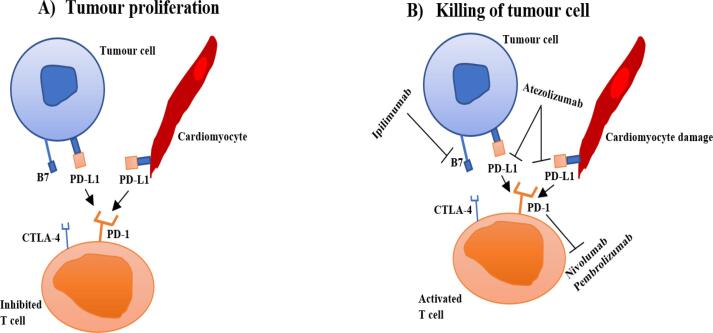
Immune Checkpoint Inhibitors (ICIs) have emerged as a leading cancer treatment modality over the last decade. Licensed ICIs in clinical practice work by targeting and inhibiting negative immune regulation receptors found on immune cells such as programmed cell death 1 (PD-1), programmed death-ligand 1 (PDL-1), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). When ICIs inhibit these checkpoints, it activates the immune system to recognise and kill tumour cells (Fig. 1). Agents licensed and used widely in the UK include inhibitors of PD-1 (Nivolumab, Pembrolizumab, Cemiplimab), PDL-1 (Atezolizumab, Durvalumab, Avelumab) and CTLA-4 (Ipilimumab). These agents have been used in various treatment settings i.e. metastatic, adjuvant and neoadjuvant and across a wide spectrum of solid tumours [1]. The most recent, but not yet extensively utilised, is Relatlimab, a lymphocyte-activation gene 3 (LAG3) inhibitor [2].
Fig. 1.
The interactions between PD-1, PD-L1 and CTLA-4 and B7 inhibits T cell proliferation, clonal expansion, cytokine production and consequently tumour cell killing. The blocking of CTLA-4, PD-1 and PD-L1 results in T cell activation and T cell mediated killing of tumour cells. Majority of tumour cells over-express a ligand e.g., PD-L1. ICIs include antibodies against for example PD-1 (nivolumab, pembrolizumab) or PD-L1 (Atezolizumab) which prevents these interactions and cause a T-cell reaction against these tumours.
Considering the broad range of processes impacted by the immune system, immune related adverse events (IRAEs) are inevitable. Moderate to severe toxicity (CTCAE grade 3 or 4) occur in 19–58 % patients receiving ICI [3]. The toxicities depend upon the type of ICI and whether it is used as a single agent or in combination with another ICI, chemotherapy, or targeted agent [4].
ICI related cardiovascular toxicities are a wide group of conditions (arrhythmia, non-inflammatory left ventricular dysfunction, ischaemic heart disease, new pulmonary artery hypertension) also having the potential to result in immune-related adverse cardiac events, such as myocarditis, pericarditis, and vasculitis [5].
ICIRM is often associated with myasthenia gravis-like syndrome and concurrent myositis [6]. Whilst rare, the consequences can be life threatening and must therefore be promptly investigated and treated aggressively. The commonest and most serious of these is ICI related myocarditis (ICIRM), with the reported incidence of severe cases being in the range of 0.04–1.14 %. and potentially a high mortality rates if left untreated i.e. 25–50 % [4]. Recent international guidelines from the European Society of Cardiology (ESC) and the European Society of Medical Oncology (ESMO) both recommend immediate treatment with high dose intravenous steroids when ICIRM is diagnosed [7]. Prompt recognition of ICIRM is recommended and remains an unmet clinical need. Underdiagnosis leads to delay or absence of appropriate therapy for ICIRM and its complications. Clarity over the diagnosis is important as the diagnosis of ICIRM usually results in the permanent cessation of ICI treatment and potentially adversely impacting cancer prognosis [4].
In 2022, the International Cardio-Oncology Society (ICOS) published criteria for the diagnosis of ICIRM [8]. The definition of ICIRM is based on pathohistological findings i.e. “multifocal inflammatory cell infiltrates with overt cardiomyocyte loss by light microscopy”, or cardiac troponin elevation associated with 1 major criterion (cardiac MRI – CMR modified diagnostic Lake Louise criteria) or with 2 minor criteria after excluding acute coronary syndrome or acute infectious myocarditis [9]. The five minor criteria include the “clinical syndrome”, ventricular arrhythmia +/− new conduction disease, decline in systolic function +/− regional wall motion abnormalities (non Takotsubo pattern), other IRAEs (myositis, myopathies) and “suggestive CMR” [10].
