Abstract
PmrA, an OmpR/PhoB-family response regulator, triggers gene transcription responsible for polymyxin resistance in bacteria by recognizing promoters where the canonical-35 element is replaced by the pmra-box, representing the PmrA recognition sequence. Here, we report a cryo-electron microscopy (cryo-EM) structure of a bacterial PmrA-dependent transcription activation complex (TAC) containing a PmrA dimer, an RNA polymerase σ70 holoenzyme (RNAPH) and the pbgP promoter DNA. Our structure reveals that the RNAPH mainly contacts the PmrA C-terminal DNA-binding domain (DBD) via electrostatic interactions and reorients the DBD three base pairs upstream of the pmra-box, resulting in a dynamic TAC conformation. In vivo assays show that the substitution of the DNA-recognition residue eliminated its transcriptional activity, while variants with altered RNAPH-interacting residues resulted in enhanced transcriptional activity. Our findings suggest that both PmrA recognition-induced DNA distortion and PmrA promoter escape play crucial roles in its transcriptional activation.
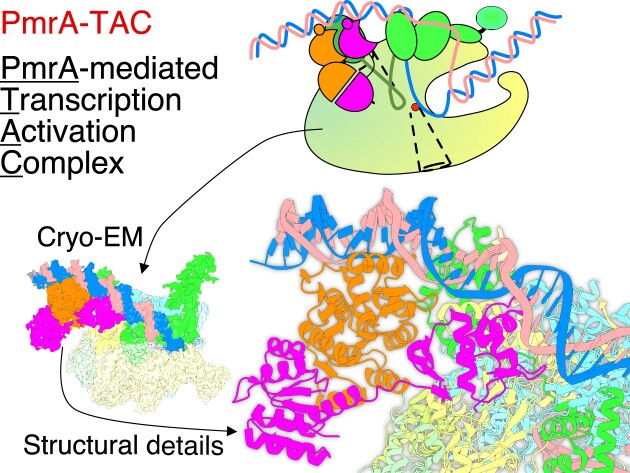
Graphical Abstract
Graphical Abstract.
INTRODUCTION
PmrA is the primary response regulator of genes responsible for polymyxin resistance in various bacterial species, such as Salmonella enterica, Escherichia coli and Klebsiella pneumoniae (1–3). The PmrA-binding promoter, termed the pmra-box and consisting of two hexanucleotide XTTAAY repeats separated by five nucleotides, overlaps with the -35 element in the pbgP, ugd and pmrCAB operons (2,4,5). PmrA binding and activation of these genes encoding enzymes that modify lipopolysaccharide in the outer membrane promote resistance to polymyxins, antimicrobial peptides and antibiotics (2,5,6). Polymyxins, especially polymyxin B and colistin (polymyxin E), are considered last-resort antibiotics against multidrug-resistant Gram-negative bacteria (7,8). However, polymyxin-resistant infections are becoming widespread, representing a significant threat to public health (9,10). Thus, understanding the structural basis of the PmrA-modulated transcriptional activation that promotes bacterial polymyxin resistance is urgently needed to aid the development of new drugs against bacterial infections.
PmrA belongs to the OmpR/PhoB family of response regulators, comprising an N-terminal receiver domain (REC) and a C-terminal winged-helix DNA-binding domain (DBD) that are connected by a flexible linker (11). For PmrA and several other family members, phosphorylation-induced dimerization of the REC allosterically enhances binding of the DBD to promoter DNA for transcriptional activation (12–14). Despite structural similarities, OmpR/PhoB family members display different modes of interaction with RNA polymerase (RNAP) to regulate transcription. Mutational studies have suggested that OmpR operates by its DBD interacting with the α subunit of RNAP (15,16), whereas the PhoB DBD interacts with the σ4 domain of the RNAP σ70 holoenzyme (RNAPH) (17–19). A low-resolution crystal structure of a PhoB subcomplex has been reported previously that revealed interactions between a chimeric σ4 domain of the σ70 RNAP cofactor, two PhoB DBDs and the promoter DNA (20). However, this subcomplex could not illustrate the full suite of interactions between PhoB and RNAPH. Moreover, a previous attempt to resolve a transcription activation complex (TAC) involving the OmpR/PhoB-family response regulator KdpE by cryogenic electron microscopy (cryo-EM) proved unsuccessful (21), with the density map for KdpE missing even though the KdpE–RNAPH–DNA ternary complex had been cross-linked with glutaraldehyde. Thus, further structural analyses are required to reveal all interactions between an OmpR/PhoB-family member and the RNAPH.
Previously, we found that beryllofluoride (BeF3-, a phosphate analog)-activated PmrA forms a dimer displaying enhanced binding affinity for the pbgP promoter (22). We also determined a 3.2 Å resolution crystal structure of BeF3–-activated PmrA-WI (a W181G/I220D double-substitution variant that exhibits high solubility and thermal stability) in complex with the pbgP promoter DNA (PDB ID 4S05) (14). In the PmrA-WI complex structure, the two RECs form a head-to-head dimer, but the two DBDs bind to the DNA in a head-to-tail orientation, resulting in an asymmetric REC–DBD interface in the upstream PmrA protomer, which was demonstrated to be transiently populated and was not critical for transcriptional activation. Our complex structure also revealed details of PmrA–DNA interactions, with the two DBDs specifically recognizing repeated ‘TAA’ sequences in the pbgP promoter and inducing a bent conformation in the promoter DNA. Furthermore, a β-galactosidase reporter assay demonstrated that the W181G/I220D double substitution did not affect PmrA’s transcriptional activity, but the DNA-recognition residues are crucial for PmrA-activated gene expression.
In this study, we determine the structure of an intact PmrA TAC ternary complex containing K. pneumoniae PmrA-WI, E. coli RNAPH and the K. pneumoniae pbgP promoter. The resulting cryo-EM map shows that contacts between the PmrA dimer and its surrounding area are dynamic, and the respective densities could be further enhanced by focused map and 3D classifications (Figure 1). The final TAC structure reveals interactions between full-length PmrA dimer, RNAPH and DNA. Combined with in vivo β-galactosidase reporter assay data, we propose a mechanism for PmrA-mediated transcriptional activation.
Figure 1.
