Abstract
Chronic diseases often lead to metabolic disorders, causing anabolic resistance and increased energy consumption, which result in cachexia. Cachexia, in turn, can lead to major clinical consequences such as impaired quality of life, shortened life expectancy, and increased healthcare expenditure. Existing international diagnostic criteria for cachexia employ thresholds derived from Western populations, which may not apply to Asians due to differing body compositions. To address this issue, the Asian Working Group for Cachexia (AWGC) was initiated. The AWGC comprises experts in cachexia research and clinical practice from various Asian countries and aims to develop a consensus on diagnostic criteria and significant clinical outcomes for cachexia in Asia. The AWGC, composed of experts in cachexia research and clinical practice from several Asian countries, undertook three‐round Delphi surveys and five meetings to reach a consensus. Discussions were held on etiological diseases, essential diagnostic items for cachexia, including subjective and objective symptoms and biomarkers, and significant clinical outcomes. The consensus highlighted the importance of multiple diagnostic factors for cachexia, including chronic diseases, either or both weight loss or low body mass index, and at least one of the following: anorexia, decreased grip strength (<28 kg in men and <18 kg in women), or elevated C‐reactive protein levels (>5 mg/L [0.5 mg/dL]). The AWGC proposed a significant weight change of 2% or more over a 3–6 month period and suggested a tentative cut‐off value of 21 kg/m2 for low body mass index in diagnosing cachexia. Critical clinical outcomes were determined to be mortality, quality of life as assessed by tools such as EQ‐5D or the Functional Assessment of Anorexia/Cachexia Therapy, and functional status as measured by the Clinical Frailty Scale or Barthel Index, with significant emphasis on patient‐reported outcomes. The AWGC consensus offers a comprehensive definition and user‐friendly diagnostic criteria for cachexia, tailored specifically for Asian populations. This consensus is set to stimulate future research and enhance the multidisciplinary approach to managing cachexia. With plans to develop further guidelines for the optimal treatment, prevention, and care of cachexia in Asians, the AWGC criteria are expected to drive research across chronic co‐morbidities and cancer in Asia, leading to future refinement of diagnostic criteria.
Keywords: Asian, Cachexia, Diagnostic criteria, Ethnicity, Expert opinion, Position paper
Introduction
Patients with chronic diseases are at risk of disease‐related undernutrition. Systemic inflammation initiates anabolic resistance and increased energy expenditure. Because protein biosynthesis requires an abundance of energy, 1 the systemic metabolic derangement that underpins inflammation associated with chronic diseases results in gradual loss of skeletal muscle. This metabolic derangement, involving loss of body protein as the dominant pathogenesis, is called cachexia. In Japan, a condition presumed to be cachexia has been known since ancient times as Kyoro (in Japanese) and Limpness. 2 Cachexia is modified by anorexia and psychological changes related to the treatment, which may exacerbate malnutrition. Patients with chronic diseases such as cancer, chronic heart failure, chronic obstructive pulmonary disease (COPD), connective tissue disease, and chronic kidney disease (CKD) experience overwhelming metabolic and nutritional disorders in addition to the direct life‐threatening effects of the diseases themselves. In addition to the treatment of the underlying diseases, metabolic and nutritional issues are important aspects of chronic inflammatory diseases that need attention.
Cachexia is a serious but under‐recognized pathology in Asia. A survey involving healthcare professionals affiliated with a nutrition society conducted by Nakahara et al. reported that only 17.4% of patients were assessed for cachexia in real‐world clinical practice. 3 Nevertheless, the prevalence of cachexia in Western countries was approximately 15% in patients with severe heart failure, 4 5% in COPD, 5 30% in rheumatoid arthritis, 6 and 30% in patients with cancer. 7 A national survey in the United States reported that cachexia was associated with increased hospitalization costs and duration of stay. 8 Although there is currently no curative treatment for cachexia, the American Society of Clinical Oncology and European Society for Medical Oncology guidelines for treating cancer cachexia recommended nutritional therapy, caregiver advice, exercise training, psychotherapy, and some pharmacotherapy. 9 , 10 In addition, anamorelin can be prescribed in Japan for cachexia caused by some cancers and is covered by health insurance. 11 Therefore, prompt identification and evaluation of cachexia are indispensable. 12
International consensus criteria for the diagnosis of cachexia include the widely cited classifications by Evans and Fearon. 13 , 14 However, these may not apply to Asian patients with different body habitus and ethnic backgrounds. Konishi et al. concluded that existing international criteria, which focus on body composition and muscle mass loss, may underestimate cachexia in Asians because their physique differs from that of their Western counterparts. 15 Indeed, a study of predominantly Caucasian participants found that patients with advanced cancer had an average BMI of 25 kg/m2 16 , 17 and patients with COPD around 27 kg/m2, 18 while a BMI of <20.7 kg/m2 was reported in only 13.6% of patients with heart failure. 19 Asians have a lower BMI on average and a differential contribution of BMI to chronic diseases compared with Caucasians. 20 An international diagnostic standard for undernutrition, the Global Leadership Initiative on Malnutrition criteria, includes separate BMI cut‐off values for Asians. 21 , 22 The Asian Working Group for Sarcopenia (AWGS) also proposed different cut‐off values of muscle mass and muscle functions that are applicable to Asian countries. 23 , 24 With an increasing population of older adults in Asian countries, the number of patients with cachexia is also expected to increase. Hence, future clinical research and practice using optimal cachexia diagnostic criteria for Asian populations should be promoted.
This provides the impetus for the Asian Working Group for Cachexia (AWGC), which was formed with the cardinal aim of improving the research and clinical practice of cachexia in Asia. In addition to some members from the AWGS, the AWGC invited leaders in the field of cachexia research and clinical practice from Asian countries. The current article is the first consensus report proposed by the AWGC on cachexia diagnostic criteria and clinical outcomes for Asians to facilitate further cachexia research and clinical practice.
Consensus building
The first AWGC meeting was held on 5 February 2021, in a web conference format with 33 participants, and involved the presentation of the results of a systematic review of studies on cachexia in Asians from 2010 to 2020 using the PubMed interface to search the MEDLINE database. Keywords used in the literature search were cachexia, cachectic, and disease‐related malnutrition. Discussion and the necessary deliberations centred on whether cachexia diagnostic criteria for Asians should be the same as those used in Caucasian populations and on the challenges in adapting recent cachexia diagnostic criteria for Asians. The members committed to publishing a consensus statement from AWGC, which will cover the definition and diagnosis of cachexia in Asians, clinical outcomes to be considered, and factors that will contribute to the understanding of the actual status of cachexia in Asians and improving outcomes. A second meeting was held on 28 April in a web conference format (34 members) to deepen the discussion and build consensus on cachexia diagnostic criteria and outcome measures for Asians using the Delphi method.