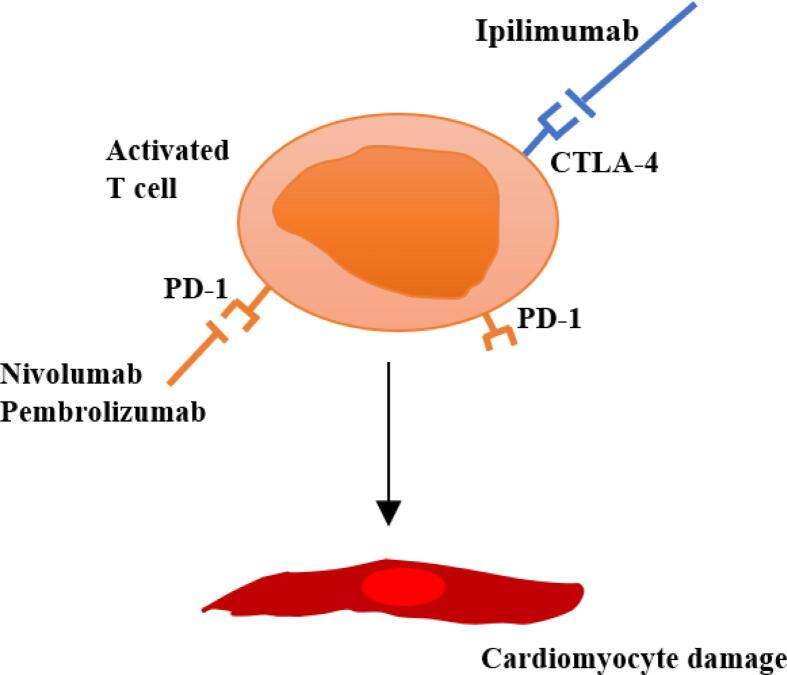
Myocarditis is characterised by inflammatory cell infiltration into the myocardium and is diagnosed using histological and immunological criteria. Inflammatory cardiomyopathy is defined as myocarditis associated with cardiac dysfunction. The molecular basis of ICIRM is not fully understood. One of the proposed explanations is that ICIs clonally reactivate CD8+ T-cells that target tumour-specific ligands such as a-myosin, so called 'self-antigens' which are also expressed in heart and peripheral muscles leading to T lymphocytic infiltration into the myocardium (Fig. 2). The release of pro-inflammatory cytokines from T-cells may also cause further myocardial injury [11]. This would suggest that perhaps multiple agents to target both T cell receptors and the cytokine tumour microenvironment, may be more effective in treating fulminant myocarditis.
Fig. 2.
The immune responses are not tumour-specific and affect the myocardium by causing autoimmune lymphocytic myocarditis through disruption of immune tolerance which is usually achieved through the presence of the PD-1/PD-L1 inhibitory pathway.
ICIRM can present with or without left ventricular impairment, with a normal ejection fraction not ruling out ICIRM. Inflammatory infiltrates affecting the conduction system can increase risk of arrythmias including heart block, atrial fibrillation, ventricular arrythmias. The mechanism in which cardiac function decreases after ICI is still a matter of ongoing research. Gergely et al. found that anti-PD-1 therapy reduced cardiac function in C57BL/6L mice without prominent myocardial immune cell infiltration, but rather by increasing IL-17 signalling in the thymus [12].
New approaches using cardiac biomarkers offer clinicians a practical method to diagnose, screen and monitor for myocarditis in patients receiving ICI therapy, enabling surveillance throughout the treatment process. Table 1 provides a summary of strengths and weaknesses of the biomarkers associated with ICI myocarditis. A recent single-centre, observational study by Vasbinder et al. consisted of 2606 adult patients who had received at least one dose of an ICI between June 2014 and December 2021 [13]. Among them, 27 individuals (1 %) were diagnosed with ICIRM. Patients with CMR confirmed myocarditis had increased levels of troponin T, creatine phosphokinase (CPK), AST, ALT and LDH, with 95 % of myocarditis patients displaying elevated levels in at least three of those biomarkers. They identified a significant correlation between elevated CPK levels and the risk of developing myocarditis i.e. for each doubling in CPK from baseline, there was an 83 % increase in the likelihood of developing myocarditis [13].