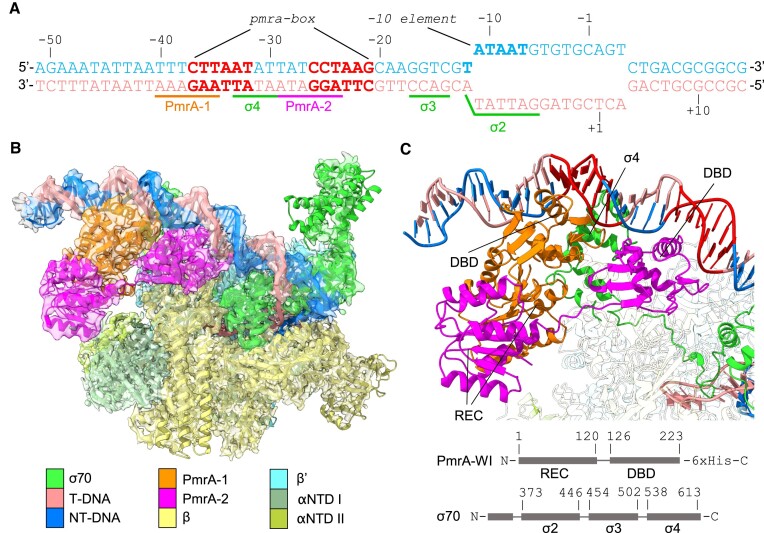
Cryo-EM structure of the PmrA-dependent TAC. (A) The synthetic promoter DNA scaffolds used in the PmrA TAC, with the pbgP promoter sequence from –62 to –13, and a transcription bubble sequence including the -10 element and discriminator from –12 to the downstream region to mimic transcription initiation. The nucleotide positions are numbered relative to the transcription start site. The template strand (T-DNA) and the non-template strand (NT-DNA) of DNA are colored in salmon and blue, respectively. Two hexanucleotide repeats in the pmra-box are colored in red. The DNA sequences associated with binding of PmrA, σ4, σ3 and σ2 are labeled and underlined. (B) A composite map containing the RNAP core and the 3.70 Å focused map (PmrA-WI, the upstream DNA and part of σ70) is overlaid with the built PmrA TAC complex structure. An EM map with partial transparency is used to present the good superposition of the map and structure. (C) PmrA-WI, the upstream DNA and part of σ70 demonstrate the interactions between PmrA and DNA. DNA regions are colored as in (A). The upstream and downstream PmrA-WI protomers are denoted as PmrA-1 and PmrA-2, and are colored in orange and magenta, respectively. The σ70 are in green. Domain architectures of PmrA and σ70 are also provided. The REC and DBD of PmrA and σ4, σ3 and σ2 of σ70 are indicated. The RNAP core proteins are uncolored.
MATERIALS AND METHODS
Preparation of the PmrA-dependent TAC
The DNA fragment encoding K. pneumoniae PmrA hosting the W181G/I220D double substitution (PmrA-WI), together with an extra methionine residue at the N-terminus and an additional LEHHHHHH tag at the C-terminus, was cloned into a pET-29b(+) (Novagen) vector and transferred into E. coli strain BL21(DE3). Recombinant PmrA-WI was expressed and purified as described (14). A synthetic promoter comprising the upstream AT-rich sequence and the pmra-box from the K. pneumoniae pbgP promoter, a consensus -10 element, a discriminator element, a transcription bubble and a 14 bp downstream extension was prepared by mixing an equal amount of two strands, heating to 95°C for 30 min and cooling slowly to room temperature. The polycistronic vector for expressing E. coli RNAP was obtained from Addgene (PVS10, plasmid #104398, with genes for rpoA, rpoB and rpoC-6×His) (23). The DNA fragment encoding the E. coli σ70 cofactor was directly amplified from E. coli DNA and cloned into a pGEX-4T1 vector with a modified Tobacco etch virus (TEV) protease cleavage sequence. The two vectors were transferred into E. coli strain BL21(DE3) for separate expression. Cells were grown in LB broth at 37°C. When the OD600 reached 0.6, protein expression was induced by means of 1 mM isopropyl-β-d-thiogalactopyransode (IPTG) at 28°C overnight. Cells were harvested, resuspended in lysis buffer (50 mM Tris, 500 mM NaCl, 5% glycerol at pH 7.3) and homogenized by using a microfluidizer. After lysis, cell debris was removed by centrifuging at 12,000 rpm for 30 min. The cell lysates containing RNAP and σ70 cofactor were incubated for 1 h and subjected to purification. First, Ni-affinity chromatography was performed with 150 ml of wash buffer (50 mM Tris–HCl, 500 mM NaCl, 20 mM imidazole, pH 7.5) and 50 ml of elution buffer (50 mM Tris–HCl, 200 mM NaCl, 300 mM imidazole, pH 7.5). Second, a GSTrap FF column was used with an elution buffer containing 50 mM Tris–HCl, 200 mM NaCl and 20 mM reduced glutathione at pH 8.0. The glutathione S-transferase (GST) tag was then removed using the TEV protease. Further purification was conducted by size-exclusion chromatography, and the resulting RNAPH sample was analyzed using a Coomassie blue-stained sodium dodecylsulfate (SDS)–polyacrylamide gel (Supplementary Figure S1). The PmrA-dependent TAC was assembled by incubating the RNAPH with a 2-fold molar excess of the pre-formed promoter DNA and PmrA-WI in final buffer (20 mM HEPES, 100 mM NaCl, 10 mM MgCl2, 200 μM ZnCl2, pH 7.0).
Cryo-EM sample, data collection, data processing and structure building
The purified PmrA TAC was thawed on ice from -80°C and diluted to 0.3–1 mg/ml for cryo-EM grid preparation. The protein complex was applied to Quantifoil holey carbon grids (Cu, 200 mesh, R2/1 μm) by using a Vitrobot Mark IV system (Thermo) with 4 s blotting time at 100% humidity and 4°C. Grids were immediately frozen by using liquid ethane and stored in a liquid nitrogen reservoir. Cryo-EM data for the PmrA TAC were collected on a 300 keV Titan Krios microscope equipped with a Gatan K2 detector. The slit width was 20 eV. EPU 1.2 was used for automatic data collection with three exposures per hole. In total, 6132 60-frame micrographs in counting mode were acquired at a physical pixel size of 0.822 Å and a nominal magnification of 165,000. The total electron dose and desired defocus ranges were 57.1 e-/Å2 and -1.0, -1.5, -2.0 and -2.2 μm, respectively.