Before initiating the Delphi rounds, we collected additional candidate aetiologies, underlying disease of cachexia, and cachexia diagnostic items in four categories: (1) subjective symptoms, (2) objective indicators, (3) biomarkers, and (4) others. Further outcome ideas were collected. The first Delphi round was conducted in May to June 2021. Participants were asked to rate their agreement on a 10‐point Likert scale (10 being the highest agreement) for the items presented. A consensus was defined when more than 70% of the panel members scored 7 points or higher. The Japanese team led by Arai H. was responsible for collating responses and reviewing the questions for the next round. The second Delphi round was conducted in June 2021. The third Delphi round, conducted from August to September 2021, examined the level of agreement on the proposed aetiology and diagnostic criteria. Another web conference was held on 11 October 2021 to discuss the final diagnosis. We presented the complete perspective in that meeting and exchanged opinions on the process. The aggregated opinions were finally reiterated, and final approval was obtained at a hybrid face‐to‐face and online meeting on 28 October 2022 and online meeting on 1 March 2023 (Figure 1).
Figure 1.
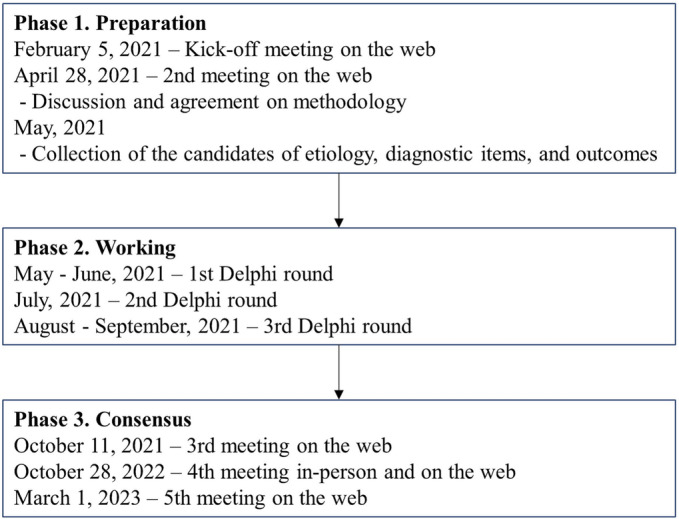
Schematic overview of the trajectory for proposing the consensus statement.
Clinical studies of cachexia in Asians
A MEDLINE search identified 13 intervention studies including 11 randomized controlled trials in Asia. 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 Approximately half (six reports) of the intervention studies included Japanese patients with cachexia. Five studies diagnosed cachexia using Fearon's criteria including three that used its modified criteria, and another five adopted Evans's criteria including two using its modified criteria. However, the other three studies used other methods to identify patients with cachexia. Additionally, 47 studies reporting the prevalence of cachexia 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 were also identified, most of which were studied in Japan (26 articles). Twenty‐two articles used the Fearon criteria, including 13 articles using its modified criteria, one article used the Evans criteria, and the majority used other criteria (24 articles). Forty‐two (89%) of the studies included patients with cancer.
Figure 2 shows a scatter plot developed from the studies that reported the BMI of the participants using three factors: sample size, mean or median age, and BMI. The mean BMI of the participants of the studies that investigated the prevalence of cachexia in Asians was around 22 kg/m2. While there may be variations across different Asian countries, due to the limitations of the studies included in our review, we were not able to comprehensively analyse the differences in average weight or BMI in patients across Asian countries. In addition, most of the intervention studies in Asian patients with cachexia involved BMI < 20 kg/m2. Most studies were conducted on older participants of age >60 years. The prevalence of cachexia in studies that diagnosed cachexia based on the rate of weight loss ranged from 42.9% to 56.4%, including six articles on patients with cancer, 40 , 42 , 66 , 68 , 81 , 82 and one study involving patients with heart failure. Many studies used BMI and weight loss rate for defining cachexia.
Figure 2.
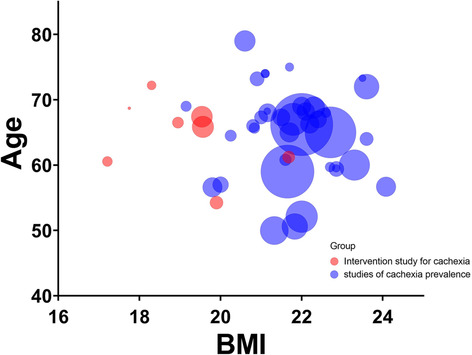
Scatter plot presenting body mass index, age, and sample size in cachexia study of Asians.
At the AWGC inaugural meeting, we agreed that the optimal cachexia diagnostic criteria for Asians might not be the same as that for non‐Asians regarding weight loss or BMI. There was also concern about the paucity of cachexia studies in Asians apart from patients with cancer. Despite the growing trend to separate non‐cancer and cancer illnesses in modern guidelines, we have chosen to incorporate both into our diagnostic criteria. This decision was made due to the overlapping nature of the metabolic imbalances and weight loss associated with cachexia in these conditions. Moreover, we believe that this comprehensive approach will provide a more inclusive understanding and treatment strategy for cachexia, addressing the syndrome's manifestations across a wide range of chronic illnesses, including cancer. We anticipate that this approach will enhance the recognition and management of cachexia in clinical settings. AWGC concluded that advocating cachexia diagnostic criteria that are easy to use and applicable to clinical practice may facilitate cachexia research in Asians with chronic diseases and cancer.
Etiologic factors of cachexia
In the first Delphi round, >70% of participants scored ≥7 points for non‐radical cancer, congestive heart failure, COPD, chronic renal failure, rheumatoid arthritis, acquired immunodeficiency syndrome, chronic respiratory failure including tuberculosis and idiopathic pulmonary fibrosis, and chronic liver failure to be included in cachexia target disease. For organizing the information, we included ‘autoimmune disease’ for rheumatoid arthritis and ‘uncontrolled chronic infectious disease’ for acquired immunodeficiency syndrome, and we were also exploring adjectives other than ‘uncontrolled’. We also conducted another round to solicit ideas for adjectives other than ‘uncontrolled’.