Table 1.
Strengths and weaknesses of Cardiac Biomarkers for ICI Related Myocarditis.
| Biomarkers | Strengths | Weaknesses |
|---|---|---|
| Troponin-I | High sensitivity Early increase to peak levels within hours of initial presentation. Good for early diagnosis. Widely available |
Can indicate myocardial injury from other causes e.g. ACS Macrotroponins can cause false positive troponin measurements Confounders e.g. renal function may complicate interpretationLow specificity |
| Troponin- T | High sensitivity Peak within days after initial presentation, increase persist for months. Good for late diagnosis.Stronger prognosticator for MACE compared to troponin I and CK |
Can indicate myocardial injury from other causes e.g. ACS Less available than Tropinin I Macrotroponins can cause false positive troponin measurements Confounders e.g. renal func-tion may complicate inter-pretationLow specificity |
| Creatinine kinase | High sensitivityEarly increase to peak levels within hours of initial presentation. Good for early diagnosis. | Low specificity |
| Natriuretic peptides | Widely availableHigh sensitivity | Low specificity e.g. can be increased in supraventricular arrhythmias |
| CK-MB | Widely available Early increase Shorter time courseMore specific for myocardial damage than CK |
Low sensitivity |
| AST | High sensitivity Routinely measured Can be helpful with detecting ICI-induced hepatotoxicityElevated in ICIRM |
Low specificity |
| ALP | High sensitivity Routinely measured Can be helpful with detecting ICI-induced hepatotoxicityElevated in ICIRM |
Low specificity |
| CPK | High sensitivity for acute myocarditis Moderate specificity Early increase to peak levels within hours of initial presentation, thus providing early indication for development of myocarditis Routinely measured Associated with rapid decline following initiation of immunosuppressive treatment, therefore useful to monitor effectiveness of treatmentElevated in ICIRM |
Decreases rapidly with acute episodes |
| LDH | Routinely measuredMarkedly elevated in ICIRM | Low specificity |
In contrast, there were cases in whom the CPK was normal but other biomarkers were elevated [13]. This would imply that CPK is a good positive predictive marker and a potential indicator for severity, although a less efficient negative predictor. In cases where CPK did not rise, myocarditis may have been underdiagnosed [13]. Moreover, this was a retrospective study, which has its own limitations. Troponin T is less cardiac specific than troponin I, particularly if CK is elevated as troponin T could reflect myositis and not myocarditis. The study also raises questions as to whether the observed increases are specifically attributable to the ICI treatment or reflective of pre-existing chronic changes.
Multiple studies and international guidelines have indicated the need for routine cardiac evaluation to include tests for B‐type natriuretic peptide (BNP) or N‐terminal pro‐B‐type natriuretic peptide (NT–proBNP), cardiac troponin and an ECG before commencing ICI therapy and during the initial 1–4 cycles or up to 12 weeks of treatment [14], [15], [16].
Cardiac troponins are both sensitive and specific for myocardial injury, and in cases in whom myocardial ischaemia is suspected, coronary angiography is advised. Cardiac troponin I is specific to myocardial injury whereas cardiac troponin T can be increased by both myocardial and skeletal muscle injury, and plasma troponin T levels are also higher in patients with chronic kidney disease. A case-control study by Mahmood et al. showed that troponin was increased in 94 % of patients with ICI-induced myocarditis, and BNP or NT-proBNP was elevated in 66 % of cases [17]. It also demonstrated that echocardiography was not particularly informative. In cancer patients, NT-proBNP may also be elevated due to inflammation [18]. A review of reported clinical cases by Zotova et al. had shown that troponin (I or T not specified) was elevated in 93.9 % of cases and CK/CK-MB elevated in 93.7 % of cases [19]. In these cases, several patients suspected of having acute coronary syndrome had a normal coronary angiogram. In a minority of ICIRM cases, cardiac biomarkers may still be normal e.g., clinically suspected cases who are troponin negative but have cardiac imaging showing inflammation and where endomyocardial biopsy confirmed myocarditis [17], [18], [19]. The primary confirmatory diagnostic test in these patients i.e., CMR may be negative in 50 % of patients with ICIRM [20].