Cryo-EM data were processed by using cryoSPARC 3.2 (24). Movie alignment was performed by full-frame motion or patch-motion correction. The contrast transfer function was estimated by using Patch CTF in cryoSPARC. Particles with 150–180 Å blob diameters were extracted from 150 micrographs for 2D classification. The well-aligned 2D averages were used for template particle selection in cryoSPARC. Through iterations of 2D classifications, 748,314 particles were finally selected for initial model generation and 3D map refinement. Another dataset containing 5644 micrographs (data acquisition condition unchanged) was processed similarly, resulting in 300,651 particles selected from 2D classifications. We applied heterogeneous refinement of five classes to separate the PmrA TAC complex from the junky signals, RNAP and RNAPH. A total of 219,627 particles belonging to the PmrA TAC complex were further refined with non-uniform sampling and per particle CTF (contrast transfer function) correction, resulting in a 2.74 Å map. We next generated a mask covering the PmrA-WI, upstream DNA and part of σ70 for local refinement and 3D classifications. A subgroup of four 3D heterogenous group containing 99,715 particles of the PmrA–DNA complex was selected and refined to 3.7 Å (Figure 1C). The two maps were used to build the RNAPH structure. Detailed PmrA and PmrA-associated interactions were refined using the 3.7 Å map.
Cryo-EM structures were built using existing template structures for E. coli RNAPH, including PDB IDs 6CA0 and 6B6H. The map was first used to remodel RNAP core subunits and σ70 cofactor by means of the real-space refinement function in Phenix 1.8 (25). Subsequently, the DNA sequences of this study were manually re-placed in the structure by using Coot 0.8.2 (26). The DBD and REC of PmrA were separately docked into the local refined PmrA–DNA map, followed by real-space refinement. The final model was manually inspected and verified in Coot.
β-Galactosidase reporter assay
The upstream region (∼500 bp) of the K. pneumoniae pbgP gene was amplified by polymerase chain reaction (PCR) and inserted in front of the lacZ gene in the placZ15 plasmid to obtain the reporter plasmid, which was then transformed by conjugation into K. pneumoniae CG43S3-ΔlacZ strain. Plasmids expressing wild-type PmrA or its variants were then transformed into the K. pneumoniae cells carrying that reporter plasmid. The cells were cultured at 37°C in LB medium containing kanamycin (50 mg/ml) and chloramphenicol (34 mg/ml) for 30 min, before adding IPTG (1 mM/ml) for PmrA or variant expression for 5 h and then harvesting by centrifugation. β-Galactosidase activity in the supernatant was measured at OD420, and values are expressed in Miller units [1000 × OD420/(T × V × A600)], where T and V are reaction time (min) and volume of culture (ml), respectively. The average values and standard errors from triplicate measurements are plotted.
RESULTS
Overall structure of the PmrA-dependent TAC
In this study, we constructed a synthetic DNA scaffold of the PmrA-dependent promoter (from base pairs -62 to +14; 76 bp in total), which contains an upstream AT-rich sequence and the pmra-box from the K. pneumoniae pbgP promoter (base pairs -62 to -13) (2), a consensus -10 element, a discriminator element, a transcription bubble and a 14 bp downstream extension (Figure 1A, B; Supplementary Figure S1A). The synthetic promoter was mixed with BeF3–-activated PmrA-WI and the E. coli RNAPH for cryo-EM grid preparation (Supplementary Figure S1B). The sequences of K. pneumoniae and E. coli RNAPH are almost identical (99, 98 and 98% for the α, β and β′ subunits, respectively, and 96% for the σ70 cofactor), implying a strong likelihood of their reasonable hybridization to the PmrA-dependent TAC. We constructed a cryo-EM map of the PmrA TAC by using single-particle reconstruction (Supplementary Figure S2). The resulting map at a resolution of 2.74 Å reveals clear EM density signals for RNAP, but the signals in the PmrA-WI region are below the average. We then performed a local focus refinement covering the PmrA-WI, the upstream double-stranded DNA and a segment of σ70, resulting in a 3.70 Å map that clearly showed the PmrA–DNA interactions (Figure 1C). By combining these two maps, we present a TAC that consists of one RNAPH (including the non-conserved region of σ70), upstream DNA and a PmrA-WI dimer (denoted PmrA-1 and PmrA-2 for the upstream and downstream PmrA-WI protomers, respectively) (Figure 1B). Our map provides insights into the PmrA-bound transcription activation machinery that reveals the structure of RNAP core subunits and the DNA region spanning base pairs -42 to 14, including the transcription bubble (Figure 1B). The detailed data collection parameters and refinement statistics are summarized in Table 1.
Table 1.
Cryo-EM data collection, refinement and validation statistics
| Accession codes (PDB/EMDB) | 8JO2/EMD-36453 |
| Data collection and processing | |
| Microscope/detector | Krios/K2 |
| Magnification | 165,000 |
| Voltage | 300 kV |
| Electron exposure (e-/Å2) | 57.1 |
| Defocus range (μm) | -1 to -2.2 |
| Pixel size (Å) | 0.822 |
| Symmetry imposed | C1 |
| Initial particle images (n) | 1,048,965 |
| Final particle images (n) | 219,627/99,715 (full map/local focused map) |
| Reconstruction method | Single particle reconstruction |
| Map resolution (Å) (FSC0.143) | 2.74/3.70 |
| Map resolution range (Å) | 2.0–7.5/ 2.0–8.0 |
| Map sharpening B factor (Å2) | -64/-68.8 |
| Refinement | |
| Initial model | 4S05, 6B6H and 6CA0 |
| Model composition | |
| Non-hydrogen atoms | 35,368 |
| Protein residues | 4163 |
| Nucleotides | 130 |
| B factor (Å2) | |
| Protein | 65.44 |
| Nucleotides | 139.55 |
| R.m.s. deviations | |
| Bond lengths (Å) | 0.011 |
| Bond angles (°) | 1.245 |
| Validation | |
| MolProbity score | 2.35 |
| Clashscore | 13.86 |
| Poor rotamers (%) | 2.22 |
| Ramachandran plot | |
| Favored (%) | 93.31 |
| Allowed (%) | 6.69 |
| Disallowed (%) | 0 |
Electrostatic interactions between PmrA-WI and the RNAPH
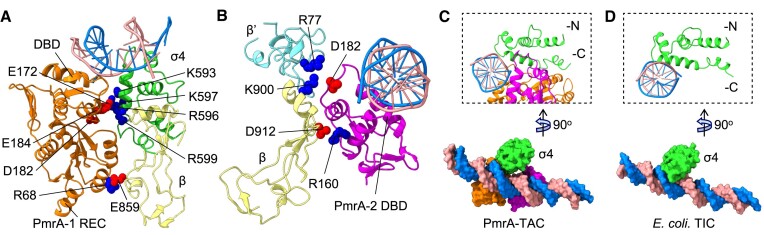
In our PmrA TAC, the E. coli-derived RNAPH adopts an overall conformation similar to that displayed by the E. coli RNAP σ70 transcription initiation complex (TIC) (21), and the conformation adopted by the PmrA-WI dimer is similar to when it is in complex with DNA alone (Supplementary Figure S3), demonstrating that the RNAPH and PmrA-WI conformations largely do not interfere with each other. First, we investigated the interactions between PmrA-WI and RNAPH, and found that the two PmrA-WI protomers are in close contact with different regions of the RNAPH (Figure 2). PmrA-1 interacts with the σ4 domain and β-flap via its DBD and REC, with an interface of 650 Å2. The DBD of PmrA-2 contacts the β-flap and β′ subunit, with an interface of 515.7 Å2. Although the resolution of our cryo-EM data does not allow precise characterization of the interacting atoms, we inferred the electrostatic interactions based on the amino acids with opposing surface charges at appropriate distances. An acidic patch (E172, D182 and E184) on the PmrA-1 DBD faces a basic patch (K593, R596, K597 and R599) of σ4 (Figure 2A). Residue D182 of the PmrA-2 DBD also forms electrostatic interactions with K900 at the β-flap-tip helix and with R77 on the β′ subunit (Figure 2B). Furthermore, two additional electrostatic interactions—between R68 on the PmrA-1 REC and E859 on the β-flap and between R160 on the PmrA-2 DBD and D912 on the β-flap—further secure PmrA to the β subunit of the RNAPH.