In the second Delphi round, all items were agreed upon, but there were many suggestions to reframe the term ‘autoimmune diseases’ as it includes many diseases that do not lead to cachexia. The words ‘progressive worsening’, ‘uncontrolled’, and ‘treatment resistant’ required consensus. Additionally, there were no adjectives suggested describing ‘chronic infections’. The third round investigated which adjective would be most appropriate. In response to these suggestions, the term was replaced with ‘collagen disease,’ and the idea was voted on again in the following round.
In the third round, a consensus was reached for ‘collagen disease’ and ‘chronic infections’. The use of adjectives like ‘progressive worsening’ and ‘uncontrolled’ for chronic infectious diseases received many votes. Subsequently, at the meeting held after three rounds of Delphi, a final consensus on the proposed aetiology was reached (Table 1).
Table 1.
Underlying diseases that cause cachexia
| Underlying diseases/aetiology |
|---|
| Cancer |
| Chronic heart failure |
| COPD |
| Chronic kidney disease |
| Rheumatoid arthritis |
| Other collagen diseases |
| Chronic respiratory failure |
| Chronic liver failure |
| Progressive worsening or uncontrolled chronic infections |
Abbreviation: COPD, chronic obstructive pulmonary disease.
Diagnosis of cachexia
Ideas for prospective items for new diagnostic criteria were collected in four categories: subjective symptoms, objective measurements, biomarkers, and other parameters. Items that were scored 7–10 points by more than 70% of respondents in the first Delphi round were (1) subjective symptoms: anorexia, fatigue, exhaustion, and weakness; (2) objective measurements: low BMI, weight loss, weight loss grading system, BMI‐adjusted weight loss, loss of skeletal muscle mass, low fat‐free mass, grip strength, low muscle strength, physical performance, and weakness; and (3) biomarkers: except for C‐reactive protein (CRP), no other biomarkers were agreed upon. While exhaustion was also a highly rated symptom, we chose to prioritize fatigue in our diagnostic criteria given its prevalence in the early stages of cachexia, which aligns with our goal of early detection in a clinical setting.
In the second round, AWGC evaluated the importance of items to be included in the new diagnostic criteria and carefully considered them from the perspective of items that could be easy to evaluate and apply clinically. In the third round, we investigated the level of agreement on the proposed combination of specific diagnostic items, and a web conference was held after the three rounds of Delphi, and we could complete the draft of a framework of the diagnostic criteria and tried to build consensus among AWGC members. Through the 3rd online, 4th hybrid (in‐person and online), and 5th online meetings, the diagnostic criteria finally reached an agreement.
The essential conditions were the presence of underlying specific diseases for cachexia and the presence of weight loss or low BMI. In addition, any of the following items are required: the presence of anorexia as a subjective symptom, decreased grip strength as an objective measurement, and elevated CRP level as a biomarker (Table 2).
Table 2.
Diagnostic criteria for cachexia in Asian patients
| (1) Presence of underlying disease |
| (2) Weight loss >2%/3–6 months or low BMI (<21 kg/m2) |
| One or more of the following: |
| (1) Subjective symptom: Anorexia |
| (2) Objective measurement: Decreased grip strength a |
| (3) Biomarker: Elevated CRP b |
Oedema, fluid retention, and imbalanced body water should be adjusted or be taken into account in assessing weight loss and low BMI.
BMI, body mass index; CRP, C‐reactive protein.
<28 kg in men and <18 kg in women.
>5 mg/L (0.5 mg/dL).
Weight loss and low BMI might reflect loss of muscle mass. AWGC discussed whether weight loss or muscle mass loss could only explain the phenotype of cachexia in Asians. There were discussions regarding a risk assessment method using a contingency table formed by two factors, weight loss and BMI with three or more categories, which is similar to the table from Martin et al. 85 A member introduced the results from the Ishida et al. study in patients with CKD. 86 Their study reported that the hazard ratio of >1 for mortality was found in all patients with BMI < 18.5 kg/m2, patients with BMI 18.5–22 kg/m2 with no weight change or weight loss during 3–6 months, and patients with BMI 22–25 kg/m2 who showed weight loss (Figure 3). 86 Concerning weight loss, using the same dataset, a member of AWGC examined the cut‐off values for determining the presence or absence of weight change and found a similar trend for conditions with hazard ratios of >1, even assuming threshold values of 1%, 2%, and 5% (Figure 3). Similar trend between weight loss and increased mortality risk was observed in the study involving >40 000 inpatients with heart failure in Japan. 87 Considering that a 1% weight gain or loss for a typical Asian weighing 50 kg is 500 g, which may be within the range of diurnal variation, AWGC proposes that a weight gain or loss of 2% or more over a 3–6 month period would be considered a significant weight gain or loss.
Figure 3.
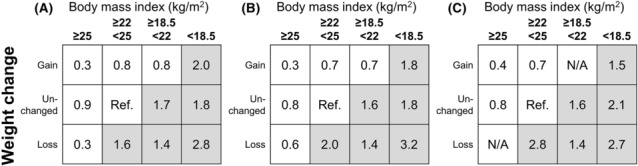
Weight change‐body mass index (BMI) contingency tables. Hazard ratios (HRs) for mortality were obtained from the dataset of the Ishida et al. study. The unchanged weight is defined by ±1% change (A), ±2% change (B), and ±5% change (C). Greyed cells are considered at high risk of mortality. N/A: not applicable.
Additionally, given patients with a low BMI were at high risk of mortality, AWGC proposes that a low BMI would also be considered as a determinant of an essential item. We conducted preliminary analyses of low BMI thresholds using an anonymized commercial claims database for more than 40 000 patients with Stage III or IV advanced cancer and more than 10 000 patients with Grade 3 or more advanced CKD. The smaller BMI values increased the hazard ratio for mortality. BMI of 22 kg/m2 or 20 kg/m2 were discussed as candidates for a threshold for mortality risk. This diagnostic criterion should not be used to diagnose cachexia by BMI alone but should be meaningful as one of the diagnostic items. Therefore, it was agreed that BMI of 21 kg/m2 should be the tentative cut‐off value. The cut‐off value for BMI would be modified by the results of future studies on Asians.
In anorexia, we recommend assessing the patient's subjective complaints of appetite with or without reduced food intake. In general, anorexia and reduced food intake are likely to coincide. However, we are concerned that some patients may be unaware of their anorexia and that some healthcare professionals may evaluate anorexia solely based on reduced food intake. AWGC considers anorexia at risk for cachexia when it is identified or when a screening tool identifies risk. Screening tools for anorexia include the Simplified Nutritional Appetite Questionnaire 88 or other validated tools.