A recent prospective cohort study by Lehmann et al. analysed the diagnostic accuracy and prognostic performances of three cardiac biomarkers: troponin T, troponin I and CK in 60 patients with ICIRM [21]. Troponin T was found to be a highly sensitive biomarker for ICI myocarditis and prediction of major adverse cardiovascular events (MACE), compared to cTnI and CK [21]. Patients with a troponin T level less than 32 times the upper limit within 72 h of ICIRM diagnosis had a lower risk for MACE, although concomitant ICI myositis may also cause raised troponin T [21]. This data contrasts guidelines recommending cTnI [22].
Elevated troponin levels and an ECG showing advanced conduction disease have been linked to higher disease severity and increased mortality rates [23], [24]. In addition to blood-based biomarkers and ECG, using other diagnostic tools such as CMR, echocardiography and prompt treatment with high-dose steroids has shown promising clinical outcomes, with a study by Andres et al. reporting zero cardiovascular mortality over 200 days of follow-up [25], [26]. A multicentre registry study by Dal’bo et al. noted that pre-existing conventional cardiovascular risk factors such as smoking and hypertension predisposed patients to ICIRM [27]. However, the reported associations were based on a univariate analysis and that the absence of these factors does not prevent the occurrence of cardiotoxicity.
Due to the rarity of ICIRM, most current evidence are derived from small case series, retrospective studies, registries, and case reports [28], [29]. To consolidate the current recommendations, large-scale clinical trials are advised, although it is challenging to conduct randomised controlled studies in this rare and complex clinical scenario. Researchers may be able to use experimental animal models to examine ICIRM in-depth and discover novel biomarkers which could lead to the early diagnosis of this condition. Abatacept has been shown in case reports and animal studies to be effective in treating ICIRM [30]. The ongoing ATRIUM study is a double-blind placebo-controlled trial where 390 patients hospitalised with ICIRM will be randomised to abatacept or placebo. Participants will be followed up for MACE [31].
Zhu et al. was a large-scale study that identified immune subsets associated with ICI myocarditis by performing time-of-flight mass cytometry on peripheral blood immune cells [32]. Confirmed by cytometry by time of flight and RNA sequencing, the study showed a significant increase in clonal Temra CD8+ cells in the blood of patients with ICIRM. In turn, Temra CD8+ cells exhibited increased expression of heart-tropic chemokines: CCL5, CCL4, and CCL4L2. Implications of the study include Temra CD8+ cells as a potential disease biomarker for ICIRM and heart-tropic chemokines expressed by Temra CD8+ cells as important therapeutic targets for ICIRM prevention and treatment.A better understanding of the relationship between ICI therapy and myocarditis, particularly at the molecular level, could help create new therapeutic strategies to safely diagnose and manage ICIRM. As the use of ICI increases, understanding how to monitor for ICIRM becomes even more relevant. Patients, commenced on ICIs should undergo baseline cardiac biomarker assessment, and if increased levels are detected the aetiology of the cardiac injury should be determined. This may identify groups more prone to developing ICI myocarditis e.g., those with coronary artery disease.
ICIRM co-occurs with other immunotherapy related adverse events and represents an infrequent but serious complication of cancer immunotherapy. Clinicians must exercise caution when utilising biomarkers to detect ICIRM, as there is not a single, completely reliable biomarker that definitively confirms the presence of this disease. Increased cardiac biomarkers during cancer treatment should be interpreted by the oncology and cardio-oncology team in the clinical setting and not immediately lead to stopping the evidence-based cancer therapy. Cardiac and noncardiac biomarkers serve as useful screening tools for the detection of ICIRM. Clinicians should monitor patients on ICIs regularly for non-cardiac biomarkers, which can prompt evaluation of sensitive cardiac markers. Elevations in these biomarkers correlate well with the diagnosis of myocarditis and should prompt more frequent cardiac monitoring and further investigation, initiation of cardioprotective treatment whilst minimising disruption to treatment. However, the optimal timing of biomarker measurements remains a topic of debate. The timing and frequency of assessing biomarkers should be tailored for each specific biomarker. Further research is needed to involve a variety of biomarkers, including novel biomarkers, to confirm their clinical utility and validate data in larger patient sets.
There is an unmet need for iterative elaboration of guidelines created by interdisciplinary teams composed of cardiologists, oncologists, and immunologists. This is to guide the interpretation of abnormal biomarker results in patients receiving ICI therapy, improve our understanding, and help identify sets of patients who may benefit from biomarker surveillance.
Author contributions
Conceptualisation and design – ST, AG, TC.
Data collection and assembly – ST, AG, TC.
Data analysis and interpretation – All Authors.
Manuscript Writing – All Authors.
