kinetics. J. Exp. Med. 1997;185:393–403. doi: 10.1084/jem.185.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Alegre M.L., Frauwirth K.A., Thompson C.B. T-cell regulation by cd28 and ctla-4. Nat. Rev. Immunol. 2001;1:220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 151.Vanhove B., Poirier N., Fakhouri F., Laurent L., t Hart B., Papotto P.H., Rizzo L.V., Zaitsu M., Issa F., Wood K., et al. Antagonist anti-cd28 therapeutics for the treatment of autoimmune disorders. Antibodies. 2017;6:19. doi: 10.3390/antib6040019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jayaraman P., Jacques M.K., Zhu C., Steblenko K.M., Stowell B.L., Madi A., Anderson A.C., Kuchroo V.K., Behar S.M. Tim3 mediates t cell exhaustion during mycobacterium tuberculosis infection. PLoS Pathog. 2016;12:e1005490. doi: 10.1371/journal.ppat.1005490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Maruhashi T., Sugiura D., Okazaki I.M., Okazaki T. Lag-3: From molecular functions to clinical applications. J. Immunother. Cancer. 2020;8:e001014. doi: 10.1136/jitc-2020-001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Triebel F., Jitsukawa S., Baixeras E., Roman-Roman S., Genevee C., Viegas-Pequignot E., Hercend T. Lag-3, a novel lymphocyte activation gene closely related to cd4. J. Exp. Med. 1990;171:1393–1405. doi: 10.1084/jem.171.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Okazaki T., Okazaki I.M., Wang J., Sugiura D., Nakaki F., Yoshida T., Kato Y., Fagarasan S., Muramatsu M., Eto T., et al. Pd-1 and lag-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J. Exp. Med. 2011;208:395–407. doi: 10.1084/jem.20100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Huard B., Prigent P., Pagès F., Bruniquel D., Triebel F. T cell major histocompatibility complex class ii molecules down-regulate cd4+ t cell clone responses following lag-3 binding. Eur. J. Immunol. 1996;26:1180–1186. doi: 10.1002/eji.1830260533. [DOI] [PubMed] [Google Scholar]
- 157.Goldberg M.V., Drake C.G. Lag-3 in cancer immunotherapy. Curr. Top. Microbiol. Immunol. 2011;344:269–278. doi: 10.1007/82_2010_114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Workman C.J., Dugger K.J., Vignali D.A.A. Cutting edge: Molecular analysis of the negative regulatory function of lymphocyte activation gene-3. J. Immunol. 2002;169:5392–5395. doi: 10.4049/jimmunol.169.10.5392. [DOI] [PubMed] [Google Scholar]
- 159.Phillips B.L., Gautam U.S., Bucsan A.N., Foreman T.W., Golden N.A., Niu T., Kaushal D., Mehra S. Lag-3 potentiates the survival of mycobacterium tuberculosis in host phagocytes by modulating mitochondrial signaling in an in-vitro granuloma model. PLoS One. 2017;12:e0180413. doi: 10.1371/journal.pone.0180413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Meyers J.H., Sabatos C.A., Chakravarti S., Kuchroo V.K. The tim gene family regulates autoimmune and allergic diseases. Trends Mol. Med. 2005;11:362–369. doi: 10.1016/j.molmed.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 161.Monney L., Sabatos C.A., Gaglia J.L., Ryu A., Waldner H., Chernova T., Manning S., Greenfield E.A., Coyle A.J., Sobel R.A., et al. Th1-specific cell surface protein tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 162.Dixon K.O., Tabaka M., Schramm M.A., Xiao S., Tang R., Dionne D., Anderson A.C., Rozenblatt-Rosen O., Regev A., Kuchroo V.K. Tim-3 restrains anti-tumour immunity by regulating inflammasome activation. Nature. 2021;595:101–106. doi: 10.1038/s41586-021-03626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tang R., Acharya N., Subramanian A., Purohit V., Tabaka M., Hou Y., He D., Dixon K.O., Lambden C., Xia J., et al. Tim-3 adapter protein bat3 acts as an endogenous regulator of tolerogenic dendritic cell function. Sci. Immunol. 2022;7:eabm0631. doi: 10.1126/sciimmunol.abm0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kuchroo V.K., Dardalhon V., Xiao S., Anderson A.C. New roles for tim family members in immune regulation. Nat. Rev. Immunol. 2008;8:577–580. doi: 10.1038/nri2366. [DOI] [PubMed] [Google Scholar]