Figure 2.
Analysis of the PmrA–RNAPH interface. Dimeric PmrA-WI interacts with the double-stranded DNA, the σ4 domain of the σ70 cofactor and the RNAP core β and β′ subunits (colored as in Figure 1B). (A) Negatively charged PmrA-1 DBD residues E172, D182 and E184 form electrostatic interactions with positively charged residues K593, R596, K597 and R599 of the σ4 domain of the σ70 cofactor. The REC residue R68 is also located near E859 of the RNAP β subunit, contributing to complex stabilization. The listed residues are presented in a single-ball mode. (B) PmrA-2 DBD, which binds to the downstream DNA, interacts electrostatically with the RNAP β and β′ subunits. (C) The weaker interaction between the σ4 domain and DNA in the PmrA TAC compared with (D) the E. coli RNAP σ70 transcription initiation complex (TIC; PDB: 6CA0) due to the binding of the two PmrA DBDs to the -35 position of the DNA. A lateral view (right top corner) shows that in the PmrA TAC, the σ4 domain is not inserted as deeply into the DNA major groove as it is in the E. coli TIC without a transcription factor.
Next, we examined the conformation of DNA in the PmrA TAC. We observed that the DNA near PmrA-binding regions shifted upon binding of that response regulator (Supplementary Figure S3), so σ4 does not enter the DNA major groove as deeply as it does in the E. coli TIC in the absence of a transcription factor. In the PmrA TAC, the interface between σ4 and the DNA covers 127.7 Å2 (Figure 2C), i.e. much smaller than the interface of 504.4 Å2 between σ4 and the -35 element DNA in the E. coli TIC (Figure 2D). Thus, during activator-dependent transcription, both the recognition affinity and specificity of σ4 are weaker than those during activator-independent transcription because the weak interaction between σ4 and the promoter DNA is compensated for by its interaction with the activator, as shown in Figure 2.
Upstream-shifted PmrA DBD–DNA recognition
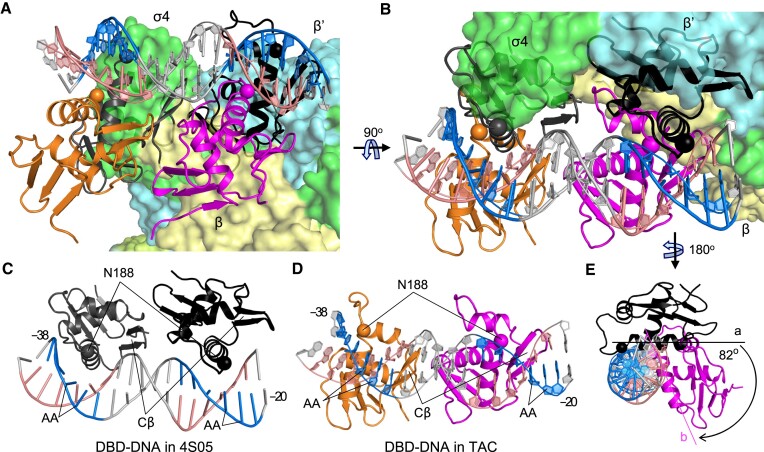
To investigate the variations in PmrA–DNA interactions between the PmrA TAC and the PmrA–DNA complex without RNAPH, we superimposed the double-stranded DNA fragment from -38T to -20C in the PmrA–DNA crystal structure onto that of the PmrA TAC (Figure 3A). A 90° rotation view (Figure 3B) and separate depictions of the DBD–DNA structures in the PmrA–DNA complex (Figure 3C) and in the PmrA TAC (Figure 3D) were generated. In both complex structures, the DNA recognition helix and the C-terminal β-hairpin (Cβ) of PmrA DBD inserted into the major and minor grooves, respectively, for DNA recognition. In the PmrA–DNA complex, two DBDs bound to the middle of the major grooves of the imperfect repeated sequences ‘CTTAAT’ and ‘CCTAAG’ in the pmra-box, with two N188 residues on the recognition helices specifically interacting with the ‘AA’ bases (Figure 3A–C). However, in the PmrA TAC, these DBD-binding sites were occupied by σ4 and the β′ subunit of the RNAPH, causing both DBDs to shift upstream, and the two N188 residues positioned in the upstream region of those ‘AA’ sequences (Figure 3A, B, D). A lateral view (Figure 3E) revealed that the DBD recognition helix binds to DNA at position ‘a’ in the absence of RNAPH, but shifted 82° along the DNA major groove to position ‘b’ upon interaction with RNAPH, resulting in an upstream shift of approximately three base pairs. In this shifted DNA binding position, the interactions between the DBD and DNA appeared to be weaker compared with those identified in the PmrA–DNA complex alone. These findings align with our initial cryo-EM map of the PmrA TAC, demonstrating a dynamic TAC conformation with clear density for the RNAPH, but weaker signals for the upstream DNA and PmrA-WI.