Grip strength measurement is a quick and simple test that can be performed in any outpatient or inpatient setting. It is an objective test that reflects skeletal muscle function and indirectly indicates skeletal muscle mass. It can be generalized to the assessment of nutritional disorders, which demonstrates its applicability to clinical practice in the Asian population. While evaluation of sarcopenia or low skeletal muscle mass is important, practical limitations might hinder its application in clinical practice. Therefore, we propose the use of grip strength measurement as a proxy for muscle function. We recommend using the AWGS 2019 cut‐off values for decreased grip strength in diagnosing cachexia in the Asian population, namely, <28 kg in men and <18 kg in women. 23 The AWGS 2019 criteria advocate for specific postures during handgrip strength measurements, recommending standing with full elbow extension when using the Smedley dynamometer and sitting with 90° elbow flexion when using the Jamar dynamometer. 23 A sitting posture is preferred if individuals are unable to stand unassisted. We adhered to the AWGS 2019 recommendation, using handgrip strength as a measure of muscular strength and employing either the spring‐type (Smedley) or hydraulic‐type (Jamar) dynamometers. The handgrip strength measurement protocol, as suggested by AWGS 2019, 23 involves taking the maximum reading from at least two trials using either both hands or the dominant hand in a maximum‐effort isometric contraction, rather than using a fixed acquisition time. In developing the diagnostic criteria, the AWGC made a deliberate choice not to include reduced muscle mass directly due to practical constraints in routine clinical settings across Asia, such as the availability and cost of sophisticated body composition measurement techniques such as dual‐energy X‐ray absorptiometry and bioelectrical impedance analysis. However, we fully recognize the relevance of sarcopenia, a condition that often co‐exists with cachexia and significantly impacts the quality of life (QOL) and clinical outcomes for these patients. The inclusion of decreased grip strength as one of the diagnostic criteria is a reflection of this recognition. Decreased grip strength is a key indicator of sarcopenia and may serve as an indirect measure of changes in body composition.
Research is also necessary for the optimization of the cut‐off for elevated CRP and other potential biomarkers such as albumin in Asians. Studies suggest that elevated CRP levels that are associated with prognosis in chronic disease are likely to use cut‐offs of 10 mg/L (1.0 mg/dL), 5 mg/L (0.5 mg/dL), or 3 mg/L (0.3 mg/dL). AWGC first advocates that a hypothetical rise of 5 mg/L (0.5 mg/dL) or more be regarded as an abnormal value for diagnosing cachexia as described in Evans's classification. Moreover, we acknowledge the comprehensive approach of the Glasgow Prognostic Score, including both CRP and albumin, which has proven its significance in several studies. 89 , 90 , 91 , 92
Definition of cachexia
Consistent with the new cachexia diagnostic criteria derived from AWGC, cachexia is defined as ‘a metabolic imbalance related to chronic diseases that are associated with weight loss, inflammatory conditions, and/or anorexia’. While some diseases such as cancer are more likely to manifest progressive weight loss, others may not necessarily show progressive weight loss in Asians who tend to have a lower BMI and to have a slower disease progression, or in conditions that present with significant fluid retention. In addition, inflammation may not always be identified by blood tests, and the subjective sensation of anorexia may not be present in some patients despite decreased food intake. In light of the heterogeneity of disease symptomatology and manifestation, the workgroup agreed that the concept of having any condition(s) that is associated with cachexia would be better for explaining this complex syndrome. A simplified definition of cachexia would also facilitate the dissemination of cachexia assessment to clinical practice and promote research.
Clinical outcome of cachexia
Clinical outcome measures that scored 7–10 points with over 70% responses in the first Delphi round were mortality, QOL, activities of daily living (ADL), mobility impairment, performance status, the feeling of well‐being, the trajectory of measurement, and hospitalization. Among these, mortality, QOL, and ADL, which received exceptionally high ratings, were investigated again in the second round. In the third round, the importance of the EQ‐5D, the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC‐QLQ)‐C30, EORTC‐QOL‐CAX24, and the Functional Assessment of Anorexia/Cachexia Therapy, which are frequently reported in cachexia studies and have been validated in multiple languages, including several Asian languages, was applied in the context of specific indicators of QOL. Regarding specific measures of ADL, the importance of the Eastern Co‐operative Oncology Group Performance Status, Clinical Frailty Scale, Barthel Index, Katz Index, and Lawton IADL scale were also studied.
After three rounds of Delphi, AWGC finally concluded that the crucial outcomes for Asian patients with cachexia were mortality, QOL, and functional status (Table 3). Functional status was determined by the Clinical Frailty Scale, a measure of frailty; the Barthel Index, a measure of activity or degree of physical function; and the Katz Index and other indicators, including the Lawton scale, 6‐min walking distance, were regarded as important indicators. The discussion after the Delphi rounds highlighted and emphasized the importance of measurement feasibility that might depend on patients' backgrounds and the circumstances of clinicians and investigators. The outcomes related to QOL and functional status can be measured using other indicators including other tools of EORTC questionnaires, ADL tools, and physical performance tests.
Table 3.
Clinical outcomes for cachexia in Asian patients
| Mortality |
| Quality of life |
| EQ‐5D |
| FAACT |
| Others (e.g., EORTC‐QLQ‐C30) |
| Functional status |
| Clinical Frailty Scale |
| Barthel Index |
| Others (e.g., Katz Index, Lawton IADL scale, and 6MWD) |
6MWD, six‐minute walking distance; EORTC‐QLQ, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; FAACT, Functional Assessment of Anorexia/Cachexia Therapy; IADL, instrumental activities of daily living.
Assessments made by healthcare providers may underestimate patients' symptoms and distress. 93 Patient‐reported outcomes, such as those represented by the QOL indicators, should be considered essential and compelling outcomes, especially in severe conditions that are difficult to treat, such as cachexia. Assessing the QOL indicators that reflect the symptoms and feelings of patients creates an opportunity for individualized treatment and care, which directly addresses their needs. In addition, improved function in life is closely related to improved QOL; assessing ADLs and objective measures of physical function can provide insight into life functions that are being impaired.
Conclusions and expectations
To facilitate cachexia research and clinical practice in Asians, the AWGC presents a new consensus report on diagnostic criteria and important clinical outcomes for cachexia in Asia. AWGC also established a definition of cachexia that incorporates innovations to facilitate clinical development. We hope that future epidemiological and intervention studies in Asia will be developed based on the proposed diagnostic criteria and outcomes, spanning cachexia studies in the areas of chronic co‐morbidities and cancer. In clinical practice, we believe the diagnostic criteria will improve case detection of cachexia, which in turn will pave the way for the implementation of a comprehensive multidisciplinary approach to cachexia, including nutritional therapy, exercise therapy, psychotherapy, and pharmacotherapy.