Final Approval of Manuscript – All Authors.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: ARL has received speaker, advisory board or consultancy fees and/or research grants from Pfizer, Novartis, Servier, Astra Zeneca, Bristol Myers Squibb, GSK, Amgen, Takeda, Roche, Janssens-Cilag Ltd, Astellas Pharma, Clinigen Group, Eli Lily, Eisai Ltd, Ferring Pharmaceuticals, Boehringer Ingelheim, Akcea Therapeutics, Myocardial Solutions, iOWNA Health and Heartfelt Technologies Ltd.
Acknowledgement
ARL is supported by the Fondation Leducq Network of Excellence in Cardio-Oncology.
References
- 1.Sharma P., Siddiqui B.A., Anandhan S., et al. The next decade of immune checkpoint therapy. Cancer Discov. 2021;11(4):838–857. doi: 10.1158/2159-8290.CD-20-1680. [DOI] [PubMed] [Google Scholar]
- 2.Amaria R.N., Postow M., Burton E.M., et al. Neoadjuvant relatlimab and nivolumab in resectable melanoma. Nature. 2022;611(7934):155–160. doi: 10.1038/s41586-022-05368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmer J.R., Abu-Sbeih H., Ascierto P.A., Brufsky J., Cappelli L.C., Cortazar F.B., et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J. Immunother. Cancer. 2021;9(6) doi: 10.1136/jitc-2021-002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palaskas N., Lopez-Mattei J., Durand J.B., Iliescu C., Deswal A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J. Am. Heart Assoc. 2020;9(2) doi: 10.1161/JAHA.119.013757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thuny F., Naidoo J., Neilan T.G. Cardiovascular complications of immune checkpoint inhibitors for cancer. Eur. Heart J. 2022;43(42):4458–4468. doi: 10.1093/eurheartj/ehac456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marco C., Simó M., Alemany M., Casasnovas C., Domínguez R., Vilariño N., et al. Myasthenia gravis induced by immune checkpoint inhibitors: an emerging neurotoxicity in neuro-oncology practice: case series. J. Clin. Med. 2022;12(1) doi: 10.3390/jcm12010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyon A.R., López-Fernández T., Couch L.S., et al. ESC Scientific Document Group. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) Eur. Heart J. 2022;43(41):4229–4361. doi: 10.1093/eurheartj/ehac244. [DOI] [PubMed] [Google Scholar]
- 8.Deharo F., Thuny F., Cadour F., Resseguier N., Meilhac A., Gaubert M., et al. Diagnostic value of the international society of cardio-oncology definition for suspected immune checkpoint inhibitor-associated myocarditis. J. Am. Heart Assoc. 2023;12(8) doi: 10.1161/JAHA.122.029211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haanen J., Obeid M., Spain L., et al. ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022;33(12):1217–1238. doi: 10.1016/j.annonc.2022.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Herrmann J., Lenihan D., Armenian S., et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur. Heart J. 2022;43(4):280–299. doi: 10.1093/eurheartj/ehab674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson D.B., Balko J.M., Compton M.L., et al. Fulminant myocarditis with combination immune checkpoint blockade. N. Engl. J. Med. 2016;375(18):1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gergely T.G., Kucsera D., Tóth V.E., Kovács T., Sayour N.V., Drobni Z.D., et al. Characterization of immune checkpoint inhibitor-induced cardiotoxicity reveals interleukin-17A as a driver of cardiac dysfunction after anti-PD-1 treatment. Br. J. Pharmacol. 2023;180(6):740–761. doi: 10.1111/bph.15984. [DOI] [PubMed] [Google Scholar]
- 13.Vasbinder A., Chen Y., Procureur A., et al. Biomarker trends, incidence, and outcomes of immune checkpoint inhibitor-induced myocarditis. JACC: CardioOncology. 2022;4(5):689–700. doi: 10.1016/j.jaccao.2022.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyon A.R., Yousaf N., Battisti N.M.L., Moslehi J., Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19(9):e447–e458. doi: 10.1016/S1470-2045(18)30457-1. [DOI] [PubMed] [Google Scholar]
- 15.Lyon A.R., Dent S., Stanway S., et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail. 2020;22(11):1945–1960. doi: 10.1002/ejhf.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pudil R., Mueller C., Čelutkienė J., et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: a position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur. J. Heart Fail. 2020;22(11):1966–1983. doi: 10.1002/ejhf.2017. [DOI] [PubMed] [Google Scholar]