Figure 3.
Upstream-shifted DBD–DNA interaction in the PmrA TAC. (A) The comparison of the PmrA DBD–DNA interactions in the TAC with the crystal structure of PmrA–DNA (PDB ID 4S05) involved superimposing the double-stranded DNA fragment from positions –38 to –20, depicted in a similar orientation to that in Figure 1C. A 90° rotation view (B) and separate depictions of the DBD–DNA structures in 4S05 (C) and in the PmrA TAC (D) provides clear visualization of their relative orientations. A lateral view (E) shows the DNA and downstream DBDs, highlighting the shifting of the DBD recognition helix in two complex structures. RNAPH is colored as in Figure 1B, with DBDs in the TAC shown in orange and magenta, and in 4S05 in dark gray and black. Residue N188 on the recognition helix is displayed as a sphere. The imperfect repeat sequences ‘CTTAAT’ and ‘CCTAAG’ in the pmra-box are emphasized in blue, their complementary sequences in salmon and other nucleotides in gray. The DNA is visualized as sticks in (C) and as rings in (D). Residue N188, the C-terminal β hairpin (Cβ) and the ‘AA’ sequences are labeled and indicated in (C) and (D).
PmrA residues responsible for promoter and RNAPH interaction are also important in transcription
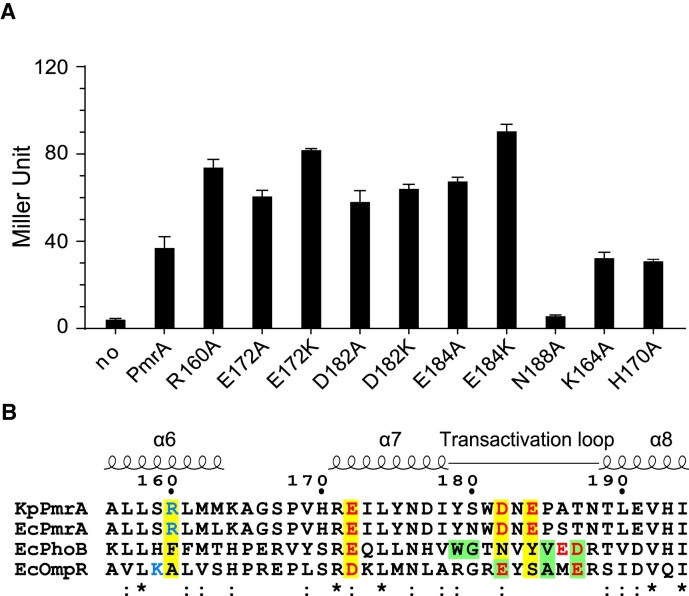
In the structure of PmrA TAC, RNAPH contacts PmrA-WI via several electrostatic interactions, causing PmrA-WI to move towards the upstream DNA sequence and leading to weaker PmrA–DNA interactions. To understand the impact of those interactions on transcriptional activation by PmrA, we conducted in vivo β-galactosidase reporter gene expression assays (Figure 4A). Mutation of PmrA residue N188 to alanine (N188A), which abolishes DNA recognition activity (14), also suppressed its transcriptional activity, indicating that DNA recognition is crucial for PmrA-mediated transcriptional activation. Notably, alanine replacement of any of the four residues involved in RNAPH interaction (R160A, E172A, D182A and E184A) increased gene expression. Furthermore, replacing any of the three negatively charged residues with the positively charged lysine (E172K, D182K and E184K) resulted in even higher gene expression. In control experiments, replacement of either K164 or H170, which are not involved in DNA or RNAPH interaction, with alanine did not alter expression or transcriptional activity (Figure 4A). The in vivo β-galactosidase reporter assay indicates that PmrA residues responsible for promoter DNA and RNAPH interaction play significant roles in transcription. This supports the findings from the cryo-EM structure of the PmrA TAC.
Figure 4.
Residues involved in transcriptional activation. (A) In vivo β-galactosidase reporter assay in K. pneumoniae carrying plasmids that express PmrA or its variants plus the reporter plasmid hosting the pbgP promoter in front of the lacZ gene. Expression of PmrA or its variants was induced by IPTG (1 mM/ml). Assay for PmrA without IPTG addition is labeled as ‘no’. Results are expressed as Miller Units. All experiments were performed in triplicate. Error bars represent the standard deviation. The horizontal dashed line indicates the value obtained from PmrA. (B) Sequence alignment of K. pneumoniae PmrA, and PmrA, PhoB and OmpR from E. coli. K. pneumoniae PmrA residues involved in RNAPH interactions are highlighted with a yellow background and are colored in blue and red for positive and negative charges, respectively. Residues for which replacement abolishes transcriptional activation by E. coli PhoB and OmpR are shown with a green background. Fully conserved or similar residues are denoted * or :, respectively.
DISCUSSION
Here, we presented the cryo-EM structure of the PmrA TAC showing that PmrA-WI contacts RNAPH via several electrostatic interactions, most notably between the two DBD domains and the σ4, β and β′ subunits of the RNAPH. In addition, compared with the previously determined PmrA–DNA complex structure, binding of RNAPH shifts the position of both DBD domains upstream by approximately three base pairs in the PmrA TAC. In vivo β-galactosidase reporter gene expression assays further showed that substituting a DNA-recognition residue in PmrA eliminated its transcriptional activity, but variants with altered RNAPH-interacting residues around the transactivation loop exhibited elevated transcriptional activity.
A sequence alignment of these residues from K. pneumoniae PmrA with those from E. coli PmrA, PhoB and OmpR reveals conserved or similar residues at proximal positions in all four response regulators (Figure 4B), suggesting that they may adopt similar mechanisms for transcription activation. Previous studies have demonstrated that substituting residues on the transactivation loop abolishes transcriptional activation in E. coli PhoB and OmpR (as indicated by the green background in Figure 4B) (15,19,27). However, in contrast, variants with altered transactivation loop residues (D182A, D182K, E184A and E184K) in K. pneumoniae PmrA led to an increase in transcriptional activity.