In summary, the consensus report on diagnostic criteria and its ease of clinical application will increase the feasibility of conducting future intervention studies in Asia. The AWGC will develop consensus or guidelines for optimal cachexia treatment/prevention and care for Asians in the future. The AWGC criteria are expected to stimulate further cachexia research in relevant clinical settings in Asia, which will provide key information for future refinement and revisitation of the criteria. We plan to revisit and update this consensus as new evidence emerges, especially pertaining to epidemiological and intervention studies in Asia.
Conflict of interest
TN reports lecture fees from Ono Pharmaceutical and institutional research funds from Otsuka Pharmaceutical and Kracie Holdings, Ltd., in relation to this work. The other authors declare that they have no conflict of interest.
Funding
The article was supported by the Japan Society for the Promotion of Science (grant number: 21H03390 to Maeda and 22K11346 to Saitoh) and the Research Funding of Longevity Sciences (grant number: 22‐4 to Maeda and Arai).
Acknowledgements
The authors of this paper certify that they have complied with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle. 94
Arai H., Maeda K., Wakabayashi H., Naito T., Konishi M., Assantachai P., et al (2023) Diagnosis and outcomes of cachexia in Asia: Working Consensus Report from the Asian Working Group for Cachexia, Journal of Cachexia, Sarcopenia and Muscle, 14, 1949–1958, 10.1002/jcsm.13323
Contributor Information
Hidenori Arai, Email: harai@ncgg.go.jp.
Liang‐Kung Chen, Email: lkchen2@vghtpe.gov.tw.
References
- 1. Karr JR, Sanghvi JC, Macklin DN, Gutschow MV, Jacobs JM, Bolival B Jr, et al. A whole‐cell computational model predicts phenotype from genotype. Cell 2012;150:389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Naito T. Evaluation of the true endpoint of clinical trials for cancer cachexia. Asia Pac J Oncol Nurs 2019;6:227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nakahara S, Wakabayashi H, Maeda K, Nishioka S, Kokura Y. Sarcopenia and cachexia evaluation in different healthcare settings: a questionnaire survey of health professionals. Asia Pac J Clin Nutr 2018;27:167–175. [DOI] [PubMed] [Google Scholar]
- 4. Carson MA, Reid J, Hill L, Dixon L, Donnelly P, Slater P, et al. Exploring the prevalence, impact and experience of cardiac cachexia in patients with advanced heart failure and their caregivers: a sequential phased study. Palliat Med 2022;36:1118–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McDonald MN, Wouters EFM, Rutten E, Casaburi R, Rennard SI, Lomas DA, et al. It's more than low BMI: prevalence of cachexia and associated mortality in COPD. Respir Res 2019;20:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Santo RCE, Fernandes KZ, Lora PS, Filippin LI, Xavier RM. Prevalence of rheumatoid cachexia in rheumatoid arthritis: a systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle 2018;9:816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anker MS, Holcomb R, Muscaritoli M, von Haehling S, Haverkamp W, Jatoi A, et al. Orphan disease status of cancer cachexia in the USA and in the European Union: a systematic review. J Cachexia Sarcopenia Muscle 2019;10:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arthur ST, Noone JM, Van Doren BA, Roy D, Blanchette CM. One‐year prevalence, comorbidities and cost of cachexia‐related inpatient admissions in the USA. Drugs Context 2014;3:212265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roeland EJ, Bohlke K, Baracos VE, Bruera E, del Fabbro E, Dixon S, et al. Management of cancer cachexia: ASCO guideline. J Clin Oncol 2020;38:2438–2453. [DOI] [PubMed] [Google Scholar]
- 10. Arends J, Strasser F, Gonella S, Solheim TS, Madeddu C, Ravasco P, et al. Cancer cachexia in adult patients: ESMO clinical practice guidelines(). ESMO Open 2021;6:100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wakabayashi H, Arai H, Inui A. The regulatory approval of anamorelin for treatment of cachexia in patients with non‐small cell lung cancer, gastric cancer, pancreatic cancer, and colorectal cancer in Japan: facts and numbers. J Cachexia Sarcopenia Muscle 2021;12:14–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baracos VE, Coats AJ, Anker SD, Sherman L, Klompenhouwer T, International Advisory B , et al. Identification and management of cancer cachexia in patients: assessment of healthcare providers' knowledge and practice gaps. J Cachexia Sarcopenia Muscle 2022;13:2683–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr 2008;27:793–799. [DOI] [PubMed] [Google Scholar]
- 14. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 15. Konishi M, Ishida J, Springer J, Anker SD, von Haehling S. Cachexia research in Japan: facts and numbers on prevalence, incidence and clinical impact. J Cachexia Sarcopenia Muscle 2016;7:515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ozola Zalite I, Zykus R, Francisco Gonzalez M, Saygili F, Pukitis A, Gaujoux S, et al. Influence of cachexia and sarcopenia on survival in pancreatic ductal adenocarcinoma: a systematic review. Pancreatology 2015;15:19–24. [DOI] [PubMed] [Google Scholar]
- 17. Ahmed O, Bolger JC, O'Neill B, Robb WB. Use of esophageal stents to relieve dysphagia during neoadjuvant therapy prior to esophageal resection: a systematic review. Dis Esophagus 2020;33:doz090. [DOI] [PubMed] [Google Scholar]
- 18. Wilson AC, Kumar PL, Lee S, Parker MM, Arora I, Morrow JD, et al. Heme metabolism genes downregulated in COPD cachexia. Respir Res 2020;21:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol 2001;38:789–795. [DOI] [PubMed] [Google Scholar]