- 165.Sánchez-Fueyo A., Tian J., Picarella D., Domenig C., Zheng X.X., Sabatos C.A., Manlongat N., Bender O., Kamradt T., Kuchroo V.K., et al. Tim-3 inhibits t helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 2003;4:1093–1101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- 166.Koguchi K., Anderson D.E., Yang L., O'Connor K.C., Kuchroo V.K., Hafler D.A. Dysregulated t cell expression of tim3 in multiple sclerosis. J. Exp. Med. 2006;203:1413–1418. doi: 10.1084/jem.20060210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Placke T., Kopp H.G., Salih H.R. Glucocorticoid-induced tnfr-related (gitr) protein and its ligand in antitumor immunity: Functional role and therapeutic modulation. Clin. Dev. Immunol. 2010;2010:239083. doi: 10.1155/2010/239083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kraehenbuehl L., Weng C.H., Eghbali S., Wolchok J.D., Merghoub T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat. Rev. Clin. Oncol. 2022;19:37–50. doi: 10.1038/s41571-021-00552-7. [DOI] [PubMed] [Google Scholar]
- 169.Tone M., Tone Y., Adams E., Yates S.F., Frewin M.R., Cobbold S.P., Waldmann H. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for t cells. Proc. Natl. Acad. Sci. USA. 2003;100:15059–15064. doi: 10.1073/pnas.2334901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shimizu J., Yamazaki S., Takahashi T., Ishida Y., Sakaguchi S. Stimulation of cd25(+)cd4(+) regulatory t cells through gitr breaks immunological self-tolerance. Nat. Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 171.Chan S., Belmar N., Ho S., Rogers B., Stickler M., Graham M., Lee E., Tran N., Zhang D., Gupta P., et al. An anti-pd-1-gitr-l bispecific agonist induces gitr clustering-mediated t cell activation for cancer immunotherapy. Nat. Cancer. 2022;3:337–354. doi: 10.1038/s43018-022-00334-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ronchetti S., Zollo O., Bruscoli S., Agostini M., Bianchini R., Nocentini G., Ayroldi E., Riccardi C. Gitr, a member of the tnf receptor superfamily, is costimulatory to mouse t lymphocyte subpopulations. Eur. J. Immunol. 2004;34:613–622. doi: 10.1002/eji.200324804. [DOI] [PubMed] [Google Scholar]
- 173.Wang S., Shi Y., Yang M., Ma J., Tian J., Chen J., Mao C., Jiao Z., Ko K.H., Baidoo S.E., et al. Glucocorticoid-induced tumor necrosis factor receptor family-related protein exacerbates collagen-induced arthritis by enhancing the expansion of th17 cells. Am. J. Pathol. 2012;180:1059–1067. doi: 10.1016/j.ajpath.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 174.Ma J., Feng D., Wei Y., Tian J., Tang X., Rui K., Lu L., Xu H., Wang S. Blockade of glucocorticoid-induced tumor necrosis factor-receptor-related protein signaling ameliorates murine collagen-induced arthritis by modulating follicular helper t cells. Am. J. Pathol. 2016;186:1559–1567. doi: 10.1016/j.ajpath.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 175.Mahmud S.A., Manlove L.S., Schmitz H.M., Xing Y., Wang Y., Owen D.L., Schenkel J.M., Boomer J.S., Green J.M., Yagita H., et al. Costimulation via the tumor-necrosis factor receptor superfamily couples tcr signal strength to the thymic differentiation of regulatory t cells. Nat. Immunol. 2014;15:473–481. doi: 10.1038/ni.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nowakowska D.J., Kissler S. Ptpn22 modifies regulatory t cell homeostasis via gitr upregulation. J. Immunol. 