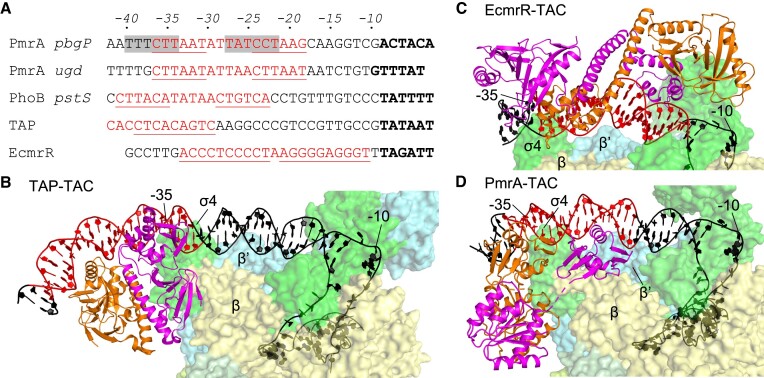
To understand why substitution of RNAPH-interacting residues on the transactivation loop exert a differential effect on transcriptional activation, we compared the promoter sequences of PmrA, PhoB and two other well-studied transcription factors, i.e. TAP (a homolog of E. coli cyclic receptor protein CAP) and EcmrR (a MerR family regulator) (Figure 5A). We excluded OmpR promoters from our analysis because OmpR binds to DNA upstream of the -35 element and interacts with the C-terminal domain of the α subunit (28). As shown in Figure 5A, it is clear that EcmrR contacts the downstream region between the -35 and -10 elements, TAP recognizes the upstream region of the -35 element, and both PmrA and PhoB bind to an intervening region. EcmrR and TAP contact different regions on the promoters and activate transcription via different mechanisms. In the class-II transcriptional activation elicited by E. coli CAP (29) or by Thermus thermophilus TAP (30), the transcription factor interacts with the RNAP β′ subunit and the σ4 domain of the σ70 cofactor to stabilize the open complex (Figure 5B). RNAPH recruitment is dominant in this mechanism, so substitution of any residues involved in RNAPH interactions inhibits transcriptional activation. In terms of transcriptional activation by the MerR family regulator, the EcmrR TAC structure (31) reveals that the RNAPH and EcmrR recognize the promoter DNA from opposite sides, without any interaction, but EcmrR binding induces significant kinks in DNA that shorten the non-optimal-35/-10 element spacers for optimal promoter recognition by the RNAPH (Figure 5C). Thus, the MerR family of regulators activate transcription by distorting DNA in an RNAPH contact-independent manner.
Figure 5.
Comparison of promoter sequences and TAC architectures. (A) Alignment of the pbgP, ugd (for PmrA), pstS (for PhoB) and promoter sequences for TAP (a T. thermophilus homolog of E. coli CAP) and EcmrR (MerR family regulator) transcription factors. The transcription factor recognition sites are marked in red and underlined. Shifted PmrA-binding sites in the PmrA TAC are highlighted with a gray background. The -10 elements are marked in bold. The structures of TAP TAC (PDB: 5I2D), EcmrR TAC (PDB: 6XL5) and PmrA TAC are shown in (B), (C) and (D), respectively. The transcription factors adopt a dimeric conformation and are colored in orange and magenta. RNAPH is colored as in Figure 1B. The DNA is colored as indicated in (A), with the -35 and -10 positions labeled.
In the PhoB-recognizing pstS promoter (19), the PhoB-binding sequence spans from positions -25 to -41, which is four base pairs upstream of the pmra-box and similar to the PmrA-binding position identified for the PmrA TAC. Hence, the RNAPH can directly contact PhoB on the promoter, in a scenario similar to the mechanism of class-II transcriptional activation with dominant RNAPH recruitment, potentially explaining why the mutants in which residues of the PhoB transactivation loop were replaced lacked transcriptional activation capability.
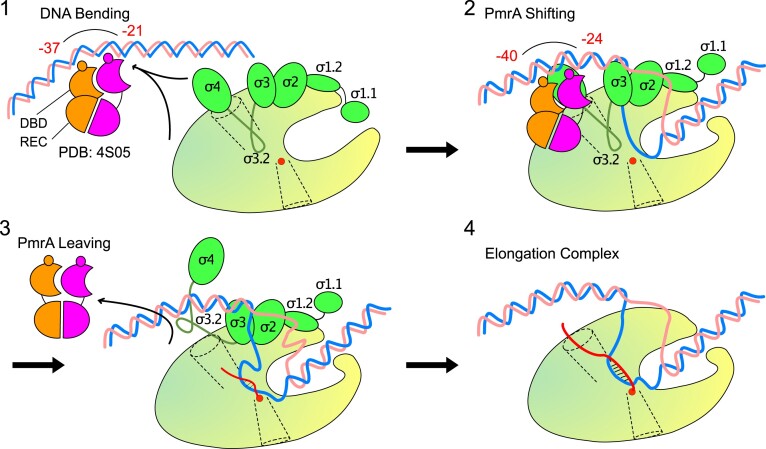
Figure 5D shows the PmrA TAC, which reveals similar yet different interactions with EcmrR TAC and TAP TAC. In Figure 6, we present a schematic illustration of the steps involved in PmrA-regulated transcriptional initiation, based on the findings from our previous and current studies. In the initial step of transcription activation of the pbgP or ugd promoters by PmrA, the BeF3–-activated PmrA-WI dimer cooperatively recognizes the imperfect repeated sequences ‘CTTAAT’ and ‘CCTAAG’ in the pmra-box (14,22). Our previous crystal structure of the PmrA–DNA complex (PDB ID 4S05) revealed that residues T187, N188, V192 and H195 establish specific contacts with the bases of the repeated ‘TAA’ sequences. In particular, N188 interacts with bases of two ‘AA’ sequences, and its substitution with alanine significantly diminishes DNA binding affinity and abolishes transcriptional activity. Binding of the PmrA-WI dimer induces DNA bending around the protein, resulting in a curvature of ∼40°. This DNA bending may destabilize the promoter structure, facilitating the formation of an open complex upon RNAPH binding.
Figure 6.
Schematic illustration of four steps in PmrA-regulated transcriptional initiation. RNAP is shown as a yellow-green oval with a main channel cleft. DNA, PmrA dimer and σ70 are colored as in Figure 1B. The catalytic Mg2+ ion is displayed as a red sphere. The nascent RNA is shown as a curvy red line. The RNA exit channel is shown as a funnel in dotted lines.
In this study (step 2 in Figure 6), we determined the PmrA TAC structure, revealing extensive interactions between RNAPH and the PmrA-WI dimer. Surprisingly, when we mutated the residues involved in RNAPH interaction (R160, E172, D182 and E184) to alanine, we observed elevated transcriptional activity (Figure 4A). Notably, mutations E172K, D182K and E184K, which repel positively charged residues on RNAPH, had the most pronounced effect on gene expression. These results suggest that RNAPH recruitment, leading to a stabilized open complex, is not essential for PmrA-mediated transcription activation.