- 20. Katz EG, Stevens J, Truesdale KP, Cai J, North KE, Steffen LM. Associations of body mass index with incident hypertension in American white, American black and Chinese Asian adults in early and middle adulthood: the Coronary Artery Risk Development in Young Adults (CARDIA) study, the Atherosclerosis Risk in Communities (ARIC) study and the People's Republic of China (PRC) study. Asia Pac J Clin Nutr 2013;22:626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maeda K, Ishida Y, Nonogaki T, Mori N. Reference body mass index values and the prevalence of malnutrition according to the Global Leadership Initiative on Malnutrition criteria. Clin Nutr 2020;39:180–184. [DOI] [PubMed] [Google Scholar]
- 22. Cederholm T, Jensen GL, Correia M, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition ‐ a consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle 2019;10:207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020;21:e2. [DOI] [PubMed] [Google Scholar]
- 24. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95–101. [DOI] [PubMed] [Google Scholar]
- 25. Yeh KY, Wang HM, Chang JW, Huang JS, Lai CH, Lan YJ, et al. Omega‐3 fatty acid‐, micronutrient‐, and probiotic‐enriched nutrition helps body weight stabilization in head and neck cancer cachexia. Oral Surg Oral Med Oral Pathol Oral Radiol 2013;116:41–48. [DOI] [PubMed] [Google Scholar]
- 26. Xie M, Chen X, Qin S, Bao Y, Bu K, Lu Y. Clinical study on thalidomide combined with cinobufagin to treat lung cancer cachexia. J Cancer Res Ther 2018;14:226–232. [DOI] [PubMed] [Google Scholar]
- 27. Wen HS, Li X, Cao YZ, Zhang CC, Yang F, Shi YM, et al. Clinical studies on the treatment of cancer cachexia with megestrol acetate plus thalidomide. Chemotherapy 2012;58:461–467. [DOI] [PubMed] [Google Scholar]
- 28. Takayama K, Katakami N, Yokoyama T, Atagi S, Yoshimori K, Kagamu H, et al. Anamorelin (ONO‐7643) in Japanese patients with non‐small cell lung cancer and cachexia: results of a randomized phase 2 trial. Support Care Cancer 2016;24:3495–3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miki K, Maekura R, Nagaya N, Nakazato M, Kimura H, Murakami S, et al. Ghrelin treatment of cachectic patients with chronic obstructive pulmonary disease: a multicenter, randomized, double‐blind, placebo‐controlled trial. PLoS ONE 2012;7:e35708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matsumoto N, Miki K, Tsubouchi H, Sakamoto A, Arimura Y, Yanagi S, et al. Ghrelin administration for chronic respiratory failure: a randomized dose‐comparison trial. Lung 2015;193:239–247. [DOI] [PubMed] [Google Scholar]
- 31. Li W, Han L, Yu P, Ma C, Wu X, Moore JE, et al. Molecular characterization of skin microbiota between cancer cachexia patients and healthy volunteers. Microb Ecol 2014;67:679–689. [DOI] [PubMed] [Google Scholar]
- 32. Kouchaki B, Janbabai G, Alipour A, Ala S, Borhani S, Salehifar E. Randomized double‐blind clinical trial of combined treatment with megestrol acetate plus celecoxib versus megestrol acetate alone in cachexia‐anorexia syndrome induced by GI cancers. Support Care Cancer 2018;26:2479–2489. [DOI] [PubMed] [Google Scholar]
- 33. Katakami N, Uchino J, Yokoyama T, Naito T, Kondo M, Yamada K, et al. Anamorelin (ONO‐7643) for the treatment of patients with non‐small cell lung cancer and cachexia: results from a randomized, double‐blind, placebo‐controlled, multicenter study of Japanese patients (ONO‐7643‐04). Cancer 2018;124:606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kapoor N, Naufahu J, Tewfik S, Bhatnagar S, Garg R, Tewfik I. A prospective randomized controlled trial to study the impact of a nutrition‐sensitive intervention on adult women with cancer cachexia undergoing palliative care in India. Integr Cancer Ther 2017;16:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hanai N, Terada H, Hirakawa H, Suzuki H, Nishikawa D, Beppu S, et al. Prospective randomized investigation implementing immunonutritional therapy using a nutritional supplement with a high blend ratio of omega‐3 fatty acids during the perioperative period for head and neck carcinomas. Jpn J Clin Oncol 2018;48:356–361. [DOI] [PubMed] [Google Scholar]
- 36. Hamauchi S, Furuse J, Takano T, Munemoto Y, Furuya K, Baba H, et al. A multicenter, open‐label, single‐arm study of anamorelin (ONO‐7643) in advanced gastrointestinal cancer patients with cancer cachexia. Cancer 2019;125:4294–4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dehghani M, Mirzaie M, Farhadi P, Rezvani A. The effect of ACE inhibitor on the quality of life amongst patients with cancer cachexia. Asian Pac J Cancer Prev 2020;21:325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou T, Yang K, Thapa S, Liu H, Wang B, Yu S. Differences in symptom burden among cancer patients with different stages of cachexia. J Pain Symptom Manage 2017;53:919–926. [DOI] [PubMed] [Google Scholar]
- 39. Zhou T, Wang B, Liu H, Yang K, Thapa S, Zhang H, et al. Development and validation of a clinically applicable score to classify cachexia stages in advanced cancer patients. J Cachexia Sarcopenia Muscle 2018;9:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang Y, Wang J, Wang X, Gao T, Tian H, Zhou D, et al. The autophagic‐lysosomal and ubiquitin proteasome systems are simultaneously activated in the skeletal muscle of gastric cancer patients with cachexia. Am J Clin Nutr 2020;111:570–579. [DOI] [PubMed] [Google Scholar]
- 41. Yoshikawa N, Naito T, Yagi T, Kawakami J. Impact of cachexia and opioid analgesic cotreatment on pregabalin pharmacokinetics and central nervous system symptoms in cancer patients. Ther Drug Monit 2019;41:591–597. [DOI] [PubMed] [Google Scholar]
- 42. Wu J, Huang C, Xiao H, Tang Q, Cai W. Weight loss and resting energy expenditure in male patients with newly diagnosed esophageal cancer. Nutrition 2013;29:1310–1314. [DOI] [PubMed] [Google Scholar]
- 43. Wakabayashi H, Sashika H. Malnutrition is associated with poor rehabilitation outcome in elderly inpatients with hospital‐associated deconditioning a prospective cohort study. J Rehabil Med 2014;46:277–282. [DOI] [PubMed] [Google Scholar]
- 44. Sun L, Quan XQ, Yu S. An epidemiological survey of cachexia in advanced cancer patients and analysis on its diagnostic and treatment status. Nutr Cancer 2015;67:1056–1062. [DOI] [PubMed] [Google Scholar]