2016;196:2145–2152. doi: 10.4049/jimmunol.1501877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Xu C., Chen Y.P., Du X.J., Liu J.Q., Huang C.L., Chen L., Zhou G.Q., Li W.F., Mao Y.P., Hsu C., et al. Comparative safety of immune checkpoint inhibitors in cancer: Systematic review and network meta-analysis. Br. Med. J. Int. Ed. 2018;363:k4226. doi: 10.1136/bmj.k4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Morelli T., Fujita K., Redelman-Sidi G., Elkington P.T. Infections due to dysregulated immunity: An emerging complication of cancer immunotherapy. Thorax. 2022;77:304–311. doi: 10.1136/thoraxjnl-2021-217260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zaemes J., Kim C. Immune checkpoint inhibitor use and tuberculosis: A systematic review of the literature. Eur. J. Cancer. 2020;132:168–175. doi: 10.1016/j.ejca.2020.03.015. [DOI] [PubMed] [Google Scholar]
- 180.Liu K., Wang D., Yao C., Qiao M., Li Q., Ren W., Li S., Gao M., Pang Y. Increased tuberculosis incidence due to immunotherapy based on pd-1 and pd-l1 blockade: A systematic review and meta-analysis. Front. Immunol. 2022;13:727220. doi: 10.3389/fimmu.2022.727220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gong W., Liang Y., Mi J., Jia Z., Xue Y., Wang J., Wang L., Zhou Y., Sun S., Wu X. Peptides-based vaccine mp3rt induced protective immunity against mycobacterium tuberculosis infection in a humanized mouse model. Front. Immunol. 2021;12:666290. doi: 10.3389/fimmu.2021.666290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Day C.L., Abrahams D.A., Bunjun R., Stone L., de Kock M., Walzl G., Wilkinson R.J., Burgers W.A., Hanekom W.A. Pd-1 expression on mycobacterium tuberculosis-specific cd4 t cells is associated with bacterial load in human tuberculosis. Front. Immunol. 2018;9:1995. doi: 10.3389/fimmu.2018.01995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tezera L.B., Bielecka M.K., Ogongo P., Walker N.F., Ellis M., Garay-Baquero D.J., Thomas K., Reichmann M.T., Johnston D.A., Wilkinson K.A., et al. Anti-pd-1 immunotherapy leads to tuberculosis reactivation via dysregulation of tnf-α. Elife. 2020;9:e52668. doi: 10.7554/eLife.52668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dhar C. Testing for latent tuberculosis before starting patients on immune checkpoint inhibitors. Indian J. Cancer. 2021;58:469–470. doi: 10.4103/ijc.IJC_283_20. [DOI] [PubMed] [Google Scholar]
- 185.Tamura A., Hebisawa A., Tanaka G., Tatsuta H., Tsuboi T., Nagai H., Hayashi K., Sagara Y., Kawabe Y., Akagawa S., et al. [Active pulmonary tuberculosis in patients with lung cancer] Kekkaku. 1999;74:797–802. [PubMed] [Google Scholar]
- 186.Watanabe A., Tokue Y., Takahashi H., Sato K., Nukiwa T., Honda Y., Fujimura S. management of mycobacteriosis in general hospital without isolation ward for tuberculosis patients. Clinical study on pulmonary tuberculosis associated with lung cancer patients. Kekkaku. 1999;74:157–162. [PubMed] [Google Scholar]
- 187.Cicenas S., Vencevicius V. Lung cancer in patients with tuberculosis. World J. Surg. Oncol. 2007;5:22. doi: 10.1186/1477-7819-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Şimşek A., Kalemci S., Mutlu N., Yapıcı İ., Acet Öztürk N.A. Lung cancer diagnosed with mycobacterium tuberculosis or nontuberculosis mycobacteria concomitantly. Tuberk. Toraks. 2017;65:291–295. doi: 10.5578/tt.59675. [DOI] [PubMed] [Google Scholar]
- 189.Tamura A., Fukami T., Hebisawa A., Takahashi F. Recent trends in the incidence of latent tuberculosis infection in japanese patients with lung cancer: A small retrospective study. J. Infect. Chemother. 2020;26:315–317. doi: 10.1016/j.jiac.2019.10.018. [DOI] [PubMed] [Google Scholar]
- 190.Vence L., Bucktrout S.L., Fernandez Curbelo I., Blando J., Smith B.M., Mahne A.E., Lin J.C., Park T., Pascua E., Sai T., et al. Characterization and comparison of gitr expression in solid tumors. Clin. Cancer Res. 2019;25:6501–6510. doi: 10.1158/1078-0432.CCR-19-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.