The PmrA TAC structure revealed that the PmrA-binding sites overlap with regions recognized by RNAPH, resulting in a three base pair upstream shift of the PmrA-WI dimer from the promoter recognition sites. In our previous study (22), we observed cooperative binding of the PmrA-WI dimer to the pbgP promoter with higher affinity (Kd = 45 ± 2.3 nM) compared with individual DBD binding to the ‘CTTAAT’ sequence (Kd = 0.13 ± 0.01 μM) or the downstream ‘CCTAAG’ sequence (Kd = 9.3 ± 1.5 μM). However, this shifted DBD–DNA interaction in the TAC disrupts cooperativity and weakens the binding affinity between the DBDs and DNA, allowing PmrA to dissociate from the promoter DNA (step 3 in Figure 6).
In general transcription initiation (32,33), after the formation of the open complex, the growth of nascent RNA below 6-mer is impeded by σ3.2 in the RNA exit channel. This obstruction causes RNAP to enter abortive synthesis cycles, releasing short RNAs while remaining bound to the promoter. Only when an RNA product of sufficient length is synthesized, effectively removing the blockage caused by σ4 and σ3.2 and crossing the exit channel, can RNAP escape from the promoter and transition into the elongation state for productive RNA synthesis (step 4 in Figure 6). Our findings suggest that the dissociation of PmrA from the promoter facilitates this transition, as evidenced by the elevated transcriptional activity observed in PmrA variants with mutations in RNAPH-interacting residues. The released PmrA can subsequently rebind to the promoters to initiate the next round of transcription.
In summary, we reveal dynamic coupling among subunits of the TAC structure containing a dimer of full-length OmpR/PhoB-family response regulator PmrA-WI, an RNAPH and the pbgP promoter. The TAC structure demonstrates extensive electrostatic interactions between PmrA-WI and RNAPH, particularly involving the two DBDs and the σ4, β and β' subunits of RNAPH. However, two notable findings emerged. Firstly, PmrA variants hosting mutations of RNAPH-interacting residues, which display weaker interaction with the RNAPH, exhibited enhanced transcriptional activity. Secondly, compared with the PmrA–DNA complex structure, RNAPH binding induces a shift of both PmrA DBD domains approximately three base pairs upstream, resulting in weaker interaction with the promoter DNA. Comparative analysis with EcmrR TAC and TAP TAC structures revealed that the mechanism of PmrA-modulated transcriptional activation is more intricate than simple DNA distortion or RNAP recruitment. Based on our previous and current studies, DNA distortion induced by PmrA binding to promoters facilitates open complex formation upon RNAPH binding, and PmrA dissociation enables progression into the elongation state, both crucial for PmrA-mediated transcriptional activation.
Supplementary Material
ACKNOWLEDGEMENTS
We appreciate Dr. Meng-Ru Ho (Biophysics Instrumentation Laboratory, Institute of Biological Chemistry, Academia Sinica) for assistance with and valuable discussions on the biophysical characterization of PmrA-related complexes. The cryo-EM experiments were performed at the Academia Sinica Cryo-EM Facility (ASCEM). ASCEM is jointly supported by Academia Sinica Core Facility and Innovative Instrument Project (AS-CFII-111–210) and Taiwan Protein Project (AS-KPQ-109-TPP2).
Contributor Information
Yuan-Chao Lou, Biomedical Translation Research Center, Academia Sinica, Taipei 11529, Taiwan.
Hsuan-Yu Huang, Institute of Biological Chemistry, Academia Sinica, Taipei 11529, Taiwan.
Hsin-Hong Yeh, Institute of Biomedical Sciences, Academia Sinica, Taipei 11529, Taiwan.
Wei-Hung Chiang, Institute of Biological Chemistry, Academia Sinica, Taipei 11529, Taiwan.
Chinpan Chen, Institute of Biomedical Sciences, Academia Sinica, Taipei 11529, Taiwan.
Kuen-Phon Wu, Institute of Biological Chemistry, Academia Sinica, Taipei 11529, Taiwan; Institute of Biochemical Sciences, College of Life Science, National Taiwan University, Taipei 10617, Taiwan.
DATA AVAILABILITY
The atomic coordinates of PmrA AC and the cryo-EM map have been deposited in PDB and EMDB with accession codes 8JO2 and EMD-36453, respectively. The focused map is provided as a supporting file of the EMD-36453. Structural coordinates for the previously determined crystal structure (4S05) and cryo-EM structures (6B6H and 6CA0) are also used in the study.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
The Ministry of Science and Technology of Taiwan [(MOST) 110-2311-B-001-046 and 111-2113-M-001-026-MY2 to K.-P.W. and 108-2311-B-001-016-MY3 to C.C.]; Academia Sinica [AS-CDA-110-L03 to K.-P.W.].
Conflict of interest statement. None declared.