- 45. Sun F, Sun Y, Zhang D, Zhang J, Song B, Zheng H. Association of interleukin‐10 gene polymorphism with cachexia in Chinese patients with gastric cancer. Ann Clin Lab Sci 2010;40:149–155. [PubMed] [Google Scholar]
- 46. Sun F, Sun Y, Yu Z, Zhang D, Zhang J, Song B, et al. Interleukin‐10 gene polymorphisms influence susceptibility to cachexia in patients with low‐third gastric cancer in a Chinese population. Mol Diagn Ther 2010;14:95–100. [DOI] [PubMed] [Google Scholar]
- 47. Seng D, Wu J, Fang Q, Liu F. Prognosis of osteosarcomas in the mandible: 15‐year experience of 55 patients. Medicine (Baltimore) 2019;98:e13875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sato H, Naito T, Ishida T, Kawakami J. Relationships between oxycodone pharmacokinetics, central symptoms, and serum interleukin‐6 in cachectic cancer patients. Eur J Clin Pharmacol 2016;72:1463–1470. [DOI] [PubMed] [Google Scholar]
- 49. Sagawa M, Yoshimatsu K, Yokomizo H, Yano Y, Okayama S, Usui T, et al. Worse preoperative status based on inflammation and host immunity is a risk factor for surgical site infections in colorectal cancer surgery. J Nippon Med Sch 2017;84:224–230. [DOI] [PubMed] [Google Scholar]
- 50. Pothirat C, Chaiwong W, Phetsuk N, Liwsrisakun C, Bumroongkit C, Deesomchok A, et al. The relationship between body composition and clinical parameters in chronic obstructive pulmonary disease. J Med Assoc Thai 2016;99:386–393. [PubMed] [Google Scholar]
- 51. Okuhara Y, Asakura M, Orihara Y, Naito Y, Tsujino T, Ishihara M, et al. Effects of weight loss in outpatients with mild chronic heart failure: findings from the J‐MELODIC study. J Card Fail 2019;25:44–50. [DOI] [PubMed] [Google Scholar]
- 52. Nakayama M, Tabuchi K, Hara A. Clinical utility of the modified Glasgow Prognostic Score in patients with advanced head and neck cancer. Head Neck 2015;37:1745–1749. [DOI] [PubMed] [Google Scholar]
- 53. Nakayama H, Suzuki M, Usuki K, Kato T. Amikacin pharmacokinetics in terminal stage of hematological malignancy. Ther Drug Monit 2019;41:533–537. [DOI] [PubMed] [Google Scholar]
- 54. Nakayama H, Suzuki M, Kato T, Echizen H. Vancomycin pharmacokinetics in patients with advanced cancer near end of life. Eur J Drug Metab Pharmacokinet 2019;44:837–843. [DOI] [PubMed] [Google Scholar]
- 55. Nakamoto R, Okuyama C, Ishizu K, Higashi T, Takahashi M, Kusano K, et al. Diffusely decreased liver uptake on FDG PET and cancer‐associated cachexia with reduced survival. Clin Nucl Med 2019;44:634–642. [DOI] [PubMed] [Google Scholar]
- 56. Naito T, Tashiro M, Yamamoto K, Ohnishi K, Kagawa Y, Kawakami J. Impact of cachexia on pharmacokinetic disposition of and clinical responses to oxycodone in cancer patients. Eur J Clin Pharmacol 2012;68:1411–1418. [DOI] [PubMed] [Google Scholar]
- 57. Naito T, Tashiro M, Ishida T, Ohnishi K, Kawakami J. Cancer cachexia raises the plasma concentration of oxymorphone through the reduction of CYP3A but not CYP2D6 in oxycodone‐treated patients. J Clin Pharmacol 2013;53:812–818. [DOI] [PubMed] [Google Scholar]
- 58. Naito T, Okayama T, Aoyama T, Ohashi T, Masuda Y, Kimura M, et al. Skeletal muscle depletion during chemotherapy has a large impact on physical function in elderly Japanese patients with advanced non‐small‐cell lung cancer. BMC Cancer 2017;17:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Naito T, Okayama T, Aoyama T, Ohashi T, Masuda Y, Kimura M, et al. Unfavorable impact of cancer cachexia on activity of daily living and need for inpatient care in elderly patients with advanced non‐small‐cell lung cancer in Japan: a prospective longitudinal observational study. BMC Cancer 2017;17:800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Naito T, Mitsunaga S, Miura S, Tatematsu N, Inano T, Mouri T, et al. Feasibility of early multimodal interventions for elderly patients with advanced pancreatic and non‐small‐cell lung cancer. J Cachexia Sarcopenia Muscle 2019;10:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mokari‐Yamchi A, Jabbari M, Sharifi A, Barati M, Kheirouri S. Low FEV1 is associated with increased risk of cachexia in COPD patients. Int J Chron Obstruct Pulmon Dis 2019;14:2433–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mohan A, Poulose R, Kulshreshtha I, Chautani AM, Madan K, Hadda V, et al. High prevalence of malnutrition and deranged relationship between energy demands and food intake in advanced non‐small cell lung cancer. Eur J Cancer Care (Engl). 2017;26:e12503. [DOI] [PubMed] [Google Scholar]
- 63. Mitsunaga S, Kasamatsu E, Machii K. Incidence and frequency of cancer cachexia during chemotherapy for advanced pancreatic ductal adenocarcinoma. Support Care Cancer 2020;28:5271–5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Matsuzuka T, Kiyota N, Mizusawa J, Akimoto T, Fujii M, Hasegawa Y, et al. Clinical impact of cachexia in unresectable locally advanced head and neck cancer: supplementary analysis of a phase II trial (JCOG0706‐S2). Jpn J Clin Oncol 2019;49:37–41. [DOI] [PubMed] [Google Scholar]
- 65. Kwon M, Kim RB, Roh JL, Lee SW, Kim SB, Choi SH, et al. Prevalence and clinical significance of cancer cachexia based on time from treatment in advanced‐stage head and neck squamous cell carcinoma. Head Neck 2017;39:716–723. [DOI] [PubMed] [Google Scholar]
- 66. Kubo Y, Naito T, Mori K, Osawa G, Aruga E. Skeletal muscle loss and prognosis of breast cancer patients. Support Care Cancer 2017;25:2221–2227. [DOI] [PubMed] [Google Scholar]
- 67. Kimura M, Naito T, Kenmotsu H, Taira T, Wakuda K, Oyakawa T, et al. Prognostic impact of cancer cachexia in patients with advanced non‐small cell lung cancer. Support Care Cancer 2015;23:1699–1708. [DOI] [PubMed] [Google Scholar]
- 68. Kim HJ, Kim HJ, Yun J, Kim KH, Kim SH, Lee SC, et al. Pathophysiological role of hormones and cytokines in cancer cachexia. J Korean Med Sci 2012;27:128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kim EY, Lee HY, Kim YS, Park I, Ahn HK, Cho EK, et al. Prognostic significance of cachexia score assessed by CT in male patients with small cell lung cancer. Eur J Cancer Care (Engl) 2018;27:e12695. [DOI] [PubMed] [Google Scholar]