- 17.Mahmood S.S., Fradley M.G., Cohen J.V., et al. Myocarditis in patients treated with immune checkpoint inhibitors. J. Am. Coll. Cardiol. 2018;71(16):1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bando S., Soeki T., Matsuura T., et al. Plasma brain natriuretic peptide levels are elevated in patients with cancer. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0178607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zotova L. Immune checkpoint inhibitors-related myocarditis: a review of reported clinical cases. Diagnostics. 2023;13(7) doi: 10.3390/diagnostics13071243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L., Awadalla M., Mahmood S.S., et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur. Heart J. 2020;41(18):1733–1743. doi: 10.1093/eurheartj/ehaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehmann L.H., Heckmann M.B., Bailly G., Finke D., Procureur A., Power J.R., et al. Cardiomuscular biomarkers in the diagnosis and prognostication of immune checkpoint inhibitor myocarditis. Circulation. 2023;148(6):473–486. doi: 10.1161/CIRCULATIONAHA.123.062405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonaca M.P., Olenchock B.A., Salem J.E., Wiviott S.D., Ederhy S., Cohen A., et al. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation. 2019;140(2):80–91. doi: 10.1161/CIRCULATIONAHA.118.034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puzanov I., Subramanian P., Yatsynovich Y.V., et al. Clinical characteristics, time course, treatment and outcomes of patients with immune checkpoint inhibitor – associated myocarditis. J. Immunother. Cancer. 2021;9(6) doi: 10.1136/jitc-2021-002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Power J.R., Alexandre J., Choudhary A., et al. International ICI-Myocarditis Registry†. Electrocardiographic manifestations of immune checkpoint inhibitor myocarditis. Circulation. 2021;144(18):1521–1523. doi: 10.1161/CIRCULATIONAHA.121.055816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andres M.S., Ramalingam S., Rosen S.D., et al. The spectrum of cardiovascular complications related to immune-checkpoint inhibitor treatment: Including myocarditis and the new entity of non inflammatory left ventricular dysfunction. Cardio-Oncology. 2022;8(1) doi: 10.1186/s40959-022-00147-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pons-Riverola A., Ghosh A.K. An update on the role of cardiac magnetic resonance imaging in cancer patients. Curr. Cardiol. Rep. 2022;24(12):2139–2147. doi: 10.1007/s11886-022-01818-x. [DOI] [PubMed] [Google Scholar]
- 27.Dal’bo N., Patel R., Parikh R., et al. Cardiotoxicity of contemporary anticancer immunotherapy. Curr. Treat. Options Cardiovsc. Med. 2020;22(12) doi: 10.1007/s11936-020-00867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Axelrod M.L., Meijers W.C., Screever E.M., Qin J., Carroll M.G., Sun X., et al. T cells specific for α-myosin drive immunotherapy-related myocarditis. Nature. 2022;611(7937):818–826. doi: 10.1038/s41586-022-05432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Won T., Kalinoski H.M., Wood M.K., Hughes D.M., Jaime C.M., Delgado P., et al. Cardiac my-osin-specific autoimmune T cells contribute to immune-checkpoint-inhibitor-associated my-ocarditis. Cell Rep. 2022;41(6) doi: 10.1016/j.celrep.2022.111611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei S.C., Meijers W.C., Axelrod M.L., Anang N.A.A.S., Screever E.M., Wescott E.C., et al. A genetic mouse model recapitulates immune checkpoint inhibitor-associated myocarditis and supports a mechanism-based therapeutic intervention. Cancer Discov. 2021;11(3):614–625. doi: 10.1158/2159-8290.CD-20-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynolds K., Mooradian M., Zlotoff D., Ridker P., Neilan T., The ATRIUM Investigators 696 Abatacept for immune checkpoint inhibitor associated myocarditis (ATRIUM): a phase 3, investigator-initiated, randomized, double blind, placebo-controlled trial. J. Immunother. Cancer. 2022;10 doi: 10.1136/jitc-2022-SITC2022.0696. [DOI] [Google Scholar]
- 32.Zhu H., Galdos F.X., Lee D., et al. Identification of pathogenic immune cell subsets associated with checkpoint inhibitor-induced myocarditis. Circulation. 2022;146(4):316–335. doi: 10.1161/CIRCULATIONAHA.121.056730. [DOI] [PMC free article] [PubMed] [Google Scholar]