REFERENCES
- 1. Chen H.D., Groisman E.A.. The biology of the PmrA/PmrB two-component system: the major regulator of lipopolysaccharide modifications. Annu. Rev. Microbiol. 2013; 67:83–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mitrophanov A.Y., Jewett M.W., Hadley T.J., Groisman E.A.. Evolution and dynamics of regulatory architectures controlling polymyxin B resistance in enteric bacteria. PLoS Genet. 2008; 4:e1000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gunn J.S. The Salmonella PmrAB regulon: lipopolysaccharide modifications, antimicrobial peptide resistance and more. Trends Microbiol. 2008; 16:284–290. [DOI] [PubMed] [Google Scholar]
- 4. Marchal K., De Keersmaecker S., Monsieurs P., van Boxel N., Lemmens K., Thijs G., Vanderleyden J., De Moor B.. In silico identification and experimental validation of PmrAB targets in Salmonella typhimurium by regulatory motif detection. Genome. Biol. 2004; 5:R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng H.Y., Chen Y.F., Peng H.L.. Molecular characterization of the PhoPQ–PmrD–PmrAB mediated pathway regulating polymyxin B resistance in Klebsiella pneumoniae CG43. J. Biomed. Sci. 2010; 17:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wosten M.M., Groisman E.A.. Molecular characterization of the PmrA regulon. J. Biol. Chem. 1999; 274:27185–27190. [DOI] [PubMed] [Google Scholar]
- 7. Olaitan A.O., Morand S., Rolain J.M.. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front. Microbiol. 2014; 5:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Storm D.R., Rosenthal K.S., Swanson P.E.. Polymyxin and related peptide antibiotics. Annu. Rev. Biochem. 1977; 46:723–763. [DOI] [PubMed] [Google Scholar]
- 9. Liu Y.Y., Wang Y., Walsh T.R., Yi L.X., Zhang R., Spencer J., Doi Y., Tian G., Dong B., Huang X.et al.. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis. 2016; 16:161–168. [DOI] [PubMed] [Google Scholar]
- 10. Wang R., van Dorp L., Shaw L.P., Bradley P., Wang Q., Wang X., Jin L., Zhang Q., Liu Y., Rieux A.et al.. The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat. Commun. 2018; 9:1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kenney L.J. Structure/function relationships in OmpR and other winged-helix transcription factors. Curr. Opin. Microbiol. 2002; 5:135–141. [DOI] [PubMed] [Google Scholar]
- 12. Narayanan A., Kumar S., Evrard A.N., Paul L.N., Yernool D.A.. An asymmetric heterodomain interface stabilizes a response regulator–DNA complex. Nat. Commun. 2014; 5:3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He X., Wang L., Wang S.. Structural basis of DNA sequence recognition by the response regulator PhoP in Mycobacterium tuberculosis. Sci. Rep. 2016; 6:24442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lou Y.C., Weng T.H., Li Y.C., Kao Y.F., Lin W.F., Peng H.L., Chou S.H., Hsiao C.D., Chen C.. Structure and dynamics of polymyxin-resistance-associated response regulator PmrA in complex with promoter DNA. Nat. Commun. 2015; 6:8838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pratt L.A., Silhavy T.J.. OmpR mutants specifically defective for transcriptional activation. J. Mol. Biol. 1994; 243:579–594. [DOI] [PubMed] [Google Scholar]
- 16. Russo F.D., Slauch J.M., Silhavy T.J.. Mutations that affect separate functions of OmpR the phosphorylated regulator of porin transcription in Escherichia coli. J. Mol. Biol. 1993; 231:261–273. [DOI] [PubMed] [Google Scholar]
- 17. Makino K., Amemura M., Kim S.K., Nakata A., Shinagawa H.. Role of the sigma 70 subunit of RNA polymerase in transcriptional activation by activator protein PhoB in Escherichia coli. Genes Dev. 1993; 7:149–160. [DOI] [PubMed] [Google Scholar]
- 18. Kim S.K., Makino K., Amemura M., Nakata A., Shinagawa H.. Mutational analysis of the role of the first helix of region 4.2 of the sigma 70 subunit of Escherichia coli RNA polymerase in transcriptional activation by activator protein PhoB. Mol. Gen. Genet. 1995; 248:1–8. [DOI] [PubMed] [Google Scholar]
- 19. Makino K., Amemura M., Kawamoto T., Kimura S., Shinagawa H., Nakata A., Suzuki M.. DNA binding of PhoB and its interaction with RNA polymerase. J. Mol. Biol. 1996; 259:15–26. [DOI] [PubMed] [Google Scholar]
- 20. Blanco A.G., Canals A., Bernues J., Sola M., Coll M.. The structure of a transcription activation subcomplex reveals how σ70 is recruited to PhoB promoters. EMBO J. 2011; 30:3776–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Narayanan A., Vago F.S., Li K., Qayyum M.Z., Yernool D., Jiang W., Murakami K.S.. Cryo-EM structure of Escherichia coli σ70 RNA polymerase and promoter DNA complex revealed a role of σ non-conserved region during the open complex formation. J. Biol. Chem. 2018; 293:7367–7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lou Y.C., Wang I., Rajasekaran M., Kao Y.F., Ho M.R., Hsu S.T., Chou S.H., Wu S.H., Chen C.. Solution structure and tandem DNA recognition of the C-terminal effector domain of PmrA from Klebsiella pneumoniae. Nucleic Acids Res. 2014; 42:4080–4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Svetlov V., Artsimovitch I.. Purification of bacterial RNA polymerase: tools and protocols. Methods Mol. Biol. 2015; 1276:13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Punjani A., Zhang H., Fleet D.J.. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods. 2020; 17:1214–1221. [DOI] [PubMed] [Google Scholar]
- 25. Liebschner D., Afonine P.V., Baker M.L., Bunkoczi G., Chen V.B., Croll T.I., Hintze B., Hung L.W., Jain S., McCoy A.J.et al.. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D Struc. Biol. 2019; 75:861–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Casanal A., Lohkamp B., Emsley P.. Current developments in Coot for macromolecular model building of electron cryo-microscopy and crystallographic data. Protein Sci. 2020; 29:1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen Y., Abdel-Fattah W.R., Hulett F.M.. Residues required for Bacillus subtilis PhoP DNA binding or RNA polymerase interaction: alanine scanning of PhoP effector domain transactivation loop and alpha helix 3. J. Bacteriol. 2004; 186:1493–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Slauch J.M., Russo F.D., Silhavy T.J.. Suppressor mutations in rpoA suggest that OmpR controls transcription by direct interaction with the alpha subunit of RNA polymerase. J. Bacteriol. 1991; 173:7501–7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Busby S., Ebright R.H.. Transcription activation by catabolite activator protein (CAP). J. Mol. Biol. 1999; 293:199–213. [DOI] [PubMed] [Google Scholar]
- 30. Feng Y., Zhang Y., Ebright R.H.. Structural basis of transcription activation. Science. 2016; 352:1330–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang Y., Liu C., Zhou W., Shi W., Chen M., Zhang B., Schatz D.G., Hu Y., Liu B.. Structural visualization of transcription activated by a multidrug-sensing MerR family regulator. Nat. Commun. 2021; 12:2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mooney R.A., Darst S.A., Landick R.. Sigma and RNA polymerase: an on-again, off-again relationship?. Mol. Cell. 2005; 20:335–345. [DOI] [PubMed] [Google Scholar]
- 33. Lee J., Borukhov S.. Bacterial RNA polymerase–DNA interaction—the driving force of gene expression and the target for drug action. Front. Mol. Biosci. 2016; 3:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The atomic coordinates of PmrA AC and the cryo-EM map have been deposited in PDB and EMDB with accession codes 8JO2 and EMD-36453, respectively. The focused map is provided as a supporting file of the EMD-36453. Structural coordinates for the previously determined crystal structure (4S05) and cryo-EM structures (6B6H and 6CA0) are also used in the study.