- 70. Kim EY, Kim YS, Seo JY, Park I, Ahn HK, Jeong YM, et al. The relationship between sarcopenia and systemic inflammatory response for cancer cachexia in small cell lung cancer. PLoS ONE 2016;11:e0161125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jiang Y, Lin J, Zhang D, Yu Z, Li Q, Jiang J, et al. Bacterial translocation contributes to cachexia and its possible pathway in patients with colon cancer. J Clin Gastroenterol 2014;48:131–137. [DOI] [PubMed] [Google Scholar]
- 72. Jiang Y, Guo C, Zhang D, Zhang J, Wang X, Geng C. The altered tight junctions: an important gateway of bacterial translocation in cachexia patients with advanced gastric cancer. J Interferon Cytokine Res 2014;34:518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ishihara H, Kondo T, Omae K, Takagi T, Iizuka J, Kobayashi H, et al. Sarcopenia and the modified Glasgow Prognostic Score are significant predictors of survival among patients with metastatic renal cell carcinoma who are receiving first‐line sunitinib treatment. Target Oncol 2016;11:605–617. [DOI] [PubMed] [Google Scholar]
- 74. Fukuta A, Saito T, Murata S, Makiura D, Inoue J, Okumura M, et al. Impact of preoperative cachexia on postoperative length of stay in elderly patients with gastrointestinal cancer. Nutrition 2019;58:65–68. [DOI] [PubMed] [Google Scholar]
- 75. Fukushima T, Nakano J, Ishii S, Natsuzako A, Hirase T, Sakamoto J, et al. Characteristics of muscle function and the effect of cachexia in patients with haematological malignancy. Eur J Cancer Care 2019;28:e12956. [DOI] [PubMed] [Google Scholar]
- 76. Fukahori M, Shibata M, Hamauchi S, Kasamatsu E, Machii K. A retrospective cohort study to investigate the incidence of cancer‐related weight loss during chemotherapy in gastric cancer patients. Support Care Cancer 2021;29:341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fujiwara Y, Kobayashi T, Chayahara N, Imamura Y, Toyoda M, Kiyota N, et al. Metabolomics evaluation of serum markers for cachexia and their intra‐day variation in patients with advanced pancreatic cancer. PLoS ONE 2014;9:e113259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fujii H, Makiyama A, Iihara H, Okumura N, Yamamoto S, Imai T, et al. Cancer cachexia reduces the efficacy of nivolumab treatment in patients with advanced gastric cancer. Anticancer Res 2020;40:7067–7075. [DOI] [PubMed] [Google Scholar]
- 79. Chen XY, Zhang XZ, Ma BW, Li B, Zhou DL, Liu ZC, et al. A comparison of four common malnutrition risk screening tools for detecting cachexia in patients with curable gastric cancer. Nutrition 2020;70:110498. [DOI] [PubMed] [Google Scholar]
- 80. Chao CT, Tang CH, Cheng RW, Wang MY, Hung KY. Protein‐energy wasting significantly increases healthcare utilization and costs among patients with chronic kidney disease: a propensity‐score matched cohort study. Curr Med Res Opin 2017;33:1705–1713. [DOI] [PubMed] [Google Scholar]
- 81. Cao DX, Wu GH, Yang ZA, Zhang B, Jiang Y, Han YS, et al. Role of beta1‐adrenoceptor in increased lipolysis in cancer cachexia. Cancer Sci 2010;101:1639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bo S, Dianliang Z, Hongmei Z, Xinxiang W, Yanbing Z, Xiaobo L. Association of interleukin‐8 gene polymorphism with cachexia from patients with gastric cancer. J Interferon Cytokine Res 2010;30:9–14. [DOI] [PubMed] [Google Scholar]
- 83. Amano K, Morita T, Miyamoto J, Uno T, Katayama H, Tatara R. Perception of need for nutritional support in advanced cancer patients with cachexia: a survey in palliative care settings. Support Care Cancer 2018;26:2793–2799. [DOI] [PubMed] [Google Scholar]
- 84. Amano K, Morita T, Koshimoto S, Uno T, Katayama H, Tatara R. Eating‐related distress in advanced cancer patients with cachexia and family members: a survey in palliative and supportive care settings. Support Care Cancer 2019;27:2869–2876. [DOI] [PubMed] [Google Scholar]
- 85. Martin L, Senesse P, Gioulbasanis I, Antoun S, Bozzetti F, Deans C, et al. Diagnostic criteria for the classification of cancer‐associated weight loss. J Clin Oncol 2015;33:90–99. [DOI] [PubMed] [Google Scholar]
- 86. Ishida Y, Maeda K, Nonogaki T, Shimizu A, Ueshima J, Nagano A, et al. Body mass index and weight loss predict mortality in older patients with chronic kidney disease. Geriatr Gerontol Int 2022;22:984–985. [DOI] [PubMed] [Google Scholar]
- 87. Konishi M, Kaneko H, Itoh H, Matsuoka S, Okada A, Kamiya K, et al. Association of weight change and in‐hospital mortality in patients with repeated hospitalization for heart failure. J Cachexia Sarcopenia Muscle 2023;14:642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hanisah R, Suzana S, Lee FS. Validation of screening tools to assess appetite among geriatric patients. J Nutr Health Aging 2012;16:660–665. [DOI] [PubMed] [Google Scholar]
- 89. Quyen TC, Angkatavanich J, Thuan TV, Xuan VV, Tuyen LD, Tu DA. Nutrition assessment and its relationship with performance and Glasgow prognostic scores in Vietnamese patients with esophageal cancer. Asia Pac J Clin Nutr 2017;26:49–58. [DOI] [PubMed] [Google Scholar]
- 90. Li X, Zhang Y, Zhao W, Liu Z, Shen Y, Li J, et al. The Glasgow Prognostic Score as a significant predictor of diffuse large B cell lymphoma treated with R‐CHOP in China. Ann Hematol 2015;94:57–63. [DOI] [PubMed] [Google Scholar]
- 91. Cao J, Xu H, Li W, Guo Z, Lin Y, Shi Y, et al. Nutritional assessment and risk factors associated to malnutrition in patients with esophageal cancer. Curr Probl Cancer 2021;45:100638. [DOI] [PubMed] [Google Scholar]
- 92. Sun P, Zhang F, Chen C, An X, Li YH, Wang FH, et al. Comparison of the prognostic values of various nutritional parameters in patients with esophageal squamous cell carcinoma from Southern China. J Thorac Dis 2013;5:484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Basch E. The missing voice of patients in drug‐safety reporting. N Engl J Med 2010;362:865–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. von Haehling S, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]


